Abstract
In recent years, diffusion-weighted magnetic resonance imaging (DW-MRI) has been increasingly used to explore the relationship between white matter structure and cognitive function. This technique uses the passive diffusion of water molecules to infer properties of the surrounding tissue. DW-MRI has been extensively employed to investigate how individual differences in behavior are related to variability in white matter microstructure on a range of different cognitive tasks and also to examine the effect experiential learning might have on brain structural connectivity. Using diffusion tensor tractography, large white matter pathways have been traced in vivo and used to explore patterns of white matter projections between different brain regions. Recent findings suggest that diffusion-weighted imaging might even be used to measure functional differences in water diffusion during task performance. This review describes some research highlights in diffusion-weighted imaging and how this technique can be employed to further our understanding of cognitive function.
Keywords: diffusion-weighted imaging, cognitive, tractography, white matter, individual differences
Diffusion-weighted magnetic resonance imaging (DW-MRI) has become an important neuroimaging tool for exploring and quantifying white matter microstructure in the living human brain. This advanced imaging technique has released enormous potential for understanding the relationship between connectivity and brain function.
This review focuses on how DW-MRI has been used to examine the relationship between brain structure and behavior and discusses the applications of this technique from voxel-based analysis to the tracing of major white matter pathways. Although a great deal of research has investigated the impact of brain pathology on white matter structure, it is beyond the scope of this review to cover this considerable literature. Instead, we provide an overview of the basis of the diffusion-weighted signal and how it is used to generate a diffusion tensor model for exploring white matter structure and connectivity in the healthy human brain.
What Is Diffusion?
DW-MRI relies on the movement of water molecules in tissue. If it were possible to closely observe the molecules within a liquid, one would be presented with a system in constant turbulence. In any fluid, each particle is continually colliding with others and follows an unpredictable “random walk” between its neighbors. If there are no restrictions to the molecules’ movement, diffusion is isotropic: equiprobable in all directions. However, if there is a barrier to movement, such as a cell wall, then diffusion becomes anisotropic: there is greater net motion in one direction (Fig. 1B and 1C).
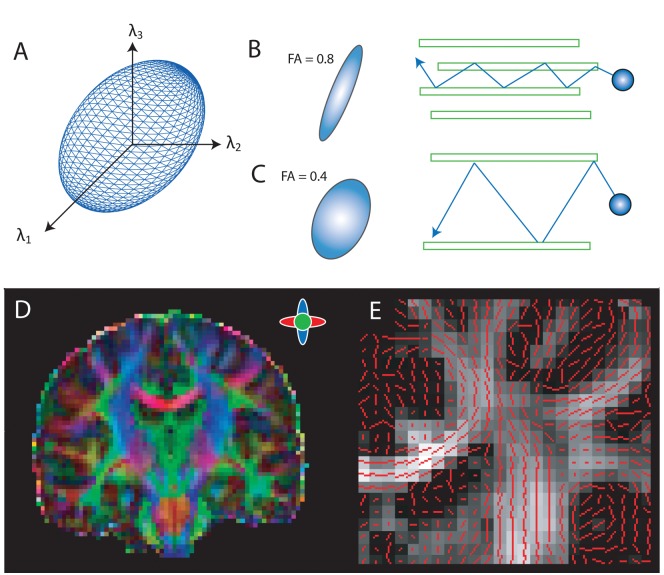
Figure 1.
Principles of diffusion. (A) The diffusion tensor ellipsoid represents the probability that water molecules within a voxel will diffuse in a given direction. (B) Fractional anisotropy (FA) is calculated from the diffusion tensor. Areas of high anisotropy have a more elongated probability distribution, reflecting the higher likelihood of diffusion in one direction. (C) Areas of lower anisotropy have a more spherical distribution. (D) This FA image has been colored to illustrate the principal directions of diffusion within different white matter pathways. (E) Here, the principal direction of diffusion within each voxel (red line) is shown overlaid on an FA map (lighter gray indicates higher anisotropy). Within white matter, the principal direction of diffusion is often aligned parallel to the length of the tract.
