Abstract
The use of Echinacea as a medicinal herb is prominent in the United States, and many studies have assessed the effectiveness of Echinacea as an immunomodulator. We hypothesized that Bauer alkamides 8, 10 and 11 and ketone 24 were absorbed similarly either as pure compounds or from Echinacea sanguinea and Echinacea pallida ethanol extracts, and that these Echinacea extracts could inhibit P-glycoprotein transporter (P-gp) in Caco-2 human intestinal epithelial cells. Using HPLC analysis, the permeation rate of Bauer alkamides by passive diffusion across Caco-2 cells corresponded with compound hydrophilicity (alkamide 8 > 10 > 11), independent of the plant extract matrix. Both Echinacea ethanol extracts stimulated apparent glucuronidation and basolateral efflux of glucuronides of alkamides 8 and 10 but not alkamide 11. Bauer ketone 24 was totally metabolized to more hydrophilic metabolites when administered as a single compound, but was also glucuronidated when present in Echinacea extracts. Bauer alkamides 8, 10 and 11 (175–230 μM) and ethanol extracts of E. sanguinea (1 mg/mL, containing ~90 μM total alkamides) and E. pallida (5 mg/mL, containing 285 μM total alkamides) decreased the efflux of the P-gp probe calcein-AM from Caco-2 cells. These results suggest that other constituents in these Echinacea extracts facilitated the metabolism and efflux of alkamides and ketones, which might improve therapeutic benefits. Alkamides and Echinacea extracts might be useful in potentiating some chemotherapeutics which are substrates for P-gp.
Keywords: Echinacea (Asteraceae), Alkamides, Ketones, Permeability, P-glycoprotein, Caco-2
Introduction
The genus Echinacea has been widely used in North America and Europe for the treatment and prevention of upper respiratory tract infections, such as the common cold and influenza [1–2]. Echinacea products were among the most commonly used dietary supplements for adults and children according to the NHIS survey in 2007 [3]. Species of Echinacea, such as E. angustifolia, E. pallida, E. sanguinea and E. purpurea, have immune modulatory, antiviral, and antibacterial activities [4–6].
Studies with single components and more complex fractions of Echinacea extracts indicate that this genus is rich in bioactive chemicals of which lipophilic alkamides, also known as alkylamides, and ketones, intermediately hydrophilic phenolic compounds (mainly caffeic-acid derivatives) and polysaccharides are the most recognized for their immunomodulatory properties [1]. Bauer alkamides 8 ((2E,4E,8Z,10Z)-N-isobutyldodeca-2,4,8,10-tetraenamide), 10 ((2E,4E,8Z)-N-isobutyldodeca-2,4,8-trienamide) and 11 ((2E,4E)-N-isobutyldodeca-2,4-dienamide) at 50 μM and ketone 24 (pentadeca-(8Z, 13Z)-diene-11-yn-2-one) at 5 μM (Fig. 1) possess anti-inflammatory properties because they significantly decrease nitric oxide and prostaglandin E (2) production in lipopolysaccharide-stimulated RAW264.7 macrophages [7].
Fig. 1.
The chemical structures of Bauer alkamides 8, 10, 11 and ketone 24.
Despite many in vitro studies ascribing biological activities to both the alkamides and ketones, these activities are possible in vivo only if they are absorbed. Woelkart et al. [8] found about 5% of the ingested dose of Bauer alkamide 8 and 1% of Bauer alkamide 10 in human blood 3 h after oral administration of a 60% ethanolic extract of E. angustifolia containing 0.4–2 mg of Bauer alkamide 8 and 10 (Fig. 1). Matthias et al. [9] showed Papp (Apparent permeability coefficients), ranging from 3 × 10−6 to 3 × 10−4 cm/s for various alkamides through Caco-2 monolayers, which were correlated to structural variations in the compounds. But few studies have focused on the metabolism of the alkamides [10], and it is essential to understand alkamide fate after ingestion because the metabolites may differ in bioactivity compared with the parent compound [11].
Caco-2 cells are immortalized, human epithelial colorectal adenocarcinoma cells and offer a standard rapid, reliable, and low-cost model for in vitro prediction of intestinal drug permeability and absorption [12]. Plant extract matrices may alter absorption or metabolism and, consequently, the bioavailability of phytochemicals [13], as Ardjomand-Woelkart et al. found that the absolute oral bioavailability of Bauer alkamide 8 with the administration of the E. purpurea extract was 1.6 fold higher compared with the treatment of the pure compound (0.75 mg/kg) in rats [14], thus, matrix effects on the uptake and metabolism of key components of Echinacea extracts deserve study. Alkamides 8, 10, 11 and ketone 24 were all present in E. pallida and E. sanguinea ethanol extracts, but not in E. angustifolia and E. simulata ethanolic extracts (data not published). Therefore, E. pallida and E. sanguinea were chosen in this study to investigate the absorption and metabolism of these compounds.
n-Hexane extracts from roots of E. pallida, E. angustifolia and E. purpurea (30 μg/mL) inhibited multidrug transporter P-glycoprotein (P-gp) activity in a human proximal tubular kidney cell line [15]. P-gp transporter plays a key role in drug absorption and distribution because it limits the permeability across the gastrointestinal tract [16] by active efflux of potentially toxic substances back into the intestinal lumen. P-gp confers resistance to anticancer chemotherapy through its over-expression in cancer cells [17]. If Echinacea extracts can block P-gp, a new paradigm for circumventing drug resistance might emerge regarding the uses of these plant materials.
Our hypotheses were that the absorbability of Bauer alkamides and ketones was dependent on the extract matrix, alkamides and ketones were glucuronidated, and that ethanolic extracts of E. pallida and E. sanguinea containing alkamides would inhibit P-gp activities in Caco-2 cells. This study was conducted to facilitate future studies of the efficacy of these herbs against inflammation and herb-drug interactions.
