Abstract
Background and Objectives
The application of fluorescent molecular imaging to surgical oncology is a developing field with the potential to reduce morbidity and mortality. However, the detection thresholds and other requirements for successful intervention remain poorly understood. Here we modeled and experimentally validated depth and size of detection of tumor deposits, trade-offs in coverage and resolution of areas of interest, and required pharmacokinetics of probes based on differing levels of tumor target presentation.
Methods
Three orthotopic tumor models were imaged by widefield epifluorescence and confocal microscopes, and the experimental results were compared with pharmacokinetic models and light scattering simulations to determine detection thresholds.
Results
Widefield epifluorescence imaging can provide sufficient contrast to visualize tumor margins and detect tumor deposits 3–5 mm deep based on labeled monoclonal antibodies at low objective magnification. At higher magnification, surface tumor deposits at cellular resolution are detectable at TBR ratios achieved with highly expressed antigens.
Conclusions
A widefield illumination system with the capability for macroscopic surveying and microscopic imaging provides the greatest utility for varying surgical goals. These results have implications for system and agent designs, which ultimately should aid complete resection in most surgical beds and provide real-time feedback to obtain clean margins.
Keywords: antibody targeting, antigen expression, fluorescence, surgery
INTRODUCTION
The pairing of molecular imaging with surgical oncology is an emerging field that stands at the interface of several disciplines [1,2]. This interdisciplinary field lies at the intersection of fluorescence illumination, intraoperative microscopy, molecular imaging agent development, and surgical oncology. The design of molecular probes to visualize cancer biomarkers is intertwined with technical equipment considerations, and both depend on surgical goals: debulking the tumor burden before chemotherapy [3], obtaining clean resection margins for a localized tumor [4], or the more challenging goal of completely resecting all tumor deposits in the body (Fig. 1A). Significant preclinical and clinical data exist demonstrating improvements in morbidity and/or mortality based on the extent of surgical resection [5–10]. This is not only the case for primary tumors, where complete resection prior to metastasis is often curative, but also for tumors that have spread. Many therapies are more effective against minimal residual disease making surgical resection a frontline treatment for numerous cancers.
Fig. 1.
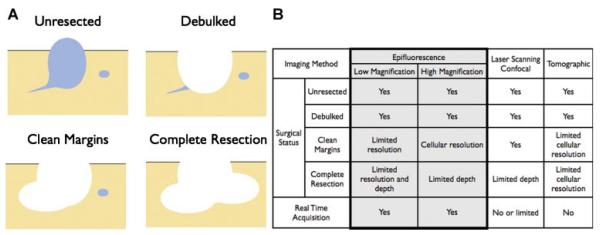
A: Imaging requirements vary depending on the surgical objective. B: The ability to image the surgical status of a resection varies with the fluorescent imaging set-up, each having strengths and limitations.
While still a nascent field, there are some obvious technical and practical prerequisites for intraoperative imaging. The imaging system must be easily incorporated into the operating room and be better/faster than current fresh frozen sectioning for determining margin status. Ideally, such systems should be able to image at video frame rates so that the operator can survey large areas, obtain real-time feedback during the surgery (>30 frames per second), and have deep tissue penetration and single cell resolution for intraoperative “molecular pathology.” Several imaging modalities meet some of these requirements, but there is no single system that incorporates all [11]. The generic imaging approaches include (a) widefield epi-illumination at variable magnifications, (b) laser scanning microscopic techniques (confocal, multiphoton, and related methods), and (c) transillumination and tomographic reconstruction methods (MFT, OPT) [12] which have yet to be adapted for facile, real-time imaging in a clinical setting [13]. Each of these methods requires a different equipment set-up and has its own strengths and limitations, as highlighted in Fig. 1B.
In this article, we focus on the widefield epi-illumination approach (using different objectives to yield different degrees of resolution) since it is most widely used and offers the most practical compromise between coverage, penetration depth, and temporal resolution while not impeding surgical access. We specifically set out to determine (a) tumor size detection thresholds, (b) maximum depth of detection, (c) effect of antigen presentation, and (d) feasibility in several intraoperative orthotopic tumor settings. We hypothesized that the detection thresholds are a function of tumor size, targeting efficiency, depth, and magnification. Using optimized approaches and integrated systems, intraoperative NIR imaging will be able to significantly improve cancer detection and aid in complete surgical resection.
