Abstract
Background
Possible beneficial effects of dietary omega-3 supplementation on patients with congestive heart failure (CHF) were investigated.
Methods and results
100 patients with CHF who had a tri-chamber pacemaker and automated defibrillator were initially recruited, and 70 agreed to participate.38 patients received 2 g/day of omega-3 and 32 received placebo capsules. BNP level, 6-min walk test and echocardiographic parameters were recorded at baseline and after 6 months of treatment.
BNP levels decreased significantly after 6 months in the omega-3 group, from 1766.2 ± 1978.1 pg/mL to 1159.4 ± 1430.9 pg/dL (P < 0.005). Tei index and late diastolic velocity index were significantly improved in treated group. Mortality and hospitalization rates did not differ.
Conclusion
The beneficial effects of omega-3 supplementation in patients with CHF were not as clear as hypothesized; however, omega-3 fatty acids can result in small changes in plasma BNP levels and modest improvements in echocardiographically assessed diastolic function (Clinical trial.gov registration: NCT01227837).
Keywords: Omega-3, Brain natriuretic peptide, Heart failure, Congestive
1. Introduction
Heart failure is considered a major problem in both developed and developing countries, as one of the leading causes of morbidity and burden of disease.1,2 It is estimated that around 23 million people are affected worldwide, with a prevalence of approximately 2% in the adult population in developed countries.3 The prevalence of heart failure increases with age, and is higher than 66 per 1000 among those aged 80–89 years.4,5 The mainstays of treatment are symptomatic therapy and efforts to slow the progression of the disease, as there is no definite treatment.6 Several therapeutic medications have been introduced with various degrees of efficacy; however, they are not all effective and each has specific side effects which sometimes worsen the patient's general condition.6 Numerous efforts have been made to develop newer and safer medications to treat heart failure symptoms.
N-3 polyunsaturated fatty acids (PUFA), also known as omega-3 fatty acids, reduce the risk of cardiovascular events in patients with chronic heart conditions.7 At doses of 3–4 g/day, they are now widely used as lipid-lowering agents that mainly reduce levels of triglycerides.8 Recently there have been reports of the beneficial effects of PUFA in patients with congestive heart failure (CHF).9–11 Omega-3 supplementation can decrease plasma levels of inflammatory markers that contribute to the exacerbation and progression of CHF, including N-terminal pro-brain natriuretic peptide (NT-proBNP).12,13 Some authors have concluded that omega-3 supplementation can prevent the development and progression of CHF, and it was shown in this connection that omega-3 fatty acids decrease the rate of sudden cardiac death in patients with CHF.14–16 Omega-3 fatty acids also decrease the incidence of atrial fibrillation in these patients.16–18
However, there is still controversy over the actual efficacy and safety of omega-3 supplements as an alternative therapeutic agent for CHF.15 In this double-blind placebo-controlled randomized clinical trial we aimed to investigate the effect of omega-3 supplementation on plasma concentrations of BNP, a diagnostic marker of CHF and its severity, along with its possible beneficial effects on the 6-min walk test and echocardiographic indicators in patients with class II or III heart failure in Shiraz, Iran.
2. Materials and methods
2.1. Participants and recruitment
This double-blind placebo-controlled randomized trial was conducted in Shiraz (southern Iran) during a 1-year period from April 2008 to April 2009. The criteria for eligibility for recruitment were class II or III CHF resulting from underlying ischemic heart disease, previous registration with or admission to hospitals affiliated with Shiraz University of Medical Sciences, recorded ejection fraction (EF) of less than 40% on echocardiography, use of cardiac resynchronization therapy (CRT) and an automatic implantable cardiac defibrillator (AICD) for at least 6 months prior to registration, and no previous omega-3 supplementation. Patients with symptomatic CHF and hospital admission in the preceding 3 months, and any rhythm other than normal sinus rhythm, were excluded. Normal functioning of CRT and AICD was confirmed in all patients prior to registration. All medications were synchronized and each patient received digoxin, an angiotensin-converting enzyme inhibitor, a diuretic, a beta-blocker and a nitro group agent as oral medications.