Materials and methods
Plant extraction
Roots of E. pallida (PI 631293) and E. sanguinea (PI 633672) were obtained from the USDA-ARS North Central Regional Plant Introduction Station (NCRPIS), in Ames, Iowa, where they were cultivated, harvested, dried and ground (vouchered as original seed samples, designated as lots PI 631293 97ncao01 SD and PI 633672 97ncao01 SD deposited at the NCRPIS, with images available at http://www.ars-grin.gov/cgi-bin/npgs/acc/search.pl?accid=PI+631293 and http://www.ars-grin.gov/cgi-bin/npgs/acc/search.pl?accid=PI+633672, respectively). Dried Echinacea root per population (6 g) were extracted with 500 mL of 95% ethanol by Soxhlet percolation for 6 h, filtered, dried by rotary evaporation and lyophilized. Then the extracts were redissolved in 0.5 mL of ethanol (77 mg of E. sanguinea extract, 50 mg of E. pallida extract) and stored at −20 °C under nitrogen. Information about the specific provenance of both accessions is available on the Germplasm Resources Information Network database at http://www.ars-grin.gov/npgs/acc/acc_queries.html.
Bauer alkamide and ketone synthesis
Chemical synthesis of Bauer alkamides and ketones [18] were conducted according to the procedures described by Wu et al. [19] and Bae [20]. Alkamide and ketone concentrations were calculated after correcting for percent purity, yielding concentrations equivalent to 100 % pure synthetic constituent. Percent purity before correction, determined by GC-MS, for Bauer alkamide 8 was 90%, Bauer alkamide 10 was 82 %, Bauer alkamide 11 was 92 %, and Bauer ketone 24 was 99%. All synthetic Bauer alkamides and ketones were dissolved in DMSO and stored at −80 °C under argon gas.
HPLC analysis
HPLC analysis was performed on a Beckman Coulter 126 HPLC, equipped with photodiode array detector model 168 and a model 508 autosampler (Beckman Coulter, Inc., Brea, CA). The mobile phase was CH3CN/H2O at a flow rate of 1.0 mL/min following a linear gradient of 40–80% CH3CN in H2O over 45 min. A reversed phase analytical YMC pack-ODS C18 column (250 mm × 4.6 mm × 5 μm, Waters Corp., Milford, MA) was used at room temperature. The detection wavelength was 260 nm and injection volume was 100 μL. The limit of detection (LOD), defined as a signal/noise ratio ≥ 3, was 0.04 μM for Bauer alkamides 8, 10 and 11, and 0.5 μM for Bauer ketone 24.
Transepithelial transfer experiment
Caco-2 cells were obtained from American Type Culture Collection (Manassas, VA) at passage 18, and all experiments were performed from passages 30 to 35. The cells were cultured according to Hubatsch et al. [12]. Cytotoxicity of pure compounds and extracts was measured according to Nasser et al. [21]. Pure Bauer alkamides and ketone 24, at 10–1000 μM, and E. pallida and E. sanguinea ethanol extracts, at 0.1– 50 mg/mL, were tested for cytotoxicity. DMSO in DMEM (Dulbecco’s modified Eagle’s medium, 0.3% v/v, Gibco Invitrogen, Carlsbad, CA) was used as control.
After the cells had grown to 90–100% confluency, they were seeded on collagen-coated polytetrafluroethylene membrane inserts (0.45 μm) fitted in bicameral chambers (Transwell-COL, 24 mm ID, Corning Inc., Corning, NY) at 1.2 × 105 cells/cm2. The transepithelial electrical resistance (TEER) was tested by Millicell ERS meter (Fisher Sci., Pittsburgh, PA) and only cells with TEER ≥ 250 Ω·cm2 were used for permeability study [12]. At 21 days post-seeding, pure Bauer alkamides and ketone 24 and extracts at non-cytotoxic concentrations, dissolved in 1.5 mL of Hank’s Buffered Salt Solution (HBSS, pH 7.4, Gibco Invitrogen, Carlsbad, CA), were added to the donor side of the chamber and 1 mL of HBSS media was added to the receiver side. After 15, 30, and 60 min, solutions were collected from the receiver side and replaced with 1 mL fresh HBSS media [22]. Samples were collected from both sides after 90 min, and the transwell membrane insert was placed in 1.5 mL of ice-cold 0.5 mol sodium hydroxide/L and sonicated with a probe-type sonic dismembrator (Biologics Inc., Manassas, VA); pH was adjusted to 7.0; all samples were injected directly to HPLC for analysis and the quantitation was based on the standard curve of alkamides and ketone 24. Transport experiments were performed at 37 °C and at 4 °C. Total cellular protein was determined by Coomassie (Bradford) assay (Pierce Laboratories, Rockford, IL).
Transepithelial transfer of pure compounds and extracts after treating with β-glucuronidase/sulfatase
Twenty μL of β-glucuronidase/sulfatase (Type H-5 from Helix pomatia, 40 units/L, Sigma-Aldrich Co., St. Louis, MO) were added to the post-experimental apical and basolateral solutions and to cell homogenates and incubated overnight at 37 °C to release the parent compounds. These samples were then injected directly to HPLC.
Apparent permeability coefficients (Papp) were determined by using the equation [12]: Papp = (dQ/dt) (1/(A × C0)); where dQ/dt is the permeability rate constant (μmol/s), A the surface area of the membrane (cm2), and C0 the initial concentration of the compound (μM).
P-gp assay
P-gp activity was evaluated with fluorimetric measurement of the intracellular accumulation of calcein produced by ester hydrolysis of the P-gp substrate calcein-AM by using a Vybrant™ Multidrug Resistance Assay Kit (Gibco Invitrogen, Carlsbad, CA). Cells were trypsinized and seeded into 96 well plates at 1.2 × 105 cells/cm2. Cells were preincubated for 15 min with pure compounds (Bauer alkamides 8, 10, and 11 and ketone 24) or extracts at non-cytotoxic concentrations; thereafter, calcein-AM was added and the fluorescence measured after 1 h with a microtiter plate reader Bio-Tek ELX 808 (Bio-Tek instruments. Inc., Winooski, VT) at 490 nm. The control wells received vehicle alone (DMSO in DMEM, 0.3% v/v). The known P-gp inhibitor, verapamil (>99%, 10 μg/mL, included in the kit), was used as a positive control.