MATERIALS AND METHODS
Cells
The human cell lines HT-29 (colon adenocarcinoma), A549 (nonsmall cell lung carcinoma) and PC3 (prostate cancer) were purchased from American Type Culture Collection (ATCC, Manassas, VA). The A549 and PC3 cell lines were transfected with GFP for cell tracking using a GFP lentivirus (SA Biosciences, Frederick, MD) and selected using 1 μg/ml puromycin (Sigma–Aldrich, St. Louis, MO).
Imaging Agents
Cetuximab (ImClone, Branchburg, NJ) and the anti-EpCAM antibody clone #158206 (R&D Systems, Minneapolis, MN) were used for imaging. These antibodies were labeled with VivoTag 680 (Visen, Bedford, MA) according to the manufacturer’s instructions. Fluorochrome-modified antibodies contained 1–2 dyes per antibody, and labeling did not have a significant effect on binding (data not shown). The vascular compartment was labeled using Angiosense-488 (Visen).
Surgically Induced Tumor Models
Experiments were performed in 12-week-old male nude mice (25–30 g) for prostate cancer and female nude mice (20–25 g) for colon and lung cancer (N = 15) obtained from COX-7 Laboratories (Massachusetts General Hospital, Boston, MA). They were cared for according to our institution’s animal facility standards and the institutional review board approved the protocol. The orthotopic implantation of colon, prostate, and lung tumor cells was done under isoflurane anesthesia (2% in 2 L/min of O2). Animals were physiologically monitored during and after all surgical procedures and kept under standard laboratory conditions with free access to water and a nonfluorescent diet in a 12-hr light/dark schedule.
For the colon tumor cell implantation, an abdominal mid-line incision was made to reach the descendent colon, where 1 × 106 HT-29 cells diluted in 5 μl of PBS and 5 μl Matrigel (BD Biosciences, San Jose, CA) were injected superficially on the colon wall. The abdominal incision was closed with Ethicon Prolene 6-0 (Johnson & Johnson, Brussels, Belgium), and the animals were allowed to recover for 2 weeks.
For the implantation of lung tumor cells, mice were placed in a left lateral position, and a 1-cm skin incision was made on the posterior side of the right thorax wall. Intercostal muscles were retracted and, visualizing the lung without opening the thoracic cavity, 1 × 106 GFP transfected A549 cells in 10 μl of PBS were injected superficially on the right inferior lobe. The skin incision was closed using Ethicon Prolene 7-0. After 7 days, a 1- to 2-mm tumor could be seen under the parietal pleura.
For orthotopic prostate tumor cell implantation, a suprapubic transversal incision was made, and the prostate was isolated extraperitoneally. GFP-transfected PC3 cells (1 × 106) diluted in 5 μl of PBS and 5 μl Matrigel were injected into the left lobe of the prostate. The abdominal incision was closed with Ethicon Prolene 6-0, and tumors reached 1–2 mm 2 weeks later.
Intraoperative Imaging
Intravenous injections of 30 μg/100 μl saline of near infrared conjugated antibodies that target EpCAM or EGFR were administered via tail vein (using a P10 tubing catheters) 72 hr prior to surgical resection.
The epifluorescence images were collected on a commercially available preclinical OV-110™ optical system (Olympus, Center Valley, PA) incorporating a Hamamatsu C9100-13 EM-CCD camera (Hamamatsu, Bridgewater, NJ). This system provides video rate images in white light or NIR channels and has several objectives to change the area of interest and resolution (0.14×, 0.56×, 0.89×, and 16×). As a control, laser scanning confocal images were collected on an IV100 microscope using a 10× objective, 0.3 NA (Olympus). Additional real-time dual white light and NIR images were collected on a clinical home built intraoperative system using an epifluorescence intraoperative microscope with a 0.65–5× variable objective.
Images were collected in the white light, green, and NIR channels (green = 475/430 ex, 530/540 em; NIR = 630/635 ex, 695/640 em) using exposure time of 280 or 33 msec (real-time).
Lung Tumor
Mice were anesthetized with isoflurane (2–3% in 2 L/min of O2), intubated, and mechanically ventilated. A 1.5-cm transversal incision was made on the right side of the thoracic wall and the thoracic cavity was opened. The ribs were gently retracted and the right lung was exposed. Animals were imaged using the white light, green, and NIR fluorescence.