The 100 patients initially considered eligible were contacted and invited to participate in the study. The study protocol was described to them, and they were informed that their anonymity would be protected and they had the right to withdraw from the study at any time during the trial. Of the 100 patients we approached, 70 agreed to participate. After contacting patients to register for the trial, the protocol and possible side effects were described in detail and written informed consent was obtained from each participant.
Physicians who were unaware of the protocol interviewed each patient with a standardized questionnaire to record personal and demographic data along with information about medications and other risk factors of CHF. A simple randomization technique was used to allocate patients to one of the two treatment protocols in the study. A nurse who was unaware of the patients' medication and condition randomized participants and delivered medications (placebo or omega-3) to patients. Medications were given to patients in the same unlabeled package, and were relabeled only after the study was completed. In group A, 38 patients received omega-3 supplements, and in group B (control), 32 patients received a placebo supplement. Participants in both groups were matched for age and sex and were unaware of which treatment arm they were assigned to. Fig. 1 shows the recruitment and allocation process.
Fig. 1.
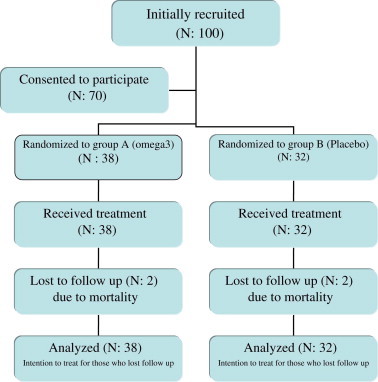
Patient allocation and study design.
2.2. Protocol definition and implementation
Group A received omega-3 supplementation in the form of capsules at a does of 2 g/day (two capsules per day each containing 1000 mg omega-3 fatty acid) for 6 months. Group B received placebo capsules that contained distilled water with color of omega-3 capsules, twice per day.
The patients' other medications were continued during the trial. All patients were followed monthly at the Faghihi Hospital echocardiography center by the same physician, who was unaware of their treatment allocation. In monthly meetings with the study nurse, who was unaware of the treatment allocation, each patient was given a month's supply of the medication and any questions they had were answered.
At baseline and before treatment began, 4 mL of blood was drawn from each individual to measure baseline plasma concentration of BNP. Patients were then asked to perform a 6-min walk test and a cardiologist performed conventional and tissue-specific echocardiographic studies (ACUSON S 2000™ Cardiovascular, Siemens, Erlangen, Germany). All data were recorded in each patient's spreadsheet. After 6 months echocardiography, BNP determination and the 6-min walk test were repeated in all participants, and outcome analysis was performed as per the protocol or according to intention to treat.
The study protocol was reviewed and approved by the Shiraz University of Medical Sciences Ethics Committee. The protocol design and reporting complied with the Consolidated Standards of Reporting Trials (CONSORT) statement.19
2.3. Outcome measures
Three different outcomes were measured to compare the efficacy of omega-3 fatty acid administration in patients with CHF. Brain natriuretic peptide, a hormonal peptide that increases significantly in CHF due to increased filling pressure of the atria, was assessed as an indicator of CHF severity and a prognostic factor. Plasma BNP concentration was measured at baseline and after 6 months of treatment with a chemiluminescent enzyme immunoassay, for which 4 mL of blood was drawn from an antecubital vein in each patient, transferred to tubes with no anticoagulant, centrifuged at 2000 rpm and frozen at −20 °C. Elecsys proBNP sandwich immunoassay kits (Roche Diagnostics, Mannheim, Germany) were used to measure NT-ProBNP concentration in each blood sample, and the results were reported in μg/mL.
Two-dimensional and conventional tissue-specific echocardiography was performed by the same cardiologist throughout the study, who was unaware of which group the participants had been assigned to. Echocardiographic studies were done at baseline and after 6 months to record wall motion, filling pressure and EF to evaluate the possible beneficial effects of omega-3 supplements.
