Abstract
Leafhoppers (Insecta, Hemiptera, Cicadellidae) actively coat their integuments with buckyball-shaped submicron proteinaceous secretory particles, called brochosomes. Here, we demonstrate that brochosomal coats, recently shown to be superhydrophobic, act as non-stick coatings and protect leafhoppers from contamination with their own sticky exudates—filtered plant sap. We exposed 137 wings of Alnetoidia alneti (Dahlbom), from half of which brochosomes were removed, to the rain of exudates under a colony of live A. alneti. One hundred and fifty-two droplets became stuck to the bared wings and only three to the intact wings. Inspection of the wings with a scanning electron microscope confirmed that the droplets that had hit the intact wings had rolled or bounced off the brochosomal coats. This is the first experimental study that tested a biological function of the brochosomal coats of leafhopper integuments. We argue that the production of brochosomes in leafhoppers and production of epidermal wax blooms in other sap-sucking hemipterans are alternative solutions, both serving to protect these insects from entrapment by their exudates.
Keywords: surface, insect, non-stick, coating, superhydrophobic, excrement
1. Introduction
Exposed surfaces of some insects and plants have superhydrophobic properties owing to their micro- and nanoscale roughness; this principle is increasingly being used for creation of artificial water-repellent and self-cleaning materials (reviewed in [1,2]). While typically the superhydrophobic roughness is just passively present on the surface, recently we demonstrated [3] that leafhoppers (Cicadellidae), a megadiverse (more than 20 000 spp.) family of sap-sucking hemipterans, actively make their integuments superhydrophobic by coating them with buckyball-shaped submicron proteinaceous particles, produced inside the body in the Malpighian tubules [4,5]. Multiple hypothetical functions of these particles, called brochosomes, have been proposed [5,6], including repellence of leafhopper exudates (filtered plant sap), but none has been tested experimentally. Our demonstration that brochosomal coats are superhydrophobic [3] suggested that they can repel aqueous exudates of leafhoppers, but the proof is lacking. Moreover, removal of brochosomes from the integument decreased the water contact angles from more than 165° to 100–130°, which is still markedly hydrophobic [3]. Do brochosomal coats provide advantage over bare integument in repelling leafhopper exudates? In the first experimental study that tested a biological function of leafhopper brochosomal coats, we addressed this question by exposing intact and artificially bared, detached leafhopper wings to a rain of sticky exudates produced by live leafhoppers.
2. Material and methods
2.1. Insects
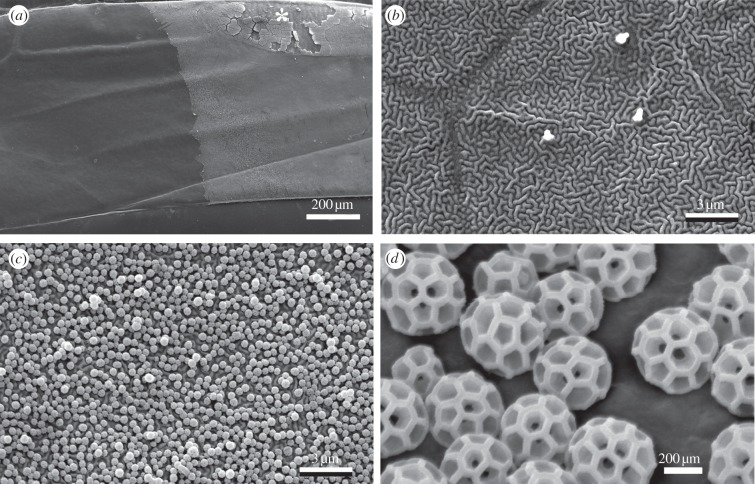
Alnetoidia alneti (Dahlbom) is a small (forewing length, 3.1–3.5 mm; figure 1a), common European leafhopper, which feeds on leaves of deciduous trees. The species belongs to the subfamily Typhlocybinae, members of which feed by sucking out mesophyll cells and produce sticky excretory droplets containing plant organelles (figure 1b). For this study, adults of both sexes were collected in Kiel, Northern Germany, from the maple Acer campestris L.
Figure 1.

Alnetoidia alneti (Dahlbom). (a) Adult insect. (b) Dried exudate spots on acrylic glass. Scale bars, 1 mm (a,b). (Online version in colour.)