Colon Tumor
Mice were anesthetized with a mixture of ketamine (90 mg/kg) and xylazine (10 mg/kg) IP and submitted to a transversal laparotomy. Macroscopic imaging of the entire mouse and microscopic imaging of descendent colon were taken using white light, green, and NIR channels on the OV-110™ system. Two nanomoles per 100 μl of a vascular contrast agent (Angiosense 488; Visen) were administered IV, and the colon was re-imaged with a confocal microscope (IV100; Olympus). Under a dissecting microscope, the orthotopic colon tumor was resected and re-imaged on the OV-110.
Prostate Tumor
Mice underwent a transversal suprapubic laparotomy under ketamine (90 mg/kg) and xylazine (10 mg/kg) anesthesia. Intraoperative macroscopic imaging of the inferior abdominal cavity and microscopic imaging of the prostate were taken using white light, green, and NIR channels on the OV-110™ system. Under the dissecting microscope, the prostate orthotopic tumor was resected and this area was re-imaged. No procedure-related deaths were noted during the intraoperative procedure, and all animals were sacrificed following imaging.
Mathematical Models and Validation
Details of the mathematical models can be found in the Supplemental Information. Briefly, the light diffusion equations [14] were simulated in cylindrical geometry with Robin boundary conditions (partial reflection) at the top, bottom, and outer surface. A no-flux Neumann boundary condition was used at zero radius. Cylindrical tumors with the height equal to the diameter were placed at the surface or varying depths in the tissue. A planar source at the surface of the tissue was used to simulate the excitation light, and this intensity was used to solve for the emission light source. The emission light intensity was then calculated with both the excitation and emission boundary value problems solved using Gaussian elimination in Matlab (Mathworks, Natick, MA). Differences in the average intensity over the tumor were compared to the background intensity to determine the tumor to background ratio. This was compared to experimentally measured fluctuations in background signal to determine the contrast to noise ratio. The maximum depth of detection was set where the contrast between tumor and background was greater than the fluctuations in background intensity.
Tumor phantoms were created using 1% Intralipid (Baxter, Deerfield, IL) and 50 ppm India ink to match typical absorption and scattering properties of human tissue. For the tumor, 100 nM VT680 fluorochrome and 2% agarose (to provide structure) were added to the Intralipid mixture, melted, and cast in centrifuge tubes cut to the proper height. Eight nanomoles of Genhance was included in the surrounding Intralipid to represent nonspecific uptake and background autofluorescence. The agarose phantom was suspended from below with the liquid level adjusted to bury the tumor under different depths of Intralipid.
The antibody pharmacokinetics were simulated using a previously validated model [15,16]. Briefly, the plasma pharmacokinetics followed biexponential decay and entered the tumor from the neovasculature and tumor surface. Inside the tumor tissue, the antibodies diffused between the cells where they associated, dissociated, and were internalized by surface antigen. The internalized tag from the degraded antibody was then lost from the cell using rates measured in vitro.
RESULTS
Imaging Modality and Magnification
Nude mice containing orthotopic HT-29 colon adenocarcinoma tumors were imaged following intravenous administration of anti-EpCAM-680. The intensity of the fluorescent tumor was sufficient to detect the tumor while imaging the entire mouse with a 0.14× objective (Fig. 2A). This macroscopic imaging enables the surgeon to survey large areas of tissue to quickly locate suspicious signals. However, under higher magnification (0.89× objective), more detail of the tumor borders could be seen (Fig. 2B). In this particular tumor example, a large cluster formed after tumor cell implantation, but several smaller deposits of cells remained in the Matrigel separate from the larger tumor. This image highlights the utility of being able to quickly focus at higher magnification on a particular region of interest during surgery. In comparison, laser scanning microscopy is able to obtain a higher signal to background ratio using the same antibody (Fig. 2C) due to the optical sectioning capability of the instrument.
Fig. 2.
A: Macroscopic view of an orthotopic colon tumor surveying the entire mouse. The arrow points to the tumor. B: A higher magnification of the tumor showing increased resolution of small cellular deposits around a larger lesion. C: A laser scanning confocal image of a colon tumor with a green fluorescent vascular probe. The optical slicing increases the signal to background ratio at the cost of slower imaging times and depth of focus. D: High magnification image of the same tumor in B after resection (top) and the resected tissue (bottom) highlighting the utility of surveying both resected and unresected tissues.
