Abstract
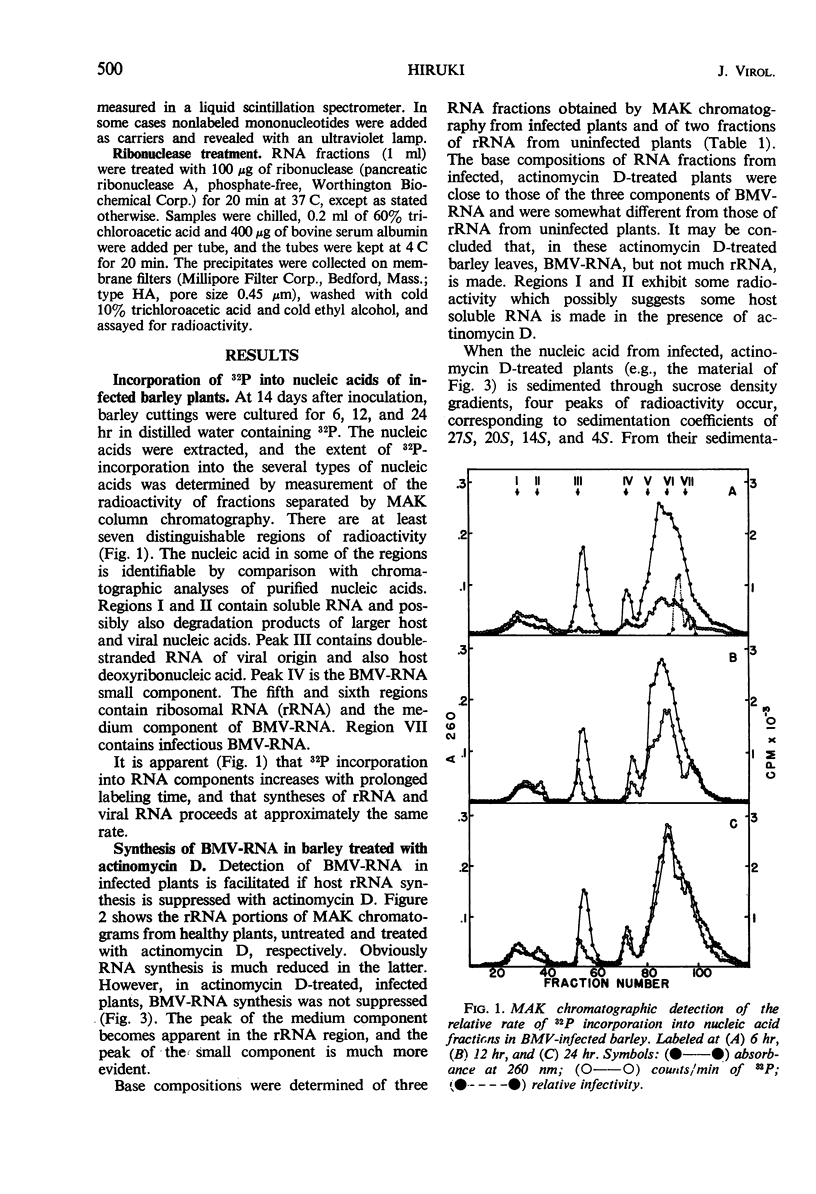
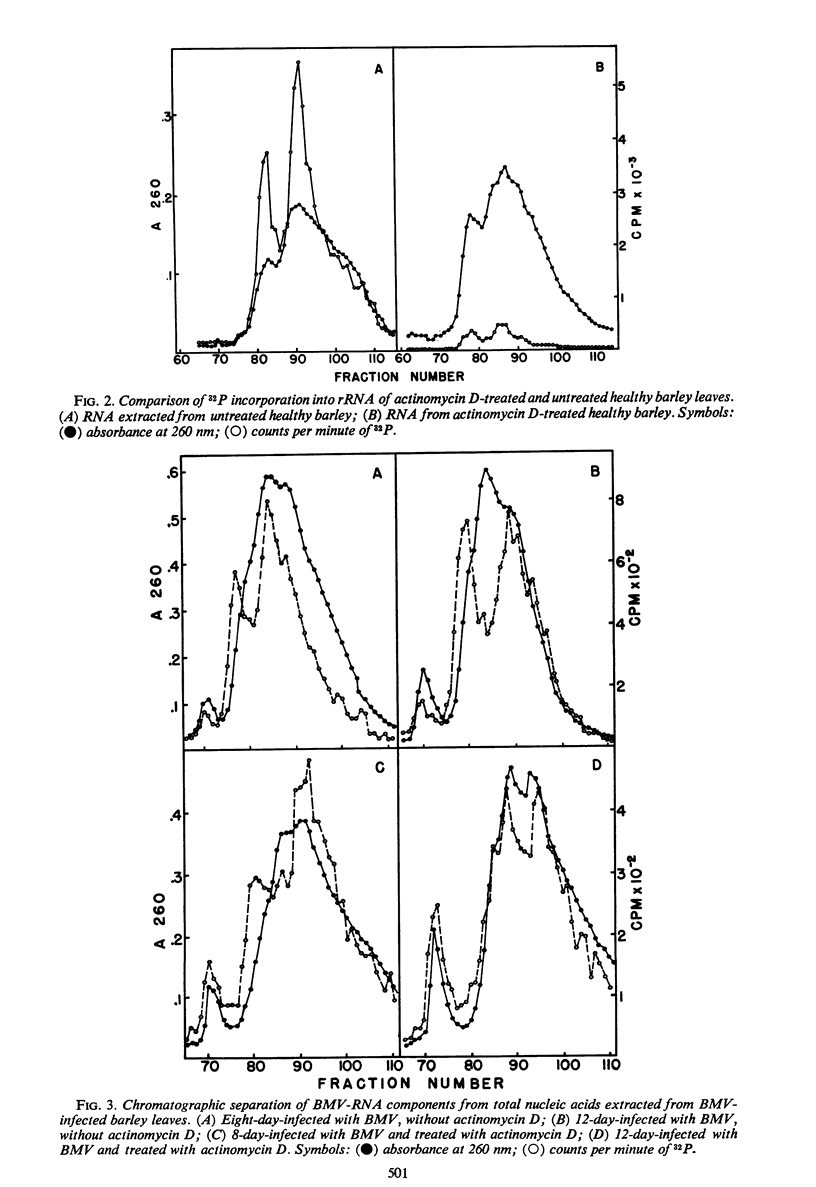
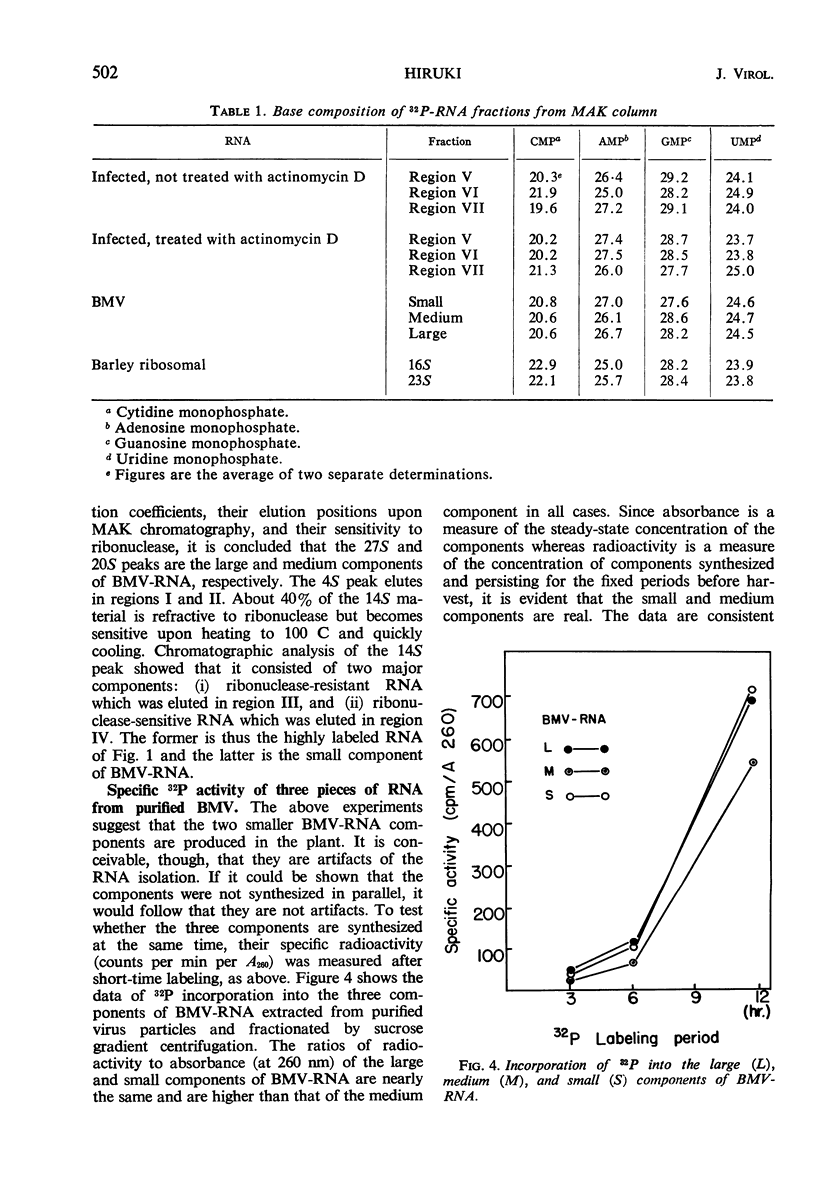
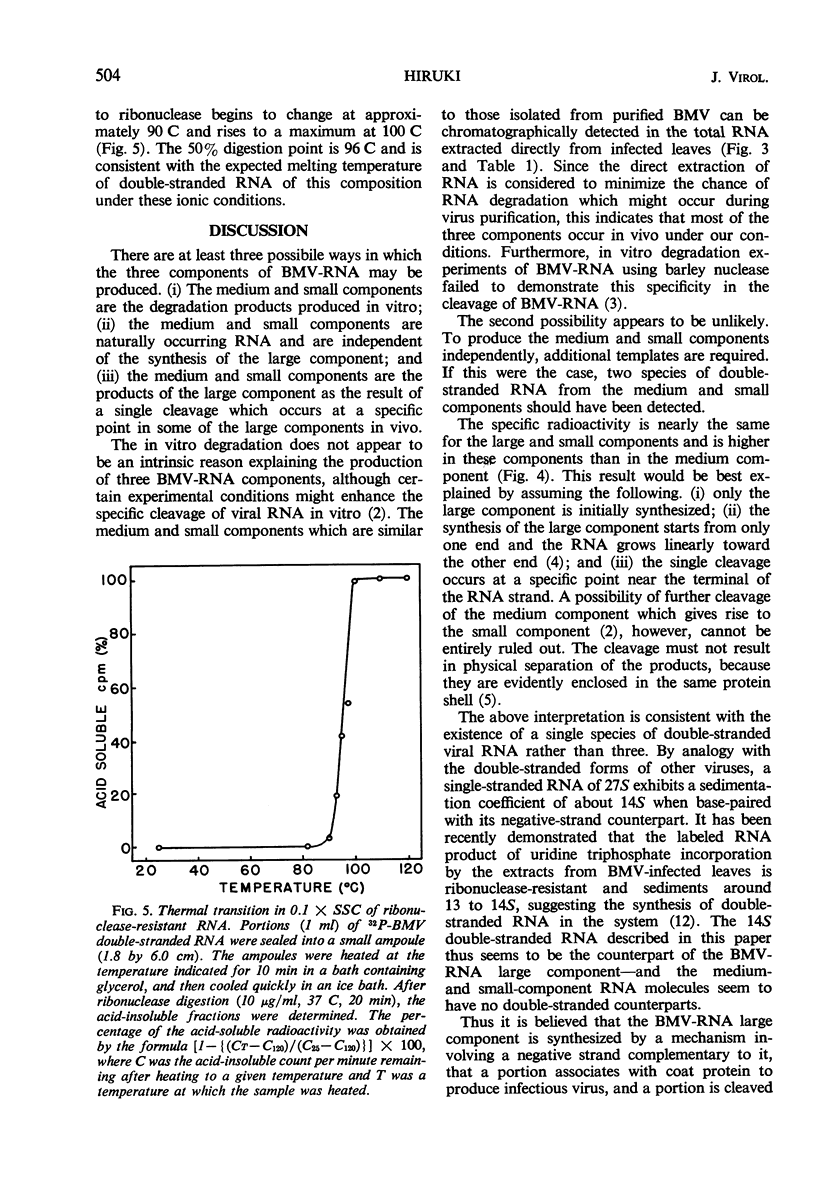
Incorporation of 32P into nucleic acids in barley plants infected with bromegrass mosaic virus (BMV) was analyzed by chromatography on methylated albumin kieselguhr (MAK) columns. Treatment with actinomycin D reduced the synthesis of ribosomal ribonucleic acid (RNA) to low levels and allowed the detection of the three components of BMV-RNA in vivo. The kinetic study on 32P incorporation into these BMV-RNA components suggested that a single cleavage occurred in some of the intact RNA shortly after completion of its synthesis, giving rise to the small and medium components. Chromatographic analyses also revealed a double-stranded, ribonuclease-resistant RNA which has been purified by differential extraction, sucrose-density gradient centrifugation, and MAK column chromatography. This RNA sediments at approximately 14S, is alkali-labile, and