Statistical analysis
Data are given as means ± S. D. Differences in cytotoxicity, Papp, transport kinetics, permeation rate, and absorbance representing calcein efflux by P-gp among treatments were evaluated statistically by using one-way analysis of variance (ANOVA) and Tukey’s multiple comparison tests in SAS 9.1 (SAS Institute Inc., Cary, NC). Differences were considered significant at p < 0.05 and p < 0.01.
Results
Bauer alkamides 8, 10 and 11, as well as ketone 24, were all present in Echinacea species studied, but with different profiles (Table 1). Echinacea sanguinea ethanol extract had ~3× greater Bauer alkamide 8 and ~6× greater Bauer ketone 24 than did the E. pallida accession on a molar basis. The amount of Bauer alkamide 11 in E. pallida was ~6× more than that of E. sanguinea, but Bauer alkamide 10 content was similar in the two species. Bauer alkamide 8 and ketone 24 were more concentrated in both Echineacea species than were Bauer alkamides 10 and 11.
Table 1.
The concentrations of alkamides and ketone in Echinacea ethanol extracts determined by HPLC.
| Bauer alkamide 8 g/L(mM) | Bauer alkamide10 g/L(mM) | Bauer alkamide11 g/L(mM) | Bauer ketone 24 g/L(mM) | |
|---|---|---|---|---|
| E. sanguinea (PI 633672, 154 g/L) | 3.23 ± 0.21 (13.07 ± 0.85) | 0.14 ± 0.01 (0.56 ± 0.04) | 0.03 ± 0.01 (0.12 ± 0.04) | 3.82 ± 0.56 (17.52 ± 2.57) |
| E. pallida (PI 631293, 100 g/L) | 1.15 ± 0.10 (4.66 ± 0.40) | 0.10 ± 0.01 (0.40 ± 0.04) | 0.26 ± 0.03 (1.03 ± 0.11) | 0.64 ± 0.04 (2.93 ± 0.18) |
E. sanguinea and E. pallida ethanolic extracts were diluted by methanol for HPLC analysis (n=9). All values are means± SD. PI, accession number.
Concentrations of Bauer alkamide 8 > 350 μM, Bauer alkamide 10 > 950 μM, Bauer alkamide 11 >460 μM, and Bauer ketone 24 >990 μM were significantly cytotoxic to Caco-2 cells when compared with the control (0.3% v/v DMSO in DMEM, p < 0.05). Ethanol extracts of E. sanguinea at 5 mg/mL (containing ~450 μM total alkamides) and E. pallida at 20 mg/mL (containing ~1150 μM total alkamides) were also significantly cytotoxic to the cells. Therefore, 10, 25, 50, 100 μM of pure Bauer alkamides, ketone 24, and E. sanguinea and E. pallida ethanol extracts containing the same concentrations of each compound were used for permeability studies.
The uptake of Bauer alkamides 8, 10 and 11 increased linearly and exhibited non-saturable transport across the tested concentrations (10 – 100 μM, Fig. 2A). Transport rates of Bauer alkamides 8, 10 and 11 in the basolateral to apical (BL–AP) direction were nearly the same as those in the apical to basolateral (AP–BL) direction at each tested time point (Fig. 2B, p > 0.05). The transepithelial transport in the two directions was not saturated within 90 min, as shown in Fig. 2B. The transport of Bauer alkamides 8, 10 and 11 across Caco-2 cell monolayers was investigated at both 37 °C and 4 °C to evaluate the effect of temperature on alkamide transport (AP-BL, Fig. 2C). No significant differences were found in the permeation rates of these three alkamides at 37 °C compared with those at 4 °C (p < 0.05).
Fig. 2.

Transport of pure Bauer alkamides 8, 10 and 11 across Caco-2 cells. A. Concentration dependency of the transport of three alkamides. B. Non-saturable transport of three alkamides at 25 μM during 90 min incubation period with no difference between apical to basolateral (AP-BL) and basolateral to apical (BL-AP) direction at each time point. C. The effect of temperature on the transport of the three alkamides (37°C vs 4°C). Means bearing different letters were significantly different among three alkamides by ANOVA and Tukey’s multiple comparison (p < 0.05). Data are presented as the mean ± S.D. (n=6).
Differences in permeability correlate with variations in alkamide structure, and the rank of the permeability of the three alkamides was Bauer alkamide 8 > 10 > 11 (Table 2, Fig. 2A, B and C). When evaluating apparent permeability coefficients before deconjugation with β-glucuronidase (Papp, Table 2), we detected no significant differences between alkamides tested as single compounds and alkamides present in both E. sanguinea or E. pallida extracts (p > 0.05).
Table 2.
Apparent permeability coefficients (Papp) for pure Bauer alkamides, ketone 24 and extracts across the Caco-2 monolayer.
| Papp (cm/s ×10−6) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Bauer alkamide 8 | Bauer alkamide 10 | Bauer alkamide 11 | Bauer ketone 24 | |||||
| Before | After | Before | After | Before | After | Before | After | |
| Single compound | 43.8 ± 11.2 a | 39.8 ± 13.4 b | 17.7 ± 8.8 a | 18.3 ± 5.9 b | 2.8 ± 1.5 a | 9.7 ± 2.3*a | ND | ND |
| E. sanguinea | 54.6 ± 13.2 a | 76.8 ± 11.7 *a | 12.9 ± 5.6 a | 28.4 ± 4.1*a | 3.7 ± 1.9 a | 8.2 ± 2.4*a | 12.2 ± 3.2 a | 22.9 ± 6.3*a |
| E. pallida | 41.3 ± 7.4 a | 65.2 ± 6.8 *a | 14.7 ± 4.2 a | 22.3 ± 7.9*a | 5.4 ± 2.5 a | 4.3 ± 1.9 b | 17.1 ± 7.8 a | 28.4 ± 6.3*a |
All values are means ± SD (n=9). TEER was 460–573 Ω·cm2. In each column, means of Papp bearing different letters were significantly different (p < 0.05). Bauer alkamides 8, 10, and 11 and ketone 24 were 10–100 μM for pure compounds. Two herb extracts were diluted to contain same concentrations of Bauer alkamides 8, 10, or 11 or ketone 24 as the pure compounds. Before: prior to deconjugation with β-glucuronidase; After: subsequent to deconjugation with β-glucuronidase.