A major advantage of fluorescent intraoperative imaging over the current technique of fresh frozen sectioning is the ability to image both the unresected tissue (Fig. 2D, top) and resected tissue (Fig. 2D, bottom) to verify clean margins. Figure 2B,D is of the same mouse before and after the surgery. Fresh frozen sectioning only looks at a small portion of resected tissue or at most the border of the resected tissue [17].
Depth of Imaging
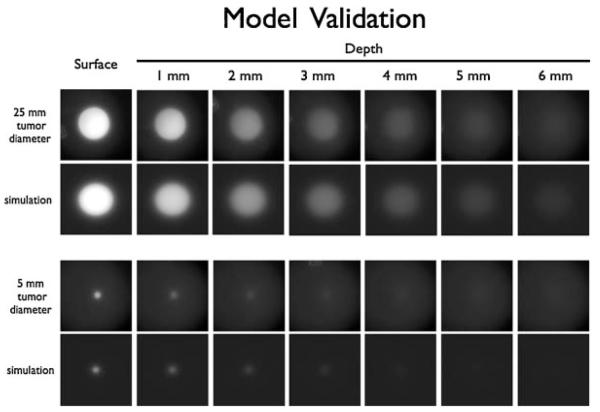
Although near infrared light can penetrate tissue more efficiently than visible light [18], there are still limitations to the depth of imaging, especially when using epifluorescence [11]. The maximum depth of detecting a tumor using epifluorescence is a complex function of the tumor size, tumor to background ratios achieved with the imaging probe, absolute concentrations of fluorophore, properties of the imaging system, the wavelength of light, and the tissue optical properties [19]. Since it is not possible to grow large tumors deeply imbedded in tissue of small animals due to size constraints, and the results from small tumors cannot be extrapolated directly, we sought to simulate the imaging of larger tumors in humans. Mathematically, the equations describing scattering and absorption of light in tissue are very similar to the equations describing the diffusion and degradation of antibodies in tumor tissue except one is an energy balance and the other a material balance (Supplemental Information). Using these previously developed equations for describing light propagation in tissue, the fluorescence emission was simulated for tumors buried under varying thicknesses of tissue. To validate this mathematical model, tumor phantoms were created using agarose gels containing varying amounts of fluorescent dye. Intralipid and dye were used to mimic the scattering and absorption properties of tissue, respectively. Figure 3 shows the simulations and tumor phantom results for two such tumor sizes. The larger tumor phantom is qualitatively visible at greater depths than the smaller tumor, in agreement with the simulations.
Fig. 3.
Comparison of tumor phantom images and light scattering simulations. A 25-mm diameter and 5 mm diameter tumor phantom were imaged on the OV110 (top row in each set) with the corresponding numerical simulation (bottom row of each set).
Simulations were carried out for a variety of tumor to background ratios, depths, and tumor sizes. For tumors less than 1 cm, the tumor size has a strong influence on the maximum depth of detection (Fig. 4A). At sizes larger than 2 cm in diameter, the tumor size has a much smaller impact than the tumor to background ratio of the imaging agent. This ratio has diminishing returns at larger values. There is a large increase in the maximum depth of detection as the TBR increases up to approximately 10, which then drops off significantly (Fig. 4B).
Fig. 4.

Maximum depth of detection as a function of (A) tumor diameter for various tumor to background ratios and (B) tumor to background ratio for a 1-cm diameter tumor. The gray shading represents typical imaging agent tumor to background ratios for large tumors. C: The maximum size of detection of surface lesions for macroscopic imaging is a function of TBR. For a saturated small lesion with a highly expressed antigen (~1.2 μM) and background of 25 nM, the smallest detectable lesion is 600 μm at low magnification (e.g., 0.14× objective). Data points represent simulations, while the lines are empirical fits to guide the eye.