In order to characterize white matter microstructure, the diffusion tensor metric was introduced (Basser and others 1994; Pierpaoli and others 1996). The diffusion tensor is calculated on a voxel-by-voxel basis to quantify the degree of anisotropy as well as provide structural orientation information (Jones and Leemans 2011). Diffusion can be characterized using six parameters that quantify the direction (eigenvectors) and size (eigenvalues) of diffusion along three orthogonal axes. This information is best described by an ellipsoid, representing the probability that a water molecule will move in a given direction within a given voxel (Fig. 1A).
The direction of maximal diffusion within a voxel (λ1) is referred to as the principal diffusion direction and within white matter is usually aligned with axonal orientation (Fig. 1D and 1E). The average of the orthogonal secondary and tertiary directions of diffusion (λ2 and λ3) provides a measure of the radial diffusivity (RD). The most popular method of combining these two measures is to calculate the fractional anisotropy (FA), given by
This index provides an estimate of the relative sizes of the eigenvalues of the diffusion ellipsoid, ranging from 0 (isotropic, cerebrospinal fluid) and low values of anisotropy in gray matter through to highly anisotropic diffusion in white matter (a value of 1 represents the theoretical limit). In white matter, diffusion is generally anisotropic due to the intrinsic directionality of the local cellular architecture.
What Does FA Mean?
What does FA and the other diffusion indices represent in the brain? The physiological basis for the anisotropic diffusion of water in white matter is currently not fully understood (Beaulieu 2002), but a number of cellular factors might contribute. These include the degree of myelination, the number and size of the axons, and properties of the cell membrane. In addition, in areas where fibers cross, recently estimated at 90% of the white matter in the brain, the FA measurement can be reduced due to axons from different pathways not being closely collimated (Jeurissen and others 2010). Previous studies have demonstrated that although the level of myelin might modulate the strength of the diffusion signal, myelin loss is most closely associated with changes in radial diffusivity (Song and others 2002), whereas the principal diffusion direction is associated with levels of axonal integrity (Budde and others 2007).
To investigate whether the FA measure has any functional significance, there has been a recent trend to combine structural diffusion-weighted imaging with transcranial magnetic stimulation (TMS). One study used paired-pulse TMS to obtain an estimate of the functional connectivity between primary and premotor cortex while participants performed a simple choice reaction time task (choose shape or size depending on the current rule). The FA in areas of white matter connecting premotor and motor cortices correlated with the degree of functional connectivity when making such action choices (Boorman and others 2007). More recently, this group has examined structure-function correlations for the reprogramming of actions (Neubert and others 2010). Based on the findings of a remarkable series of studies, the different effects of presupplementary motor area (pre-SMA) and the right inferior frontal gyrus on motor cortex excitability appear to be mediated by two different white matter pathways that operate at two different latencies. The first short latency pathway was predominantly corticocortical, while the longer route went via subcortical structures, including potentially the subthalamic nucleus (STN).
These data indicate that diffusion parameters might provide an index of functional connectivity. However, higher FA has not always been associated with superior performance (Imfeld and others 2009). Therefore, as uncertainty remains over how indices such as FA and RD relate to physical properties of white matter, interpretation of results should ideally be placed in the context of other neuroimaging and anatomical findings to reduce the likelihood of erroneous conclusions.
Individual Differences and White Matter Structure
In recent years, diffusion tensor imaging (DTI) has been used to examine how individual differences in behavior might be related to white matter structure. In particular, indices such as FA and RD have been correlated with performance on a range of motor and cognitive tasks. For example, choice reaction time on a visuospatial task correlates positively with FA in the right optic radiation, right posterior thalamus, and right medial precuneus and negatively with FA in left superior temporal sulcus and left parietal operculum (Tuch and others 2005). Within-session improvement was positively correlated with FA in the left posterior thalamus and right precuneus and negatively correlated with FA in right superior cuneus and right superior temporal sulcus. This pattern of findings has been suggested to mirror a network of brain regions thought to be part of the visuospatial attention network.