2.2. Preparation of wing specimens
Forewings and hindwings of etherized A. alneti were detached at their bases with fine-tipped forceps and glued dorsal side up onto aluminium scanning electron microscopy (SEM) mushroom-shaped stubs (diameter, 9 mm) with a thin layer of commercial Rimmel 60-seconds nail polish (Coty Inc., UK). Each stub carried four to eight wings of the same type, arranged in a daisy pattern (figure 2a). Then we removed brochosomes from half of the wings by applying fluid polyvinylsiloxane and removing polymerized polyvinylsiloxane film with trapped brochosomes [3] (figure 3a–d) to obtain an alternating pattern of intact and bared wings on each stub (figure 2a). The stubs were secured in the vertical position on two 125 × 82 mm plates of acrylic glass. All procedures were performed with maximum care to avoid damage to the brochosomal coats. A total of 137 wings were prepared, including 32 intact forewings, 32 bared forewings, 36 intact hindwings and 37 bared hindwings.
Figure 2.
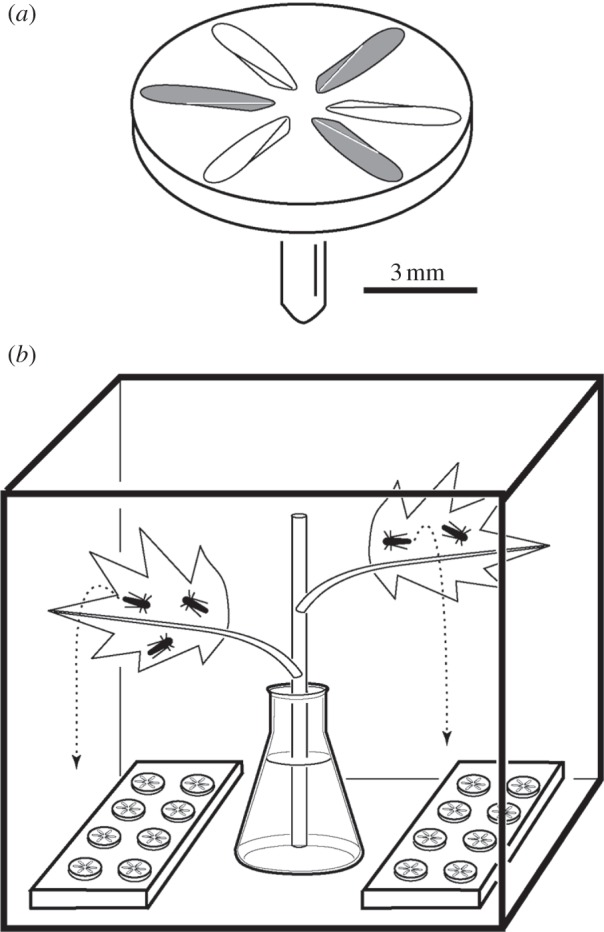
Schematic of the experiment (not to scale). (a) Aluminium SEM stub with six forewings of Alnetoidia alneti glued on its top; from three wings (shaded) brochosomes were removed with polyvinylsiloxane. (b) Experiment cage containing two plates with wing-carrying stubs and a maple branch cutting standing in water. Alnetoidia alneti feed at the abaxial surfaces of the leaves and produce a rain of exudates (dashed trajectories).
Figure 3.
Forewings of Alnetoidia alneti: intact and bared with polyvinylsiloxane. (a) Central part of a forewing the left half of which was bared for the purpose of illustration; note that the bare integument appears darker. The partly chipped area (asterisk) is specialized in Typhlocybinae to store a thick layer of brochosomes prior to further distribution across the integument. (b) Bared integument, displaying microtrichia (three protuberances) and vermiculate microsculpture. (c) Intact, brochosome-coated integument. (d) Close-up of (c) showing individual brochosomes.
2.3. Experiment
The experiment (figure 2b) was carried out inside a 30 × 30 × 30 cm meshed cage (LiveMonarch, USA) containing a cut branch of the maple A. campestris standing in a water-filled flask. Approximately 100 freshly caught adult A. alneti fed predominantly on the lower (abaxial) sides of the maple leaves and produced a rain of exudates falling down onto the cage bottom, where plates with the wing-carrying stubs were placed. The average distance between the wings and the feeding leafhoppers was ca 17 cm. We believe that this is within the potential hazard range in natural populations of the leafhopper. Because the intact and bared wings were placed closely together in an alternating fashion, and we had no control over the trajectories of the falling exudates, the probabilities of being hit by a droplet can be considered equal for both types.
The experiment continued for 6 days, during that time the plant was renewed twice. The average temperature and relative humidity were 23.6°C and 50.3%, respectively. At the end, the number of dry excrement spots stuck to the wings was counted under a stereomicroscope. The wing bases, where the brochosomal coats were damaged by forceps, were excluded from consideration. For closer examination, the wing-carrying stubs were sputtered with gold and examined in a Tescan Vega XMU scanning electron microscope (Tescan, Brno, Czech Republic).
