Abstract
Susceptibility to inflammatory bowel diseases depends upon interactions between the genetics of the individual and induction of chronic mucosal inflammation. We hypothesized that administration of dietary phenolics, caffeic acid and rutin, would suppress upregulation of inflammatory markers and intestinal damage in a mouse model of colitis. Colitis was induced in C3H/HeOuJ mice (8 wk old, 6 male/6 female per treatment) with 1.25% dextran sulfate sodium (DSS) for 6 d in their drinking water. Rutin (1.0 mmol (524 mg)/kg in diet), caffeic acid (1.0 mmol (179 mg)/kg in diet), and hypoxoside extract (15 mg/d, an anticolitic phenolic control) were fed for 7 d before and during DSS treatment, as well as without DSS treatment. Body weight loss was prevented by rutin and caffeic acid during DSS treatment. Colon lengths in mice fed caffeic acid and hypoxoside during DSS treatment were similar to DSS-negative control. Food intake was improved and myeloperoxidase (MPO) was decreased with each phenolic treatment in DSS-treated mice compared with DSS treatment alone. Colonic mRNA expression of IL-17 and iNOS were inhibited when IL-4 was increased by each phenolic treatment combined with DSS, whereas CYP4B1 mRNA was increased only by caffeic acid in DSS-treated mice, compared with DSS treatment alone. Colonic and cecal histopathology scores of DSS-treated mice were significantly more severe (P< 0.01) than in mice fed caffeic acid before and during DSS treatment based on mucosal height, necrosis, edema, erosion, and inflammatory cell infiltration. Although both rutin and caffeic acid suppressed the expression of selected inflammatory markers, only caffeic acid protected against DSS induced colitis, in association with normalization of CYP4B1 expression. The inhibition of DSS-induced colitic pathology by caffeic acid was mediated by mechanisms in addition to anti-inflammatory effects that deserve further study.
Keywords: caffeic acid, rutin, colitis, CYP4B1, inflammation, mouse
Introduction
The pathogenesis of inflammatory bowel diseases (IBDs; e.g., Crohn’s disease, ulcerative colitis) is not fully understood. Genes, environment, enteric microbiota, and other factors alter disease risk (1). Nutritional factors (e.g., elemental diets, sucrose or other specific carbohydrate diets, other dietary components that alter gut microbial populations) modulate these diseases, and play a significant role in the treatment of IBD and influence the disease course and prognosis (2). Increased colon cancer risk is a key concern associated with colitis, as evident in a Danish epidemiological study of more than 5,500 ulcerative colitis patients who showed greater risk for colon cancer than controls (3). IBD models induced by enteric bacteria are well established (4, 5). Colitis in mice also may be induced by dextran sodium sulfate (DSS) in drinking water (6) causing weight loss, diarrhea with blood and/or mucus, shortening of the colon, erosion of the mucosal epithelium, and acute neutrophilic infiltration (7). Experimental colitis models are characterized by up-regulated nuclear factor kappa B (NF-κB) and pro-inflammatory cytokines (e.g., interleukin-1β (IL-1β), tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ)) resulting in tissue damage (8, 9). Despite adverse side effects, glucocorticosteroids are used to treat IBD (1, 10). Immunosuppressive and immunoregulatory agents (cyclosporine, aminosalicylates and azathioprine) have also been used to control severe disease, however, serious complications and toxic side effects were associated with them (11). Thus, dietary phenolics may be an alternative to control this disease. Caffeic acid phenethyl ester (CAPE) decreased colonic NF-κB and prevented colitis in peptidoglycan-polysaccharide (PG-PS)-treated rats injected with 30 mg CAPE /kg for seven days (12). Dietary rutin (feeding 0.1% rutin diet for 2 weeks) prevented DSS-induced colitis and possible colorectal carcinogenesis by suppressing pro-inflammatory cytokines (TNF-α, IL-1β, 13). Using DSS- and Brachyspira hyodysenteriae-induced colitis, hypoxoside extract showed protection (Wannemuehler laboratory, unpublished). Hypoxoside Extract, a known anti-inflammatory component of African potato (14, 15), decreased inflammatory damage, was possibly related to the down-regulation of NF-κB pathways in mucosa. Dietary caffeic acid, a major phenolic acid widely distributed in plant foods and herbs, has not been studied for its effect on DSS-induced mouse colitis and the relationship between specific inflammation-related cytokine mRNA expression, myeloperoxidase and mucosal histopathology. In the present study, commonly occurring dietary phenolics, rutin and caffeic acid, were compared with hypoxoside extract, for protective efficacy in a C3H/HeOuJ mouse model of colitis. This work may lead to improvement of the therapeutic and prophylactic benefits of plant foods and botanical supplements. Our long term goals are to identify novel anti-inflammatory phenolics that may be used to prevent or treat human colitis and to establish and validate a screening assay for dietary components that may prevent colitis and colon cancer.
