Abstract
Rival conspecifics often produce stereotyped sequences of signals as agonistic interactions escalate. Successive signals in sequence are thought to convey increasingly pronounced levels of aggressive motivation. Here, we propose and test a model of aggressive escalation in black-throated blue warblers, presenting subjects with two sequential and increasingly elevated levels of threat. From a speaker outside the territorial boundary, we initiated an interaction (low-threat level), and from a second speaker inside the territory, accompanied by a taxidermic mount, we subsequently simulated a territorial intrusion (escalated threat level). Our two main predictions were that signalling behaviours in response to low-threat boundary playback would predict signalling responses to the escalated within-territory threat, and that these latter signalling behaviours would in turn reliably predict attack. We find clear support for both predictions: (i) specific song types (type II songs) produced early in the simulated interaction, in response to boundary playback, predicted later use of low-amplitude ‘soft’ song, in response to within-territory playback; and (ii) soft song, in turn, predicted attack of the mount. Unexpectedly, use of the early-stage signal (type II song) itself did not predict attack, despite its apparent role in aggressive escalation. This raises the intriguing question of whether type II song can actually be considered a reliable aggressive signal. Overall, our results provide new empirical insights into how songbirds may use progressive vocal signalling to convey increasing levels of threat.
Keywords: aggressive signalling, birdsong, black-throated blue warbler, escalation, Setophaga caerulescens, soft song
1. Introduction
Communication signals can help animals settle conflicts quickly and efficiently, by making available information about potential rivals' fighting ability and/or motivational state [1,2]. Many animal species produce specific sequences of signalling behaviours as agonistic interactions escalate [3–7]. In the classic example of red deer, to illustrate, males initiate agonistic signalling interactions with roaring. If a challenger subsequently approaches after roaring, then opponents often proceed to a ‘parallel walk’. If neither male retreats during the parallel walk, then the interaction may escalate into combat [3]. A possible functional explanation for signalling sequences in agonistic interactions is that successive signals convey increasing levels of aggressive motivation, with intermediate stages allowing animals multiple opportunities to escalate or de-escalate, and to convey more precisely their aggressive state and willingness to risk combat [5,8–10].
Aggressive signals were once considered susceptible to widespread bluffing [11–14], but are now more typically presumed to be reliable [15–17]. For aggressive signals to be reliable, they should predict subsequent aggressive behaviour such as attacks by signal senders [10]. A traditional method for assessing aggressive signal function is through playback studies, in which signals are presented and receiver responses documented. A presumption in many such studies is that signals with greater aggressive content will elicit stronger subject responses. Such studies, however, are limited because they focus on the behaviour of signal receivers and not signal senders [18,19]. Only recently has research on aggressive signalling begun to focus on the sender's perspective, asking how signals might predict an animal's own future aggression [20–23]. To answer this question, researchers are applying a new experimental approach, as follows: subjects are provoked through playback of a rival's signal, the subject's signalling behaviour is documented and the subject is subsequently given a chance to attack a conspecific model. Attack is considered an unambiguous assay of aggression, and prior signalling behaviours that predicted attack are thus regarded as reliable indicators of aggressive motivation [8,21]. Using this approach, several recent studies have identified specific signalling patterns that predict attack reliably [21,23–27].
While attack is a direct measure of aggression, it is probably predicted most strongly by the most recent signal(s) in stereotyped signalling progressions, when compared with signals produced earlier in progression [28]. Multiple stages of signalling before actual attack might allow contestants to escalate or de-escalate, thus diminishing the value of initial signals as ultimate predictors of attack. However, signals could still indicate animals' motivation if they predict escalation to the next highest signalling stage [10]. One notable limitation of traditional playback studies is that experimental trials are typically initiated at extreme levels of threat, i.e. as staged intrusions within subjects' territories. Such trials probably bypass earlier stages of signalling that may occur in the natural escalation process [28]. Therefore, to assess properly the predictive content of signals produced earlier in a progressive sequence, it would be useful to simulate more natural interactions in which playback stimuli begin at low levels of threat and subsequently escalate to higher levels of threat. Towards this end, we implement a sequential two-speaker playback design that aimed to elevate perceived levels of threat more gradually, and which might thus better reveal patterns of progressive signal escalation. This is an approach originally suggested by Beecher et al. [29] and also implemented in a recent study on song sparrows, Melospiza melodia, by Akçay et al. [27], a study closely parallel to ours (see discussion) of which we became aware as our study was in review.
Our study focused on the black-throated blue warbler, Setophaga caerulescens, another songbird species in which reliable signalling of aggressive motivation has been investigated [25]. We ask two main questions: (i) do signalling patterns in early stages of an interaction predict signalling patterns in later stages of an interaction? and (ii) do these later-stage signalling patterns provide reliable predictors of ultimate attack? Available evidence suggests that black-throated blue warblers can use two vocal signal attributes to convey information about aggressive motivation. First, they can sing songs at varying amplitudes, and a prior experimental study showed that low-amplitude ‘soft’ song in this species is an extremely reliable predictor of attack [25], a result parallel to those emerging in studies of other birds [21,24,30] as well as anurans [31,32]. Second, black-throated blue warblers might convey information about aggressive motivation by modulating their use of distinct song types, as suggested for other avian species [33–37]. More specifically, within the wood-warblers (Parulidae), many species sing two discrete song type categories, produced in distinct contexts (reviewed in [38]). Songs in one category (often referred to as type I) are typically sung from the centre of a territory and near females, whereas songs in the second category (type II) are typically sung near the territory edge and while interacting aggressively with other males. Consistent with this putative role for type II song, Byers [39] found that in chestnut-sided warblers, type II songs were associated with approaches to other males and/or engaging them in fights. Our own work on black-throated blue warblers suggests that males similarly vary the usage of type I and type II songs to convey information about varying levels of aggression. More specifically, (i) type I and type II song types are acoustically distinct; (ii) type I to type II switches generally occur as males engage in close range interactions including fights; (iii) type I to type II switches generally precede the production of soft songs; and (iv) type II to type I switches generally occur as males retreat from aggressive interactions (D. Hof 2010, unpublished data). Based on these observations and the results from Hof & Hazlett [25], we here propose and test a model, adapted from that of Searcy & Beecher [10], for how male black-throated blue warblers might convey, during signalling sequences, precise and increasingly pronounced levels of aggressive motivation (figure 1).