In contrast, more efficient performance on the Go/No-Go task has been associated with reduced RD in white matter tracts connecting ventral prefrontal cortex and subcortical striatum (Liston and others 2006). Recently, Forstmann and others (2010) found that participants who were able to adapt to task demands—to prioritize either speed or accuracy—had higher levels of FA in the pathways connecting pre-SMA and the striatum. Further, individual posterror slowing was found to be related to white matter integrity in pathways connecting posterior-medial-frontal cortical regions to brain areas involved in motor inhibition (Danielmeier and others 2011). These investigations illustrate that although current evidence suggests higher FA is associated with a superior physical connection, it is impossible to discern whether a pathway is inhibitory or facilitatory. Therefore, lower or higher FA must be considered in the context of the known functions of a pathway and connecting regions.
The integrity of white matter microstructure in the corpus callosum (CC)—the largest white matter structure in the human brain that connects the right and left hemispheres—appears to predict performance on a variety of different tasks. For example, shorter reaction times on an object recognition task have been associated with higher organization (or FA) in the posterior aspect of the CC (splenium), whereas longer reaction times were correlated with increased organization in the anterior aspect of the CC (genu) (Baird and others 2005). The FA in an area of the CC where interhemispheric connections between supplementary motor areas and the caudal cingulate motor area pass through was also found to correlate with bimanual motor coordination (Johansen-Berg and others 2007).
Speech and language processing has been a popular area of inquiry for diffusion imaging research. Reading age correlated with FA in the white matter of the left temporal region in children and adults (Klingberg and others 2000; Deutsch and others 2005), with the specific location of such correlations lying along the border of the superior longitudinal fasciculus, part of which—the arcuate fasciculus—connects Broca and Wernicke areas. Further, FA in the left parietal-temporal region correlated positively with performance on a sound-to-word learning task and appears to be related to a ventral pathway mediating auditory comprehension (Wong and others 2011).
Other “higher” cognitive functions have also been investigated using DTI. Interindividual variation in the ability to introspect about self-performance has been linked to white matter microstructure in the prefrontal cortex (Fig. 2A), namely the genu of the CC, a region containing fibers that connect anterior prefrontal and orbitofrontal cortices (Fleming and others 2010). These studies demonstrate how DTI can be used to probe the structural basis for individual differences in performance.
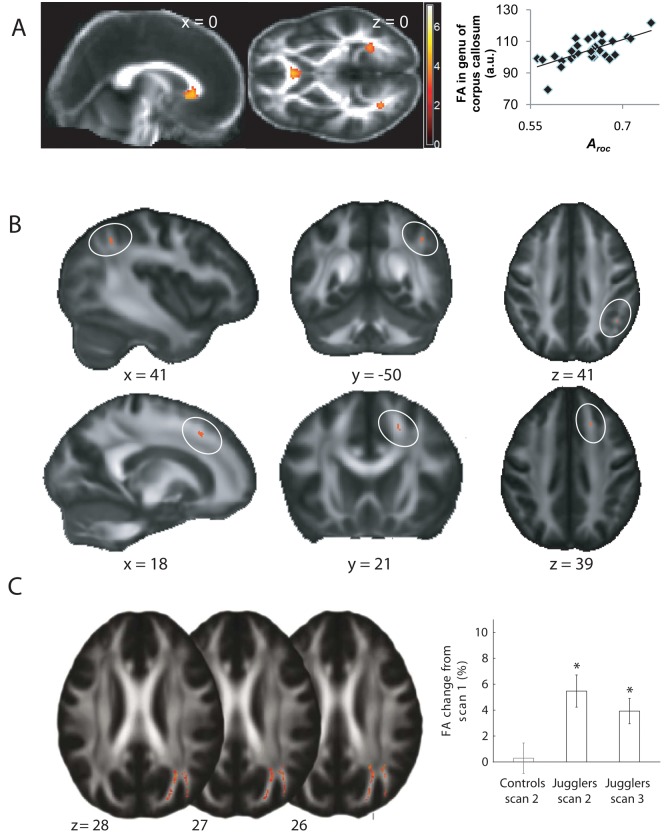
Figure 2.