3. Results
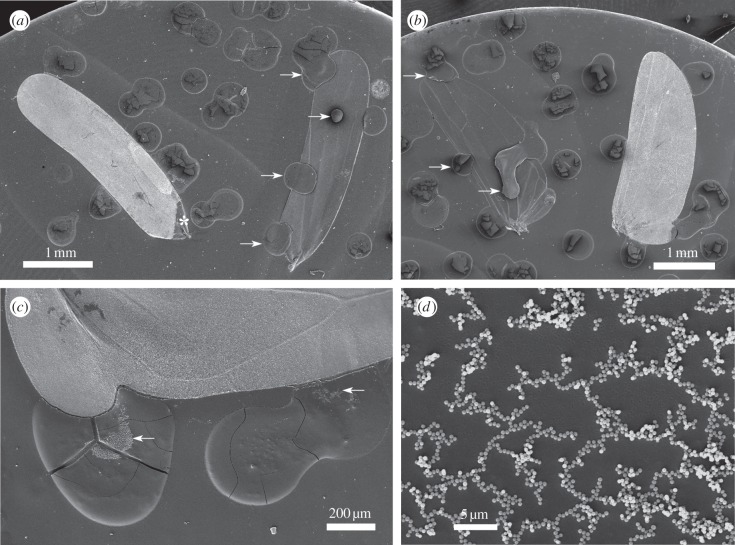
From the total of 155 dried exudate spots stuck to the wings, 152 were recorded on the artificially bared wings and only three on the intact, brochosome-coated wings (figure 4a,b). Each of the latter three spots was located mostly outside and only marginally extended onto the wing surface. The number of spots per wing was 2.2 ± 1.52 (mean ± s.d.) for the bared and 0.0 ± 0.20 for the intact wings (figure 5 shows these numbers separately for the fore- and the hindwings). This difference was highly significant (one-way ANOVA, F1,135 = 132.9, p < 0.00001). For exact spot counts across all studied wings, see the electronic supplementary material, table S1.
Figure 4.
Contamination of Alnetoidia alneti wings, arranged on tops of SEM stubs, with A. alneti exudates. (a) Intact (left) and bared (right) forewings. At the base of the intact wing the brochosomal coat was damaged with forceps (asterisk) during handling; arrows point out dried exudate spots on the bared wing. (b) Intact (right) and bared (left) hindwings; arrows point out dried exudate spots on the bared wing. (c) Dried exudate spots near the margin of an intact hindwing display brochosomes (arrows) stuck to their surface. (d) Close-up of a dried exudate spot showing brochosomes forming a meshwork.
Figure 5.
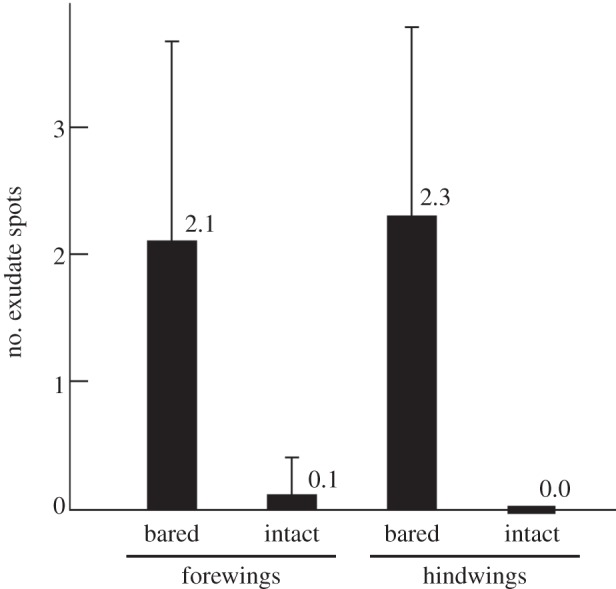
Contamination of intact versus bared wings of Alnetoidia alneti after 6 days of exposure to the rain of conspecific liquid exudates: mean number of dried exudate spots per wing (±s.d.).
SEM revealed that most exudate spots adjacent to the intact wings had brochosomes embedded into their surface (figure 4c,d), indicating that the droplet first hit the brochosomal coat and then bounced or rolled off. No bald spots were noted on such wings (figure 4a,b). The brochosomes sticking to surfaces of dried exudate spots were interconnected in long chains that included hundreds of particles (figure 4d).
