Abstract
Polyploidy (or whole-genome doubling) is a key mechanism for plant speciation leading to new evolutionary lineages. Several lines of evidence show that most species among flowering plants had polyploidy ancestry, but it is virtually unknown for conifers. Here, we study variability in pollen tetrad morphology and the size of the conifer pollen type Classopollis extracted from sediments of the Triassic–Jurassic transition, 200 Ma. Classopollis producing Cheirolepidiaceae were one of the most dominant and diverse groups of conifers during the Mesozoic. We show that aberrant pollen Classopollis tetrads, triads and dyads, and the large variation in pollen size indicates the presence of unreduced (2n) pollen, which is one of the main mechanisms in modern polyploid formation. Polyploid speciation may explain the high variability of growth forms and adaptation of these conifers to different environments and their resistance to extreme growth conditions. We suggest that polyploidy may have also reduced the extinction risk of these conifers during the End-Triassic biotic crisis.
Keywords: palynology, polyploidy, Cheirolepidiaceae, evolution, mass extinctions
1. Introduction
Polyploidy (or whole-genome doubling (WGD)) is a key mechanism for plant speciation leading to new evolutionary lineages [1,2]. Several lines of evidence show that most species among flowering plants had polyploidy ancestry [3,4]. Molecular dating of ancient whole-genome doubling events suggests that the rise of seed plants and angiosperms were associated with a series of WGD events at around 319 and 192 Ma [5]. While polyploidy is ubiquitous among flowering plants, it is virtually unknown for extant gymnosperms [6]. It is only reported within one species of the Cupressaceae, Sequoia sempervirens, and one species of Cycads, Encepharlatos hildebrandtii, while it is more common among species of Ephedra [7,8]. In angiosperms, the formation of unreduced gametes and pollen is regarded as the main mechanism leading to polyploids [9,10]. In modern gymnosperms, unreduced gametes are less common and have been reported for only one species of the Cupressaceae, Cupressus dupreziana [11]. However, extant gymnosperms represent only a small group of a much more diverse group of gymnosperms which were outcompeted by angiosperms during the Cretaceous [12]. It has been suggested that differences in life strategies and genome dynamics may have favoured the evolutionary success of angiosperms over conifers [3,6,13,14]. However, the Cheirolepidiaceae represent one of the most prominent and diverse groups of Mesozoic conifers. This extinct conifer family is presumably related to the Cupressaceae or Araucariaceae and occupied a wide range of ecological niches and possessed a highly variable growth habit [15,16]. It had an exceptional long geological record ranging from the Late Triassic until the End-Cretaceous, from approximately 225 Ma until 65 Ma.
In this paper, we test the hypothesis that this conifer family produced unreduced pollen. Polyploidy may have been an important evolutionary mechanism explaining the exceptional ecological diversity of this successful conifer family. As cell size correlates with the DNA content [1], stomatal size of living and fossil leaf remains has been used to infer ploidy history in angiosperms during the Caenozoic [17,18]. Similar to the stomatal size, pollen size and volume also varies with the ploidy level of a plant [9,10,19–22]. In order to test our hypothesis, we examined the morphology of the fossil pollen Classopollis, which was produced by the Cheirolepidiaceae.
While polyploidy is a widespread phenomenon in flowering plants, it is in modern gymnosperms, despite their large genome size, rare [1,6]. We show that tetrad and pollen size analysis of the fossil conifer pollen Classopollis reveals evidence of hitherto unknown WGD events within gymnosperms. Our results suggest that WGD events may be more widespread within this clade than has been inferred before and that palaeobotanical and palynological analysis may be a key to elucidating this history of genome evolution.
2. Material and methods
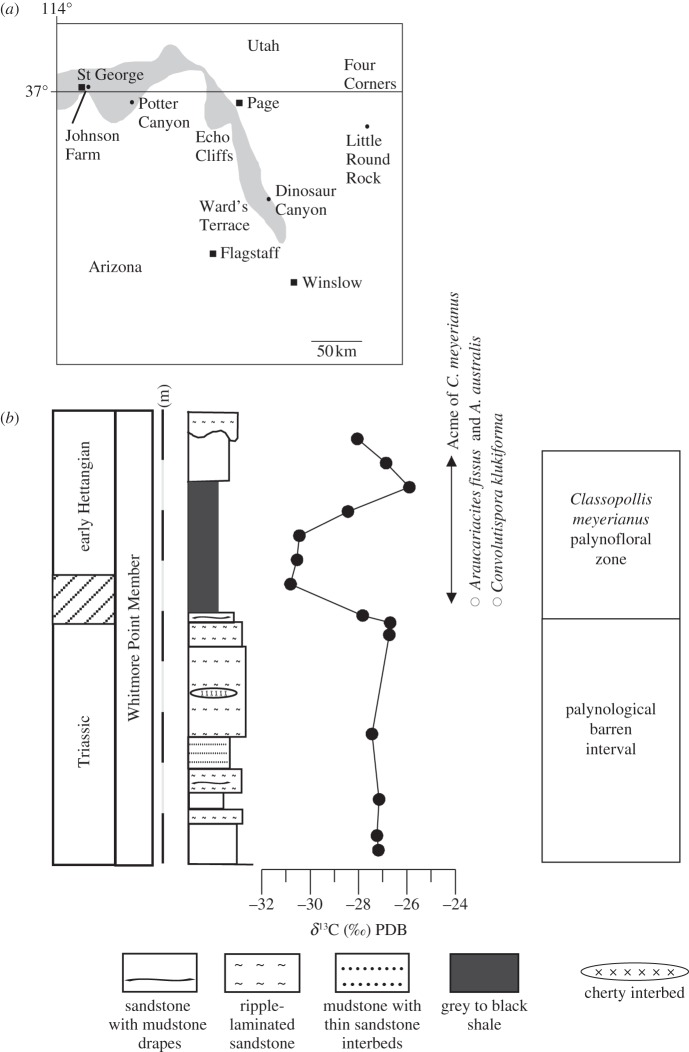
The Potter Canyon section is a south-facing promontory in Mohave County in northern Arizona at 36°52.872′ N, 112°52.083′ W. Fourteen rock samples were taken from the lower part of the Whitmore Point Member for palynological and bulk C-isotope analysis according to Utrecht laboratory standard protocols [23,24].