2.4. 6-min walk test
Patients were asked to perform the 6-min walk test on a flat surface at baseline and after 6 months. Walking distance was measured in meters and distances were recorded in each participant's spreadsheet.
2.5. Statistical analysis
All data are reported as the mean ± standard deviation. Baseline and 6-month echocardiographic findings, the results of the 6-min walk test and plasma BNP concentrations were compared within each treatment group and between groups A and B with the Kolmogorov–Smirnov one-sample test. All statistical analyses were done with SPSSv. 11.5 software. P values less than 0.05 were considered statistically significant.
3. Results
During the course of the trial 3 patients in group A and 1 patient in group B withdrew from the study. Another 3 patients (1 in group B and 2 in group A) died during the trial. These patients were included in the intention to treat analysis. Table 1 summarizes the demographic characteristics, baseline BNP concentrations and echocardiographic features in participants in both groups at the start of the study.
Table 1.
Demographic features of patients in both groups.
| Demographic features | Treatment group | Placebo group | P value* |
|---|---|---|---|
| Age (years) | 56 | 58 | 0.4 |
| Male gender (%) | 58 | 61 | 0.34 |
| Smoking (%) | 34 | 28 | 0.2 |
| Diabetes (%) | 39 | 32 | 0.38 |
| Hypercholesterolemia (%) | 24 | 30 | 0.8 |
| High blood pressure (%) | 23 | 34 | 0.5 |
*Indicate the level of significance of P.
The average distance walked at baseline was 281.7 ± 108.4 m in group A and 264.2 ± 117.5 m in group B (P = 0.83). However, after 6 months no significant increase was seen in patients who received omega-3 supplements compared to the placebo group (319 ± 103.8 vs. 274.1 ± 127.9, P = 0.06) (Table 2 and Table 3).
Table 2.
Plasma BNP level, 6-min walk test results and echocardiographic parameters at baseline and after 6 months in the two groups.
| Variables | Group | Baseline | After 6 months | P value* |
|---|---|---|---|---|
| Plasma BNP (pg/mL) | Omega-3 | 1766.23 ± 1978 | 1159.4 ± 1430.9 | 0.0005 |
| Placebo | 1802 ± 1670 | 2121.5 ± 2703.9 | 0.32 | |
| 6-min walk test (m) | Omega-3 | 284.2 ± 111.57 | 319.27 ± 103.8 | 0.0005 |
| Placebo | 262 ± 121.74 | 274.15 ± 127.9 | 0.49 | |
| End systolic diameter (mm) | Omega-3 | 50.56 ± 12.37 | 53.35 ± 10.81 | 0.01 |
| Placebo | 50.42 ± 13.69 | 53.76 ± 12.41 | 0.02 | |
| End diastolic diameter (mm) | Omega-3 | 61.83 ± 9.61 | 64.32 ± 11.65 | 0.03 |
| Placebo | 61.15 ± 11.42 | 64.15 ± 12.23 | 0.04 | |
| Ejection fraction (%) | Omega-3 | 30.59 ± 9.23 | 31.75 ± 9.40 | 0.13 |
| Placebo | 31.69 ± 9.12 | 34.26 ± 9.09 | 0.01 | |
| Left ventricular mass (gr) | Omega-3 | 284.51 ± 106.41 | 242.91 ± 89.58 | 0.004 |
| Placebo | 269.3 ± 87.33 | 256.3 ± 95.18 | 0.42 | |
| End systolic volume (cc) | Omega-3 | 101.51 ± 38.21 | 99.29 ± 42.23 | 0.37 |
| Placebo | 101.11 ± 52.88 | 111.19 ± 71.3 | 0.22 | |
| EM index (cm/s) | Omega-3 | 14.91 ± 6.33 | 13.43 ± 4.2 | 0.06 |
| Placebo | 15.61 ± 10.47 | 11.88 ± 4.87 | 0.08 | |
| AM index (cm/s) | Omega-3 | 11.41 ± 4.22 | 10.25 ± 4.36 | 0.025 |
| Placebo | 12.06 ± 6.05 | 11.22 ± 4.37 | 0.52 | |
| SM (cm/s) | Omega-3 | 12.02 ± 3.96 | 9.4 ± 3.57 | 0.000 |
| Placebo | 11.13 ± 4.01 | 9.33 ± 4.08 | 0.08 | |
| Tei index | Omega-3 | 0.9 ± 0.35 | 0.8 ± 0.25 | 0.04 |
| Placebo | 0.8 ± 0.31 | 0.9 ± 0.36 | 0.31 |
*P < 0.05 is considered statistically significant. BNP: brain natriuretic peptide; Em: early diastolic velocity; Am: late diastolic velocity; Sm: systolic velocity.