Figure 1.
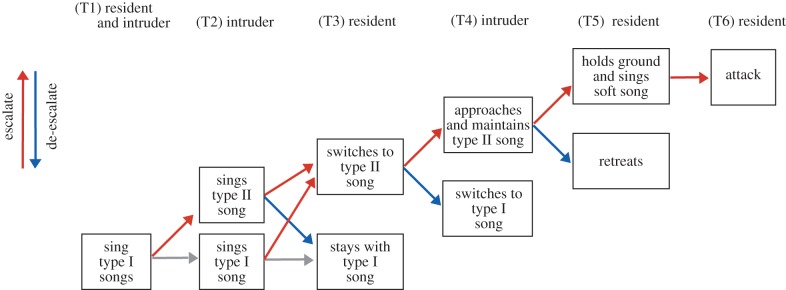
Schematic diagram showing the proposed model of aggressive escalation between two male black-throated blue warblers. In this model, type I, type II and soft songs form a progressive sequence of increasingly aggressive signals. Males usually sing type I songs from inside their territories (T1). A challenger may approach the territorial boundary and switch to type II songs (T2). The resident can continue to sing type I songs or escalate the interaction by switching to type II songs (T3). The challenger might not be deterred, and breach the territorial boundary while continuing to sing type II songs (T4). At this point, the resident may retreat or hold his ground and sing soft songs (T5). Soft song, in turn, signals a high likelihood of subsequent attack [25]. Model structure based on Searcy & Beecher [10]. (Online version in colour.)
Our test of the proposed model uses a sequential two-speaker design. The first speaker initiates an interaction with song playback from outside the territory boundary, and the second speaker, coupled with presentation of a male taxidermic mount, subsequently simulates a territorial intrusion. We make two primary predictions, following figure 1: (i) males that sing type II songs, in response to playback from beyond their territory boundary, should be more likely to sing soft songs in response to within-territory playback (figure 1, T3–T5); and (ii) males that sing soft songs during within-territory playback should be more likely to attack the mount (T5–T6), as in Hof & Hazlett [25]. Our dataset also allows us to explore the relationship between an early-stage threat signal (type II song) and the probability of attack. Reliability theory suggests that type II songs should predict attack, although we may not expect these early-stage signals to be as strongly predictive of attack as later-stage signals, i.e. soft song [10,28].
2. Methods
(a). Study site and subjects
Experimental trials were conducted from 9 June to 15 July 2011 at a study plot in Green Mountain National Forest, Ripton, VT, Addison County, USA. A 650 × 450 m grid, marked out with flagging every 25 m, was found to be occupied by 27 breeding pairs of black-throated blue warblers. Twenty-one of these males served as subjects in playback trials, 17 of which had been previously captured and fitted with unique combinations of colour bands to allow individual identification. The additional four were identified readily by their songs and because all of their territorial neighbours were banded. Experimental trials (see below) were conducted during the subjects' incubation or nestling breeding stages, or occasionally soon after nests fledged.
We began mapping territories on 8 May 2011, once most males had arrived on site. Each male was visited once every 1–3 days by one of six observers. Observers followed male movements, and notated on a blank map of the study grid the locations of singing and aggressive interactions with other males. A cumulative map of defended space for all males was maintained, and extensions of observed singing locations were updated daily. This map provided a guide to approximate territory boundary locations. To identify boundary locations with greater precision pre-trial, each focal male and one of its neighbours were observed simultaneously by two observers for 2–4 h, 1–2 days prior to their trial. These observers placed flagging (differing in colour for the two males) at the extreme singing locations of each male along their shared boundary, and at locations where the two males interacted. This allowed us to identify specific locations of territory boundaries.
(b). Playback stimuli
Playback songs were selected from recordings of males obtained either at the same field site in prior years (n = 18 birds) or at another field site in New Hampshire (n = 5 birds). We selected a total of 30 playback songs, 15 type I and 15 type II. Using Signal v. 4.0 [40], these recordings were high-pass frequency filtered (above 1 kHz) to reduce background noise, standardized to a common peak amplitude and then placed in stimulus sequences. Two stimulus sequences were generated for each trial, one ‘boundary’ sequence and one ‘within-territory’ sequence. The boundary sequence began with a type I song repeated once every 10 s for 2 min, followed by a type II song repeated once every 10 s for 2 min. The within-territory sequence began with 250 s of silence corresponding to the boundary playback, followed by the selected type II song from the ‘boundary sequence’ repeated once every 10 s for 2 min, followed by 2 min of silence, an additional 2 min of the same type II song, an additional 2 min of silence and finally four sputter vocalizations repeated once every 5 s. Type I and type II songs were paired randomly without replacement, and no type I and II song combinations were recorded from the same male or used in more than one experimental trial.
(c). Playback protocol
The playback protocol was identical to Hof & Hazlett [25], but with the addition of a preceding playback sequence, presented from outside the focal bird's territory boundary, i.e. the ‘boundary’ speaker. During playback trials, the boundary speaker was placed approximately 10 m outside of the focal male's territory, thus generally in space defended by a neighbouring male. Occasionally, this speaker was placed in undefended space between neighbouring territories. To ensure that this speaker was placed outside of the focal bird's territory, we always erred on placing it further outside the territory boundary. This speaker was set up to broadcast stimuli directionally towards the focal bird's territory [41,42]. To do this, the speaker was placed inside an opened plastic box packed with sound dampening foam on the back and sides, and mounted on two tripods approximately 1 m above the ground. A ‘within-territory’ speaker was placed approximately 20 m into the focal male's territory. This speaker was mounted on a tripod 1 m above the ground, and a taxidermic mount attached to a standardized perch was positioned approximately 1 m above the speaker. The mount was initially covered with a cloth attached to a long string.