Classopollis-dominated pollen assemblages were extracted from rocks of the Whitmore Point Member of the upper part of the Moenave Formation at the Potter Canyon section on the Southern Colorado Plateau, northwest Arizona (figure 1). Bio- and magnetostratigraphic data suggest that the Whitmore Point Member of the upper part of the Moenave Formation encompasses the Triassic–Jurassic transition [25]. The C-isotope record shows in the middle part of Whitmore Point Member a marked negative C-isotope excursion of −4‰ (figure 1). We correlate this part of the Whitmore Point Member with the lower part of the Classopollis meyerianus palynofloral zone of the Newark Supergroup [26]. In accordance with previous stratigraphic correlations [25], we correlate the negative C-isotope excursion with the main isotope excursion at the Triassic–Jurassic transition [23,24].
Figure 1.
(a) Location map of the Potter canyon section in AZ, USA and (b) summary diagram with lithology, C-isotope ratios of bulk sedimentary organic matter and main palynological events from the lower Whitmore Point Member of the Moenave Formation. C-isotope ratios are reported in standard delta notation relative to Vienna PDB standard (PDB, Pee Dee Belemnite).
Classopollis pollen diameters were measured (in total n = 3754) with an image analyser (analySIS program of Olympus Soft Imaging Solutions), attached to a light microscope from one interval with Classopollis-dominated pollen assemblages extracted from the same sediment samples. Also, pollen tetrad configurations were analysed in these Classopollis-dominated pollen assemblages. Histograms of Classopollis size-frequency distributions were constructed using R [27]. Statistical analyses were performed using the R package ‘moments’ [28]. In a first series, 1400 Classopollis pollen grains were measured. The size-frequency distribution of the first series of measurements on Classopollis pollen (n = 1400) indicates that the majority of specimens measure 28–38 µm and is positively skewed which was statistically significant using a D'Agostino test of skewness (skew = 1.140; p < 0.0001) [29]. In order to examine the frequency of pollen grains in the right-hand tail of the distribution, a second round of measurements (n = 2354) were undertaken on specimens of Classopollis pollen larger than 34 µm from the same rock sample.
As all measurements were carried out from the same population, we can exclude the confounding bias of pollen size during sample preparation. If pollen size was eventually influenced by shrinking during fossilization, then all Classopollis pollen should be affected in the same way because they all possess the same basic shape, structure and morphology. Classopollis is a robust and thick-walled pollen grain that is well preserved in the sediments and approximates the shape of an ellipsoid. The volume can be calculated by the formula V = 4/3 × π × a × b × c, in which a, b and c represent the axes of the ellipsoid.
3. Results
(a). Classopollis pollen and tetrad morphology
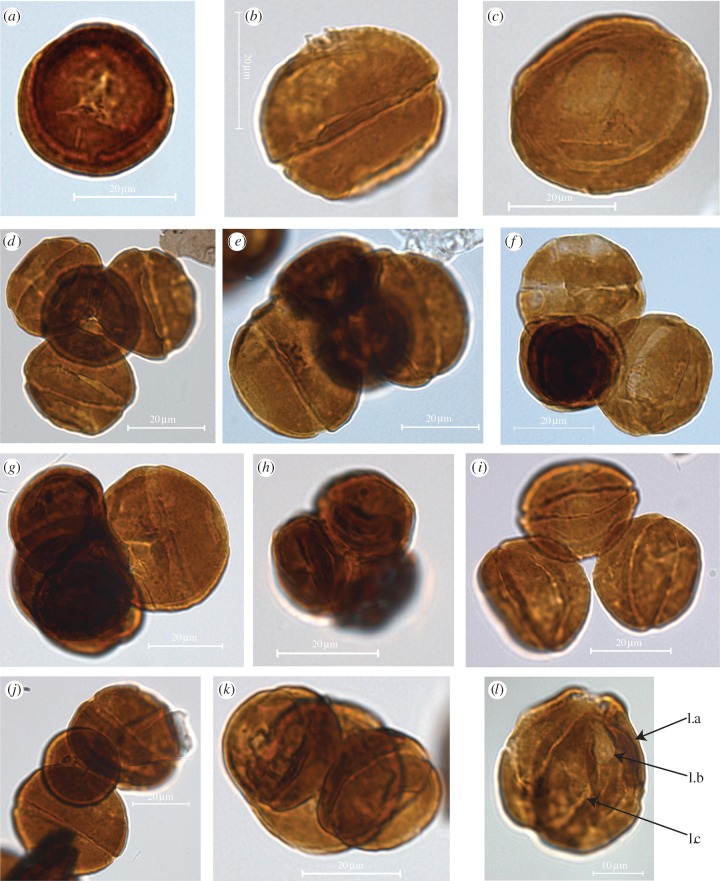
Well-preserved dispersed pollen of Classopollis dominated the palynofloral assemblages (figure 2a–c). Also, clustered pollen tetrads, triads and dyads were encountered, which show a remarkable variation. We found three types of tetrads. The normal multiplanar Classopollis clusters form tetrahedral tetrads in which each Classopollis pollen grain is in contact with three others (figure 2d). A second type of multiplanar tetrads shows Classopollis pollen arranged in two pairs lying at right angles across one other (figure 2e,f). One pollen pair in these unbalanced tetrads is usually larger than in the normal tetrahedral tetrads, whereas the second pollen pair is frequently smaller. There are also uneven-sized tetrads with three small Classopollis attached to one giant Classopollis pollen grain (figure 2g). A third variant of tetrads shows four evenly reduced Classopollis pollen grains (figure 2h). As a third kind of pollen clusters, we also found pollen triads with even and uneven pollen size in several combinations of different-sized Classopollis pollen (figure 2i–k). As a fourth category of pollen cluster, we found pollen dyads (figure 2l).