Size of Detection
For large tumors, tumor to background ratios typically range from 3 to 12 depending on the imaging probe. Small tumors often have better uptake of imaging agents due to more efficient diffusion in from the surface resulting in much higher ratios. For example, a saturated lesion with 1–2 million antigens/cell and background of 25 nM will have a TBR around 50. For lesions located at the surface, the detection threshold is a function of TBR (which depends on antigen expression for antibodies in small lesions) and magnification. For macroscopic imaging, where the diffuse light simulations are valid, the smallest lesion detectable with a highly expressed antigen (e.g., several million antigens/cell) is approximately 600 μm (Fig. 4C). This is approaching the pixel resolution of the macroscopic images (110 μm/pixel). Conversely, at higher magnification of the same tumor, much smaller lesions can be imaged even down to the single cell level (Supplemental Fig. 1) due to higher resolution.
These detection limits are determined using an experimentally measured fluctuation in background auto fluorescence, but this can vary significantly with the tissue, fluorescent wavelength, and type of imaging agent used. Tumors that do not achieve the detection threshold can often still be seen; however, their intensity is not sufficiently greater than the background autofluorescence to positively identify it as a tumor. Likewise, the absorption and scattering of light vary significantly between tissues [20]. Although the absolute magnitude of depth may vary with the tissue, the relative trends will exist across different operating conditions.
Real-Time Imaging
Another advantage of widefield epifluorescence imaging is that the entire field of view is excited and captured simultaneously. The major limitation for imaging at faster frame rates is the exposure time for each frame. With sensitive cameras, such as EMCCD cameras, rapid frame rates can still capture a significant signal with low levels of noise (from reduced photon counts and instrumentation noise). To test real-time imaging capabilities, a GFP A549 orthotopic lung cancer tumor was imaged due to the potential for extensive movement artifacts. At three frames per second, the respiratory and cardiac movement resulted in a blurred image (Fig. 5). By increasing the frame rate 10-fold to 30 frames per second (video frame rate), a clear image of the tumor and fluorescent image was captured. This type of frame rate is not possible with laser scanning confocal microscopy, where the image is built up from scanning one pixel at a time. While spinning disk confocal microscopes maintain fast frame rates, they are currently not designed for imaging very thick sections where out of focus fluorescence is not completely blocked due to pinhole cross-talk.
Fig. 5.

Imaging of an orthotopic A549 lung carcinoma. The GFP channel (left) and infrared channel (right) show a blurred image when viewed at a slow frame rate due to cardiac and respiratory movements (top). These artifacts are significantly reduced when imaging at video frame rates (bottom) without significant degradation in picture quality.
Target Antigen Expression
Target expression also impacts the overall ability to achieve surgical objectives with a given piece of imaging equipment. The signal from large tumors is often limited by delivery of the probe, so there is not a dramatic difference in uptake for a moderately to highly expressed antigen [16]. However, for small tumors, on the order of a couple millimeters or less in size, the total amount of fluorophore in the whole tumor may be limited. Even if the surface of all the tumor cells is saturated, the total amount of fluorophore in the small tumor may be low. Here, delivery is less of a concern due to diffusion from the surrounding tissue (and generally better vascularization and less necrosis in small tumors). Targeting a highly expressed antigen allows more fluorophore to accumulate, resulting in higher concentrations and signal (Fig. 6A). When the absolute amount of fluorophore is limiting, a lower expression antigen may not be significantly brighter than the surrounding tissue (Fig. 6B). This can be seen when targeting EpCAM in an orthotopic model of prostate cancer using PC3 cells versus an HT-29 orthotopic colon cancer model (Fig. 6C). This effect of target expression on TBR is one reason antibodies were chosen as a representative molecular imaging agent. Due to the heterogeneity found in human tumors, no single antibody will be appropriate for every patient. The clinical development of dozens of antibodies against many targets (primarily for therapeutics) can be utilized to select an appropriate antibody and target for a given patient. The two targets used in this work, EpCAM and EGFR, were chosen due to their high expression in a variety of human tumors. EpCAM is expressed by epithelial cancers such as colon, lung, prostate, breast, ovarian, cervical, head and neck, esophageal, and gastric carcinomas [21,22]. EGFR is also overexpressed in many carcinomas including pancreatic, renal, bladder, colon, lung, prostate, breast, ovarian, head and neck, and esophageal [23]. In addition, it is overexpressed in several important sarcomas (osteosarcomas and soft tissue sarcomas) [24,25] and glioblastomas [26,27].
Fig. 6.