Stimulus sequences were selected randomly for each focal male with the stipulation that stimulus songs were not recorded within four territories of a given focal male in the present or previous two breeding seasons. Therefore, stimulus songs were probably unfamiliar to subjects, and did not represent territorial neighbours. Both playback speakers were set to broadcast stimuli at peak amplitudes of 88 dB SPL at 1 m, an estimated normal singing amplitude for this species. Trials were initiated only when two conditions were met: (i) the focal male was singing type I songs near the centre of his territory and within 25 m of the within-territory speaker, and (ii) the neighbour sharing the boundary in which the boundary speaker was positioned was in the centre or on the far side of his territory. Trials in which neighbouring males approached either playback speaker, or interacted with the focal male, were aborted and excluded from further analysis. This occurred during eight trials, and these focal males were tested again on a different day. Trials were not attempted for the same focal male more than twice.
We describe the experimental design and expectations with reference to figure 1. Trials were initiated by commencing both playback sequences simultaneously. Focal birds first heard playback of a 2 min period of type I song broadcast from the boundary speaker, corresponding to normal baseline singing behaviour where neighbours sing type I songs from inside their territories (T1 in figure 1). The stimulus then switched to a 2 min period of type II songs from the boundary speaker (T2 in figure 1). Possible vocal responses of subjects during this 2 min period, stay on type I or switch to type II, correspond to T3 in figure 1. After this 2 min period plus 10 s of silence, the within-territory speaker began to broadcast the same type II song for 2 min followed by 2 min of silence. The 2 min of playback corresponds to T4 in figure 1, and subjects' responses during this playback plus silent period correspond to T5 (figure 1). This switch of playback location simulated what the focal bird would have heard had an aggressive challenging male flown into the resident's territory undeterred. At this point in the trial, the taxidermic mount was revealed by removing the cloth via a string, providing subjects the opportunity to attack. Presentation of the mount was accompanied by an additional 2 min period of type II song playback followed by 2 min of silence and then finally by the sputter vocalizations, as in Hof & Hazlett [25]. Subjects were then given an additional 8 min opportunity to attack the mount. Also as in Hof & Hazlett [25], attacks were defined as when a male made direct contact with the mount, or made a direct flight or dive to within 1 m of the mount (see also [24]).
One observer was positioned approximately 15 m from the mount. This observer recorded focal bird vocal behaviour with a Marantz PMD 660 stereo digital recorder connected to two Sennheiser ME62 omnidirectional microphones. One of these microphones was mounted in a Telinga parabolic reflector held by the observer. The second microphone was placed below the mount to help document the occurrence of soft songs, which are typically sung near an intruder. A second observer was initially positioned approximately 15 m from the boundary speaker and 25 m from the within-territory speaker, and recorded vocal responses with a Sennheiser ME66 directional microphone connected to a Marantz PMD 660 digital recorder. When the playback stimuli switched from the boundary to the within-territory speaker, this observer moved quietly to a position approximately 15 m from the within-territory speaker on a different side from the other observer. Both observers recorded vocal responses for the entire trial duration (up to approx. 20 min), and each observer quietly dictated behavioural observations into their microphones noting all vocalizations and movements detected from focal subjects, whether songs sung were soft or broadcast, and whether an attack ultimately occurred. Both observers were well experienced at discriminating soft and broadcast songs, and songs that were perceived to be intermediate in amplitude were classified as broadcast songs. For other details of the playback protocol, see Hof & Hazlett [25].
(d). Analysis
Trial recordings from both observers were perused simultaneously as real-time spectrograms in Audacity 1.3.3-beta (http://audacity.sourceforge.net/), and subjects' behaviour transcribed onto flow sheets. We scored each song sung by focal males as type I or type II, and as soft or broadcast. Type I and type II songs are acoustically discrete in this species and are distinguished readily during field trials (D. Hof 2010, unpublished data). These classifications were confirmed while viewing spectrograms. Additionally, we had recorded vocal repertoires of focal males intensively prior to experimental trials, and had prior knowledge about the type I and II song types sung by subjects (D. Hof 2011, unpublished data). Note that both type I and type II songs can be sung as either soft or broadcast.
Trials were divided into four analysis periods: (i) the 2 min period after the boundary speaker began to broadcast type II songs (T3 in figure 1); (ii) the initial 4 min after the stimulus switched to the within-territory speaker (T4 and T5 in figure 1); (iii) the first 4 min after the mount was revealed; and (iv) 1 min before an attack, when one occurred. To generate a comparable time period with the 1 min before attack in non-attackers, we followed the method of Searcy et al. [21] and Ballentine et al. [24]: we first determined the attack times for trials in which attacks did occur, randomly assigned these attack times to each non-attacker, and used the preceding minute for analysis. For each analysis period, we tallied the total numbers of type I and II songs, the total numbers of soft and broadcast songs, and the numbers of soft songs of each song type category.
To test our first prediction, that males responding to the boundary speaker with type II songs should subsequently sing more soft songs in response to the within-territory speaker, we performed a non-parametric rank-based regression with the number of type II songs during analysis period 1 as the predictor variable, and the number of soft songs during analysis period 2 as the response variable. As an additional test of this prediction, we ran a chi-squared test asking whether males that switched from type I to type II song during boundary playback were more likely to sing soft song during within-territory playback. To test our second prediction, that soft song will reliably predict attack, we performed a forward stepwise multiple logistic regression for each analysis period, with vocal response variables as predictors and attack/no attack as the response variable. This latter analysis also allowed us to assess the statistical relationship between type II song during the boundary playback and the likelihood of attack. Analyses were performed using JMP 5.0 software (SAS Institute, Cary, NC, USA).