Figure 2.
Light-microscope photographs of Classopollis meyerianus pollen: (a,b) dispersed normal-sized pollen and (c) giant variant, (d) normal-sized multiplanar Classopollis clusters form tetrahedral tetrad, (e,f) multiplanar tetrads with two uneven-sized pairs lying at right angles across one other, (g) uneven-sized tetrad with three small Classopollis attached to one giant Classopollis pollen grain, (h) small even-sized Classopollis cluster form tetrahedral tetrad, (i) even-sized planar triad, (j–k) uneven-sized triads. (l) Dyads showing the furrow (l.a), the proximal tetrad scar (l.b) and the distal cryptopore (l.c). (Online version in colour.)
Another striking feature of the dispersed Classopollis population is their variability in diameter. No other conifer pollen from this palynofloral assemblage shows a similar variability of size. Variability in size and morphology of Classopollis pollen from this location has previously been used to identify different morphological ‘species’ of Classopollis, such as C. meyerianus, Classopollis torosus as well as Classopollis itunensis [29]. According to our light microscopy and scanning electron microscopy analyses, however, most of the morphotypes of Classopollis exactly resemble the morphology and surface structure of C. meyerianus, except for the variability in size (see the electronic supplementary material). The surface structures of both the normal and the giant C. meyerianus pollen are rather smooth and show no ornamentation or striation. The relative size proportions of these pollen types are equal. As small and large Classopollis morphotypes form together tetrads or triads, they cannot be separate taxa as suggested by previous authors [30], but belong to the same mother plant.
(b). Classopollis pollen size analysis
We systematically measured the variability of C. meyerianus pollen diameter with an image analyser in order to assess its size distribution (figure 3). The size-frequency distribution of Classopollis pollen from the Whitmore Point Member indicates that the majority of specimens measure 28–38 µm (figure 3). However, several specimens are much larger, measuring in excess of 50 µm, and the distribution is positively skewed (figure 3). This observation is statistically significant using a D'Agostino test of skewness (skew = 1.140; p < 0.0001) [29].
Figure 3.
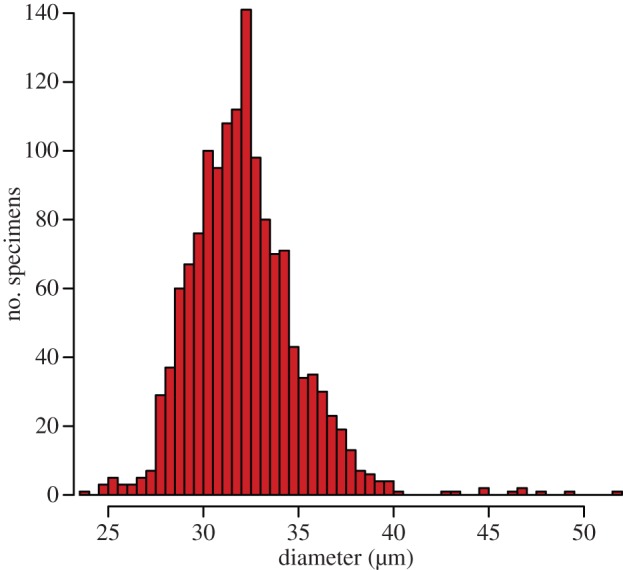
Histogram showing the overall distribution of Classopollis pollen size from the Whitmore Point Member (n = 1400). Data binned at 0.5 µm intervals. (Online version in colour.)