4. Discussion
We demonstrated that the integuments of A. alneti are completely repellent to the exudates of that insect owing to their brochosomal coats. The observed difference in contaminability between the intact and bare wings—three versus 152 spots—is striking. Moreover, the three spots recorded on the intact wings only marginally extended onto the wing and probably resulted from lateral spreading of exudate from the adjacent stub, which can be considered as an experimental artefact.
The observed effect is explained by superhydrophobic properties of brochosomal coats, recently demonstrated for A. alneti and two other leafhopper species through static contact angle measurements [3]. These properties appear to result from the complex surface geometry of brochosomal layers, forming hierarchical roughness at the micro- and nanoscales (figure 3c,d), as is typical of natural and artificial superhydrophobic and self-cleaning surfaces (reviewed in [1,2]). Our extremely low estimates of the surface free energy of brochosomal coats (less than 1.0 mN m−1) predicted that these must be repellent to high surface tension liquids in general, including aqueous solutions, and we suggested that leafhopper exudates fall within that category [3]. The current study confirmed this for the exudates of typhlocybine leafhoppers, which feed on the contents of mesophyll cells.
Most studies of natural and artificial superhydrophobic surfaces focused on rigid microsculptures [1,2]. By contrast, brochosomal coats consist of loose powder, which partly comes off and sticks to the impacting liquid droplet (figure 4c,d). This observation is not at odds with the superhydrophobic properties of the brochosomal powder. If their adhesion to the solid surface is sufficiently weak, even hydrophobic particles adhere to aqueous droplets, producing the well-known Lotus effect [7] and causing chunks of hydrophobic wax to coat exudate droplets of some gall-inhabiting aphids [8]. In our experiment, impacting droplets removed only small amounts of the brochosomal powder (figure 4c,d), leaving no bald spots in the coats (figure 4a,b). The forces that keep brochosomes on the integument remain to be studied. Interestingly, many if not most individual brochosomes are interconnected into chains, sometimes branched (figure 4d; see [3]). The nature and origin of the bridges between particles are unknown, but they are likely to increase cohesion within the brochosomal coats and, consequently, their robustness to damage. Additionally, bouts of grooming, during which leafhoppers vigorously scrub their integuments with their legs [4,6], may repair accidental damage to the coats by rearranging brochosomes.
We demonstrated that, despite being distinctly hydrophobic, bare integuments perform poorly at repelling the exudates, whereas brochosomes increase such repellence dramatically. Such function of brochosomes has been suspected [5,6], but this study is the first one to test any biological function of the brochosomal coats of leafhopper integuments experimentally. The effect we observed using wing preparations is clearly of high importance for the survival of leafhoppers. For small and delicate insects, such as A. alneti and the majority of leafhoppers, contamination with their sticky exudates must result in the insect becoming entrapped or, no less lethal, its body parts becoming glued together. This hazard sufficiently explains why the integuments of these exclusively terrestrial insects display some of the largest superhydrophobic contact angles known among organisms [3], but protection against rain and against entrapment by plant glandular trichomes and spider webs may be additional benefits.
Sap-sucking hemipterans display diverse protective mechanisms against harmful contacts with their sticky exudates [9]. Forceful shooting of exudates away from the body is widespread, but it does not protect either from discharges of other individuals or from getting stuck to contaminated plant surfaces. Some galling aphids (Hemiptera, Aphidoidea) escape entrapment by the honeydew inside the narrow space of their galls by coating their exudate droplets with loose superhydrophobic wax powder [8]. Particulate waxes secreted by integuments of free-living aphids and other sap-sucking hemipterans display intricate structure at the submicron level [10–15], which probably (direct measurements are absent) makes them water repellent, similar to better studied cuticular waxes of plants [7]. One difference—and possible advantage—of brochosomal coats compared with such waxes is that brochosomes are applied by leafhoppers actively and the resulting coats apparently can be quickly modified, renewed or repaired on demand. Remarkably, whiteflies (Hemiptera, Aleyrodoidea) apply wax to their integument also in special behaviours [14,15]. Together with the fact that few leafhoppers produce waxes, and only in small quantities [3,16,17], these observations indicate that brochosomes of leafhoppers and waxes of other sap-sucking Hemiptera are alternative solutions of the same biological problem, both protecting these insects against their liquid exudates (for additional discussion, see [5,6]). Given the ubiquity of surface waxes in insects, it is intriguing that leafhoppers have evolved brochosomes rather than particulate wax coats. One possible explanation is that application of the Malpighian tubule products onto the integument had evolved in the leafhopper lineage prior to the origin of brochosomes and for a different function [18].