Exploring individual differences using diffusion-weighted imaging. (A) Fractional anisotropy (FA) in white matter of a region within the genu of the anterior corpus callosum showed a correlation between FA and metacognitive ability that was statistically significant. Correlations are shown overlaid on the average FA image of all participants (adapted from Fleming and others 2010). (B) On a mean FA map (gray scale), the locations of differences in radial diffusivity between Royal Air Force pilots and controls are shown in red and circled in white. In the parietal region of interest (ROI), higher values of radial diffusivity were found in the control group, whereas in the dorsomedial frontal ROI, higher values of radial diffusivity occurred in the pilot group. All participants were assessed using the Eriksen flanker task. In the parietal cluster, mean radial diffusivity was negatively correlated with accuracy, incongruence cost, and pure cost across the group. In the medial frontal cluster, mean radial diffusivity was positively correlated with incongruence cost and benefit and negatively with postconflict adaptation change in pure cost. (C) After a six-week training period on a visuomotor task (juggling), a significant increase in FA was found in the white matter adjacent to the posterior parietal cortex (adapted from Scholz and others 2009).
White Matter and Expertise
Another approach to understanding the role of white matter integrity on behavioral performance is to compare the brain structure of individuals who are highly skilled, or “expert,” in a particular discipline with that of nonexperts. Such studies have sought to identify whether changes in white matter occur after long-term training in specific motor or cognitive skills, the inference being that any differences found will highlight specific areas, or networks, of the brain that have become optimized for performing particular behavioral tasks.
In a study of concert pianists, the amount of piano training positively correlated with fiber tract organization in regionally specific areas (Bengtsson and others 2005). The number of hours of practice during childhood correlates positively with FA in the CC and posterior limb of the internal capsule, in addition to areas of superior and inferior frontal white matter. More focal correlations, primarily in the CC, were detected with respect to the number of hours of practice in adolescence. By contrast, the amount of training in adulthood correlated positively with FA in the arcuate fasciculus and anterior limb of the internal capsule. This is an excellent example of how DTI can be used to measure changes in brain structure and plasticity associated with development.
Differences in white matter microstructure have also been found in other expert groups. An analysis of expert players of the attentionally demanding Korean game Baduk, or “Go,” reported increased FA in frontal areas including cingulum and striatothalamic regions, whereas lower FA was found in dorsolateral premotor and parietal areas (Lee and others 2010). A recent study of Royal Air Force fighter pilots found that RD of white matter adjacent to parietal and premotor cortex in the right hemisphere correlated with performance on the Eriksen flanker task (Fig. 2B) (Roberts and others 2010). This investigation revealed that the level of RD in these regions was associated with increased sensitivity to the distracters on the task but also with superior accuracy compared to controls. These studies illustrate how expert groups can be used to examine adaptations in brain networks associated with optimal functionality.
White Matter Plasticity and Experiential Learning
To date, DTI investigations have largely reported correlations but not causal relationships between behavior and brain structure. However, recent investigations into white matter plasticity have provided strong evidence to suggest that indices of white matter microstructure alter as a result of motor learning. Using a complex visuospatial coordination task (juggling), the level of FA in the white matter underlying the medial-parietal cortex was found to significantly increase after a six-week training period (Fig. 2C) (Scholz and others 2009). There is also evidence to suggest that even short periods of meditation can have a detectable effect on FA in the corona radiata (Tang and others 2010). These findings demonstrate that in addition to changes in brain activity, experiential learning can result in adaptation of white matter structure.
Tractography: The Anatomy of White Matter Pathways in the Brain
So far, we have considered how looking at local differences in brain microstructure might inform our view of cognitive function. A different approach is to examine larger scale patterns of connectivity and differences in the major association pathways of the brain. Diffusion tensor imaging tractography (DTT) provides the opportunity to trace such pathways. DTT reconstructs the trajectories of white matter fibers by measuring the principal direction of water diffusivity on a voxel-by-voxel basis and piecing together information from contiguous voxels. The parallel orientation of nerve axons within white matter constrains water molecules to move preferentially along the main direction of a nerve fiber.
There are two main types of tractography algorithm: deterministic and probabilistic. Both techniques assume that if the principal diffusion direction within two contiguous voxels has a significant degree of alignment, then it is likely that they form part of the same pathway. However, these approaches differ in how they apply a threshold to determine the likelihood of a pathway. Deterministic tractography is designed to trace a single path between two regions of interest. By selecting a starting point, or “seed” region, the algorithm will trace a path between voxels based on criteria such as the relative alignment of the principal diffusion direction and the level of FA. If these principles are violated, for example, if the level of FA falls below a certain value, the tracing algorithm will terminate. Differences in connectivity can be compared between individuals by calculating the number of successfully traced pathways (streamlines) between a seed region and a target region. Probabilistic tractography employs the same initial principle but instead calculates every possible connection between a seed voxel and all target voxels to create a probability connectivity distribution. A lower threshold is initially used to calculate the connectivity distribution, and then a subsequent threshold is applied to remove connections that have a low probability.