Materials and Methods
Chemicals and Reagents
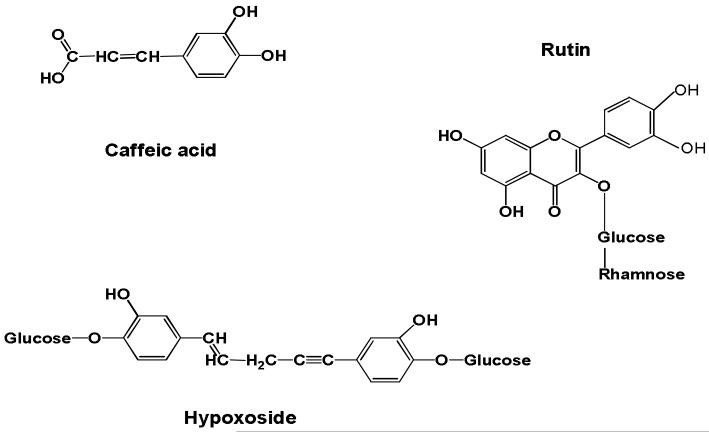
Purified caffeic acid and rutin were purchased from Chroma Dex ™, Inc. Santa Ana, CA. Hypoxoside extract was a gift from Allison AC, Dawa Corp., Belmont, CA (Figure 1). Dextran sulphate sodium was purchased from Fisher Scientific (Pittsburgh, PA). RNAlater® Tissue Collection solution was purchased from Applied Biosystems Business (Foster City, CA). Working solutions of 3, 3′, 5, 5′-tetramethylbenzidine hydrochloride (TMB, Sigma; 2.5 mM in water) and hydrogen peroxide (5 mM in water) were prepared immediately before use. Sulfuric acid (Fisher Scientific; 2 M) was used as a reaction stop solution. The detergent cetyltrimethylammonium bromide (CTAB, Sigma; 0.02% in water) was used as a lysing agent for determining total myeloperoxidase content of neutrophils. Phenylmethylsulfonyl fluoride (PMSF, Sigma), dimethyl sulfoxide (DMSO, Sigma) and phosphate buffered saline (PBS, pH 7.4) were used for tissue preparation.
Figure 1.
Structures of caffeic acid, rutin and hypoxoside.
Diets
In the experimental period, C3H/HeOuJ mice were fed AIN 93 G (Harlan Teklad, Madison, WI) diet with or without DSS or treatment diets based on AIN 93 G containing rutin (1.0 mmol/kg or 524 mg/kg in diet) or caffeic acid (1.0 mmol/kg or 179 mg/kg in diet). For the remaining treatment, mice were fed AIN 93 G diet and gavaged with hypoxoside extract (15 mg/d, 60 mg solid crude hypoxoside extract/mL sterile saline, each mouse given 0.25 mL daily using a 22 gauge feeding needle) with or without DSS. Experimental diets were prepared and stored at 4°C.