Table 3.
Mean changes in plasma BNP levels, 6-min walk test results and echocardiography parameters after 6 months in the omega-3 and placebo groups.
| Variables | Treatment group (%) | Placebo group (%) | P value* |
|---|---|---|---|
| Plasma BNP levels pg/mL (% decline from baseline) | 606.8 ± 1325.6 pg/mL (1.1% ± 100%) | 1100.6 ± 4899 pg/mL (−0.3% ± 56%) | 0.47 |
| 6-min walk test (% change from baseline in m) | 35 ± 61.2 (18% ± 29%) | 12.1 ± 90.2 (5% ± 35%) | 0.06 |
| Aortic orifice (mm) | 29 ± 4.5 | 30 ± 4 | 0.07 |
| Left atrial size (mm) | 42 ± 7 | 40 ± 6 | 0.17 |
| PAT (msec) | 118 ± 30 | 126 ± 3 | 0.14 |
| End systolic diameter (mm) | −8 ± 17 | −9 ± 15 | 0.41 |
| End diastolic diameter (mm) | −4 ± 13 | −5 ± 13 | 0.36 |
| Ejection fraction (%) | 7 ± 26 | 10 ± 24 | 0.28 |
| Left ventricular mass (cc) | 10 ± 30 | 2 ± 25 | 0.14 |
| End systolic volume (cc) | −1 ± 40 | −13 ± 45 | 0.15 |
| EM index (cm/s) | −0.1 ± 35 | −9 ± 39 | 0.16 |
| AM index (cm/s) | −7 ± 30 | 19 ± 91 | 0.04 |
| SM index (cm/s) | −16 ± 39 | −6 ± 43 | 0.18 |
| Tei index | 4 ± 33 | −23 ± 57 | 0.011 |
*P < 0.05 is considered statistically significant. PAT: pulmonary acceleration; Em: early diastolic velocity; Am: late diastolic velocity; Sm: systolic velocity.
After 6 months, a significant decrease in plasma BNP concentration was observed in patients who received omega-3 compared to baseline (1766.2 ± 1978.1 pg/mL vs. 1159.4 ± 1430.9 pg/mL, P < 0.005). In the placebo group the decline from baseline levels was not statistically significant after 6 months (Table 2).
The Tei index decreased significantly more after 6 months in group A than in group B. The late diastolic velocity (Am) index improved significantly more in the omega-3 group than in the placebo group. Almost all echocardiographic indices improved significantly after 6 months in participants who received omega-3. However, the changes compared to the placebo group were statistically significant only for Tei index and Am index. Table 3 summarizes the mean changes in echocardiographic parameters in both groups after 6 months.
No significant differences between groups were found for the incidence of myocardial infarction or hospitalization due to worsening CHF. Mortality rate did not differ significantly between groups during the 6-month study period. In group A, 2 patients (4.2%) died after a major cardiovascular event (including myocardial infarction), and in group B, 1 patient died (3.6%, P = 0.73).