Deterministic tractography is more suited to the examination of large white matter pathways, as it is more likely to terminate when reaching areas of crossing fibers or low anisotropy. This method has been used to investigate hemispheric asymmetries in the human brain (Fig. 3A). Individuals with symmetrical direct connections between Broca and Wernicke areas in both hemispheres have been found to be more adept at remembering words using semantic association (Catani and others 2007). A recent study used functional imaging to define brain areas involved in saccadic eye movements and then employed deterministic tractography to demonstrate a significant right hemisphere lateralization of white matter pathways connecting frontal eye fields and supplementary eye fields (Fig. 3B). This study demonstrates how tractography can be combined with functional imaging to elucidate brain networks in terms of both structure and function (Anderson and others 2011).
Figure 3.
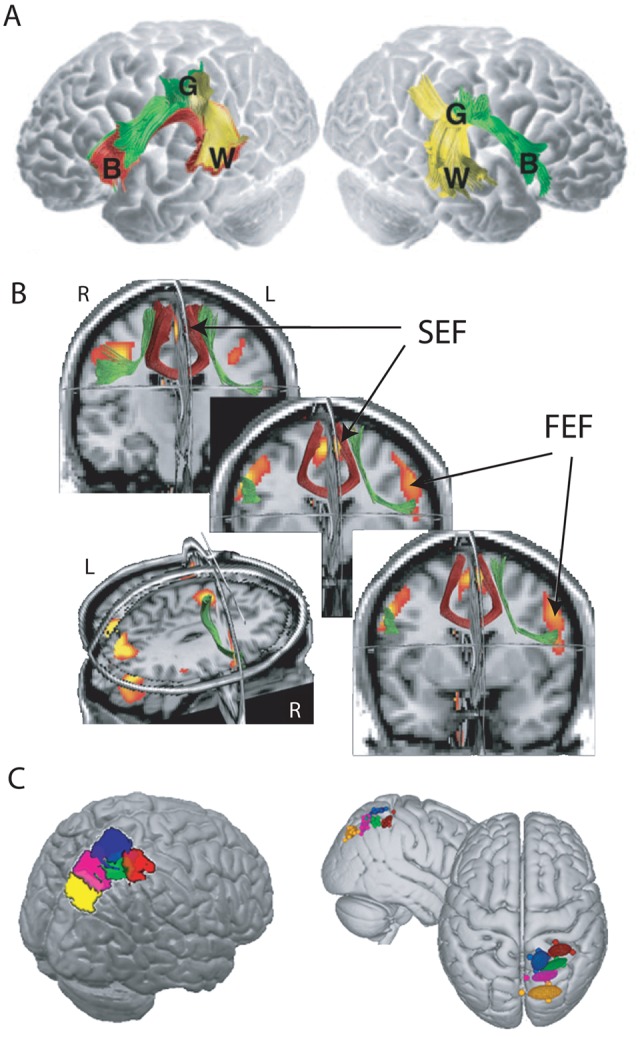
Applications of diffusion tensor tractography. (A) Analysis of pathways connecting Broca area (B), Wernicke area (W), and Geschwind area (G) revealed a significant left hemisphere lateralization in the majority of participants. Those individuals with Broca-Wernicke pathways in the right hemisphere were superior at recalling words based on semantic associations (adapted from Catani and others 2007). (B) Group-averaged tractography results, normalized and co-registered in MNI space, superimposed on coronal, axial, and sagittal slices of the SPM T1 template image, together with the group functional magnetic resonance imaging results, illustrating the locations of frontal cortical eye fields: supplementary eye field (SEF) and frontal eye field (FEF). Tract connecting contralateral SEF shown in red and ipsilateral SEF to FEF connections shown in green (adapted from Anderson and others 2011). (C) Probabilistic tractography was used to parcellate the posterior parietal cortex by identifying brain areas with similar cortical projections. Colors correspond to specific cortical projection sites (adapted from Neubert and others 2010).
