Abstract
Objective(s): Drug resistant Acinetobacter baumannii have emerged as a major problem in many hospitals and intensive care units. Various types of extended spectrum beta-lactamases (ESBLs) are responsible for resistance to beta-lactam antibiotics in different parts of the world. The objective of this study was to determine the prevalence of integron class1 (INT 1) and ESBL types PER-1, PER-2 and VEB-1 among A. baumannii strains isolated from Tabriz, North-West of Iran.
Material and Methods: A total of 100 A. baumannii isolates collected from different clinical samples were included in the study. Antimicrobial susceptibility profiles were determined using the Kirby Bauer disk diffusion method. Production of ESBL was investigated by testing resistance against ceftazidime, cefotaxime, ceftriaxone and verified by Double Disk Synergy Test. DNA was extracted from the isolates and the frequency of INT 1 and ESBL types PER-1, PER-2 and VEB-1 were determined by PCR using specific primers.
Results: Among 100 A. baumannii isolates screened, 80 isolates were multidrug-resistant and 70 isolates were positive for ESBL production. PCR screening revealed that 74 % of the isolates contained class 1 integron, 51% were positive for PER-1 gene, 10% positive for VEB1 whereas none of the isolates were positive for PER2 type gene.
Conclusion: This is the first report of ESBL types VEB and PER in A. baumannii from North West of Iran. The results of this study demonstrated high prevalence of PER-1 and VEB-1 type ESBLs among A. baumannii isolates in the study region and reminded the necessity of appropriate infection control strategy to prevent further spread of infection by these organisms.
Key Words: Acinetobacter baumannii, Extended spectrum betalactamases, Integron class I
Introduction
Acinetobacter is a Gram-negative, aerobic, non-fermentative cocobacilli that has gained special attentions as a nosocomial opportunistic pathogen. Nutritional requirements of this bacterium are simple and they easily grow on most environmental conditions. Acinetobacter can survive on different medical equipments and even on healthy human skin (1). The multidrug-resistant A. baumannii (MDRAB) are defined as A. baumannii isolates that are resistant to at least three different classes of antimicrobial agents mainly betalactams, aminoglycosides, fluoroquinolones and carbapenems (2). There are increasing reports of MDRAB outbreaks in various clinical settings worldwide (3, 4). Carbapenems are currently the drugs of choice in the treatment of severe infections caused by this organism; however, carbapenem resistant A. baumannii is now reported increasingly throughout the world (5-8).
Extended spectrum beta lactamases (ESBLs) are a class of group A beta lactamases which results in hydrolysis of first, second, and third generation cephalosporines but are inhibited by beta-lactamase inhibitors like Acid clavulanic (9-11). Classic extended spectrum beta lactamases are originated from the plasmid encoding ESBLs of TEM (Temoneira) /SHV (sulphydril variable), and OXA (oxacillinase) families. But in recent years, new families of extended spectrum beta lactamases have also emerged all over the world (12), including PER (for Pseudomonas extended resistance) and VEB (for Vietnamese extended-spectrum beta–lactamase) families (13). Plasmid is responsible for the distribution of most beta lactamases, however, the gene encoding for these enzymes may also be on the chromosomes or transposable elements, integrons (14). Five different classes of integrons have been found among which INT-1 is the most common type found in the gram negative bacilli and clinical isolates of A. baumannii (2, 15). It has shown that carriage on integrones facilitates the spread of resistance genes among bacteria.
This study was carried out to determine the prevalence of integron class I, PER and VEB type ESBLs among A. baumannii strains isolated from patients hospitalized in Imam Reza Hospital of Tabriz, in Northwest of Iran.
Materials and Methods
Bacterial isolation and identification
In this study, a total of 100 non-duplicate isolates of A. baumannii were collected from a University Hospital in Tabriz city located in Northwest of Iran between March 2008 and June 2009. The isolates were from different clinical samples including tracheal secretion, bronchial lavage, blood, wound, sputum, abscess drainage, peritoneal fluid, and urine. Identification of the isolates were performed using standard microbiological tests such as; Gram stain, oxidase test, growth at 44°C and O/F test.
