Abstract
Background
Rheumatic heart disease (RHD) is still a public health issue in many countries in the world, and particularly in Southeast Asia. India, for example, contributes 25%–50% of the global burden of RHD. Clinic-based and epidemiological studies on RHD in India have used different methodologies and clinical criteria to estimate RHD burden in India. The present study employs strict clinical criteria, including echocardiography, to estimate RHD prevalence and associated clinical complications in a large unique rural population in southern India covered through a governmental health insurance scheme.
Materials and methods
Total 44,164 eligible patients were screened from 238 primary care health centers in rural southern India between October 2007 and March 2012 using strict clinical criteria and objective ascertainment. A total of 403 patients aged 15 years or above were finally analyzed based on both the inclusion and exclusion criteria. Detailed information on both demographic and clinical characteristics was obtained through personal interviews and clinical examinations. Descriptive analyses were performed, including age standardization.
Results
The age-standardized RHD prevalence rate was 9.7/1000 populations—more common in younger age groups (<44 years) and relatively high among females. Pulmonary hypertension was the most common clinical complication followed by CHF, tricuspid regurgitation, as well as infective endocarditis. More than two-thirds had no past history of RHD or penicillin prophylaxis.
Conclusions
RHD rates are still high in rural India among populations covered through governmental health insurance scheme. Both primary and secondary preventive measures, including widespread coverage of penicillin prophylaxis, must be considered mainstay tools to both prevent and reduce RHD burden in endemic populations, including rural India.
Keywords: Prevalence, RHD, South India
1. Introduction
Rheumatic fever and rheumatic heart disease are non-suppurative complications of Group A streptococcal pharyngitis due to a delayed immune response. The diagnosis of rheumatic fever (RF) is based on Jones criteria, which include major and minor clinical manifestations, laboratory manifestations and evidence of preceding streptococcal infection. In the context of a preceding streptococcal infection, two major manifestations, or a combination of one major and two minor manifestations, provide reasonable evidence for the diagnosis of RF.1
Rheumatic heart disease (RHD) remains a major public health problem in many developing countries, including India. India contributes to nearly 25%–50% of the global burden of RHD.1,2 According to WHO estimates nearly 1.33 lakh deaths occur from RF/RHD annually in the Southeast Asia compared with 10,000 and 30,000 deaths in the Americas and Europe, respectively. In 1994, an estimated 7.6 deaths and 173.4 (disability adjusted life years) DALYS for 100,000 population were attributable to RHD in south Asia.1 A recent survey across various tertiary care hospitals reported hospital admission rates for RHD varying between 5% and 26% of cardiac admissions.3 A study from Orissa, one of the poorly developed states in India, shows that RHD still accounts for 45% of cardiac admissions in a tertiary care teaching hospital.4 A survey of secondary care hospitals shows that nearly 30% of cardiac cases are associated with RHD.5 Similarly, a population survey done in the year 2000 in northern India reported a high prevalence of RHD (6.4/1000).6 The surveys conducted by the Indian Council of Medical Research (ICMR) from 2002 to 2005 indicate a decline in the prevalence of RHD (0.43–1.47/1000).7 The incidence of RF was 0.4/1000 among a population >15 years of age.8
Most of the studies in India are based on school surveys and although school surveys are regarded as ideal, conclusions about prevalence of rheumatic heart disease cannot be drawn solely from these surveys as RHD represents the cumulative heart damage of previous acute rheumatic fever episodes.9 RHD peaks in the third and fourth decades of life; therefore although ARF is a disease of the childhood, its effects are felt throughout adulthood, especially in the young adult years when patients are in most productive period of their lives. The above studies indicate that RHD decline in India is not uniform and continues unabated in several parts of the country. Poor hygiene, poverty, overcrowding, under nutrition, low level of awareness about disease and shortage of resources for health care all contribute to higher prevalence rates of RHD in India.10 Studies regarding the prevalence of RHD in the above school-age populations are scarce in India. The objective of the present study was to examine the prevalence of RHD and their associated main clinical complications in the above 15 years age group, in a rural population of south India covered by a governmental health insurance scheme.