Significantly different compared with the Papp before deconjugation with β-glucuronidase by ANOVA and Tukey’s multiple comparison, p < 0.05.
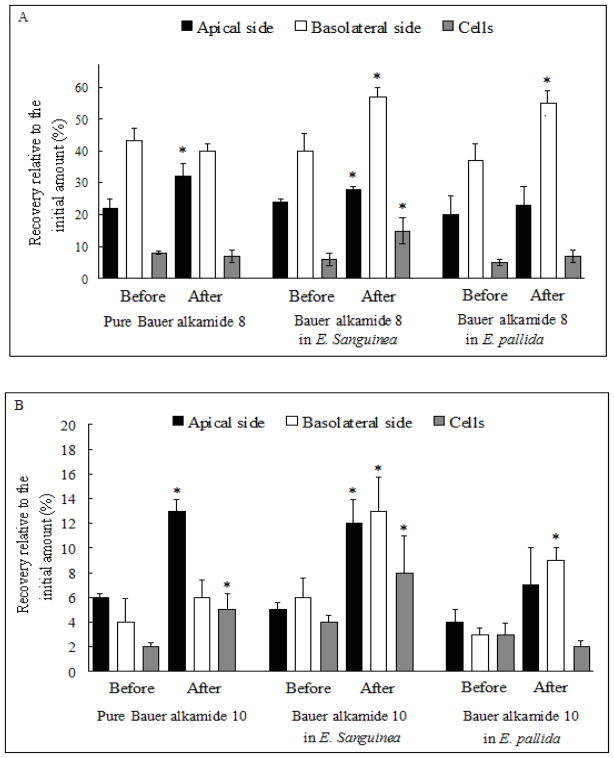
After addition of the alkamides to the apical side of Caco-2 monolayers, glucuronide metabolites of three alkamides in the E. sanguinea extract were found in all compartments, including apical and basolateral sides as well as cell lysates, compared with only in the apical side for pure Bauer alkamide 8, in the apical side and cell lysates for pure Bauer alkamide 10 as well as in the basolateral side and cell lysates for pure Bauer alkamide 11 (Fig. 3A, B and C). Interestingly, glucuronide conjugates were only detected basolaterally for Bauer alkamide 8 and 10 from E. pallida extract (Fig. 3A and B), and only in the cell lysates for Bauer alkamide 11 from E. pallida extract (p < 0.05, Fig. 3C).
Fig. 3.
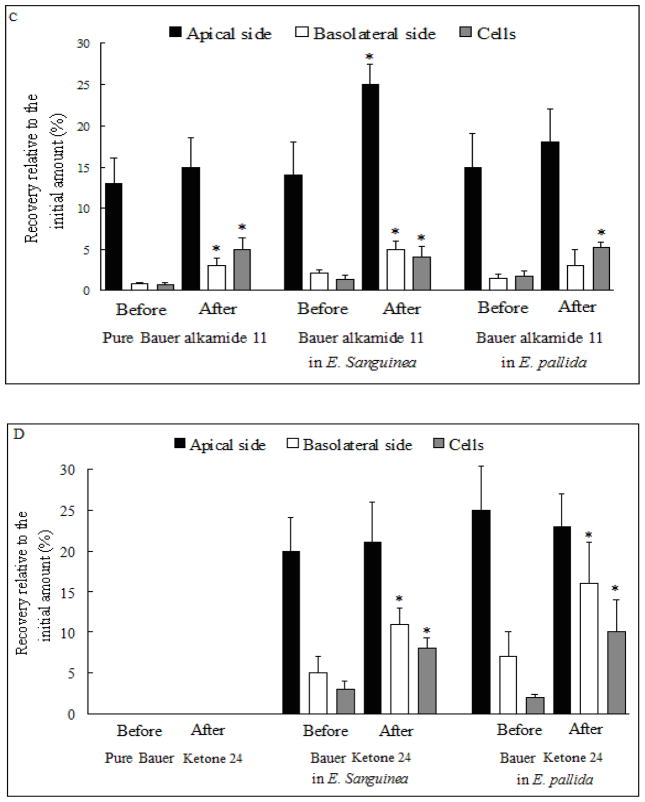
Glucuronidation of Bauer alkamides 8, 10, 11 and ketone 24 for pure compounds or from plant extracts by Caco-2 cells. Before: prior to deconjugation with β-glucuronidase; After: subsequent to deconjugation with β-glucuronidase. The concentration of three Bauer alkamides and ketone 24 was 25 μM both for pure compounds or as found in the two Echinacea extracts. A. Bauer alkamide 8. B. Bauer alkamide 10. C. Bauer alkamide 11. D. Bauer ketone 24. * Significantly different compared with the recovery relative to the initial amount before the enzyme treatment by two-sample t-test (p < 0.05). Data are presented as the mean ± S.D. (n=6).
The Papp for Bauer ketone 24 in E. sanguinea and E. pallida ethanol extracts was 12.2 ± 3.2 or 17.1 ± 7.8 cm/s ×10−6, respectively, with no significant difference between them (p > 0.05, Table 2), while Bauer ketone 24 was not found apically, basolaterally, or in cell lysates when applied as pure compound at any time point (15–90 min) across tested concentrations (10–100 μM). Two unknown peaks were detected (retention times of 13.3 and 15.4 min) in both apical and basolateral chambers and in cell lysates after treating the Caco-2 cells with pure Bauer ketone 24, but not for this same compound when present as a component in the two plant extracts (Fig. 4B, C and D). After β-glucuronidase treatment, Bauer ketone 24 recovery, relative to the applied amount, was significantly increased both basolaterally and in cell lysates for this ketone when contained in E. sanguinea and E. pallida extracts (Fig. 3D). Papp was significantly increased for the three alkamides and Bauer ketone 24 in E. sanguinea extract and for Bauer alkamides 8 and 10 and ketone 24 in E. pallida extract when compared with single compounds after deconjugation with β-glucuronidase (p < 0.05, Table 2).