Effect of target expression on detection. A: Antibody uptake in a 1-mm tumor was simulated to determine the TBR achieved with varying antigen expression. Light scattering simulations used this TBR to determine the contrast to noise ratio for the tumor. B: Light scattering simulations show the contrast for low TBR (left) and high TBR (right) compared to the heterogeneity in background autofluorescence (middle). C: The lower expression of EpCAM in an orthotopic PC3 prostate cancer model (left, arrow points to tumor) results in a lower CNR compared to the high expression in HT29 orthotopic colon tumors (right). Simulations details are found in the Supplemental Information.
DISCUSSION
Fluorescent intraoperative imaging can increase the success of surgery by enabling the operator to ensure clean margins and remove a significantly greater portion of cancerous tissue, find additional including metastatic lesions, and avoid complications in difficult to operate surgical beds. In the case of complete resection, this can achieve a cure [28,29].
There are four main considerations involved in intraoperative molecular imaging: (1) imaging equipment, (2) fluorescent targeting agents, (3) tumor type, and (4) the surgical intention. There are a variety of fluorescent imaging modalities and equipment, each with its own strengths and weaknesses. This includes widefield epi-illumination at variable magnifications, laser scanning microscopic techniques (confocal, multiphoton, and related methods), and transillumination and tomographic reconstruction methods (MFT, OPT). Each of these methods requires a different equipment set-up. Extensive software and data processing can also be included for spectral deconvolution of signal versus autofluorescence and tomographic reconstruction [11,12]. Some of these are entering the market, and several sophisticated systems have been described [2,30–32]. Fluorescent agents used for imaging range from passive accumulation due to poor vascularization and increased vascular permeability (enhanced permeation and retention or EPR) effects [33], binding interactions [34–37], proteolytic cleavage [6,38], and metabolically processed tags [39]. These probes accumulate based on the local physiology (e.g., indocyanine green) or targeting the host tissue (e.g., macrophages, neovasculature), cancer cells (e.g. cetuximab, trastuzumab, anti-EpCAM antibodies), or specific enzymes (e.g., cathepsins, MMPs). Common imaging targets in tumors include cell specific targets (such as EpCAM and EGFR used here) or host response targets (TAM, CTL, microvasculature). Often, these agents can yield complementary information, making the case for multichannel imaging akin to multicolor immunohistochemistry [16]. The presence or ability of the tumor to metastasize also impacts the surgical goals. Surgical objectives include debulking the tumor, such as prior to chemotherapy for ovarian cancer [10], obtaining clean resection margins [4], and/or complete resection. This final goal is often the desired objective, although it remains exceedingly difficult to achieve in practice.
Based on the data presented here, a widefield fluorescence imaging system capable of transitioning from macroscopic imaging to survey the tissue down to microscopic imaging to detect individual cancer cells will provide considerable flexibility to the operator. The macroscopic imaging is capable of detecting large tumors imbedded in the tissue, while the microscopic mode can verify clean resection margins by detecting lesions down to the cellular level (Supplemental Fig. 1). Provided that the imaging agent accumulates to sufficient levels above tissue autofluorescence, a true laser scanning confocal system may not be necessary for detection. The widefield epifluorescent setup is capable of imaging in real time with minimal interruptions to the operator. In the future, faster tomographic reconstruction and transillumination may play a role, but currently these are not capable of facile imaging in real-time.
In conclusion, fluorescent intraoperative imaging stands to dramatically improve surgical outcomes for tumor resection. Only when the surgical objectives, imaging equipment, and targeting agent selection are considered together will the full potential be realized. Widefield macroscopic to microscopic real-time imaging provides a flexible platform for multiple surgical objectives when utilized with a sufficiently sensitive and specific imaging agent.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Joshua Dunham for help in creating the tumor phantoms, Rabi Upadhyay and Claudio Vinegoni for helpful discussion and review of the manuscript, and Yvonna Fisher-Jeffes for manuscript review. Grant support was provided in part by P50 CA86355, U24 CA092782, and T32 CA079443.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Greg M. Thurber and Jose-Luiz Figueiredo contributed equally to this work.