2. Materials and methods
This cross-sectional study in rural southern India of Andhra Pradesh covers 238 rural primary health centers, each catering to 30,000 people, was conducted between October 2007 and March 2012. From an eligible population of 44,164 subjects screened through health camps and clinically assessed for CRHD based on history of rheumatic fever, clinical examination and echocardiography, 429 subjects were CRHD diagnosed but 403 got admitted as inpatients for additional clinical ascertainment and medical and surgical management. The Ethics Committee of the GSL Medical College and General Hospital, Rajahmundry, India approved the present study. Informed consent was also obtained from each participant before the collection of any data. For participants who could not read or write, the participant information sheet and consent form were explained by the Arogyamithra (health volunteer) and a thumb print was taken to mark consent.
2.1. Inclusion criteria
All subjects above the age of 15 years were included in the study.
2.2. Clinical and echocardiographic examination
Clinical examination was performed by cardiologists with experience in the diagnosis of rheumatic heart disease. Cardiac auscultation was performed with the patient in the supine and left lateral decubitus positions. Subjects in whom an organic murmur was detected clinically underwent echocardiography for confirmation of rheumatic heart disease.
Rheumatic heart disease was defined by the presence of any definite evidence of mitral- or aortic-valve regurgitation seen in two planes by Doppler echocardiography, accompanied by at least two of the following three morphologic abnormalities of the regurgitant valve: restricted leaflet mobility, focal or generalized valvular thickening, and abnormal subvalvular thickening. Echocardiography findings were interpreted independently by two cardiologists with experience in treating rheumatic heart disease.
2.3. Statistical analysis
Statistical analyses were performed using SPSS software trial version 16 and MS Excel 2007. Values were presented as mean ± SD and in percentages. Chi-square test was used for analysis of qualitative data. For all statistical analyses, P < 0.05 was considered as statistically significant. Direct age-standardization method was adopted in this study taking the age and population distribution of a nationally representative sample survey, namely, the 2005 NFHS, as the ‘standard’ population. A recent study in India used a similar approach for calculation of age-standardized rates.11
3. Results
The total number of subjects screened was 44,164 out of which 12,302 were males and 29,362 were females. Age and gender distribution of the screened population are shown in Table 1. A total of 403 patients were finally included for analyses [Fig. 1]. The mean age was 33 ± 4.2 years [70% were females (31 ± 4.8) and 30% were males (35 ± 3.6)]. The age-standardized prevalence of RHD in this study was 9.7/1000 population (95% CI: 8.8/1000–10.7/1000), 9.8/1000 (95% CI: 7.9/1000–11.5/1000) for males and 10.2/1000 (95% CI: 8.9/1000–11.4/1000) for females.
Table 1.
Age-specific and age-standardized prevalence of RHD subjects.
| Total study subjects |
RHD subjects |
P value | ||||
|---|---|---|---|---|---|---|
| Age | Male | Female | Male no (%) | Female no (%) | Total no (%) | |
| 15–24 | 2272 | 3835 | 28 (1.2) | 65 (1.7) | 93 (1.5) | 0.687 |
| 25–34 | 4353 | 9860 | 43 (1) | 90 (1) | 133 (1) | |
| 35–44 | 2460 | 8436 | 32 (1.3) | 77 (1) | 109 (1) | |
| 45–54 | 1706 | 5917 | 10 (0.6) | 38 (0.6) | 48 (0.6) | |
| 55–64 | 1511 | 1314 | 6 (0.4) | 14 (1.1) | 20 (0.7) | |
| Total | 12,302 | 29,362 | 119 (0.9) | 284 (0.9) | 403 (0.9) | |
Fig. 1.