Experimental Design and Animals
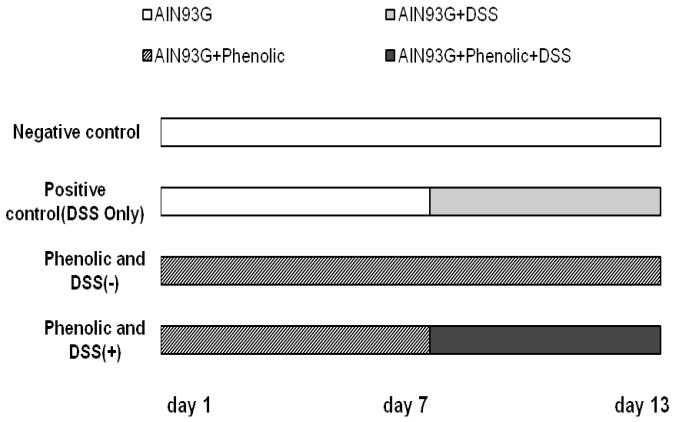
Forty eight male and 48 female mice at 6 weeks of age were obtained from Jackson Laboratory (Bar Harbor, ME). All mice were acclimated for 2 week before starting the experiment and were randomly assigned to eight treatment groups in order to achieve similar mean body weight/group. Mice were individually housed in microisolater cages with wood chip bedding and consumed standard rodent lab chow and tap water ad libitum during the acclimation period. The animal room was maintained at 23°C with a 12-h light/dark cycle during the experimental period. The experiment was a 4 × 2 factorial design: 8 treatment groups (control, caffeic acid, rutin and hypoxoside) and 2 disease statuses (with and without DSS, Figure 2). Colitis was induced in groups of 12 C3H/HeOuJ mice (6 males and 6 females) with DSS in their drinking water, a method previously reported with some modifications (6, 16, 17), namely 1.25% DSS was used, and only for 6 days rather than repeated DSS administration over longer time periods. The various phenolic treatments were fed to the mice for 7 days prior to DSS exposure and continually during DSS treatment (Figure 2). Food intake was measured weekly over 2-3 consecutive days per week. Body weights were measured twice a week. Signs of disease (weight loss, diarrhea, dehydration) were observed daily. The supplemented diets and the DSS-containing drinking water were provided to the mice continually until the experiment was terminated. All animal procedures were performed in accordance with the experimental protocol approved by the Iowa State University Institutional Animal Care and Use Committee.
Figure 2.
Experimental design. Phenolics fed were rutin (1.0 mmol/kg diet), hypoxoside extract (15 mg/d by gavage) and caffeic acid (1.0 mmol/kg diet). The study included eight groups of C3H/HeOuJ mice: DSS (−) without and with each of the 3 phenolics; DSS-only control: DSS (+); Rutin/DSS (+); Caffeic acid/DSS (+); Hypoxoside/DSS (+).
Tissue Sample Collection and Preparation
The colon and cecum were removed after euthanasia. After washing in phosphate-buffered saline (PBS), they were placed on filter papers to measure colonic lengths, score macroscopic cecal lesions and obtain photographs of each tissue. The colonic and cecal contents were removed, and the colon and cecum from each animal were fixed in formalin and six of the 12 tissue samples were randomly selected from each group for histopathological analyses. Gross cecal lesions were scored using published criteria (18). Macroscopic cecal lesions were scored 0-4 as follows: no gross lesions (grade 0, normal); evidence of atrophy (grade 1, mild); excess intraluminal mucus with atrophy localized to the cecal apex (grade 2, moderate); generalized cecal atrophy with increased intraluminal mucus and no cecal contents (grade 3, severe); score 3 plus bloody cecal content (grade 4, most severe). Colonic tissues for myeloperoxidase activity were put in 15% DMSO and 0.1 mM PMSF in cryovials (Corning Company, Corning, New York). A portion of colon (approximate 1.5 cm) for each sample was placed into RNALater (1.2 mL) for subsequent RT-PCR analysis of cytokine-specific mRNA expression. All above samples were stored at −85°C until analysis.