Fig. 4.

HPLC chromatograms of Bauer ketone 24 across Caco-2 cell monolayers as pure compound or from Echinacea species. A. Bauer ketone 24 standard at 100 μM (retention time 17.6 min). B. Bauer ketone 24 (100 μM) was not detected in the basolateral supernatant fluid, but two more hydrophilic metabolites were detected (retention time 13.3 and 15.4 min). C. Bauer ketone 24 was shown in the basolateral side after apically applied E. sanguinea containing 100 μM of ketone 24 (retention time 17.6 min). D. Bauer ketone 24 was shown in the basolateral side after apically applied E. pallida containing 100 μM of ketone 24 (retention time 17.6 min).
As shown in Fig. 5, Bauer alkamide 8 at 175 μM, Bauer alkamide 10 at 205 μM, and Bauer alkamide 11 at 230 μM, as single compounds, significantly inhibited P-gp activity (p < 0.05), but Bauer ketone 24 was not active across the tested concentrations (1–250 μM). The ethanolic extracts of E. sanguinea and E. pallida significantly inhibited P-gp at 1 mg/mL (containing 85 μM of alkamide 8, 2 μM of alkamide 10, and 0.7 μM of alkamide 11) and 5 mg/mL (containing 215 μM of alkamide 8, 25 μM of alkamide 10, and 45 μM of alkamide 11), respectively (Fig. 5).
Fig. 5.
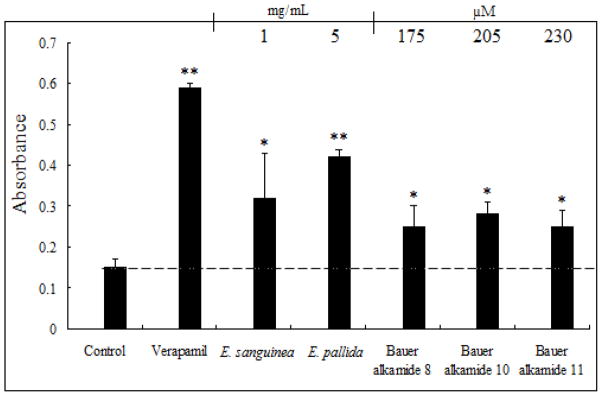
The effects of Bauer alkamides 8, 10, 11, ketone 24 on P-glycoprotein transporter activity. Control was 0.3% v/v DMSO in PBS. Verapamil was used as positive control at 10 μg/mL. *, ** Significantly different compared with control by two-sample t-test (p < 0.05, 0.01, respectively). Data are presented as the mean ± S.D. (n=6). The ethanolic extract of E. sanguinea at 1 mg/mL contained 85 μM of alkamide 8, 2 μM of alkamide 10, and 0.7 μM of alkamide 11. The ethanolic extract of 5 mg/mL of E. pallida extract contained 215 μM of alkamide 8, 25 μM of alkamide 10, and 45 μM of alkamide 11.
Discussion
This study investigated the uptake and metabolism of three alkamides and a ketone, when introduced to cultures of the human intestinal epithelial cell line, Caco-2, either as pure compounds or as components of complex ethanolic extracts of Echinacea. Because Bauer alkamide 8 was absorbed by passive diffusion [35], we expected that other alkamides would also be absorbed by a similar mechanism across Caco-2 cells. This is supported by the finding that the rate of membrane permeation of these three alkamides increased linearly with concentration, was not saturable during the tested incubation period (15–90 min), and was not different in either direction of transfer (AP-BL vs. BL-AP, Fig. 2A and B). Further, the transport of three alkamides was temperature independent (Fig. 2C), indicative of passive diffusion through the Caco-2 cells [23].
The order of the uptake of the three alkamides was Bauer alkamide 8 > 10 > 11, consistent with Papp for Bauer alkamide 8 of ~2.5× and ~15× greater than that of Bauer alkamides 10 and 11, respectively (Table 2). This trend for increases in hydrophilicity giving rise to increases in their apparent permeability has also been noted across the family of alkamides [9, 24].
Monohydroxylated, monoepoxidized, and N-dealkylated metabolites were reported after incubation of parent Bauer alkamide 8 with NADPH and human liver microsomes [25]. But few studies have investigated Phase II biotransformation of alkamides [14]. In our study, the amount of the parent compound increased by ~1.5 to 4.2× for Bauer alkamides 8, 10 and 11 on the apical and basolateral sides, or in cell lysates after β-glucuronidase incubation, indicating that alkamides were seemingly N-glucuronidated to some extent based on the amide structure (O-glucuronide might not form because of steric hindrance, Fig. 1), revealing another important pathway for alkamide metabolism besides cytochrome P450 (CYP) [10]. This finding is different from that of Ardjomand-Woelkart et al. [14] showing that no glucuronide or sulfate metabolite was present in the urine after oral administration of Bauer alkamide 8 both as pure compound or in E. purpurea root extract in rats [14]. It may be that components not found in E. purpurea that are specific to the Echinacea species studied facilitate this metabolism. In our study, alkamide 8 as a pure compound was not found as a glucuronide metabolite basolaterally but only apically. This suggests that there may be glucuronidation of pure alkamide 8 in vivo but it might not be readily detected if it is entirely transported to the intestinal lumen, because gut microbial β-glucuronidases would likely transform this metabolite to the parent compound. Further study of alkamide and ketone glucuronides (N- or O-) and their interaction with intestinal cell transporters and other Echinacea constituents is warranted.