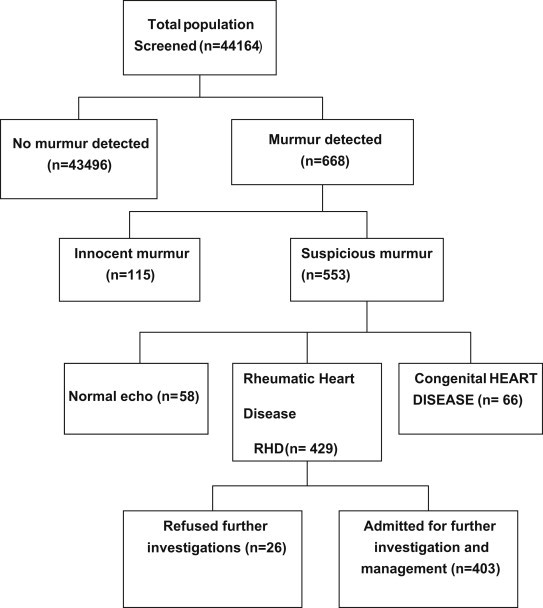
Outcome of screening for RHD in the study population.
RHD was more common in those below 44 years of age [Table 2]. Mitral regurgitation was the predominant lesion occurring in 175 (43.4%) patients followed by mitral stenosis in 171 (42.4%) patients [Fig. 2]. Aortic stenosis was seen in 16 (4%) patients and aortic regurgitation was seen in 14 (3.5%). Both mitral and aortic valve lesions were seen in 26 (6.5%) patients and there was one case of pulmonary stenosis [Fig. 2]. History of previous rheumatic fever was present in only 31% patients and only 40% were on penicillin prophylaxis [Table 2]. Pulmonary hypertension was seen in 52% patients, functional tricuspid regurgitation in 34% patients and 31% patients presented with congestive heart failure. Transesophageal echocardiography revealed left atrial thrombus in four cases. Atrial fibrillation was found in 18% patients, 4% patients suffered from embolic cerebrovascular events and 2.7% had infective endocarditis [Fig. 3].
Table 2.
Age distribution of rheumatic fever, penicillin prophylaxis and RHD.
| Age (years) | Total no of subjects | History of RF no (%) | Penicillin prophylaxis no (%) | Mitral valve | Aortic valve | Mitral valve + aortic valve | Pulmonary valve |
|---|---|---|---|---|---|---|---|
| 15–24 | 93 | 41 (44) | 59 (63) | 84 | 5 | 4 | 0 |
| 25–34 | 133 | 56 (42) | 67 (50) | 113 | 9 | 10 | 1 |
| 35–44 | 109 | 18 (17) | 29 (27) | 97 | 7 | 5 | 0 |
| 45–54 | 48 | 7 (15) | 6 (13) | 37 | 6 | 5 | 0 |
| 55–64 | 20 | 3 (15) | 0 (0) | 15 | 3 | 2 | 0 |
RF = rheumatic fever.
Fig. 2.
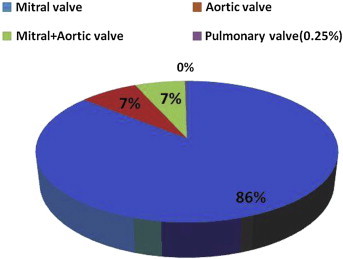
Valvular involvement in RHD in the study population.
Fig. 3.
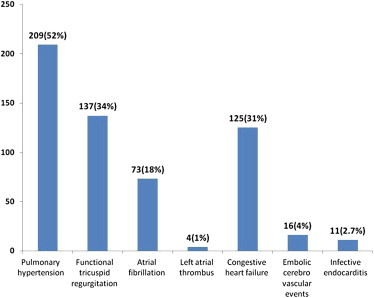
Complications of rheumatic heart disease.
4. Discussion
In spite of declining trends in the prevalence of rheumatic fever/rheumatic heart disease in India [Table 3]; RHD still remains a major public health problem. The prevalence of RHD in this study was 9.7/1000 population and this estimate is seven times higher than what was documented in a recent ICMR study in school-going children (0.43–1.4). Recent studies indicate that RHD is an ongoing problem in many developing countries and the projected disease burden is an underestimate as most of the studies are based on clinical criteria alone and did not include echocardiography. According to calculated estimates, the actual disease burden in the population is 5.5–7.2 times higher than what can be found in the school children of 5–14 year-old age group.12 Many recent studies suggest a 10-fold rise in the prevalence rates of RHD when echocardiography was used for the diagnosis along with clinical criteria.13–16 A recent study from rural Pakistan using household survey and rigorous methodology found 5.7 RHD cases per 1000 population.17 In a recent Chinese study, the prevalence of RHD was 11/1000 population. All these studies strongly indicate that RHD is still a major public health problem in Asia.18
Table 3.