Antimicrobial susceptibility of isolates
Antimicrobial susceptibility of isolates was determined by Kirby Bauer disk diffusion method using Clinical Laboratory Standard Institute (CLSI) guidelines. A single pure colony was inoculated into 3 ml of normal saline and vortexed to mix well. The inoculum size was standardized by comparison of the turbidity of the bacterial suspension with 0.5 McFarland Standard. A sterile cotton-tipped swab was dipped into the suspension and used to lawn the bacterial suspension onto the Muller Hinton agar plate. The antibiotic discs cefotaxime (30 μg), cefixime (5 μg), ceftizoxime (30 μg), ceftazidime (30 μg), ceftriaxon (30 μg), cephalexin (30 μg), cephalotin (30 μg), tetracycline (30 μg), ciprofloxacin (5 μg), cotrimoxazole (25 μg), kanamycin (30 μg), ticarcilin (75 mg), polymixin B (300 μg), tobramycin (10 μg), choloramphenicol (30 μg), norfloxacin (10 μg), ofloxacin (5 μg), amikacin (30 μg), gentamicin (10 μg), ampicillin (10 μg), carbenicillin (100 μg), rifampin (5 μg), colistin (110 μg ) (MAST, Merseyside, UK) were then placed on the plates and incubated at 37°C overnight. The diameter of inhibition zones was measured using the CLSI guidelines. Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as controls.
Detection of ESBL production by double disc synergy test
After inoculation of the isolates on Muller Hinton agar media an amoxicillin-clavulanic acid disc was placed in the center of plate and the cefotaxim, ceftazidim, cefpime and azteronam discs were placed in a distance of 10 mm from the central disc and 20 mm from each other. Plates were incubated at 37°C for 18 hr and the increase in the sensitivity zone and its intend toward amoxicillin-clavulanic acid disc was considered as positive for production of ESBL.
DNA Extraction and PCR amplification
All A. baumannii isolates were grown for 18 hr at 37°C in MacConkey agar and DNA was extracted by SDS-Proteinase K phenol chloroform method as described (16). Briefly, 4-5 fresh colonies was resuspended in 300 µl of TE buffer, SDS (1%) and proteinase K (10 μg/ml), and incubated at 40°C for 3 hrs followed by phenol-chloroform extraction and ethanol precipitation. Finally, DNA was dissolved in distilled water and quality and concentration of the DNA was checked with a spectrometer and on a 1% agarose gel.
Detection of PER1, PER-2, VEB-1 and class I integron in clinical isolates of A. baumannii was carried out by PCR with primers illustrated in the Table 1. OXA51 PCR was also performed for confirmation of the identity of isolates. PCR master mix components were as follows; 10X PCR buffer in final concentration of 1X, MgCl2 (50mM) in a final concentration of 1.5 mM, dNTP mix, 10 mM in a final concentration of 0.2 mM and forward and reverse primers in a final concentration of 0.4μM. PCR amplification was performed in a total volume of 25 μl (24 μl of PCR master mix plus 1 μl of template DNA).
PCR amplification conditions were as follows: initial denaturation at 94°C for 4 min followed by 35 cycles of 60 sec denaturation at 94°C, 60 sec annealing at primers annealing temperature (Table 1) and 45 sec extension at 72°C with a final extension at 72°C for 7 min. PCR products were analyzed by electrophoresis in 1.2% agarose gel in a TAE buffer at 90 volts alongside with DNA ladder. The gel was then stained with ethidum bromide for 20 min and finally visualized in gel documentation system.
Table 1.
The sequence of primers accompanied with their annealing temperatures and expected PCR product sizes
| Primer | Primer sequences | Annealing temperature |
Product size (bp) |
|---|---|---|---|
| VEB-F VEB-R |
5'-GAAACAACTTTGACGATTGA-3' 5'-CCCTGTTTTATGAGCAACAA-3' |
50°C | 370 |
| PER1-F PER1-R |
5'-ATGAATGTCATTATAAAAGC-3' 5'-AATTTGGGCTTAGGGCAGAA-3' |
50°C | 925 |
| PER2-F PER2-R |
5'-GTAGTATCAGCCCAATCCCC-3' 5'- CCAATAAAGGCCGTCCATCA-3' |
50°C | 738 |
| INT-1-F INT-1-R |
5’-GGTGTGGCGGGCTTCGTG-3' 5’-GCATCCTCGGTTTTCTGG-3' |
55°C | 456 |
| OXA-51F OXA-51R |
5’-ACAAGCGCTATTTTTATTTCAG-3' 5’-CCCATCCCCAACCACTTTT-3' |
51°C | 641 |
Results
Patients and Bacterial isolation
A total of 100 A. baumannii isolates were obtained from patients that had been admitted to the university hospital of Imam Reza in Tabriz, North-West of Iran. The isolates were obtained from different clinical samples including the respiratory system specimens (54%), urine (21%), blood (7%), catheter (6%), and other sources (12%) (Table 2). 37% of the isolates were from hospitalized patients in the intensive care units (ICU). The age range of the patients was from 14 to 86 years old. The isolates were obtained from patients belonging to different age groups: 20-39 years (n = 25), 40-59 (n= 39), 60-90 years (n = 33) and three isolates were from patients less than 20 years old. 29 % of patients sustained trauma and invasive procedures such as intubation and tracheostomy were used for 63% of patients.