The plant extracts did not affect transfer of alkamides as single compounds before deconjugation with β-glucuronidase (Table 2), establishing that the transfer of alkamides was passive and independent of the plant extract matrix for the two Echinacea species studied. Alkamide 8 in the plant extracts is seemingly a non-separable mixture of (2E,4E,8Z,10 E/Z)-N-isobutyldodeca-2,4,8,10-tetraenamide isomers, but this apparent mixture behaves similarly to the 90% pure (2E,4E,8Z,10Z)-N-isobutyldodeca-2,4,8,10-tetraenamide isoform in transferability before reaction with β-glucuronidase (Fig. 3A, Table 2). After β-glucuronidase hydrolysis, the plant extract alkamide 8 isomer mixture behaves similarly to alkamide 10 (Fig. 3AB, Table 2), suggesting that the plant extract alkamide 8 isomers may be similar to each other in their metabolism and transfer; this remains to be determined by comparing the pure alkamide 8 isomers. After β-glucuronidase hydrolysis, recovery relative to initial amounts was significantly increased in all compartments of the system, including the apical and basolateral sides as well as the cell lysates, for all three alkamides studied in E. sanguinea extract (Figure 3). These results suggest that other constituents in Echinacea species might regulate the expression of MRP (multidrug resistance-associated protein) or OATP (organic anion transporter protein) transporters, which in turn affect the permeation of glucuronidated alkamides, because anionic conjugates (glutathione, glucuronide or sulfate) cannot exit cells unless an MRP or OATP transporter is present [26].
Pure Bauer ketone 24 might be metabolized by CYPs based on its diene structure and the position of its ketone group. About 5% of the Bauer ketone 24 was recovered in the basolateral side for the compound as contained in two Echinacea species extracts (Table 2). Bauer ketone 24 transferred across the Caco-2 monolayers with apparent permeability of 10 ± 3 cm/s ×10−6, as extracted and isolated from E. pallida roots [27], similar to that seen in the present study from E. sanguinea or E. pallida ; Papp for Bauer ketone 24 was 12–17 cm/s ×10−6 (Table 2). The difference in permeability of Bauer ketone 24 as a pure compound versus that in extracts might be due to the effects of other extract constituents, because several Echinacea species extracts (11.2 – 2447 μg/mL) inhibited CYP 2C19, 2D6, and 3A4 by 20–100 %, and alkamides contributed to CYP inhibitory effects seen with Echinacea preparations [28]. E. purpurea root selectively modulated the catalytic activity of CYP3A and CYP1A2 at hepatic and intestinal sites by the interaction between Echinacea and enzyme substrates [29], although Gurley et al. reported minor activities of E. purpurea on CYP1A2, CYP2E1, CYP2D6, or CYP3A4 phenotypes [30]. In our study, Bauer alkamides 8, 10 and 11 were incubated with ketone 24 at 10–100 μM, but ketone 24 was still totally metabolized (data not shown). This implies that the CYP isoforms inhibited by those alkamides were not involved in the biotransformation of ketone 24, although we cannot rule out the possibility that the doses of those alkamides were too low to exhibit inhibitory effects. Although E. pallida polyacetylenes and polyenes are considered to have low chemical stability, after 72 h, 100 μM ketone 24 at 37°C in RPMI 1640 medium, hydroperoxide intermediate formation was about 25% of the parent compound, and hydroxylated derivatives were not observed [31]. Therefore, the unknown more hydrophilic metabolites of ketone 24 were formed by Caco-2 cell metabolism during 90 min incubation period, rather than non-metabolic oxidation. The identification of the ketone metabolites and herbal constituents that inhibit ketone metabolizing enzymes is required.
P-gp actively effluxes a wide range of structurally diverse anticancer agents, and P-gp-mediated multidrug resistance (MDR) has been associated with inhibition of caspase-dependent tumor cell apoptosis [32]. One strategy for reversal of MDR in cells expressing ATP-Binding Cassette (ABC) transporters is combined use of anticancer drugs with modulators [33]. In our study, extracts of E. sanguinea and E. pallida significantly inhibited P-gp-mediated efflux of calcein AM, a substrate for P-gp, indicating that alkamides were potentiated in the inhibition of P-gp by other extract constituents (Figure 5). For example, quercetin at 100 μM inhibited 30 % of P-gp mediated efflux of [3H]-taxol in Caco-2 cells [34]. The concentrations of Echinacea plant extracts that inhibited P-gp in our study were greater than shown previously in human proximal tubular kidney cells, showing that E. pallida extract was the most active at 3 μg/mL compared with E. angustifolia and E. purpurea both at 30 μg/mL [15]. More interestingly, Bauer ketone 24 was found to be the most efficient constituent isolated from E. pallida to inhibit P-gp at 3–200 μg/mL, equivalent to 14 μM-933 μM [15]. But in our study, Bauer ketone 24 was ineffective, which might be due to the difference in the cell lines used, to the metabolism of Bauer ketone 24, or to extract preparation. The extraction ratio (raw material weight: final solution volume) was 12:1 for the ethanol extract in our study vs. 125:1 for the n-hexane extract in the study of Romiti et al. [15]. Although alkamides are probably mostly absorbed in the small intestine, because we have shown that alkamides are apparently glucuronidated, the alkamide glucuronides will be effluxed to the lumen to some extent in intestinal cells, (except in the case of the alkamides in E. pallida), and these metabolites would also be susceptible to biliary excretion into the intestine. Although N-glucuronides are more slowly hydrolyzed by gut bacterial β-glucuronidase, than are O- or S-glucuronides [35], the parent alkamides would still be available to act in the large intestine. In addition, alkamides were highly permeable, which permits their interaction with P-gp, since its binding sites are either inside the bilayer or at the inner leaflet of the cell membrane [36]. Although glucuronidation may prevent tissue uptake of the alkamides, glucuronidase produced by liver and neutrophils [37–38] may convert the glucuronide conjugates back to the parent compounds, and alkamides may enter the hepatic portal vein at relative high concentrations, possibly permitting alkamide inhibition of P-gp in tissues throughout the body.
In conclusion, Bauer alkamides 8, 10 and 11 were transferred across Caco-2 cells, independent of extract matrix, thus the results of experiments testing efficacy of pure compounds may reasonably be extrapolated to results obtained from cruder extracts of plant materials containing these compounds. Alkamides and Bauer ketone 24 in Echinacea species extracts had a more extensive metabolism in the Caco-2 cells than did pure compounds, and extract matrices may have facilitated the metabolism of alkamides and ketones. Bauer alkamides 8, 10 and 11, as well as E. sanguinea and E. pallida extracts, inhibited P-gp mediated efflux in Caco-2 cells. Considering the increasing knowledge about the role of P-gp in cancer resistance and the global increase in the use of Echinacea preparations, further studies in humans are required to specifically investigate the uptake and metabolism of alkamides and ketones derived from natural plant products. Mechanisms for enhanced metabolism by accompanying constituents in plant extracts should also be established. Because Echinacea supplements are usually ingested on a chronic basis, their long-term effects on the metabolizing enzymes and expression of efflux transporters need to be investigated, especially for the ability to potentiate drug efficacy.