Rheumatic heart disease (RHD) prevalence in school and community studies in the last two decades.
| Author | Time period | Place | Population | Age (years) | Prevalence (per 1000) | Type of survey |
|---|---|---|---|---|---|---|
| Grover et al22 | 1988–91 | Haryana | 31,200 | 5–15 | 2.1 | Community survey |
| Gupta et al23 | 1991 | Jammu | 10,263 | 6–16 | 1.4 | School survey |
| Agarwal et al6 | 1991–92 | Uttar Pradesh | 3760 | 0–15 | 6.4 | Community survey |
| Thakur et al24 | 1992–93 | Shimla | 15,080 | 5–16 | 1.98–4.8 | School survey |
| Jose3 | 2001–02 | Vellore | 229,829 | 6–18 | 0.68 | School survey |
| ICMR7 | 2002–05 | Kochi, Vellore, Chandigarh, Indore | 100,269 | _ | 0.43–1.47 | School survey |
| Misra et al4 | 2003–06 | Gorakhpur | 118,212 | 4–18 | 0.5 | School survey |
| Periwal et al25 | 2006 | Bikaner | 3292 | 5–14 | 0.67 | School survey |
| Bharani26 | 2009 | Indore | 25,676 | 5–15 | 0.46 | School survey |
| Present study | 2007–12 | Andhra Pradesh | 44,164 | >15 | 9.7 | Community survey |
ICMR = Indian Council of Medical Research.
In this study, the RHD prevalence rates were slightly higher among female subjects (10.2%) compared to male subjects (9.8%). Various other studies also revealed higher prevalence rates among females.10,19 The present study also documented clinical complications associated with RHD. Mitral valve disease accounted for 86% of cases, aortic valve involvement was seen in 7% of cases, and both mitral and aortic valve involvement were seen in 7% cases. There was only one case of pulmonary valve stenosis. Mitral valve disease particularly mitral stenosis was responsible for atrial fibrillation and associated embolic complications. Embolic cerebrovascular events occurred in 4% cases in the present study and such complications contributed to seeking medical attention by a large number of patients. Majority of the congestive heart failure cases presented with New York Heart Association functional class III symptoms. Infective endocarditis was seen in 11 cases with mitral valve involvement in 7 cases and aortic valve involvement in 4 cases. Half of the cases of infective endocarditis and substantial proportion of strokes in Asian countries were attributed to underlying rheumatic heart disease.12
Contrary to the general observation that RHD is declining, the high prevalence rate in this study shows that RHD is still a major public health problem in rural populations. A meta-analysis and few other studies suggest that there is no decline in the prevalence of RHD over the last 20 years.4,12 In one study, clinical examination revealed only 0.8 RHD cases/1000 population, whereas echocardiography revealed 20.4 RHD cases/1000 population.19 The reported differences in the prevalence of RHD might be due to different diagnostic methods adopted in various studies or due to non-uniformity of the populations studied.
Unfortunately, there are few comparable studies in India determining the prevalence of RHD in rural communities in the above 15 years age groups. Warm and humid climatic conditions in this coastal part of Andhra Pradesh where this study was conducted might be responsible for higher prevalence of rheumatic fever and RHD in this region. Because of the non-availability of such data comparisons cannot be made with other studies. The reason for higher prevalence rates among women in our study is also not clear. Women are home bound and get exposed to indoor smoke from household use of solid fuels, which predisposes them to higher rates of respiratory infections. The delay in the diagnosis of subclinical disease in the population leads to advanced stages of RHD, although all subclinical cases may not progress to clinical RHD.19 The large number of cases (>80%) in the age group below 44 years is also a cause for concern. One significant finding of this study was 69% of the cases with past history of rheumatic fever were absent and 60% of them were not on penicillin prophylaxis (medical records were available for only 40% of the screened population). Fear of adverse reactions to penicillin and pain from injections besides ignorance about the consequences of ARF may partly explain these low penicillin prophylaxis rates. Health education and proper counseling of masses where literacy is low may help in overcoming this problem.