REFERENCES
- 1.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirsch DG, Dinulescu DM, Miller JB, et al. A spatially and temporally restricted mouse model of soft tissue sarcoma. Nat Med. 2007;13:992–997. doi: 10.1038/nm1602. [DOI] [PubMed] [Google Scholar]
- 3.Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J Clin Oncol. 2002;20:1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 4.Fatouros M, Roukos DH, Arampatzis I, et al. Factors increasing local recurrence in breast-conserving surgery. Expert Rev Anticancer Ther. 2005;5:737–745. doi: 10.1586/14737140.5.4.737. [DOI] [PubMed] [Google Scholar]
- 5.Stummer W, Reulen HJ, Meinel T, et al. Extent of resection and survival on glioblastoma multiforme-identification of and adjustment for bias. Neurosurgery. 2008;62:564–574. doi: 10.1227/01.neu.0000317304.31579.17. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen QT, Olson ES, Aguilera TA, et al. Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival. Proc Natl Acad Sci USA. 2010;107:4317–4322. doi: 10.1073/pnas.0910261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerrand CH, Wunder JS, Kandel RA, et al. Classification of positive margins after resection of soft-tissue sarcoma of the limb predicts the risk of local recurrence. J Bone Joint Surg [Br] 2001;83B:1149–1155. doi: 10.1302/0301-620x.83b8.12028. [DOI] [PubMed] [Google Scholar]
- 8.Lewis JJ, Leung D, Heslin M, et al. Association of local recurrence with subsequent survival in extremity soft tissue sarcoma. J Clin Oncol. 1997;15:646–652. doi: 10.1200/JCO.1997.15.2.646. [DOI] [PubMed] [Google Scholar]
- 9.Wasserberg N, Gutman H. Resection margins in modern rectal cancer surgery. J Surg Oncol. 2008;98:611–615. doi: 10.1002/jso.21036. [DOI] [PubMed] [Google Scholar]
- 10.Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian-carcinoma. Am J Obstet Gynecol. 1994;170:974–980. doi: 10.1016/s0002-9378(94)70090-7. [DOI] [PubMed] [Google Scholar]
- 11.Leblond F, Davis SC, Valdes PA, et al. Pre-clinical whole-body fluorescence imaging: Review of instruments, methods and applications. J Photochem Photobiol B Biol. 2010;98:77–94. doi: 10.1016/j.jphotobiol.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graves EE, Weissleder R, Ntziachristos V. Fluorescence molecular imaging of small animal tumor models. Curr Mol Med. 2004;4:419–430. doi: 10.2174/1566524043360555. [DOI] [PubMed] [Google Scholar]
- 13.Ntziachristos V, Ripoll J, Weissleder R. Would near-infrared fluorescence signals propagate through large human organs for clinical studies? Opt Lett. 2002;27:333–335. doi: 10.1364/ol.27.000333. [DOI] [PubMed] [Google Scholar]
- 14.Wang L, Wu H. Biomedical optics. Wiley; Hoboken, NJ: 2007. [Google Scholar]
- 15.Thurber GM, Zajic SC, Wittrup KD. Theoretic criteria for antibody penetration into solid tumors and micrometastases. J Nucl Med. 2007;48:995–999. doi: 10.2967/jnumed.106.037069. [DOI] [PubMed] [Google Scholar]
- 16.Thurber G, Figueiredo J, Weissleder R. Multicolor fluorescent intravital live microscopy (FILM) for surgical tumor resection in a mouse xenograft model. PLoS ONE. 2009;4:e8053. doi: 10.1371/journal.pone.0008053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagi C, O’Grady TC, Izadpanah A. Mohs micrographically controlled surgery and the treatment of malignant melanoma. Semin Oncol. 2002;29:336–340. doi: 10.1053/sonc.2002.34111. [DOI] [PubMed] [Google Scholar]
- 18.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003;9:123–128. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 19.Kepshire D, Davis SC, Dehghani H, et al. Fluorescence tomography characterization for sub-surface imaging with protoporphyrin IX. Opt Express. 2008;16:8581–8593. doi: 10.1364/oe.16.008581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheong WF, Prahl SA, Welch AJ. A review of the optical properties of biological tissues. IEEE J Quantum Electr. 1990;26:2166–2185. [Google Scholar]