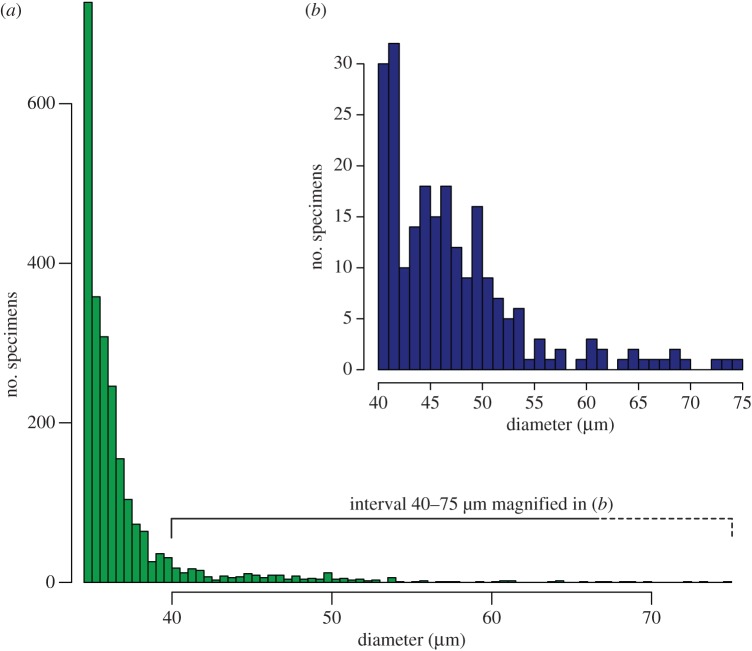
The right-hand tail of the frequency distribution of C. meyerianus pollen with diameters larger than 34 μm was further investigated with a second round of measurements (figure 4a). Within the frequency distribution of the larger diameters, distinct peaks can be observed (figure 4a). Using the main peak in the pollen diameter frequency diagram of 32 μm as the base value, we calculated the pollen volume as a function of the measured diameters. Some of the distinct increments in the pollen diameter histogram (figure 4b), such as the peaks at 45, 49, 55 and 60 μm, represent discrete multiplications of the pollen volume such as two, three and five times the average pollen volume.
Figure 4.
Histograms showing the size-frequency distribution of Classopollis pollen greater than 34 µm measured in a detailed investigation of large Classopollis from the Whitmore Point Member. (a) The overall distribution of Classopollis pollen greater than 34 µm (n = 2354). Data binned at 0.5 µm intervals. (b) Magnification of the 40–75 µm interval to show the shape of the distribution of Classopollis pollen around 43–53 µm, and to highlight the frequency of very large Classopollis pollen grains (n = 226). Data binned at 1 µm intervals. (Online version in colour.)
Pollen tetrad formation has been previously described for Classopollis pollen [31]. Typically, Classopollis forms multiplanar tetrahedral tetrads in which each Classopollis pollen grain is in contact with three others (figure 2d). The pollen diameter of the individual Classopollis pollen in these normal tetrads is constant and is equal to the main diameter of the dispersed Classopollis pollen of 32 µm. In this study also aberrant, uneven-sized multiplanar tetrads occur where Classopollis pollen grains are arranged in two pairs lying across one other (figure 2e,f). Pollen grains in the abnormal tetrads are notably larger than in the normal tetrahedral tetrads. One pollen pair is frequently significantly reduced in size (figure 2e,f). A second type of uneven-sized multiplanar tetrahedral tedrads is formed by a combination of three small and one giant Classopollis pollen grain (figure 2g). Besides these combinations of uneven-sized tetrads, we also found tetrads with equally reduced Classopollis pollen grains (figure 2h). Triads occur in combination of three normal-sized pollen grains (figure 2i) or in uneven-sized combinations of two normal-sized and one smaller pollen grain (figure 2j) or one normal-sized pollen grain and two smaller pollen grains (figure 2k). It is possible that these pollen triads and dyads represent damaged pollen tetrads where one or two pollen grains were lost. However, in a multiplanar tetrahedral configuration, pollen grains are aligned at a different angle to each other than in a planar triad or dyad configuration. The orientation of the openings or the furrow of the Classopollis pollen indicates the alignment of the pollen and helps to distinguish a primary planar or multiplanar orientation. In the studied material, the orientation of the furrow indicates the planar alignment of the Classopollis pollen in most of the triads and dyads.
The observed variability in the formation of Classopollis tetrads is unusual and has not, to our knowledge, been described before. Principally, the observed pattern can be ascribed to the formation of anucleate, haploid and unreduced 2n pollen. In modern plants, pollen size is related to the number of chromosomes with increasing pollen volume at higher ploidy levels [9,19–22,32–35]. While the number of pollen openings has been reported to vary in some polyploidy angiosperms [36], in this study, the number of pores and the furrows of Classopollis remains constant.
The pollen diameter in the normal even-sized Classopollis tetrads is in agreement with the pollen measuring 28–38 µm, with the mode at 32–33 µm, in the dispersed Classopollis assemblages. They represent the majority of the specimens measured in this study (figure 3). Pollen grains from this size class are interpreted as haploid (n) pollen grains dispersed at maturity following successful meiosis. Therefore, the normal even-sized Classopollis tetrads (figure 2d) are interpreted as clusters of unripe haploid (n) pollen that were released before separation. Specimens of Classopollis pollen smaller than the majority, around 25 µm and below, are interpreted as anucleate pollen grains (figure 3). Aborted pollen development is also recognized in the tetrahedral Classopollis tetrads with evenly reduced pollen (figure 2h) of the same size, approximately less than or equal to 25 µm. Specimens of Classopollis pollen from the right-hand tail of the positively skewed distribution are interpreted as diploid (2n) and higher polyploid pollen grains (figure 3).