Table 2.
Sources of Acinetobacter baumannii isolates
| Source | Frequency (%) |
|---|---|
| Tracheal | 37% |
| Urine | %21 |
| Sputum | 9% |
| Blood | %7 |
| Catheter | %6 |
| Bronchial washing | %6 |
| wound | %5 |
| Abscess drainage | %3 |
| CSF | 2% |
| Pleural effusion | %2 |
| Ascites fluid | %2 |
| Total | % 100 |
Bacterial identification
Biochemical tests revealed that all isolates belonged to A. baumannii species. Analysis for presence of blaOXA-51-like gene demonstrated that all isolates were positive for blaOXA-51-like gene that confirmed their identity as A. baumannii.
Antibiotic susceptibility testing
The drug resistance pattern of isolates has been shown in Table 3. The highest resistance rate was against cefixime and ceftizoxim whereas the lowest resistances were to polymixin B (16%), colicitin (19%) and ampicillin-sulbactam (55%). 62% and 63% of isolates were resistant to imipenem and meroprnem, respectively. Double disc synergy test analysis revealed that 70% of isolates were ESBL positive.
ESBL Genotypes
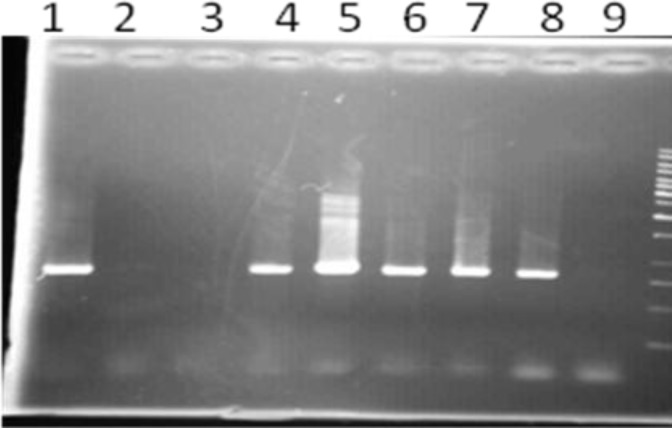
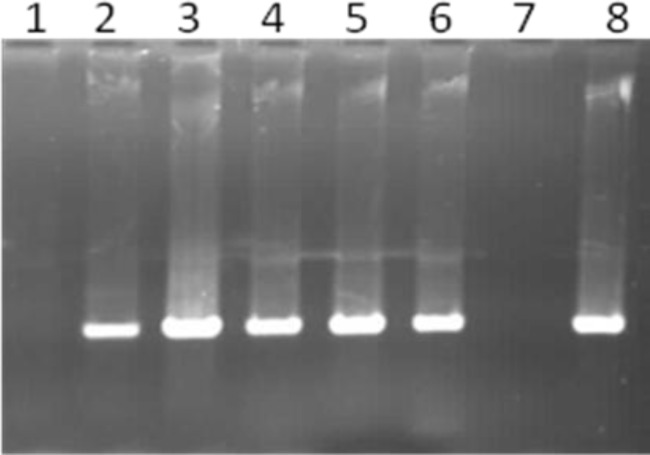
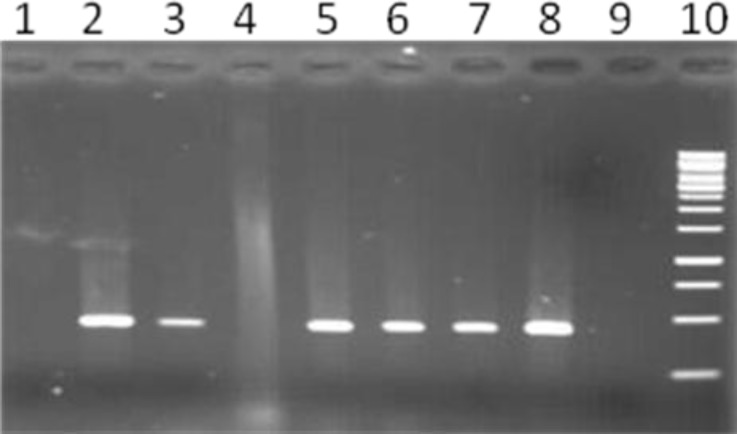
Screening for PER-1, PER-2 and VEB-1 genotypes by PCR among ESBL positive isolates revealed that 51% of isolates were positive for PER1 gene (Figure 1) whereas VEB-1 determinant were positive in 10 % of isolates (Figure 2). None of the isolates were positive for PER-2 ESBL gene. PCR amplification for INT-1 gene showed that 73% of isolates contained the INT-1 insertion sequence (Figure 3).