Probabilistic tractography is more robust to areas of lower anisotropy or crossing fibers but is still susceptible to distance effects, as closer voxels are more likely to have a large number of local and long-range connections than voxels further from the seed region. Probabilistic tractography can also be used to provide a quantitative measure of connectivity between brain areas based on the probability of a connection, taking into account that a single voxel might connect with more than one target voxel. Probabilistic tractography has been used to analyze distributed connectivity patterns between the thalamus and the cortex (Behrens and others 2003), leading to some fascinating findings that demonstrate how thalamic nuclei can be visualized using probabilistic tractography.
This technique has also been used ingeniously to examine homologies between humans and macaque monkeys based on the connectivity profile of posterior parietal cortex and cross-correlations with resting-state functional connectivity data (Mars and others 2011). The findings led to a parcellation of parietal regions and the suggestion that humans differ from macaques in the strength of interaction between a specific inferior parietal region and prefrontal cortex (Fig. 3C).
Future Directions
This review has focused on how individual differences in behavior can be related to brain structure. Although evidence of white matter changes associated with learning and expertise has been discussed, it has been implicit that DW-MRI measures an essentially static system. However, there is some evidence to suggest that indices of white matter structure can change in response to stimuli on shorter time scales than those observed using conventional functional imaging. For example, significant differences in water diffusion within the visual cortex have been reported when participants viewed a flickering dartboard stimulus (Le Bihan and others 2006), mirroring changes in the BOLD response measured using functional magnetic resonance imaging. Alterations in FA along white matter tracts have also been observed during both tactile and visual stimulation (Mandl and others 2008). These studies demonstrate both the exciting possibility that DW-MRI measures could be used to investigate functional activation and that our understanding of what FA means in terms of brain structure and function is still at an early stage.
This review has focused on studies employing the diffusion tensor model to provide information about white matter structure. This model has however been shown to be inadequate in regions of “crossing fibers,” where multiple fiber orientations exist within the same voxel. Hence, considerable efforts are currently being made to develop higher order models of diffusion to more fully characterize the information provided by DW-MRI (Tournier and others 2011).
Conclusions
Diffusion-weighted imaging has enabled researchers to probe the relationship between in vivo white matter structure and a wide range of human behaviors. Variations at both the individual and group level have provided greater insight into the effect that experience, ability, and development have on white matter integrity. Recent findings even suggest that brain structure can dynamically change in response to relatively short periods of training. Hence, DW-MRI has enormous potential for enhancing our understanding of the role of connectivity and plasticity in human brain function and behavior.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported by a grant from Wellcome Trust, Medical Research Council UK, Economic & Social Research Council UK.
References
- Anderson EJ, Jones DK, O’Gorman RL, Leemans A, Catani M, Husain M. 2011. Cortical network for gaze control in humans revealed using multimodal MRI. Cereb Cortex June 21 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird AA, Colvin MK, VanHorn JD, Inati S, Gazzaniga MS. 2005. Functional connectivity: integrating behavioral, diffusion tensor imaging, and functional magnetic resonance imaging data sets. J Cogn Neurosci 17:687–93 [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, Lebihan D. 1994. Estimation of the effective self-diffusion tensor from the Nmr spin-echo. J Magn Reson B 103:247–54 [DOI] [PubMed] [Google Scholar]
- Beaulieu C. 2002. The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed 15:435–55 [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CAM, Boulby PA, and others. 2003. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6:750–7 [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullén F. 2005. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci 8:1148–50 [DOI] [PubMed] [Google Scholar]
- Boorman ED, O’Shea J, Sebastian C, Rushworth MFS, Johansen-Berg H. 2007. Individual differences in white-matter microstructure reflect variation in functional connectivity during choice. Curr Biol 17:1426–31 [DOI] [PubMed] [Google Scholar]