In order to examine the frequency of pollen grains in the right-hand tail of the distribution, a second round of measurements were undertaken on specimens of Classopollis pollen larger than 34 µm from the same rock sample. These measurements increase the maximum size of Classopollis pollen found in the Whitmore Point Member from 52 to 75 µm (figure 4a,b). They also highlight a group of specimens that measure between 43 and 53 µm (figure 4b). Classopollis specimens in this group are interpreted as diploid (2n) pollen grains. Specimens that are larger than this may represent higher orders of polyploidy, including 4n and perhaps even 8n although a linear relationship between pollen volume and ploidy level needs to be tested.
Subpopulations within larger populations can be difficult to separate and define [37]. Partly, this is because the upper and lower tails of each subpopulation can overlap with one another. Such overlap has been observed in studies of pollen diameter from polyploid Arabidopsis plants, in which the right-hand tail of pollen from triploid (3n) plants overlaps with the left-hand tail of pollen from hexaploid (6n) plants [22]. Such overlap in the diameter of pollen from polyploids can be shown clearly in studies of modern plants because the ploidy of the parent plant is known. The boundaries between different populations in fossil material are much harder to define, although a Mann–Whitney test indicates that the population of Classopollis pollen shown in figure 3 and the population of Classopollis pollen shown in figure 4b have significantly different size-frequency distributions (p < 0.0001).
4. Discussion
Unreduced gametes have been observed in many modern polyploid mutants and can be generated by a variety of cytological mechanisms [9,10,32]. In plants where unreduced gametes were found, unreduced pollen is formed by anomalies occurring during meiotic cell division. These aberrations include abnormal spindle orientation, defective synapsis, omission of meiosis I or II and impaired cytokinesis [33–35]. Cytological studies also frequently document aberrant unequal-sized tetrads, triads and dyads during the formation of unreduced pollen. The exact cytological mechanism leading to unreduced pollen in the Cheirolepidiaceae awaits further investigations. It should be noted, however, that unbalanced tetrads, normal and unbalanced triads, as well as dyads were also found as a result of abnormal spindle orientation at male meiosis II in Arabidopsis thaliana (AtPS1) mutants leading to diplogamete formation and diploid pollen grains. The presence of aberrant pollen tetrads, triads and dyads is one line of evidence for unreduced pollen in the fossil Classopollis assemblage.
While polyploidy is a widespread phenomenon in flowering plants, it is uncommon in modern gymnosperms [1,6]. Yet, gymnosperms have a large genome size compared with most other land plants, and ancient polyploidy events may have played an important role in their evolution [6]. Two lines of evidence in our study, the presence of aberrant pollen tetrads, triads and dyads as well as the distinct variation in pollen size within the dispersed Classopollis pollen assemblage suggests the formation of unreduced 2n pollen by the Cheirolepidiaceae about 200 Ma. Our data represent the oldest evidence for unreduced pollen yet documented from the fossil record of vascular plants. We suggest that polyploidy events did not only play a pivotal role in the evolution of flowering plants but were also a more widespread phenomenon in the evolutionary history of extinct gymnosperms.
It should be noted that Cheirolepidiaceae are probably related to the Araucariaceae or Cupressaceae [15,16], the latter representing one of the few modern conifer families reported to have polyploids [6]. They represent an exceptional successful group of gymnosperms including growth forms from small shrubs to large trees, which are adapted to a wide range of environments, from wet to arid (sub)tropical environments including mangroves [15,16]. During their long evolutionary history starting in the Late Triassic and lasting until the end of the Cretaceous, they inhabited many of the niches now dominated by angiosperms. The unusual exine structure of the Classopollis pollen has attracted much interest because its tectate collumellate structure resembles to some degree the pollen wall structure of angiosperms [38,39]. It has been suggested that the complex wall structure may have served as a biochemical recognition mechanism for taxonomical compatibility [40]. Moreover, seeds of the Cheirolepidiaceae are covered by a double integumentum that opened proximally on the scale [41]. This feature, uncommon in gymnosperms would imply that the pollen did not have direct access to the ovule micropyle and may have germinated on the scale. Further studies need to investigate whether the unusual pollen structure and pollination biology of the Cheirolepidiaceae may have had an effect on the formation of unreduced pollen and polyploids.