Figure 1.
PCR amplification of PER-1 resistance gene among ESBL positive Acinetobacter baumannii isolates.
Figure 2.
PCR amplification of VEB-1 resistance gene among ESBL positive Acinetobacter baumannii isolates
Figure 3.
PCR amplification of Int-1 mobile element among Acinetobacter baumannii isolates
Table 3.
Antimicrobial resistance pattern of Acinetobacter baumannii against different antibiotics
| Antimicrobial agent | Resistance (%) | Antimicrobial agent | Resistance (%) |
|---|---|---|---|
| Cefpodoxime Ceftizoxime Cefixime Ticarcilin Aztreonam Ceftriaxone Ceftazidime Ampicillin Cefepime |
100 100 100 100 97 94 92 91 88 |
Co-Trimixazole Gentamicin Ciprofloxacin Levofloxacin Meropenem Imipenem Ampicillin-sulbactam Colisitin Polymixin B |
79 78 78 76 63 62 55 19 16 |
Discussion
Nosocomial infections are a major source of mortality and morbidity in hospitalized patients (17) and A. baumannii plays an important role in these infections due to its resistance against multiple classes of antibiotics (18). Production of ESBLs is one of the main mechanisms for antibiotic resistance
in gram negative bacteria including A. baumannii and detection of ESBL production and related genotypes is critical for surveillance of drug resistance in different geographic regions.
In this study, the rate of ESBL production, the prevalence of ESBL genotypes PER1, PER-2, VEB-1 and the presence of INT-1 gene were investigated in A. baumannii isolates recovered from patients hospitalized in Emam-Reza Hospital of Tabriz which is the largest university hospital in North-west region of Iran.
Findings of the present study showed that 70% of the studied Acinetobacter isolates were positive for ESBL. This indicates a high frequency of resistance due to ESBL in the studied cases. Previous reports from Iran showed the ESBL production in 39 % of A. baumannii (19) which indicates an increase in the rate of antibiotic resistance due to ESBL.
Screening for PER-1 genotype revealed that 51% of A. baumannii isolates contained PER-1 gene. This results is similar to the reports which were published by researchers from Turkey (46%) (19) and South Korea (54.6%) (20) and indicates that PER-1 is the most common ESBL genotype in A. baumannii.
Investigation through PCR for VEB-1 type ESBL showed that among 70 ESBL positive isolates, VEB-1 gene was positive in 10% of cases that all belonged to MDRAB isolates. Literature review for VEB-1 shows that the prevalence of this ESBL varies in different part of the world. VEB-1 type ESBL in A. baumannii was reported for the first time by Laurent Poirel in 58.33% of isolates in France in 2003 (21). In a study performed by Thierry Naas and his colleagues in Belgium in 2006, 6 out of 8 isolates of A. baumannii were positive for VEB-1 (22) whereas Pasteran and Rapopot was reported VEB-1 in 47.61% of isolates in the USA (23). Also, VEB type
ESBL reported in 4 out of 6 clinical A. baumannii isolates (66 %) in Argentin (24). It has been shown that integrons are one of the main factors in carriage and distribution of drug resistant genes among bacteria. In this study, the screening for INT-1 gene by PCR showed that among 70 ESBL positive isolates the INT-1 gene were positive in 63.3% of cases. In a study carried out by Francesca Gomac and his colleagues in Italy, 16 out of 36 A. baumannii isolates (44%) contained integron I (25) whereas Javier Ariza and his colleagues found integron I in 27.53% of A. baumannii isolates in Spain (15). A recent report from China (2) showed integron class I in 71.4% (202 out of 283) of A. baumannii isolates, which is consistent with our findings in this research.