has a sharp thermal transition with a Tm of 96 C in 0.1 × standard saline citrate buffer, as determined by susceptibility to ribonuclease. The RNA is absent in uninfected barley plants.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMMANN J., DELIUS H., HOFSCHNEIDER P. H. ISOLATION AND PROPERTIES OF AN INTACT PHAGE-SPECIFIC REPLICATIVE FORM OF RNA PHAGE M12. J Mol Biol. 1964 Dec;10:557–561. doi: 10.1016/s0022-2836(64)80079-6. [DOI] [PubMed] [Google Scholar]
- Bancroft J. B., Hiebert E., Rees M. W., Markham R. Properties of cowpea chlorotic mottle virus, its protein and nucleic acid. Virology. 1968 Feb;34(2):224–239. doi: 10.1016/0042-6822(68)90232-8. [DOI] [PubMed] [Google Scholar]
- Bockstahler L. E., Kaesberg P. Isolation and properties of RNA from bromegrass mosaic virus. J Mol Biol. 1965 Aug;13(1):127–137. doi: 10.1016/s0022-2836(65)80084-5. [DOI] [PubMed] [Google Scholar]
- COMMONER B., SYMINGTON J. Linear biosynthesis of tobacco mosaic virus: incorporation of C14 into rods of different lengths. Proc Natl Acad Sci U S A. 1962 Nov 15;48:1984–1991. doi: 10.1073/pnas.48.11.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert E., Bancroft J. B., Bracker C. E. The assembly in vitro of some small spherical viruses, hybrid viruses, and other nucleoproteins. Virology. 1968 Mar;34(3):492–508. doi: 10.1016/0042-6822(68)90069-x. [DOI] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- MONTAGNIER L., SANDERS F. K. REPLICATIVE FORM OF ENCEPHALOMYOCARDITIS VIRUS RIBONUCLEIC ACID. Nature. 1963 Aug 17;199:664–667. doi: 10.1038/199664a0. [DOI] [PubMed] [Google Scholar]
- REDDI K. K. STUDIES ON THE FORMATION OF TOBACCO MOSAIC VIRUS RIBONUCLEIC ACID. III. UTILIZATION OF RIBONUCLEOSIDES OF HOST RIBONUCLEIC ACID. Proc Natl Acad Sci U S A. 1963 Sep;50:419–425. doi: 10.1073/pnas.50.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDDI K. K. Studies on the formation of tobacco mosaic virus ribonucleic acid. II. Degradation of host ribonucleic acid following infection. Proc Natl Acad Sci U S A. 1963 Jul;50:75–81. doi: 10.1073/pnas.50.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIPP W., HASELKORN R. DOUBLE-STRANDED RNA FROM TOBACCO LEAVES INFECTED WITH TMV. Proc Natl Acad Sci U S A. 1964 Aug;52:401–408. doi: 10.1073/pnas.52.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semal J., Hamilton R. I. RNA synthesis in cell-free extracts of barley leaves infected with bromegrass mosaic virus. Virology. 1968 Oct;36(2):293–302. doi: 10.1016/0042-6822(68)90147-5. [DOI] [PubMed] [Google Scholar]
- WEISSMANN C., BORST P., BURDON R. H., BILLETER M. A., OCHOA S. REPLICATION OF VIRAL RNA, III. DOUBLE-STRANDED REPLICATIVE FORM OF MSW PHAGE RNA. Proc Natl Acad Sci U S A. 1964 Apr;51:682–690. doi: 10.1073/pnas.51.4.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. E., Vlamis J. The Effect of Silicon on Yield and Manganese-54 Uptake and Distribution in the Leaves of Barley Plants Grown in Culture Solutions. Plant Physiol. 1957 Sep;32(5):404–409. doi: 10.1104/pp.32.5.404. [DOI] [PMC free article] [PubMed] [Google Scholar]