4. Discussion
Earlier research found that omega-3 supplementation has beneficial effects on mortality and hospital admissions in patients with CHF.11,20,21 This fatty acid is associated with a reduced response of myocytes to noradrenaline, which plays an important role in worsening CHF as a neurohormonal response.16 The effect of omega-3 was attributed to its ability to lower intracellular calcium and inhibit myocyte activity. Several trials have demonstrated the antiarrhythmic properties of omega-3 PUFA in patients with a history of myocardial infarction.21,22 Other research has reported the antiarrhythmic potential of omega-3 acid ethyl esters, which can prevent recurrent atrial fibrillation in patients with no structural heart disease.7,18 Fiaccavento et al noted that omega-3 PUFA prolonged survival and inhibited myocardial pathologies in hamsters with cardiomyopathy.10
To our knowledge no studies have been done to evaluate the effect of omega-3 supplementation on echocardiographic parameters in CHF patients. We investigated the effect of 2 g/day of omega-3 on echocardiographic parameters in patients with class II or III heart failure due to ischemic causes. We saw a significant improvement in most echocardiographic parameters after 6 months; however, these changes were not significant compared to those in patient who received the placebo. Although the improvements in the omega-3 supplementation group were small, the beneficial effects cannot be overlooked, and included improvements in the Tei and late diastolic velocity indices as markers of diastolic left ventricular function. Omega-3 PUFA had no significant impact on the 6-min walk results or plasma BNP levels compared to the placebo, although some beneficial effects were observed in both groups. The lack of a statistically significant improvement in patients with class II and III heart failure in this study may be a result of the small number of patients enrolled, and as found in earlier studies, the degree of improvement with any therapy for patients with class II and II heart failure is not large. For example, improvements in survival with angiotensin-converting enzyme inhibitors in patients with heart failure were seen in only 16% of the patients treated in all classes of heart failure not in refractory heart failure patients like ours.23
Plasma BNP concentration is an indicator of the severity of heart failure, and increases exponentially as cardiac condition worsens.24–28 Zhao et al studied 76 patients with CHF and reported that omega-3 supplements significantly reduced inflammatory markers and NT-ProBNP. They concluded that omega-3 supplementation may reduce mortality in patients with heart failure.12 We used the same dose of omega-3 as Zhao and colleagues (2 g/day). They reported a mean decrease in plasma NT-ProBNP levels of 138 ± 82 pg/mL after 3 months, compared to a decrease of 606 ± 1325.6 pg/mL in our patients after 6 months. Unlike us, Zhao and colleagues found a significant decrease in BNP levels in their treatment group compared to their placebo group. However, like us, they found no significant improvement in EF after treatment with the PUFA (2% increase in EF, P > 0.05).
Omega-3 supplementation did not change the echocardiographic parameters, and did not result in any improvement in systolic function markers. However, it significantly improved the Tei index, a sensitive marker of diastolic function. In the late stages of CHF any minor change in echocardiographic indexes may significantly affect the course of the disease, although changes in echocardiographic parameters are small and do not predict the survival benefit with any therapies. Additional long-term studies in larger series of patients are thus needed to determine the significance of these changes in response to omega-3 PUFA supplementation.
Our findings showed some advantages of dietary omega-3 supplementation in patients with class II and III heart failure who did not respond to routine medical therapy, and who had a biventricular pacemaker for mechanical support. In particular, omega-3 fatty acid improved echocardiographic parameters and lowered plasma BNP concentrations in the short term. Although the effects were small, these potential benefits in patients with severe heart failure merit further attention considering that omega-3 supplementation is safe and has few side effects.18
5. Limitations
The small sample size could have affected the results, especially with regard to BNP plasma levels. Therefore a larger sample size could help measure the beneficial effects of omega-3 fatty acids on plasma BNP levels more accurately. This trial showed an overall beneficial effect of omega-3 supplementation in patients with CHF, especially in echocardiographic parameters. However, larger multicenter clinical trials should be conducted with longer follow-up periods to more accurately determine the benefits of omega-3 supplementation with regard to BNP levels, and to characterize its influence on long-term morbidity and mortality in patients with CHF.