In this study, none of the isolates were positive for PER-2 gene. Few studies have been carried out about PER-2 gene in the world. This type of ESBL was identified for the first time in A. baumannii isolates in the U.S.A in 2006 (23). Also, Poirel and his colleagues (2007) reported PER-2 gene in one out of 6 (16.6%) A. baumannii isolates in Argentina. It seems that either PER-2 type ESBL distribution is restricted to some specific geographic regions or it may not have been studied in other parts of the world.
The results of this study also revealed that 37% of A. baumannii isolates were from patients hospitalized in the ICU wards most of whom (79%) were ESBL positive. It may not be surprising since it has already been shown that ICU stay and long term antibiotic use in these patients is one of the risk factors that predispose patients for A. baumannii infections ( 26).
Conclusion
This study revealed high antimicrobial resistance among A. baumannii isolates in the study region. The ESBL type PER and VEB played an important role in this antimicrobial resistance reminding the necessity of appropriate preventive policy in hospitals to control further spreading of these highly resistant strains.
References
- 1.Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH. Manual of Clinical Microbiology. 7th ed. Washington,D.C: ASM Press; 1999. pp. 517–525. [Google Scholar]
- 2.Huang LY, Chen TL, Lu PL, Tsai CA, Cho WL, Chang FY, et al. Dissemination of multidrug-resistant, class 1 integron-carrying Acinetobacter baumannii isolates in Taiwan. Clin Microbiol Infect. 2008;14:1010–1019. doi: 10.1111/j.1469-0691.2008.02077.x. [DOI] [PubMed] [Google Scholar]
- 3.Nowak-Zaleska A, Krawczyk B, Kotłowski R, Mikucka A, Gospodarek E. Amplification of a single-locus variable-number direct repeats with restriction fragment length polymorphism (DR-PCR/RFLP) for genetic typing of Acinetobacter baumannii strains. Pol J Microbiol. 2008;57:11–17. [PubMed] [Google Scholar]
- 4.Peleg AY, Seifert H, Paterson DL. Successful Pathogen Acinetobacter baumannii: Emergence of a10.1128/CMR.00058-07. Clin Microbiol Rev. 2008;21:538. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marais E, G de Jong, Ferraz V, Maloba B, Duse AG. Interhospital transfer of pan-resistant Acinetobacter strains in Johannesburg,South Africa. Am J Infect Control. 2004;32:278–281. doi: 10.1016/j.ajic.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Merkier AK, Catalano M, Ramírez MS, Quiroga C, Orman B, Ratier L, et al. Polyclonal spread of blaOXA-23 and blaOXA-58 in Acinetobacter baumannii isolates from Argentina. J Infect Dev Ctries. 2008;2:235–240. doi: 10.3855/jidc.269. [DOI] [PubMed] [Google Scholar]
- 7.Coelho J, Woodford N, Afzal-Shah M, Livermore D. Occurrence of OXA-58-Like Carbapenemases in Acinetobacter spp. collected over 10 years in three continents. Antimicrob Agents Chemother. 2006;50:756–758. doi: 10.1128/AAC.50.2.756-758.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 9.Bradford PA. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001;14:933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacoby GA, Munoz-Price LS. Mechanisms of disease the new ß-Lactamases. N Engl J Med. 2005;352:380–3891. doi: 10.1056/NEJMra041359. [DOI] [PubMed] [Google Scholar]
- 11.Gniadkowski M. Evolution and epidemiology of extended-spectrum b-lactamases (ESBLs) and ESBL-producing microorganisms. Clin Microbiol Infect. 2001;7:597–608. doi: 10.1046/j.1198-743x.2001.00330.x. [DOI] [PubMed] [Google Scholar]
- 12.Bush k. New beta-lactamases in gram negative bacteria: diversity and impact on the selection of antimicrobial therapy. Clin Infect Dis. 2001;32:1085–1089. doi: 10.1086/319610. [DOI] [PubMed] [Google Scholar]
- 13.Stürenburg E, Mack D. Extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory, therapy, and infection control. J Infect . 2003;47:273–295. doi: 10.1016/s0163-4453(03)00096-3. [DOI] [PubMed] [Google Scholar]