Acknowledgments
The research described herein was supported by Award Number P50AT004155 from the National Center for Complementary & Alternative Medicine.
Footnotes
Conflict of Interest
There are no conflicts of interest to disclose.
References
- 1.Barnes J, Anderson LA, Gibbons S, Phillipson JD. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L) Moench): A review of their chemistry, pharmacology and clinical properties. J Pharm Pharmacol. 2005;57:929–954. doi: 10.1211/0022357056127. [DOI] [PubMed] [Google Scholar]
- 2.Kligler B. Echinacea. Am Fam Physician. 2003;67:77–80. [PubMed] [Google Scholar]
- 3.Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States. National Health Statistics Reports. 2007 [PubMed] [Google Scholar]
- 4.Borchers AT, Keen CL, Stern JS, Gershwin ME. Inflammation and Native American medicine: The role of botanicals. Am J Clin Nutr. 2000;72:339–347. doi: 10.1093/ajcn/72.2.339. [DOI] [PubMed] [Google Scholar]
- 5.Pleschka S, Stein M, Schoop R, Hudson JB. Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus (H5N1, H7N7) and swine-origin H1N1 (S-OIV) Virol J. 2009;6:197. doi: 10.1186/1743-422X-6-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma SM, Anderson M, Schoop SR, Hudson JB. Bactericidal and anti-inflammatory properties of a standardized Echinacea extract (Echinaforce): Dual actions against respiratory bacteria. Phytomedicine. 2010;17:563–568. doi: 10.1016/j.phymed.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 7.LaLone CA, Hammer KD, Wu L, Bae J, Leyva N, Liu Y, Solco AK, Kraus GA, Murphy PA, Wurtele ES, Kim OK, Seo KI, Widrlechner MP, Birt DF. Echinacea species and alkamides inhibit prostaglandin E(2) production in RAW264. 7 mouse macrophage cells. J Agric Food Chem. 2007;55:7314–7322. doi: 10.1021/jf063711a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woelkart K, Koidl C, Grisold A, Gangemi JD, Turner RB, Marth E, Bauer R. Bioavailability and pharmacokinetics of alkamides from the roots of Echinacea angustifolia in humans. J Clin Pharmacol. 2005;45:683–689. doi: 10.1177/0091270004273493. [DOI] [PubMed] [Google Scholar]
- 9.Matthias A, Blanchfield JT, Penman KG, Toth I, Lang CS, De Voss JJ, Lehmann RP. Permeability studies of alkylamides and caffeic acid conjugates from Echinacea using a Caco-2 cell monolayer model. J Clin Pharm Ther. 2004;29:7–13. doi: 10.1046/j.1365-2710.2003.00530.x. [DOI] [PubMed] [Google Scholar]
- 10.Toselli F, Matthias A, Bone KM, Gillam EM, Lehmann RP. Metabolism of the major Echinacea alkylamide N-isobutyldodeca-2E, 4E, 8Z, 10Z-tetraenamide by human recombinant cytochrome P450 enzymes and human liver microsomes. Phytother Res. 2010;24:1195–1201. doi: 10.1002/ptr.3111. [DOI] [PubMed] [Google Scholar]
- 11.Cech NB, Tutor K, Doty BA, Spelman K, Sasagawa M, Raner GM, Wenner CA. Liver enzyme-mediated oxidation of Echinacea purpurea alkylamides: Production of novel metabolites and changes in immunomodulatory activity. Planta Med. 2006;72:1372–1377. doi: 10.1055/s-2006-951718. [DOI] [PubMed] [Google Scholar]
- 12.Hubatsch I, Ragnarsson EG, Artursson P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat Protoc. 2007;2:2111–2119. doi: 10.1038/nprot.2007.303. [DOI] [PubMed] [Google Scholar]
- 13.Manach C, Donovan JL. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radic Res. 2004;38:771–785. doi: 10.1080/10715760410001727858. [DOI] [PubMed] [Google Scholar]
- 14.Ardjomand-Woelkart K, Kollroser M, Magnes C, Sinner F, Frye RF, Derendorf H, Bauer R, Butterweck V. Absolute/relative bioavailability and metabolism of dodeca-2E,4E,8Z,10E/Z-tetraenoic acid isobutylamides (tetraenes) after intravenous and oral single doses to rats. Planta Med. 2011;77:1794–1799. doi: 10.1055/s-0030-1271120. [DOI] [PubMed] [Google Scholar]
- 15.Romiti N, Pellati F, Nieri P, Benvenuti S, Adinolfi B, Chieli E. P-glycoprotein inhibitory activity of lipophilic constituents of Echinacea pallida roots in a human proximal tubular cell line. Planta Med. 2008;74:264–266. doi: 10.1055/s-2008-1034308. [DOI] [PubMed] [Google Scholar]
- 16.Hunter J, Hirst BH. Intestinal secretion of drugs. The role of P-glycoprotein and related drug efflux systems in limiting oral drug absorption. Adv Drug Deliv Rev. 1997;25:129–157. [Google Scholar]
- 17.O’Connor R. The pharmacology of cancer resistance. Anticancer Res. 2007;27:1267–1272. [PubMed] [Google Scholar]
- 18.Bauer R, Jurcic K, Puhlmann J, Wagner H. Immunological in vivo and in vitro examinations of Echinacea extracts. Arzneim Forsch/Drug Res. 1988;38:276–281. [PubMed] [Google Scholar]
- 19.Wu L, Bae J, Kraus G, Wurtele E. Diacetylenic isobutylamides of Echinacea: Synthesis and natural distribution. Phytochemistry. 2004;65:2477–2484. doi: 10.1016/j.phytochem.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 20.Bae J. Doctoral Thesis. Iowa State University; 2006. Synthesis of the natural compounds in Echinacea. [Google Scholar]