Colonic Mucosal Histopathological Analysis
Cecum and proximal colon in 10% neutral buffered formalin were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Sections of the cecum and proximal colon were scored by a pathologist (Dr. J. Hostetter) who was blinded to the treatment group (19). Briefly, histological scores were evaluated based on the severity of mucosal epithelial damage, architectural/glandular alterations, and the magnitude/character of lamina propria cellular infiltration. Scoring system for the histopathological evaluation of gastrointestinal inflammation included mucosal height, erosions, inflammatory score, edema score and inflammatory cells. Parameters were scored 1-5, resulting in a maximal total histological score of 20 based on the four evaluation parameters.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR) for Colonic Inflammation-Related Cytokine Gene Expression
Primers for IL-1β, IL-4, IL-6, IL-10, IL-17, GAPDH, IFN-γ, TNF-α, iNOS (inducible nitric oxide synthase), ICAM-1 (intercellular adhesion molecule-l), CYP4B1 (cytochromes P450, family 4B1) were analyzed in pooled colonic samples from each treatment group as a preliminary screen of gene expression (Table 1). Reverse transcription–polymerase chain reaction (RT–PCR) analysis of mRNA in each pooled sample was performed as previously 0 described with some modifications (20, 21). 20 mg samples were prepared from each colonic tissues and total mRNA was extracted using a Qiagen RNeasy mini Kit (Ambion, Austin, TX) for dissected tissue homogenization. The mRNA concentrations were detected by NanoDrop® ND-1000 UV-Vis Spectrophotometer (NanoDrop Technologies, Inc. Wilmington, DE) in 2.0μl mRNA samples using RNA program. Then the mRNA extractions were treated with TURBO DNA-free DNase (Ambion, Austin, TX) to remove genomic DNA. SYBR green (Invitrogen, San Diego, CA) real-time polymerase chain reaction (the same method as described below) was used for testing DNA contamination in mRNA samples to determine the mRNA sample purity. The mRNA extraction was reverse transcribed into cDNA by SuperScript™ III First-Strand Synthesis System (Invitrogen, San Diego, CA). The cDNA converted from 20ng mRNA was amplified using Platinum® SYBR® Green qPCR SuperMix-UDG (Invitrogen, San Diego, CA). Real time PCR was used to provide quantitative assessment of mucosal cytokine expression. The PCR condition was 95°C for 10 minutes, followed by 40 cycles of amplification (95°C for 10 seconds, 60°C for 15 seconds) run in Rotor-Gene 3000 (Corbett Research, Mortlake, Australia). Then the specific primers were used to evaluate the gene expression of IL-17, IL-4, iNOS and CYP4B1 in six individual colon tissue samples from eight treatment groups (3 males and 3 females). Standard curves of specific genes and housekeeping gene GAPDH were made by two-fold serial dilutions of cDNA using real-time PCR. The relative mRNA quantity was normalized to GAPDH.
Table 1.