Polyploid plants are more stress tolerant than their diploid relatives [1,6,42]. Growth experiments showed that 2n pollen production is stimulated by environmental factors such as temperature, herbivory, wounding, and water and nutrient stress [43,44]. Plants in their natural habitats experience many of the environmental factors that influence 2n gamete production in growth experiments. This suggests that natural environmental variation, as well as large-scale climate change, could substantially alter the frequency of polyploid evolution. The high incidence of polyploidy at high latitudes, high altitudes, and recently glaciated areas may be related to the tendency of harsh environmental conditions to induce 2n gametes and polyploid formation [45]. Moreover, several geological periods during the Phanerozoic are characterized by large-scale environmental stress, which caused unusual high extinction rates [46]. WGD events may have lowered extinction risks during mass extinction events, such as at the Cretaceous–Paleogene boundary some 65 Ma [47]. We suggest that similar to aberrant lycophyte spores [48] and conifer pollen [49], during the end-Permian mass extinction aberrant Classopollis pollen may indicate environmental mutagenesis during the end-Triassic mass extinction. Extensive volcanism of the Central Atlantic magmatic province during the Triassic–Jurassic transition is generally viewed as a trigger mechanism of this global biotic crisis by extreme climate transitions [50]. Evidence for rapid climate cooling followed by extreme warming comes from geochemical proxy [51] as well as biological proxy records [52]. Palynological records across the Triassic–Jurassic transition become completely dominated by Classopollis pollen in many parts of the world [53] indicating a widespread proliferation of the Cheirolepidiaceae forests in the aftermath of the biotic crisis. Based on our findings, we suggest that the unusual evolutionary success of this conifer family may be related to their ability to form unreduced 2n pollen and thus polyploid hybrids, which were more stress tolerant. Polyploidy may have reduced the extinction risk of the Cheirolepidiaceae during the end-Triassic environmental crisis. To corroborate the concept of polyploidy within this conifer family, we need to collect additional morphological evidence of polyploidy within the sporophyte. Complementary to pollen, variation in stomatal size of Cheirolepidiaceae shoots, such as Brachyphyllum and Pagiophyllum is another potential source of palaeobotanical evidence that verifies the presence of polyploidy in the sporophyte. It should be noted that on Brachyphyllum/Pagiophyllum shoots from the Newark Supergroup show a distinct trend from small to large leaf sizes across the Triassic–Jurassic transition (B. Cornet 1977, unpublished data). These preliminary observations await quantification by cuticle analysis but points to the existence of polyploidy among the conifer sporophyte generation. Finally, our results imply that the fossil plant record holds key evidence for a much more prominent role of WGD events in extinct lineages which is of importance because, aside angiosperms, so much of green plant diversity and disparity is unrepresented in the modern flora.
Acknowledgements
W.M.K. initiated this research at Utrecht University. W.M.K. designed the research, W.M.K. and S.B. performed research, W.M.K., S.B. and L.M. analysed data and wrote the paper. Constructive comments by P. Donoghue and two anonymous reviewers are acknowledged and significantly improved the manuscript. We thank S. G. Lucas (New Mexico NHM, Albuquerque, USA) for introducing us into this fieldwork area and logistic support.
Funding statement
W.M.K. acknowledges financial support from a ‘High Potential Award’ of the board of Utrecht University. L.M.'s contribution to this research was supported by a Marie Curie International Incoming Fellowship, within the 7th European Community Framework Programme (PIIF-GA-2012-328245).
References
- 1.Stebbins GL. 1971. Chromosomal evolution in higher plants. London, UK: Edward Arnold [Google Scholar]
- 2.Wood TE, Takebayashi N, Barker MS, Mayrose I, Greenspoon PB, Rieseberg LH. 2009. The frequency of polyploidy speciation in vascular plants. Proc. Natl Acad. Sci. USA 106, 13 875–13 879 (doi:10.1073/pnas.0811575106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Bodt S, Maere S, van de Peer Y. 2005. Genome duplication and the origin of angiosperms. Trends Ecol. Evol. 20, 591–597 (doi:10.1016/j.tree.2005.07.008) [DOI] [PubMed] [Google Scholar]
- 4.Soltis DE, et al. 2009. Polyploidy and angiosperm diversification. Am. J. Bot. 96, 336–348 (doi:10.3732/ajb.0800079) [DOI] [PubMed] [Google Scholar]