3. Results
(a). Does type II song predict escalation to soft song?
As predicted, the number of type II songs birds sang, in response to the type II portion of the boundary playback, significantly predicted the number of soft songs they sang later, during the initial 4 min period of within-territory playback (Spearman's ρ = 0.5158, p = 0.017; figure 2a). Additionally, males that switched from type I to type II song in response to the boundary playback were also more likely to sing soft song during the initial within-territory playback, when compared with males that did not switch to type II song (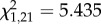 , p = 0.02; electronic supplementary material, table S2).
, p = 0.02; electronic supplementary material, table S2).
Figure 2.
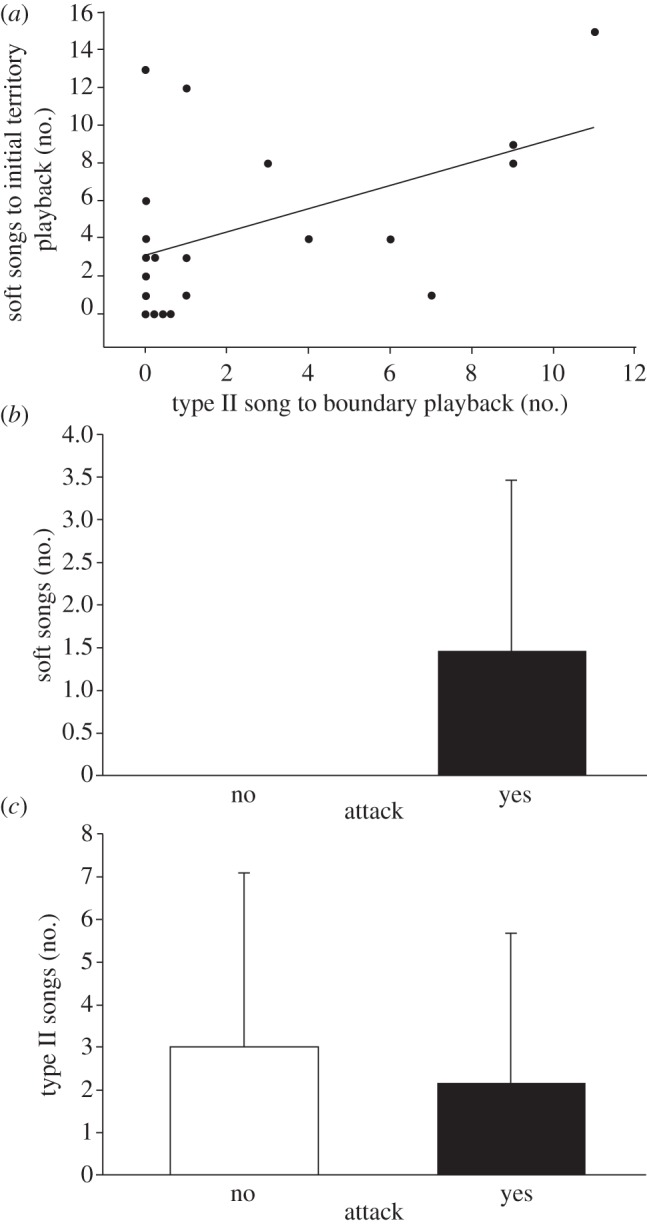
Vocal responses of male black-throated blue warblers showing (a) the number of soft songs sung during the initial 4 min of the within-territory portion of trials as a function of the number of type II songs sung during the type II portion of boundary playback (Spearman's ρ = 0.52, p = 0.017); note that soft song was also predicted by whether birds switched from type I to type II song, see text. (b) The number of soft songs (means±s.d.) 1 min before attack by males that attacked (n = 13) and did not attack (n = 8) the mount (Wilcoxon two-sample test: 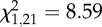 , p = 0.0034), and (c) the number of type II songs (means ± s.d.) during the type II portion of boundary playback by males that attacked (n = 13) and did not attack (n = 8) the mount (Wilcoxon two-sample test:
, p = 0.0034), and (c) the number of type II songs (means ± s.d.) during the type II portion of boundary playback by males that attacked (n = 13) and did not attack (n = 8) the mount (Wilcoxon two-sample test: 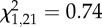 , p = 0.389). Statistical conclusions were based on tests described in the text.
, p = 0.389). Statistical conclusions were based on tests described in the text.
(b). Predictors of attack
Our second main prediction in this study was that soft song would predict attack, as in Hof & Hazlett [25]. The statistical approach we applied here, multiple logistic regression, also allowed us to assess the potential relationship between the putative early-stage escalation signal, type II song, and the probability of attack. As we indicated in the introduction, reliability theory would suggest that early-stage signals should predict attack, although the strength of this relationship has not been assessed in prior studies of aggressive escalation (cf. [27]).
During playback trials, 13 males attacked the mount and eight males did not, and the average latency to attack for birds that did attack was 3 m 13 s after the mount was revealed. No vocal display behaviours given in response to the boundary speaker entered the multiple logistic regression model (α-level < 0.25) as predictors of eventual attack.
During the initial 4 min of playback inside territories, just before the mount was revealed, males that later attacked the mount sang more soft songs yet fewer type II songs, when compared with males that did not attack. Both the numbers of soft songs and type II songs (negatively) entered the logistic regression model as predictors of attack, yet neither were significant predictors of eventual attack (logistic regression; soft songs; 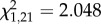 , p = 0.152; type II songs;
, p = 0.152; type II songs; 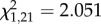 , p = 0.152).
, p = 0.152).