Acknowledgements
The work of the first author in Germany was made possible by a grant from the German Academic Exchange Service (DAAD) to R.R. and the SPP 1420 priority programme of the German Science Foundation (DFG) ‘Biomimetic Materials Research: Functionality by Hierarchical Structuring of Materials’ (project GO 995/9–1) to S.N.G.
References
- 1.Nosonovsky M, Bhushan B. 2008. Multiscale dissipative mechanisms and hierarchical surfaces. Friction, superhydrophobicity, and biomimetics. Berlin, Germany: Springer. [Google Scholar]
- 2.Nosonovsky M, Rohatgi PK. 2012. Biomimetics in materials science: self-healing, self-lubricating, and self-cleaning materials. New York, NY: Springer. [Google Scholar]
- 3.Rakitov R, Gorb SN. 2013. Brochosomal coats turn leafhopper (Insecta, Hemiptera, Cicadellidae) integument to superhydrophobic state. Proc. R. Soc. B 280, 20122391 ( 10.1098/rspb.2012.2391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rakitov RA. 1996. Post-moulting behaviour associated with Malpighian tubule secretions in leafhoppers and treehoppers (Auchenorrhyncha: Membracoidea). Eur. J. Entomol. 93, 167–184. [Google Scholar]
- 5.Rakitov RA. 2002. What are brochosomes for? An enigma of leafhoppers (Hemiptera, Cicadellidae). Denisia 4, 411–432. [Google Scholar]
- 6.Rakitov RA. 2009. Brochosomal coatings of the integument of leafhoppers (Hemiptera, Cicadellidae). In Functional surfaces in biology, vol. 1 (ed. Gorb SN.), pp. 113–137. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 7.Barthlott W, Neinhuis C. 1997. Purity of the sacred lotus or escape from contamination in biological surfaces. Planta 202, 1–8. ( 10.1007/s004250050096) [DOI] [Google Scholar]
- 8.Pike N, Richards D, Foster W, Mahadevan L. 2002. How aphids lose their marbles. Proc. R. Soc. Lond. B 269, 1211–1215. ( 10.1098/rspb.2002.1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss MR. 2006. Defecation behavior and ecology of insects. Annu. Rev. Entomol. 51, 635–661. ( 10.1146/annurev.ento.49.061802.123212) [DOI] [PubMed] [Google Scholar]
- 10.Pope RD. 1983. Some aphid waxes, their form and function (Homoptera: Aphididae). J. Nat. Hist. 17, 489–506. ( 10.1080/00222938300770431) [DOI] [Google Scholar]
- 11.Pope RD. 1985. Visible insect waxes: form, function and classification. Antenna 9, 4–8. [Google Scholar]
- 12.Foldi I. 1991. The wax glands in scale insects: comparative ultrastructure, secretion, function and evolution (Homoptera: Coccoidea). Ann. Soc. Entomol. Fr. 27, 163–188. [Google Scholar]
- 13.Smith RG. 1999. Wax glands, wax production and the functional significance of wax use in three aphid species (Homoptera: Aphididae). J. Nat. Hist. 33, 513–530. ( 10.1080/002229399300227) [DOI] [Google Scholar]
- 14.Navone P. 1987. Origine, struttura e funzioni di escreti e secreti entomatici di aspetto ceroso distribuiti sul corpo mediante zampe. Ann. Fac. Sci. Agr. Univ. Torino 14, 237–294. [Google Scholar]
- 15.Byrne DN, Hadley NF. 1988. Particulate surface waxes of whiteflies: morphology, composition and waxing behaviour. Physiol. Entomol. 13, 267–276. ( 10.1111/j.1365-3032.1988.tb00478.x) [DOI] [Google Scholar]
- 16.Rakitov RA. 1995. The covering formed by brochosomes on the cuticle of leafhoppers (Homoptera, Cicadellidae). Entomol. Rev. 74, 90–103. [Google Scholar]
- 17.Gaestel J, Mühlethaler R. 2012. Vorkommen und Verteilung von Brochosomen und Wachs bei Graphocephala fennahi und Cicadella viridis (Hemiptera: Cicadellidae). Mitt. Dtsch. Ges. Allg. Angew. Entomol. 18, 95–98. [Google Scholar]
- 18.Rakitov RA. 2002. Structure and function of the Malpighian tubules, and related behaviors of juvenile cicadas: evidence of homology with spittlebugs (Hemiptera, Cicadoidea & Cercopoidea). Zool. Anz. 241, 117–130. ( 10.1078/0044-5231-00025) [DOI] [Google Scholar]