Rheumatic heart disease and its complications are responsible for preventable morbidity and mortality in the younger age groups in endemic regions. There is a need for better clinical and echocardiography-based prevalence studies from regions where RHD is still endemic.12 Primary prevention strategies though ideal are not entirely feasible for large populations such as rural India, but tertiary prevention measures that include both medical and surgical care may be an alternative cost-effective approach for RHD.20 Secondary prevention measures, such as early and correct diagnosis of ARF, as well as penicillin prophylaxis are both effective and cost-effective, and such approaches should be strongly considered to prevent and to reduce RHD-associated clinical complications.1 Establishment of registries of known patients and integrating secondary prevention measures with existing primary health care services must certainly prove effective in reducing morbidity and mortality, as well as to gain additional insights into the epidemiology of RHD in populations where RHD is still endemic.21
In conclusion, the present study determines the high prevalence of RHD in rural India associated with main clinical complications that are mostly preventive if both primary and secondary preventive measures are in place and properly implemented. This observational cross-sectional study has its inherent methodological limitations but RHD diagnosis based on strict clinical criteria, including echocardiography is an underlying strength of this study. Nevertheless, the fact that more than two-thirds of the patients studied had no past history of rheumatic fever or penicillin prophylaxis is intriguing, especially when the population studied were covered through a government health insurance scheme. Such observations deem further explorations.
Funding
The authors disclose that this article received no funding from any source.
Conflicts of interest
All authors have none to declare.
Acknowledgments
The authors would like to acknowledge the encouragement of the management and administration, GSL Medical College and General Hospital and Arogyasri Department for their help in preparing this manuscript. The authors are particularly thankful to N. Laksmanrao Assistant Professor of community medicine for statistical analysis.
References
- 1.Rheumatic Fever and Rheumatic Heart Disease. World Health Organization; Geneva, Switzerland: 2004. (Rheumatic Fever and Rheumatic Heart Disease: Report of a WHO Expert Consultation, Geneva, 29 October–1 November 2001). [Google Scholar]
- 2.Carapetis J.R. Rheumatic heart disease in developing countries. N Engl J Med. 2007;357:439–441. doi: 10.1056/NEJMp078039. [DOI] [PubMed] [Google Scholar]
- 3.Jose V.J. Changes in profile and presentation of rheumatic heart disease. In: Das S., editor. vol. 13. Association of Physicians of India (API); Mumbai: 2003. pp. 564–567. (Medicine Update). [Google Scholar]
- 4.Mishra T.K., Routray S.N., Behera M., Pattniak U.K., Satpathy C. Has the prevalence of rheumatic fever/rheumatic heart disease really changed? A hospital-based study. Indian Heart J. 2003;55:152–157. [PubMed] [Google Scholar]
- 5.Shrivastava S. Rheumatic heart disease: is it declining in India? Indian Heart J. 2007;59:9–10. [PubMed] [Google Scholar]
- 6.Agarwal A.K., Yunus M., Ahmad J., Khan A. Rheumatic heart disease in India. J R Soc Health. 1995;115:303–304. doi: 10.1177/146642409511500509. 309. [DOI] [PubMed] [Google Scholar]
- 7.Jai Vigyan Mission mode project on community control of RHD. Non-communicable diseases. Indian Council Med Res Annu Rep 2007–08. 2008:63–64. New Delhi. [Google Scholar]
- 8.Lalchandani A., Kumar H.R., Alam S.M. Prevalence of rheumatic heart disease in rural and urban school children of district Kanpur. Indian Heart J. 2000;52:672. [Google Scholar]
- 9.Ramakrishnan S., Shyam S.K., Juneja R., Bharghava B., Saxena A., Bahl V.K. Prevalence of rheumatic heart disease: has it declined in India? Natl Med J India. 2009;22:72–74. [PubMed] [Google Scholar]