- 21.Winter MJ, Nagtegaal ID, van Krieken J, et al. The epithelial cell adhesion molecule (Ep-CAM) as a morphoregulatory molecule is a tool in surgical pathology. Am J Pathol. 2003;163:2139–2148. doi: 10.1016/S0002-9440(10)63570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pande AU, Iyer RV, Rani A, et al. Epidermal growth factor receptor-directed therapy in esophageal cancer. Oncology. 2007;73:281–289. doi: 10.1159/000132393. [DOI] [PubMed] [Google Scholar]
- 23.Herbst RS, Shin DM. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors—A new paradigm for cancer therapy. Cancer. 2002;94:1593–1611. doi: 10.1002/cncr.10372. [DOI] [PubMed] [Google Scholar]
- 24.Wen YH, Koeppen H, Garcia R, et al. Epidermal growth factor receptor in osteosarcoma: Expression and mutational analysis. Hum Pathol. 2007;38:1184–1191. doi: 10.1016/j.humpath.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Yang JL, Hannan MT, Russell PJ, et al. Expression of HER1/EGFR protein in human soft tissue sarcomas. Eur J Surg Oncol. 2006;32:466–468. doi: 10.1016/j.ejso.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Andersson U, Guo D, Malmer B, et al. Epidermal growth factor receptor family (EGFR, ErbB2-4) in gliomas and meningiomas. Acta Neuropathol. 2004;108:135–142. doi: 10.1007/s00401-004-0875-6. [DOI] [PubMed] [Google Scholar]
- 27.Heimberger AB, Suki D, Yang D, et al. The natural history of EGFR and EGFRvIII in glioblastoma patients. J Translat Med. 2005;3:6. doi: 10.1186/1479-5876-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wind J, Smit EJ, Senan S, et al. Residual disease at the bronchial stump after curative resection for lung cancer. Eur J Cardiothorac Surg. 2007;32:29–34. doi: 10.1016/j.ejcts.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Renehan AG, Egger M, Saunders MP, et al. Impact on survival of intensive follow up after curative resection for colorectal cancer: Systematic review and meta-analysis of randomised trials. Br Med J. 2002;324:813–816. doi: 10.1136/bmj.324.7341.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Themelis G, Yoo JS, Soh KS, et al. Real-time intraoperative fluorescence imaging system using light-absorption correction. J Biomed Opt. 2009;14:9. doi: 10.1117/1.3259362. [DOI] [PubMed] [Google Scholar]
- 31.Sheth RA, Upadhyay R, Stangenberg L, et al. Improved detection of ovarian cancer metastases by intraoperative quantitative fluorescence protease imaging in a pre-clinical model. Gynecol Oncol. 2009;112:616–622. doi: 10.1016/j.ygyno.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Grand AM, Frangioni JV. An operational near-infrared fluorescence imaging system prototype for large animal surgery. Technol Cancer Res Treat. 2003;2:553–562. doi: 10.1177/153303460300200607. [DOI] [PubMed] [Google Scholar]
- 33.Ishizawa T, Fukushima N, Shibahara J, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer. 2009;115:2491–2504. doi: 10.1002/cncr.24291. [DOI] [PubMed] [Google Scholar]
- 34.Kaushal S, McElroy MK, Luiken GA, et al. Fluorophore-conjugated anti-CEA antibody for the intraoperative imaging of pancreatic and colorectal cancer. J Gastrointest Surg. 2008;12:1938–1950. doi: 10.1007/s11605-008-0581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou P, Xu SB, Povoski SP, et al. Near-infrared fluorescence labeled anti-TAG-72 monoclonal antibodies for tumor imaging in colorectal cancer xenograft mice. Mol Pharm. 2009;6:428–440. doi: 10.1021/mp9000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Longmire MR, Gunn AJ, Morgan NY, et al. Real-time fluorescence-enhanced imaging as an aid to surgery in ovarian cancer. IEEE J Selected Top Quantum Electr. 2007;13:1602–1609. [Google Scholar]
- 37.Veiseh M, Gabikian P, Bahrami SB, et al. Tumor paint: A chlorotoxin: Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res. 2007;67:6882–6888. doi: 10.1158/0008-5472.CAN-06-3948. [DOI] [PubMed] [Google Scholar]
- 38.Weissleder R, Tung CH, Mahmood U, et al. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 39.Bogaards A, Varma A, Collens SP, et al. Increased brain tumor resection using fluorescence image guidance in a preclinical model. Lasers Surg Med. 2004;35:181–190. doi: 10.1002/lsm.20088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.