- 21.Nasser B, Moustaid K, Moukha S, Mobio TA, Essamadi A, Creppy EE. Evaluation of the cytotoxicity and genotoxicity of extracts of mussels originating from Moroccan Atlantic coast, in human colonic epithelial cells Caco-2. Environmental Toxicology. 2008;23:539–547. doi: 10.1002/tox.20364. [DOI] [PubMed] [Google Scholar]
- 22.Qiang Z, Ye Z, Hauck C, Murphy PA, McCoy JA, Widrlechner MP, Reddy MB, Hendrich S. Permeability of rosmarinic acid in Prunella vulgaris and ursolic acid in Salvia officinalis extracts across Caco-2 cell monolayers. J Ethnopharmacol. 2011;137:1107–1112. doi: 10.1016/j.jep.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Said HM, Ortiz A, Ma TY. A carrier-mediated mechanism for pyridoxine uptake by human intestinal epithelial Caco-2 cells: Regulation by a PKA-mediated pathway. Am J Physiol Cell Physiol. 2003;285:1219–1225. doi: 10.1152/ajpcell.00204.2003. [DOI] [PubMed] [Google Scholar]
- 24.Jager H, Meinel L, Dietz B, Lapke C, Bauer R, Merkle HP, Heilmann J. Transport of alkamides from Echinacea species through Caco-2 monolayers. Planta Med. 2002;68:469–471. doi: 10.1055/s-2002-32076. [DOI] [PubMed] [Google Scholar]
- 25.Matthias A, Gillam EM, Penman KG, Matovic NJ, Bone KM, De Voss JJ, Lehmann RP. Cytochrome P450 enzyme-mediated degradation of Echinacea alkylamides in human liver microsomes. Chem Biol Interact. 2005;155:62–70. doi: 10.1016/j.cbi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Peng KC, Cluzeaud F, Bens M, Duong Van Huyen JP, Wioland MA, Lacave R, Vandewalle A. Tissue and cell distribution of the multidrug resistance-associated protein (MRP) in mouse intestine and kidney. J Histochem Cytochem. 1999;47:757–768. doi: 10.1177/002215549904700605. [DOI] [PubMed] [Google Scholar]
- 27.Chicca A, Pellati F, Adinolfi B, Matthias A, Massarelli I, Benvenuti S, Martinotti E, Bianucci AM, Bone K, Lehmann R, Nieri P. Cytotoxic activity of polyacetylenes and polyenes isolated from roots of Echinacea pallida. Br J Pharmacol. 2008;153:879–885. doi: 10.1038/sj.bjp.0707639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Modarai M, Gertsch J, Suter A, Heinrich M, Kortenkamp A. Cytochrome P450 inhibitory action of Echinacea preparations differs widely and co-varies with alkylamide content. J Pharm Pharmacol. 2007;59:567–573. doi: 10.1211/jpp.59.4.0012. [DOI] [PubMed] [Google Scholar]
- 29.Gorski JC, Huang SM, Pinto A, Hamman MA, Hilligoss JK, Zaheer NA, Desai M, Miller M, Hall SD. The effect of Echinacea (Echinacea purpurea root) on cytochrome P450 activity in vivo. Clin Pharmacol Ther. 2004;75:89–100. doi: 10.1016/j.clpt.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Carrier J, Khan IA, Edwards DJ, Shah A. In vivo assessment of botanical supplementation on human cytochrome P450 phenotypes: Citrus aurantium, Echinacea purpurea, milk thistle, and saw palmetto. Clin Pharmacol Ther. 2004;76:428–440. doi: 10.1016/j.clpt.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Chicca A, Adinolfi B, Pellati F, Orlandini G, Benvenuti S, Nieri P. Cytotoxic activity and G1 cell cycle arrest of a Dienynone from Echinacea pallida. Planta Med. 2010;76:444–446. doi: 10.1055/s-0029-1186224. [DOI] [PubMed] [Google Scholar]
- 32.Smyth MJ, Krasovskis E, Sutton VR, Johnstone RW. The drug efflux protein, P-glycoprotein, additionally protects drug-resistant tumor cells from multiple forms of caspase-dependent apoptosis. Proc Natl Acad Sci USA. 1998;95:7024–7029. doi: 10.1073/pnas.95.12.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi CH. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005;5:30–43. doi: 10.1186/1475-2867-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayeshi R, Masimirembwa C, Mukanganyama S, Ungell AL. The potential inhibitory effect of antiparasitic drugs and natural products on P-glycoprotein mediated efflux. Eur J Pharm Sci. 2006;29:70–81. doi: 10.1016/j.ejps.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Casarett LJ, Doull J, Klaassen CD. Casarett and Doull’s toxicology: The basic science of poisons. 7. McGraw-Hill Press; USA: 2008. p. 257. [Google Scholar]
- 36.Loo TW, Clarke DM. Do drug substrates enter the common drug-binding pocket of P-glycoprotein through “gates”? Biochem Biophys Res Commun. 2005;329:419–422. doi: 10.1016/j.bbrc.2005.01.134. [DOI] [PubMed] [Google Scholar]
- 37.O’Leary KA, Day AJ, Needs PW, Sly WS, O’Brien NM, Williamson G. Flavonoid glucuronides are substrates for human liver beta-glucuronidase. FEBS Lett. 2001;503:103–106. doi: 10.1016/s0014-5793(01)02684-9. [DOI] [PubMed] [Google Scholar]
- 38.Bartholomé R, Haenen G, Hollman CH, Bast A, Dagnelie PC, Roos D, Keijer J, Kroon PA, Needs PW, Arts CW. Deconjugation kinetics of glucuronidated phase II flavonoid metabolites by beta-glucuronidase from neutrophils. Drug Metab Pharmacokinet. 2010;25:379–387. doi: 10.2133/dmpk.dmpk-10-rg-002. [DOI] [PubMed] [Google Scholar]