- 10.Padmavati S. Rheumatic heart disease: prevalence and preventive measures in the Indian subcontinent. Heart. 2001;86:127. doi: 10.1136/heart.86.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prasad D.S., Kabir Z., Dash A.K., Das B.C. Prevalence and risk factors for metabolic syndrome in Asian Indians: community study from urban eastern India. J Cardiovasc Dis Res. 2012;3:204–211. doi: 10.4103/0975-3583.98895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carapetis J.R. Rheumatic heart disease in Asia. Circulation. 2008;118:2748–2753. doi: 10.1161/CIRCULATIONAHA.108.774307. [DOI] [PubMed] [Google Scholar]
- 13.Marijon E., Ou P., Celermajer D.S. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med. 2007;357:470–476. doi: 10.1056/NEJMoa065085. [DOI] [PubMed] [Google Scholar]
- 14.Steer A.C., Kado J., Wilson N. High prevalence of rheumatic heart disease by clinical and echocardiographic screening among children in Fiji. J Heart Valve Dis. 2009;18:327–336. [PubMed] [Google Scholar]
- 15.Bhaya M., Panwar S., Beniwal R., Panwar R.B. High prevalence of rheumatic heart disease detected by echocardiography in school children. Echocardiography. 2010;27:448–453. doi: 10.1111/j.1540-8175.2009.01055.x. [DOI] [PubMed] [Google Scholar]
- 16.Figueroa F.E., Fernadez M.S., Valdes P. Prospective comparison of clinical and echocardiographic diagnosis of rheumatic carditis: long term follow up of patients with subclinical disease. Heart. 2001;85:407–410. doi: 10.1136/heart.85.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rizvi S.F., Khan M.A., Kundi A., Marsh D.R., Samad A., Pasha O. Status of rheumatic heart disease in rural Pakistan. Heart. 2004;90:394–399. doi: 10.1136/hrt.2003.025981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X., Zhang M., Huang D. An epidemiologic investigation of acute rheumatic fever and rheumatic heart disease among students aged 5–18 in west area of Sichuan Province. Sichuan Da Xue Xue Bao Yi Xue Ban. 2003;34:533–535. [PubMed] [Google Scholar]
- 19.Saxena A., Sivasubramanian R., Roy A. Prevalence and outcome of subclinical rheumatic heart disease in India (rheumatic heart echo utilisation and monitoring actuarial trends in Indian children) study. Heart. 2011;97:2018–2022. doi: 10.1136/heartjnl-2011-300792. [DOI] [PubMed] [Google Scholar]
- 20.McDonald M., Brown A., Noonan S., Carapetis J.R. Preventing recurrent rheumatic fever: the role of register based programmes. Heart. 2005;91:1131–1133. doi: 10.1136/hrt.2004.057570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar R., Raizada A., Aggarwal A.K., Ganguly N.K. A community-based rheumatic fever/rheumatic heart disease cohort: twelve-year experience. Indian Heart J. 2002;54:54–58. [PubMed] [Google Scholar]
- 22.Grover A., Dhawan A., Iyengar S.D., Anand I.S., Wahi P.L., Ganguly N.K. Epidemiology of rheumatic fever and rheumatic heart disease in a rural community in northern India. Bull World Health Organ. 1993;71:59–66. [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta I., Gupta M.L., Parihar A., Gupta C.D. Epidemiology of rheumatic and congenital heart diseases in school children. J Indian Med Assoc. 1992;90:57–59. [PubMed] [Google Scholar]
- 24.Thakur J.S., Negi P.C., Ahluwalia S.K., Vaidya N.K. Epidemiological survey of rheumatic heart disease among school children in the Shimla Hills of northern India: prevalence and risk factors. J Epidemiol Community Health. 1996;50:62–67. doi: 10.1136/jech.50.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Periwal K.L., Gupta B.K., Panwar R.B., Khatri P.C., Raja S., Gupta R. Prevalence of rheumatic heart disease in school children in Bikaner: an echocardiographic study. J Assoc Physicians India. 2006;54:279–282. [PubMed] [Google Scholar]
- 26.Bharani A. Prevalence of rheumatic fever/rheumatic heart disease in India: lessons from active surveillance and a passive registry. J Am Coll Cardiol. 2010;55(10A suppl 1):E1415. [Google Scholar]