- 5.Jiao Y, et al. 2011. Ancestral polyploidy in seed plants and angiosperms. Nature 473, 97–100 (doi:10.1038/nature09916) [DOI] [PubMed] [Google Scholar]
- 6.Leitch AR, Leitch IJ. 2012. Ecological and genetic factors linked to contrasting genome dynamics in seed plants. New Phytol. 194, 629–646 (doi:10.1111/j.1469-8137.2012.04105.x) [DOI] [PubMed] [Google Scholar]
- 7.Delevoryas T. 1980. Polyploidy in gymnosperms. In Polyploidy: biological relevance (ed. Lewis W.), pp. 215–218 New York, NY: Plenum Press [Google Scholar]
- 8.Ahuja MR. 2005. Polyploidy in gymnosperms: revisited. Silvae Genetica 54, 59–69 [Google Scholar]
- 9.Ramsey J, Schemske DW. 1998. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu. Rev. Ecol. Syst. 29, 467–501 (doi:10.1146/annurev.ecolsys.29.1.467) [Google Scholar]
- 10.Brownfield L, Köhler C. 2011. Unreduced gamete formation in plants: mechanisms and prospects. J. Exp. Bot. 62, 1659–1668 (doi:10.1093/jxb/erq371) [DOI] [PubMed] [Google Scholar]
- 11.El Maataoui M, Pichot C. 1999. Nuclear and cell fusion cause polyploidy in the megagametophyte of common cypress, Cupressus sempervirens L. Planta 208, 345–351 (doi:10.1007/s004250050568) [Google Scholar]
- 12.Niklas KJ, Tiffney BH, Knoll AH. 1983. Patterns in vascular plant diversification. Nature 303, 614–616 (doi:10.1038/303614a0) [Google Scholar]
- 13.Wing SL, Boucher LD. 1989. Ecological aspects of the Cretaceous flowering plant radiation. Ann. Rev. Earth Planet. Sci. 26, 379–421 (doi:10.1146/annurev.earth.26.1.379) [Google Scholar]
- 14.Crane PR, Friis EM, Pedersen KR. 1995. The origin and early diversification of angiosperms. Nature 374, 27–33 (doi:10.1038/374027a0) [Google Scholar]
- 15.Alvin KL. 1982. Cheirolepidiaceae: biology, structure and palaeoecology. Rev. Palaeobot. Palynol. 37, 71–98 (doi:10.1016/0034-6667(82)90038-0) [Google Scholar]
- 16.Watson J. 1988. The Cheirolepidiaceae. In Origin and evolution of gymnosperms (ed. Beck CH.), pp. 382–447 New York, NY: Columbia University Press [Google Scholar]
- 17.Beck SL, Dunlop RW, Fossey A. 2003. Stomatal length and frequency as a measure of ploidy level in black wattle, Acacia mearnsii (de Wild). Bot. J. Linn. Soc. 141, 177–181 (doi:10.1046/j.1095-8339.2003.00132.x) [Google Scholar]
- 18.Masterson J. 1994. Stomatal size in fossil plants: evidence for polyploidy in majority of angiosperms. Science 264, 421–424 (doi:10.1126/science.264.5157.421) [DOI] [PubMed] [Google Scholar]
- 19.Altmann T, Damm B, Frommer WB, Martin T, Morris PC, Schweizer D, Willmitzer L, Schmidt R. 1994. Easy determination of ploidy level in Arabidopsis thaliana plants by means of pollen size measurement. Plant Cell Rep. 13, 652–656 (doi:10.1007/BF00232939) [DOI] [PubMed] [Google Scholar]
- 20.Becerra Lopez-Lavalle LA, Orjeda G. 2002. Occurrence and cytological mechanism of 2n pollen formation in tetraploid accession of Ipomoea batatas (sweet potato). J. Hered. 93, 185–192 (doi:10.1093/jhered/93.3.185) [DOI] [PubMed] [Google Scholar]
- 21.De Storme N, van Labeke M-C, Geelen D. 2007. Formation of unreduced pollen in Arabidopsis thaliana. Comm. Appl. Biol. Sci. (Ghent University) 72, 159–163 [PubMed] [Google Scholar]
- 22.De Storme N, Zamariola L, Mau M, Sharbel TF, Geelen D. 2013. Volume-based pollen size analysis: an advanced method to assess somatic and gametophytic ploidy in flowering plants. Plant Reprod. 26, 65–81 (doi:10.1007/s00497–012-0209-0) [DOI] [PubMed] [Google Scholar]
- 23.Ruhl M, Kürschner WM, Krystyn L. 2009. Triassic–Jurassic organic carbon isotope stratigraphy of key sections in the western Tethys realm (Austria). Earth Planet. Sci. Lett. 281, 169–187 (doi:10.1016/j.epsl.2009.02.020) [Google Scholar]
- 24.Bonis NR, Ruhl M, Kürschner WM. 2010. Milankovitch-scale palynological turnover across the Triassic–Jurassic transition at St. Audrie's Bay, SW UK. J. Geol. Soc. Lond. 167, 877–888 (doi:10.1144/0016-76492009-141) [Google Scholar]
- 25.Lucas SG, Tanner LH, Donohoo-Hurley LL, Geissman JW, Kozur HW, Heckert AB, Weems RE. 2011. Position of the Triassic–Jurassic boundary and timing of the End-Triassic extinctions on land: date from the Moenave Formation on the southern Colorado Plateau, USA. Palaeogeo. Palaeoecol. Palaeoclim. 302, 194–205 (doi:10.1016/j.palaeo.2011.01.009) [Google Scholar]
- 26.Cornet B, Traverse A. 1975. Palynological contributions to the chronology and stratigraphy of the Hartford basin in Connecticut and Massachusetts. Geosci. Man 11, 1–33 (doi:10.1080/00721395.1975.9989753) [Google Scholar]
- 27.R.D.C.T 2007. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 28.Komsta L. 2012. R Package ‘moments’, v. 0.13 See http://cran.r-project.org/web/packages/.