During the first 4 min after the mount was revealed, attackers sang more soft songs and fewer type II songs than birds that did not attack. Both the number of soft songs and the number of type II songs entered the logistic regression model as predictors of attack. Of these two singing behaviours, only soft song provided a significant predictor of attack (logistic regression; 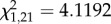 , p = 0.042).
, p = 0.042).
As in the previous two analysis periods, attackers during the 1 min before attack sang more soft songs than did non-attackers (figure 2b). Soft song was the only vocal display variable to differ between attackers and non-attackers (figure 2b). Non-attackers did not produce a single soft song during this period, so logistic regression could not be used for this analysis owing to a lack of variance in non-attackers. Therefore, we used a chi-squared test, with attack as the response variable, to test whether presence or absence of soft song was a significant predictor variable. This analysis revealed that males which sang at least one soft song were significantly more likely to attack than males that did not sing any soft songs (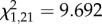 , p = 0.002).
, p = 0.002).
To summarize, the results for soft song are all highly consistent with those reported in Hof & Hazlett [25], and support prediction 2, that soft song is a strong predictor of attack. By contrast, use of type II song during all analysis periods did not provide a significant predictor of attack, contrary to the expectations of reliability theory (figure 2c).
(c). Type I versus type II soft song as predictors of attack
Our data also offered an opportunity to reconstruct post hoc whether type I and type II soft songs differed in their reliability as predictors of attack. Results from this analysis are presented in the electronic supplementary material, figure S1.
4. Discussion
A primary aim of this study was to test the hypothesis that agonistic signalling interactions escalate along a progressive sequence of signals leading up to attack. We derived two main predictions from this hypothesis: that signalling patterns produced early in interactions should contain reliable information about signallers' motivation to escalate to later stages of signalling; and that signalling patterns produced later in interactions should reliably predict attack. Our results support both predictions. First, we find that early-stage type II song, both in terms of the number of songs produced and the tendency to switch from type I song to type II song, predicted the later use of another vocal signalling pattern, soft song (prediction 1; figure 2a and the electronic supplementary material, table S2). Second, soft song in response to simulated intrusions, in turn, predicted attack of a model opponent (prediction 2; figure 2b; see also [25]).
The idea that animals ‘escalate’ aggressive interactions pervades animal behaviour research, and implies that animals use interactive sequences of signalling behaviours to negotiate potential conflicts. Many prior studies have assessed escalation by observing naturally occurring agonistic interactions and calculating transition probabilities of one signalling behaviour to the next, and have shown that particular signalling sequences are more likely than others [3,4,6,7,43]. Use of stereotyped signal sequences is typically interpreted as providing a means for opponents to assess each other's fighting ability, with each subsequent signal in sequence providing information that is increasingly accurate [7,44–46]. For example, roaring and parallel walking in red deer are thought to help opponents to assess each other's stamina, strength and/or size, important determinants of contest outcome ([47], see also [48]). One line of evidence that supports this interpretation is that equally matched opponents are often more likely to advance to higher stages of signalling than opponents that are less symmetrically matched [47–52].
While most research on escalation and the role of signals therein has focused on fighting ability, some researchers have posited that signalling behaviours in sequence can also convey information about levels of aggressive motivation [5,8–10]. It is important to note that fighting ability and aggressive motivation might not correlate with each other [10]; aggressive motivation, in particular, can be highly context-dependent and variable over time [53–55]. Moreover, information about fighting ability and aggressive motivational state might be conveyed independently by different signal components that are, in turn, mediated by different types of costs [10,13].
Understanding animals' aggressive motivational state has been enhanced in recent years through experimental studies that have used attack of a model as an assay of aggression [21,23–26]. One limitation of these studies was that subjects were only presented with high threat stimuli, which thus did not give subjects the chance to engage in gradual aggressive escalation. Towards this end, in a study on song sparrows, Akçay et al. [27] applied a two-speaker sequential playback design in which songs were first played outside the territorial boundary and then played inside the territory. This provided subjects an opportunity to escalate their signals of aggressive motivation. The authors report that one signal attribute, type matching, reliably predicts two subsequent signal attributes, soft song and wing waves, and that all three attributes predict attack. We applied the same basic two-speaker design, and our main results parallel those of Akçay et al. [27]: an early-stage signalling pattern (type II song) predicts a late-stage signalling pattern (soft song) which predicts attack.
Our study design also allowed us to ask whether early-stage signals of aggressive escalation (type II song) would predict eventual attack. Reliability theory suggests that early-stage signals, or any aggressive signals for that matter, should indeed predict attack, because otherwise they would bear no consequence to signal receivers, and receivers would then be selected to ignore them [15,17]. Consistent with this expectation, Akçay et al. [27] report that in song sparrows an early-stage signal of aggression (song type matching) indeed predicted attack. Yet, in our study, we failed to uncover any relationship between the early-stage signal (type II song) and ultimate attack (figure 2c). How do we reconcile this finding with the expectations of reliability theory?
A first set of possible explanations is statistical. Perhaps type II songs in black-throated blue warblers do indeed predict attack, but our sample size was insufficient to detect this relationship. More specifically, while we were able to detect expected relationships between escalation steps in immediate succession, one might require greater statistical power to detect relationships between non-successive escalation steps. Particularly telling in this regard were individuals that followed only partially the expected steps of escalation. For instance, some study subjects produced limited type II song responses to boundary playback yet later responded very aggressively to within-territory playback by singing soft songs and attacking the mount. The two most extreme data points herein (figure 2a) were birds that sang many (12–13) soft songs during the initial period of within-territory playback, and eventually attacked the mount, yet that had produced either zero or one type II songs in response to the earlier boundary playback. While this limited vocal response might imply that these two birds were initially unmotivated as responders, on the contrary supplemental observations revealed that they responded to the boundary playback with soft songs and aggressive flights. We thus regard these individuals as having escalated to a high level of aggression unusually quickly, without following the usual progression. This kind of immediate escalation would obscure the expected relationship between early-stage signals and attack.