- 14.Rasheed JK, Jay C, Metchock B, Berkowitz F, Weigel L, Crellin J, etal Evolution of extended-spectrum b-lactam resistance (SHV-8) in a strain of Escherichia coli during multiple episodes of bacteremia. Antimicrob Agents Chemother. 1997;41:647–653. doi: 10.1128/aac.41.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ribera A, Vila J, Fernández-Cuenca F, Martínez-Martínez L, Pascual A, Beceiro A, et al. Spanish Group for Nosocomial Infection (GEIH). Type 1 integrons in epidemiologically unrelated acinetobacter baumannii isolates collected at spanish hospitals, American Society for Microbiology. Antimicrob Agents Chemother . 2004;48:364–365. doi: 10.1128/AAC.48.1.364-365.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yousefi S, Farajnia S, Nahaei MR, Akhi MT, Ghotaslou R, Soroush MH, et al. Detection of metallo-β-lactamase-encoding genes among clinical isolates of Pseudomonas aeruginosa in northwest of Iran. Diagn Microbiol Infect Dis . 2010;68:322–5. doi: 10.1016/j.diagmicrobio.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 17.García-Garmendia JL, Carlos OL, José GM, Francisco-Javier J-J, Carmen P-P, Ana EBA, et al. Risk Factors for Acinetobacter baumannii Nosocomial Bacteremia in Critically Ill Patients :A Cohort Study. Clin Infect Dis. 2001;33:939–946. doi: 10.1086/322584. [DOI] [PubMed] [Google Scholar]
- 18.Perez F, Hujer AM, Hujer KM, Decker BK, Rather PhN, Bonomo RA. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2007;51:3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vahaboglu H, Oztürk R, Aygün G, Coşkunkan F, Yaman A, Kaygusuz A, et al. Wide- spread detection of PER-1-type extended-spectrum beta-lactamases among nosocomial Acinetobacter and Pseudomonas aeruginosa isolates in Turkey: A nationwide multi-center study. Antimicrob Agents Chemother. 1997;41:2265–2269. doi: 10.1128/aac.41.10.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yong D, Shin JH, Kim S, Lim Y, Yum JH, Lee K, et al. High prevalence of PER-1 extended-spectrum beta-lactamase-producing Acinetobacter spp. in Korea. Antimicrob Agents Chemother. 2003;47:1749–1751. doi: 10.1128/AAC.47.5.1749-1751.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poirel L, Menuteau O, Agoli N, Cattoen C, Nordmann P. Outbreak of extended-spectrum beta-lactamase VEB-1-producing isolates of acinetobacter baumannii in a French Hospital. J Clin Microbiol. 2003;41:3542–3547. doi: 10.1128/JCM.41.8.3542-3547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naas T, Bogaerts P, Bauraing C, Degheldre Y, Glupczynski Y, Nordmann P. Emergence of PER and VEB extended-spectrum beta-lactamases in Acinetobacter baumannii in Belgium. J Antimicrob Chemother. 2006;58:178–82. doi: 10.1093/jac/dkl178. [DOI] [PubMed] [Google Scholar]
- 23.Pasterán F, Rapoport M, Petroni A, Faccone D, Corso A, Galas M, et al. Emergence of PER-2 and VEB-1a in Acinetobacter baumannii Strains in the Americas. Antimicrob Agents Chemother. 2006;50:3222–3224. doi: 10.1128/AAC.00284-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poirel L, Corvec S, Rapoport M, Mugnier P, Petroni A, Pasteran F, et al. Identification of the novel narrow-spectrum ß-lactamase SCO-1 in Acinetobacter spp. from Argentina. Antimicrob Agents Chemother. 2007;51:2179–2184. doi: 10.1128/AAC.01600-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gombac F, Riccio ML, Rossolini GM, Lagatolla C, Tonin E, Monti-Bragadin C, et al. Molecular characterization of integrons in epidemiologically unrelated clinical isolates of acinetobacter baumannii from Italian hospitals reveals a limited diversity of gene cassette arrays. Antimicrob Agents Chemother. 2002;46:3665–3668. doi: 10.1128/AAC.46.11.3665-3668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis. 2006;42:692–699. doi: 10.1086/500202. [DOI] [PubMed] [Google Scholar]