Funding source
This research was supported by a grant from Shiraz University of Medical Sciences.
Disclosures
Authors of this article have no conflict of interest and the research was granted by research faculty of Shiraz University of Medical Sciences.
Dr Javad Kojuri, Mohammas Ali Ostovan, Gholam Reza Rezaian, Archin Dialameh, Nima Zamiri and Mohammad Bagher Sharifkazemi have no conflict of interest or any financial ties to disclose.
Conflicts of Interest
All authors have none to declare.
Acknowledgments
We thank K. Shashok (Author AID in the Eastern Mediterranean) for improving the use of English in the manuscript.
References
- 1.Zannad F., Agrinier N., Alla F. Heart failure burden and therapy. Europace. 2009 Nov;11(suppl 5):v1–v9. doi: 10.1093/europace/eup304. [DOI] [PubMed] [Google Scholar]
- 2.Usher B.M., Cammarata K. Heart failure and family caregiver burden: an update. Progr Cardiovasc Nurs. 2009 Sep;24(3):113–114. doi: 10.1111/j.1751-7117.2009.00046.x. [DOI] [PubMed] [Google Scholar]
- 3.American Heart Association . American Heart Association; Dallas, Texas: 2007. Heart Disease and Stroke Statistics – 2007 Update. [Google Scholar]
- 4.Jessup M., Brozena S. Heart failure. N Engl J Med. 2003 May 15;348(20):2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 5.Ho K.K., Pinsky J.L., Kannel W.B., Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993 Oct;22(4 Suppl A):6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 6.Gardner R.S., McDonagh T.A. The treatment of chronic heart failure due to left ventricular systolic dysfunction. Clin Med (London) 2004 Jan–Feb;4(1):18–22. doi: 10.7861/clinmedicine.4-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witte K.K., Clark A.L. Fish oils – adjuvant therapy in chronic heart failure? Eur J Cardiovasc Prev Rehabil. 2004 Aug;11(4):267–274. doi: 10.1097/01.hjr.0000136728.27524.f5. [DOI] [PubMed] [Google Scholar]
- 8.Boak L., ChiuDusting J.P. Hypercholesterolemia and endothelium dysfunction: role of dietary supplementation as vascular protective agents. Curr Vasc Pharmacol. 2004;2(1):45–52. doi: 10.2174/1570161043476546. [DOI] [PubMed] [Google Scholar]
- 9.Duda M.K., O'Shea K.M., Stanley W.C. Omega-3 polyunsaturated fatty acid supplementation for the treatment of heart failure: mechanisms and clinical potential. Cardiovasc Res. 2009 Oct 1;84(1):33–41. doi: 10.1093/cvr/cvp169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiaccavento R., Carotenuto F., Minieri M. Alpha-linolenic acid-enriched diet prevents myocardial damage and expands longevity in cardiomyopathic hamsters. Am J Pathol. 2006 Dec;169(6):1913–1924. doi: 10.2353/ajpath.2006.051320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marchioli R., Silletta M.G., Levantesi G., Pioggiarella R. Omega-3 fatty acids and heart failure. Curr Atheroscler Rep. 2009 Nov;11(6):440–447. doi: 10.1007/s11883-009-0066-y. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y.T., Shao L., Teng L.L. Effects of n-3 polyunsaturated fatty acid therapy on plasma inflammatory markers and N-terminal pro-brain natriuretic peptide in elderly patients with chronic heart failure. J Int Med Res. 2009 Nov-Dec;37(6):1831–1841. doi: 10.1177/147323000903700619. [DOI] [PubMed] [Google Scholar]