Some features in our experimental design may also have made it difficult to detect the expected relationship. Prominent among these was the extended temporal window that separated the initial presentation of playback stimuli from the presentation of the taxidermic mount. More specifically, in every trial, the mount was revealed 8 min 10 s after the initiation of playback. This introduced ample opportunity for external contingencies to arise between the start of a trial and the opportunity to attack, factors that would weaken the expected association between early signals and attack probabilities. Consistent with this possibility, our own data show several trends. First, we find that half of birds (five of 10) that produced type II songs in response to boundary playback did not ultimately attack the mount. Second, during within-territory playback, we find that late soft song predicted attack, whereas early soft song did not. In both scenarios, external contingencies not immediately apparent to us may have interfered with full escalation to attack, a possibility that was enhanced by the prolonged time window of our trials.
One more possibility is that our data do indeed reflect a true lack of direct relationship between early-stage type II song and probabilities of attack. In other words, perhaps birds that initially signalled aggressive motivation later de-escalated as trials progressed. Would we therefore conclude that type II songs are unreliable as signals of aggressive motivation? Answering this question may depend on one's willingness to consider the reliability of signals that predict attack only indirectly. If an early-stage signal predicts a late-stage signal, and if that late-stage signal predicts attack, then the reliability of the early-stage signal might be maintained if animals learn the association and respond accordingly. This would be consistent with a definition of aggressive signals as signals that predict attack or escalation towards attack, i.e. signals that predict ‘any step higher up the chain of escalation, whether or not it reliably predicts actual attack’ [10, p. 1282]. According to this definition, type II song in black-throated blue warblers would be considered a reliable aggressive signal not because it predicts eventual attack, but because it predicts future escalation to soft song.
In conclusion, sequential playback studies that simulate aggressive escalation, such as that employed here and in Akçay et al. [27], have the potential to enable more refined and nuanced inferences about signal use and function during aggressive interactions [28]. In some of the most well-studied systems, signals long presumed to be aggressive, such as frequency matching and song overlapping in black-capped chickadees [56,57], are proving to be poor predictors of actual attack ([26], see also [58]). Yet some types of singing behaviours, such as type II songs in black-throated blue warblers and song type matching in song sparrows, may be important in mediating early stages of aggressive interactions, indicating the likelihood of further escalation. Investigating the signal value of these behaviours will ultimately require expanding from the traditional ‘in territory’ playback design to also using playback from outside the focal territory, simulating more closely how these behaviours are used in natural contexts.
Acknowledgements
We are especially grateful to Heather Ewing for field assistance and for serving as the second observer during playback trials, and to Travis Collingwood, Nicole Hazlett, Sara Weaver and Meghan Strange for additional field assistance. Bruce Byers, Ethan Clotfelter, Sheila Patek, Dana Moseley, Sarah Goodwin and Jesse McClure provided helpful discussion on the experimental design, statistical analysis and/or comments on an earlier version of the manuscript. We also thank Ted Gerow for providing housing near the study site, Mike Sease and GMNF for permission to use the study site, Mike Webster, Nick Rodenhouse and Scott Sillett for providing the taxidermic mount, and Jasper for serving as the taxidermic mount and for withstanding many attacks.
This research was conducted in accordance with the ASAP/ABS Guidelines for the Use of Animals in Research and was approved by the University of Massachusetts Institutional Animal Care and Use Committee (protocol 2009-12).
Funding statement
This research was supported by a National Science Foundation Doctoral Dissertation Improvement Grant to D.H. and an NSF grant no. IOS-1028964 to J.P.
References
- 1.Logue DM, Abiola IO, Rains D, Bailey NW, Zuk M, Cade WH. 2010. Does signaling mitigate the costs of agonistic interactions? A test in a cricket that has lost its song. Proc. R. Soc. B 277, 2171–2175 (doi:10.1098/rspb.2010.0421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santer RD, Hebets EA. 2011. Evidence for air movement signals in the agonistic behaviour of a nocturnal arachnid (order: Amblypygi). PLoS ONE 6, e22473 (doi:10.1371/journal.pone.0022473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clutton-Brock TH, Albon SD. 1979. The roaring of red deer and the evolution of honest advertisement. Behaviour 69, 145–170 (doi:10.1163/156853979X00449) [Google Scholar]
- 4.Popp JW. 1987. Risk and effectiveness in the use of agonistic displays by American goldfinches. Behaviour 103, 141–156 (doi:10.1163/156853987X00314) [Google Scholar]