- 29.D'Agostino RB. 1970. Transformation to normality of the null distribution of G1. Biometrika 57, 679–681 (doi:10.1093/biomet/57.3.679) [Google Scholar]
- 30.Cornet B, Waanders G. 2006. Palynomorphs indicate Hettangian (Early Jurassic) age for the middle Whitmore Point Member of the Moenave Formation, Utah and Arizona. New Mex. Mus. Nat. Hist. Sci. Bull. 37, 390–406 [Google Scholar]
- 31.Courtinat B. 1980. Structure d'adhérence des grains de pollen en tetrad du genre Classopollis Pflug, 1953, de l'Hettangian de Saint Fortmont, Manche (France). Géobios 13, 209–229 (doi:10.1016/S0016-6995(80)80029-5) [Google Scholar]
- 32.Bretagnolle F, Thompson JD. 1995. Tansley review no-78: gametes with the somatic chromosome number: mechanisms of their formation and role in the evolution of autopolyploid plants. New Phytol. 129, 1–22 (doi:10.1111/j.1469-8137.1995.tb03005.x) [DOI] [PubMed] [Google Scholar]
- 33.d'Erfurth I, et al. 2008. Mutations in AtPS1 (Arabidopsis thaliana Parallel Spindle 1) lead to the production of diploid pollen grains. PLoS Genet. 4, e1000274 (doi:10.1371/journal.pgen.1000274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Storme N, Geelen D. 2011. The Arabidopsis mutant Jason produces unreduced first division restitution male gametes through a parallel/fused spindle mechanism. Plant Physiol. 155, 1403–1415 (doi:10.1104/pp.110.170415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bohac JR, Jones A. 1994. Unreduced pollen in hexaploid sweetpotato (Ipomoea batatas). J. Hered. 85, 162–166 [Google Scholar]
- 36.Thanikaimoni G. 1986. Pollen apertures: form and function. In Pollen and spores: form and function (eds Blackmore S, Ferguson IK.) Linnean Society Symposium Series no. 12, pp. 119–136 London, UK: Academic Press [Google Scholar]
- 37.Hammer Ø, Harper DAT. 2006. Palaeontological data analysis. Oxford, UK: Blackwell Publishing [Google Scholar]
- 38.Pettitt JM, Chaloner WG. 1964. The ultrastructure of the Mesozoic pollen Classopollis. Pollen et Spores 6, 611–620 [Google Scholar]
- 39.Chaloner WG. 1976. The evolution of adaptive features in fossil exines. In The evolutionary significance of the exine (eds Ferguson IK, Muller J.), pp. 1–14 London, UK: Academic Press [Google Scholar]
- 40.Taylor TN, Alvin KL. 1984. Ultrastructure and development of Mesozoic pollen: Classopollis. Am. J. Bot. 71, 575–587 (doi:10.2307/2443333) [Google Scholar]
- 41.Clement-Westerhof JA, van Konijnenburg-van Cittert JHA. 1991. Hirmeriella muensteri: new data on the fertile organs leading to a revised concept of the Cheirolepidiaceae. Rev. Palaeobot. Palynol. 68, 147–179 (doi:10.1016/0034-6667(91)90062-8) [Google Scholar]
- 42.Thompson JD, Lumaret R. 1992. The evolutionary dynamics of polyploid plants: origins, establishment and persistence. Trends Ecol. Evol. 7, 302–307 (doi:10.1016/0169-5347(92)90228-4) [DOI] [PubMed] [Google Scholar]
- 43.McHale NA. 1983. Environmental induction of high frequency 2n pollen formation in diploid Solanum. Can. J. Genet. Cytol. 25, 609–615 [Google Scholar]
- 44.De Storme N, Copenhaver GP, Geelen D. 2012. Production of diploid male gametes in Arabidopsis by cold-induced destabilization of postmeiotic radial microtubule arrays. Plant Physiol. 160, 1808–1826 (doi:10.1104/pp.112.208611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunter KL, Betancourt JL, Riddle BR, van Devender TR, Cole KL, Spaudling WG. 2001. Ploidy race distributions since the Last Glacial Maximum in the North American desert shrub, Larrea tridentata. Glob. Ecol. Biogeogr. 10, 521–533 (doi:10.1046/j.1466-822X.2001.00254.x) [Google Scholar]
- 46.Benton MJ. 1995. Diversification and extinction in the history of life. Science 268, 52–58 (doi:10.1126/science.7701342) [DOI] [PubMed] [Google Scholar]
- 47.Fawcett JA, Maere S, van de Peer Y. 2009. Plants with double genomes might have had a better chance to survive the K/T extinction event. Proc. Natl Acad. Sci. USA 106, 5737–5742 (doi:10.1073/pnas.0900906106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Visscher H, Looy CV, Collinson ME, Brinkhuis H, van Konijnenburg-van Cittert JHA, Kürschner WM, Sephton M. 2004. Environmental mutagenesis during the end-Permian ecological crisis. Proc. Natl Acad. Sci. USA 101, 12 952–12 956 (doi:10.1073/pnas.0404472101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foster CB, Afonin SA. 2005. Abnormal pollen grains: an outcome of deteriorating atmospheric conditions around the Permian–Triassic boundary. J. Geol. Soc. Lond. 162, 653–659 (doi:10.1144/0016-764904-047) [Google Scholar]
- 50.Blackburn TJ, Olsen PE, Bowring SA, McLean NM, Kent DV, Puffer J, Rasbury ET, Et-Touhami M. 2013. Zirkon U-Pb geochronology links the End-Triassic extinction with the Central Magmatic Province. Science 340, 941–945 (doi:10.1126/science.1234204) [DOI] [PubMed] [Google Scholar]
- 51.Korte C, Hesselbo SP, Jenkyns HC, Rickaby REM, Spoetl C. 2009. Palaeoenvironmental significance of carbon- and oxygen-isotope stratigraphy of marine Triassic–Jurassic boundary sections in SW Britain. J. Geol. Soc. Lond. 166, 431–445 (doi:10.1144/0016-76492007-177) [Google Scholar]
- 52.McElwain JC, Popa ME, Hesselbo SP, Haworth M, Surlyk F. 2007. Macroecological responses of terrestrial vegetation to climatic and atmospheric change across the Triassic/Jurassic boundary in East Greenland. Paleobiology 33, 547–573 (doi:10.1666/06026.1) [Google Scholar]
- 53.Bonis NR, Kürschner WM. 2012. Vegetation history, diversity patterns, and climate change across the Triassic/Jurassic boundary. Paleobiology 38, 240–264 (doi:10.1666/09071.1) [Google Scholar]