- Budde MD, Kim JH, Liang HF, Schmidt RE, Russell JH, Cross AH, and others. 2007. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med 57:688–95 [DOI] [PubMed] [Google Scholar]
- Catani M, Allin MPG, Husain M, Pugliese L, Mesulam MM, Murray RM, and others. 2007. Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci U S A 104:17163–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielmeier C, Eichele T, Forstmann BU, Tittgemeyer M, Ullsperger M. 2011. Posterior medial frontal cortex activity predicts post-error adaptations in task-related visual and motor areas. J Neurosci 31:1780–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JDE, Wandell B. 2005. Children’s reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex 41:354–63 [DOI] [PubMed] [Google Scholar]
- Fleming SM, Weil RS, Nagy Z, Dolan RJ, Rees G. 2010. Relating introspective accuracy to individual differences in brain structure. Science 329:1541–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, Anwander A, Schäfer A, Neumann J, Brown S, Wagenmakers EJ, and others. 2010. Cortico-striatal connections predict control over speed and accuracy in perceptual decision making. Proc Natl Acad Sci U S A 107:15916–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imfeld A, Oechslin MS, Meyer M, Loenneker T, Jancke L. 2009. White matter plasticity in the corticospinal tract of musicians: a diffusion tensor imaging study. Neuroimage 46:600–7 [DOI] [PubMed] [Google Scholar]
- Jeurissen B, Leemans A, Tournier JD, Jones DK, Sijbers J. 2010. Estimating the Number of Fiber Orientations in Diffusion MRI Voxels: A Constrained Spherical Deconvolution Study. Stockholm: International Society for Magnetic Resonance in Medicine [Google Scholar]
- Johansen-Berg H, Della-Maggiore V, Behrens TEJ, Smith SM, Paus T. 2007. Integrity of white matter in the corpus callosum correlates with bimanual co-ordination skills. Neuroimage 36:16–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Leemans A. 2011. Diffusion tensor imaging. Methods Mol Biol 711:127–44 [DOI] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JDE, Moseley ME, and others. 2000. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron 25:493–500 [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Urayama S, Aso T, Hanakawa T, Fukuyama H. 2006. Direct and fast detection of neuronal activation in the human brain with diffusion MRI. Proc Natl Acad Sci U S A 103:8263–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Park JY, Jung WH, Kim HS, Oh JS, Choi CH, and others. 2010. White matter neuroplastic changes in long-term trained players of the game of. Neuroimage 52:9–19 [DOI] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, and others. 2006. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex 16:553–60 [DOI] [PubMed] [Google Scholar]
- Mandl RC, Schnack HG, Zwiers MP, Van Der Schaaf A, Kahn RS, Hulshoff-Pol H, and others. 2008. Functional diffusion tensor imaging: measuring task-related fractional anisotropy changes in the human brain along white matter tracts. PLoS One 3:e3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Jbabdi S, Sallet J, O’Reilly JX, Croxson PL, Olivier E, and others. 2011. Diffusion-weighted imaging tractography-based parcellation of the human parietal cortex and comparison with human and macaque resting-state functional connectivity. J Neurosci 31:4087–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubert FX, Mars RB, Buch ER, Olivier E, Rushworth MF. 2010. Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proc Natl Acad Sci U S A 107:13240–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. 1996. Diffusion tensor MR imaging of the human brain. Radiology 201:637–48 [DOI] [PubMed] [Google Scholar]
- Roberts RE, Anderson EJ, Husain M. 2010. Expert cognitive control and individual differences associated with frontal and parietal white matter microstructure. J Neurosci 30:17063–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. 2009. Training induces changes in white-matter architecture. Nat Neurosci 12:1370–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. 2002. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17:1429–36 [DOI] [PubMed] [Google Scholar]
- Tang YY, Lu Q, Geng X, Stein EA, Yang Y, Posner MI. 2010. Short-term meditation induces white matter changes in the anterior cingulate. Proc Natl Acad Sci U S A 107: 15649–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier JD, Mori S, Leemans A. 2011. Diffusion tensor imaging and beyond. Magn Reson Med 65:1532–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch DS, Salat DH, Wisco JJ, Zaleta AK, Hevelone ND, Rosas HD. 2005. Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visuospatial attention. Proc Natl Acad Sci U S A 102:12212–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong FC, Chandrasekaran B, Garibaldi K, Wong P. 2011. White matter anisotropy in the ventral language pathway predicts sound-to-word learning success. J Neurosci 31:8780–5 [DOI] [PMC free article] [PubMed] [Google Scholar]