- 5.Waas JR. 1991. The risks and benefits of signaling aggressive motivation: a study of cave-dwelling little blue penguins. Behav. Ecol. Sociobiol. 29, 139–146 (doi:10.1007/BF00166489) [Google Scholar]
- 6.Fowler-Finn KD, Hebets EA. 2006. An examination of agonistic interactions in the whip spider Phrynus marginemaculatus. J. Arachnol. 34, 62–76 (doi:10.1636/S04-104.1) [Google Scholar]
- 7.Egge AR, Brandt Y, Swallow JG. 2011. Sequential analysis of aggressive interactions in the stalk-eyed fly Teleopsis dalmanni. Behav. Ecol. Sociobiol. 65, 369–379 (doi:10.1007/s00265-010-1054-5) [Google Scholar]
- 8.Waas JR. 1991. Do little blue penguins signal their intentions during aggressive interactions with strangers? Anim. Behav. 41, 375–382 (doi:10.1016/S0003-3472(05)80838-3) [Google Scholar]
- 9.Beecher MD, Campbell SE. 2005. The role of unshared songs in singing interactions between neighboring song sparrows. Anim. Behav. 70, 1297–1304 (doi:10.1016/j.anbehav.2005.03.008) [Google Scholar]
- 10.Searcy WA, Beecher MD. 2009. Song as an aggressive signal in songbirds. Anim. Behav. 78, 1281–1292 (doi:10.1016/j.anbehav.2009.08.011) [Google Scholar]
- 11.Maynard Smith J, Price GR. 1973. The logic of animal conflict. Nature 246, 15–18 (doi:10.1038/246015a0) [Google Scholar]
- 12.Maynard Smith J. 1974. The theory of games and the evolution of animal conflicts. J. Theor. Biol. 47, 209–221 (doi:10.1016/0022-5193(74)90110-6) [DOI] [PubMed] [Google Scholar]
- 13.Maynard Smith J. 1982. Do animals convey information about their intentions? J. Theor. Biol. 97, 1–5 (doi:10.1016/0022-5193(82)90271-5) [Google Scholar]
- 14.Dawkins R, Krebs JR. 1978. Animal signals: information or manipulation? In Behavioral ecology (eds Krebs JR, Davies NB.), pp. 282–309 Oxford, UK: Blackwell Scientific Publications [Google Scholar]
- 15.Grafen A. 1990. Biological signals as handicaps. J. Theor. Biol. 144, 517–546 (doi:10.1016/S0022-5193(05)80088-8) [DOI] [PubMed] [Google Scholar]
- 16.Johnstone RA, Grafen A. 1993. Dishonesty and the handicap principle. Anim. Behav. 46, 759–764 (doi:10.1006/anbe.1993.1253) [Google Scholar]
- 17.Searcy WA, Nowicki S. 2005. The evolution of animal communication: reliability and deception in signaling systems. Princeton, NJ: Princeton University Press [Google Scholar]
- 18.Searcy WA, Nowicki S. 2000. Male-male competition and female choice in the evolution of signaling systems. In Animal signals: signaling and signal design in animal communication (eds Espmark Y, Amundsen T, Rosenqvist G.), pp. 301–315 Trondheim, Norway: Tapir Academic Press [Google Scholar]
- 19.Collins S. 2004. Vocal fighting and flirting: the functions of birdsong. In Nature's music: the science of birdsong (eds Marler P, Slabbekoorn H.), pp. 33–79 San Diego, CA: Academic Press [Google Scholar]
- 20.Vehrencamp SL. 2001. Is song-type matching a conventional signal of aggressive intentions? Proc. R. Soc. Lond. B 268, 1637–1642 (doi:10.1098/rspb.2001.1714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Searcy WA, Anderson RC, Nowicki S. 2006. Bird song as a signal of aggressive intent. Behav. Ecol. Sociobiol. 60, 234–241 (doi:10.1007/s00265-006-0161-9) [Google Scholar]
- 22.Vehrencamp SL, Hall ML, Bohman RB, Depeine CD, Dalziell AH. 2007. Song matching, overlapping, and switching in the banded wren: the sender's perspective. Behav. Ecol. 18, 849–859 (doi:10.1093/beheco/arm054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laidre ME. 2009. How often do animals lie about their intentions? An experimental test. Am. Nat. 173, 337–346 (doi:10.1086/596530) [DOI] [PubMed] [Google Scholar]
- 24.Ballentine B, Searcy WA, Nowicki S. 2008. Reliable aggressive signaling in swamp sparrows. Anim. Behav. 75, 693–703 (doi:10.1016/j.anbehav.2007.07.025) [Google Scholar]
- 25.Hof D, Hazlett N. 2010. Low-amplitude song predicts attack in a North American wood warbler. Anim. Behav. 80, 821–828 (doi:10.1016/j.anbehav.2010.07.017) [Google Scholar]
- 26.Baker TM, Wilson DR, Mennill DJ. 2012. Vocal signals predict attack during aggressive interactions in black-capped chickadees. Anim. Behav. 84, 965–974 (doi:10.1016/j.anbehav.2012.07.022) [Google Scholar]
- 27.Akçay Ç, Tom ME, Campbell SE, Beecher MD. 2013. Song type matching is an honest early threat signal in a hierarchical animal communication system. Proc. R. Soc. B 280, 20122517 (doi:10.1098/rspb.2012.2517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laidre ME, Vehrencamp SL. 2008. Is bird song a reliable signal of aggressive intent? Behav. Ecol. Sociobiol. 62, 1207–1211 (doi:10.1007/s00265-007-0539-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beecher MD, Campbell SE, Burt JM, Hill CE, Nordby JC. 2000. Song-type matching between neighboring song sparrows. Anim. Behav. 59, 21–27 (doi:10.1006/anbe.1999.1276) [DOI] [PubMed] [Google Scholar]
- 30.Rek P, Osiejuk TS. 2011. Nonpasserine bird produces soft calls and pays retaliation cost. Behav. Ecol. 22, 657–662 (doi:10.1093/beheco/arr027) [Google Scholar]
- 31.Wagner WE. 1992. Deceptive or honest signalling of fighting ability? A test of alternative hypotheses for the function of changes in call dominant frequency by male cricket frogs. Anim. Behav. 44, 449–462 (doi:10.1016/0003-3472(92)90055-E) [Google Scholar]