- 13.Mehra M.R., lavie C.J., Ventura H.O., Milani R.V. Fish oils produce antiinflammatory effects and improve body weight in sever heart failure. J Heart Lung Transplant. 2006;25(7):834–838. doi: 10.1016/j.healun.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Yamagishi K., Nettleton J.A., Folsom A.R. Plasma fatty acid composition and incident heart failure in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2008 Nov;156(5):965–974. doi: 10.1016/j.ahj.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witte K.K., Clark A.L. Marine polyunsaturated fatty acids in heart failure. Are the theoretical benefits matched by the clinical data? Pol Arch Med Wewn. 2009 Mar;119(3):162–169. [PubMed] [Google Scholar]
- 16.Den Ruijter H.M., Berecki G., Verkerk A.O. Acute administration of fish oil inhibits triggered activity in isolated myocytes from rabbits and patients with heart failure. Circulation. 2008 Jan 29;117(4):536–544. doi: 10.1161/CIRCULATIONAHA.107.733329. [DOI] [PubMed] [Google Scholar]
- 17.Den Ruijter H.M., Verkerk A.O., Berecki G., Bakker D., van Ginneken A.C., Coronel R. Dietary fish oil reduces the occurrence of early after depolarizations in pig ventricular myocytes. J Mol Cell Cardiol. 2006 Nov;41(5):914–917. doi: 10.1016/j.yjmcc.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Pratt C.M., Reiffel J.A., Ellenbogen K.A., Naccarelli G.V., Kowey P.R. Efficacy and safety of prescription omega-3-acid ethyl esters for the prevention of recurrent symptomatic atrial fibrillation: a prospective study. Am Heart J. 2009 Aug;158(2):163–169. doi: 10.1016/j.ahj.2009.05.024. e1–3. [DOI] [PubMed] [Google Scholar]
- 19.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. (Clinical research ed.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tavazzi L., Maggioni A.P., Marchioli R. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008 Oct 4;372(9645):1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 21.Brouwer I.A., Zock P.L., Camm A.J. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: the Study on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) randomized trial. JAMA. 2006 Jun 14;295(22):2613–2619. doi: 10.1001/jama.295.22.2613. [DOI] [PubMed] [Google Scholar]
- 22.Puttmann M., Krug H., von Ochsenstein E., Kattermann R. Fast HPLC determination of serum free fatty acids in the picomole range. Clin Chem. 1993 May;39(5):825–832. [PubMed] [Google Scholar]
- 23.The CONSENSUS Trial Study Group Effects of enalapril on mortality in severe congestive heart failure. N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 24.Koglin J., Pehlivanli S., Schwaiblmair M., Vogeser M., Cremer P., von Scheidt W. Role of brain natriuretic peptide in risk stratification of patients with congestive heart failure. J Am Coll Cardiol. 2001 Dec;38(7):1934–1941. doi: 10.1016/s0735-1097(01)01672-2. [DOI] [PubMed] [Google Scholar]
- 25.Anand I.S., Fisher L.D., Chiang Y.T., Val-HeFT Investigatorsl . Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2003 Mar 11;107(9):1278–1283. doi: 10.1161/01.cir.0000054164.99881.00. [DOI] [PubMed] [Google Scholar]
- 26.de Groote P., Dagorn J., Soudan B., Lamblin N., McFadden E., Bauters C. B-type natriuretic peptide and peak exercise oxygen consumption provide independent information for risk stratification in patients with stable congestive heart failure. J Am Coll Cardiol. 2004 May 5;43(9):1584–1589. doi: 10.1016/j.jacc.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 27.Stanek B., Frey B., Hulsmann M. Prognostic evaluation of neurohumoral plasma levels before and during beta-blocker therapy in advanced left ventricular dysfunction. J Am Coll Cardiol. 2001 Aug;38(2):436–442. doi: 10.1016/s0735-1097(01)01383-3. [DOI] [PubMed] [Google Scholar]
- 28.Berger R., Huelsman M., Strecker K. B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation. 2002 May 21;105(20):2392–2397. doi: 10.1161/01.cir.0000016642.15031.34. [DOI] [PubMed] [Google Scholar]