- 32.Burmeister SS, Ophir AG, Ryan MJ, Wilczynski W. 2002. Information transfer during cricket frog interactions. Anim. Behav. 64, 715–725 (doi:10.1006/anbe.2002.4012) [Google Scholar]
- 33.Lein MR. 1978. Song variation in a population of chestnut-sided warblers (Dendroica pensylvanica): its nature and suggested significance. Can. J. Zool. 56, 1266–1283 (doi:10.1139/z78-182) [Google Scholar]
- 34.Nelson DA, Croner LJ. 1991. Song categories and their functions in the field sparrow (Spizella pusilla). Auk 108, 42–52 [Google Scholar]
- 35.Duguay JP, Ritchison G. 1999. A contextual analysis of singing behavior in male tufted titmice. J. Field Ornithol. 69, 85–94 [Google Scholar]
- 36.Wiebe MO, Lein MR. 1999. Use of song types by mountain chickadees (Poecile gambeli). Wilson Bull. 111, 368–375 [Google Scholar]
- 37.Trillo PA, Vehrencamp SL. 2005. Song types and their structural features are associated with specific contexts in the banded wren. Anim. Behav. 70, 921–935 (doi:10.1016/j.anbehav.2005.02.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spector DA. 1992. Wood-warbler song systems. A review of paruline singing behaviors. Curr. Ornithol. 9, 199–238 (doi:10.1007/978-1-4757-9921-7_6) [Google Scholar]
- 39.Byers BE. 1996. Messages encoded in the songs of chestnut-sided warblers. Anim. Behav. 52, 691–705 (doi:10.1006/anbe.1996.0214) [Google Scholar]
- 40.Beeman K. 2002. Signal v. 4.0. Belmont, MA: Engineering Design [Google Scholar]
- 41.Burt JM, Campbell SE, Beecher MD. 2001. Song type matching as threat: a test using interactive playback. Anim. Behav. 62, 1163–1170 (doi:10.1006/anbe.2001.1847) [Google Scholar]
- 42.Akçay C, Reed VA, Campbell SE, Templeton CN, Beecher MD. 2010. Indirect reciprocity: song sparrows distrust aggressive neighbors based on eavesdropping. Anim. Behav. 80, 1041–1047 (doi:10.1016/j.anbehav.2010.09.009) [Google Scholar]
- 43.Bartos L, Fricova B, Bartosova-Vichova J, Panama J, Sustr P, Smidova E. 2007. Estimation of the probability of fighting in fallow deer during the rut. Aggression Behav. 33, 7–13 (doi:10.1002/ab.20162) [DOI] [PubMed] [Google Scholar]
- 44.Enquist M, Leimar O. 1983. Evolution of fighting behaviour: decision rules and assessment of relative strength. J. Theor. Biol. 102, 387–410 (doi:10.1016/0022-5193(83)90376-4) [Google Scholar]
- 45.Enquist M, Leimar O, Ljungberg T, Mallner Y, Segerdahl N. 1990. A test of the sequential assessment game: fighting in cichlid fish Nannacara anomala. Anim. Behav. 40, 1–14 (doi:10.1016/S0003-3472(05)80660-8) [Google Scholar]
- 46.Payne RJH. 1998. Gradually escalating fights and displays: the cumulative assessment model. Anim. Behav. 56, 651–662 (doi:10.1006/anbe.1998.0835) [DOI] [PubMed] [Google Scholar]
- 47.Clutton-Brock TH, Albon SD, Gibson RM, Guiness FE. 1979. The logical stag: adaptive aspects of fighting in red deer (Cervus elaphus L.). Anim. Behav. 27, 211–224 (doi:10.1016/0003-3472(79)90141-6) [Google Scholar]
- 48.Chen S, Yeelin LA, Bowens NM, Huber R, Kravitz EA. 2002. Fighting in fruit flies: a model system for the study of aggression. Proc. Natl Acad. Sci. USA 99, 5664–5668 (doi:10.1073/pnas.082102599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DiMarco FP, Hanlon RT. 1997. Agonistic behavior in the squid Loligo plei (Loliginidae, Teuthoidea): fighting tactics and the effects of size and resource value. Ethology 103, 89–108 (doi:10.1111/j.1439-0310.1997.tb00010.x) [Google Scholar]
- 50.Panhuis T, Wilkinson G. 1999. Exaggerated male eye span influences contest outcome in stalk-eyed flies (Diopsidae). Behav. Ecol. Sociobiol. 46, 221–227 (doi:10.1007/s002650050613) [Google Scholar]
- 51.Terleph TA. 2004. The function of agonistic display behaviours in Gnathonemus petersii. J. Fish Biol. 64, 1373–1385 (doi:10.1111/j.0022-1112.2004.00401.x) [Google Scholar]
- 52.Triefenbach FA, Zakon HH. 2008. Changes in signaling during agonistic interactions between male weakly electric knifefish, Apteronotus leptorhynchus. Anim. Behav. 75, 1263–1272 (doi:10.1016/j.anbehav.2007.09.027) [Google Scholar]
- 53.Popp JW. 1987. Resource value and dominance among American goldfinches. Bird Behav. 7, 73–77 (doi:10.3727/015613887791918088) [Google Scholar]
- 54.Lemel J, Wallin K. 1993. Status signalling, motivational condition and dominance: an experimental study in the great tit, Parus major L. Anim. Behav. 45, 549–558 (doi:10.1006/anbe.1993.1065) [Google Scholar]
- 55.Arnott G, Elwood RW. 2007. Fighting for shells: how private information about resource value changes hermit crab pre-fight displays and escalated fight behaviour. Proc. R. Soc. B 274, 3011–3017 (doi:10.1098/rspb.2007.1196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Otter KA, Ratcliffe L, Njegovan M, Fotheringham J. 2002. Importance of frequency and temporal song matching in black-capped chickadees: evidence from interactive playback. Ethology 108, 181–191 (doi:10.1046/j.1439-0310.2002.00764.x) [Google Scholar]
- 57.Mennill DJ, Ratcliffe LM. 2004. Overlapping and matching in the song contests of black-capped chickadees. Anim. Behav. 67, 441–450 (doi:10.1016/j.anbehav.2003.04.010) [Google Scholar]
- 58.Hof D, Hazlett N. 2012. Mortal combat: an apparent intraspecific killing by a male black-capped chickadee. J. Field Ornithol. 83, 290–294 (doi:10.1111/j.1557-9263.2012.00377.x) [Google Scholar]