Sequences of Primers Were Used for Reverse Transcription Polymerase Chain Reaction Amplification
| Gene— Object |
Primer | Sequence (5′–3′) | Amplification product size (bp) |
Accession |
|---|---|---|---|---|
| GAPDH | Forward | TCACCACCATGGAGAAGGC | 168 | |
| Reverse | GCTAAGCAGTTGGTGGTGCA | |||
| IL-1-beta | Forward | CAACCAACAAGTGATATTCTCCATG | 152 | M15131 |
| Reverse | GATCCACACTCTCCAGCTGCA | X04964 | ||
| IL-4 | Forward | ACAGGAGAAGGGACGCCAT | 95 | M25892 |
| Reverse | GAAGCCCTACAGACGAGCTCA | X05253 | ||
| IL-6 | Forward | GAGGATACCACTCCCAACAGACC | 141 | X54542 |
| Reverse | AAGTGCATCATCGTTGTTCATACA | M20572 | ||
| IL-10 | Forward | GGTTGCCAAGCCTTATCGGA | 191 | M37897 |
| Reverse | ACCTGCTCCACTGCCTTGCT | M84340 | ||
| IL-17 | Forward | GCTCCAGAAGGCCCTCAGA | 142 | NM_010552 |
| Reverse | AGCTTTCCCTCCGCATTGA | U35108 | ||
| IFN-gamma | Forward | TCAAGTGGCATAGATGTGGAAGAA | 92 | K00083 |
| Reverse | TGGCTCTGCAGGATTTTCATG | M74466 | ||
| TNF-alpha | Forward | CATCTTCTCAAAATTCGAGTGACAA | 175 | M13049 |
| Reverse | TGGGAGTAGACAAGGTACAACCC | Y00467 | ||
| iNOS | Forward | CAGCTGGGCTGTACAAACCTT | 95 | U43428 |
| Reverse | CATTGGAAGTGAAGCGTTTCG | L23806 | ||
| ICAM-1 | Forward | CCGCAGGTCCAATTCACACT | 143 | X52264 |
| Reverse | TCCAGCCGAGGACCATACAG | X15372 | ||
| CYP4B1 | Forward | CACCTGGACTTCCTCGACAT | 233 | |
| Reverse | TCATCCCACTGGAAGGAGTC |
Myeloperoxidase Assay Method in Colonic Tissue
Myeloperoxidase (MPO) activity which was used to quantify neutrophil accumulation in tissues was assessed using 96-well flat bottom microtiter plates (Linbro/Titertek, USA) and was previously described with some modifications (22, 23). Colonic tissues were thawed and blotted on paper towel at room temperature to absorb as much as moisture as possible. Tissue samples were trimmed to approximately 35mg. Each sample was homogenized in l mL PBS and PMSF (0.1mM) and the probe was washed 5 times with PBS. Each sample was sonicated on pulse (output control set to 2; 50% duty cycle setting; 5-10 pulse per sample, Sonicator 3000, Misonix, Inc. Farmingdale, NY). Samples were centrifuged 15 minutes at 1200 rpm (Eppendorf® Micro Centrifuge Model, USA). At least 150 μl supernatant was collected. Each sample was measured by NanoDrop® ND-1000 UV-Vis Spectrophotometer (NanoDrop Technologies, Inc. Wilmington, DE) to measure total protein concentrations. 150 μl of each sample supernatant was plated in triplicate wells (per sample). TMB (50 μL) was added, followed immediately with 50 μL 10 μM H2O2. The color change reaction was allowed to proceed for 2 min, and 50 μL of 2 M sulfuric acid was added to stop the reaction. The optical density (OD) in each well was determined at 405 nM using a microtiter plate spectrophotometer (V-Max, Molecular Devices, USA) with SOFTmax PRO 4.0 software. The total myeloperoxidase content was calculated from OD of lysed neutrophils using a standard curve. Standard suspensions of mouse neutrophils (3×106 cells mL−1) with PMSF (1 mM in absolute ethanol) were prepared and stored at −85 °C. Two-fold serial dilutions of a standard neutrophil suspension were lysed with CTAB. The MPO content of these known concentrations of neutrophils was compared to the OD values and a standard curve was calculated. Total myeloperoxidase content in mouse colonic tissue were normalized for number of neutrophils. The MPO activity was expressed as the units of enzyme per gram of wet weight of tissue.
Statistical Analysis
Data were analyzed by the difference between means and statistical significance was calculated using three-way ANOVA followed by Tukey method as a Post Hoc test (SAS Institute, 2003, Cary, NC). The equal variance and normality of residuals assumptions are verified by a residual vs. predicted values plot and a histogram of residuals. Both group and male/female of colon length, food intake, body weight change, MPO activity, total histopathology scores, and cytokine/enzyme RT-PCR products were reported as means ± SEM. Statistical significance was set at P < 0.05. Pearson correlation analysis was used for relationship between MPO activity/cytokine gene expression and colonic histopathology score.
Results
General Effects of Phenolic Treatments on DSS-Induced Colitis
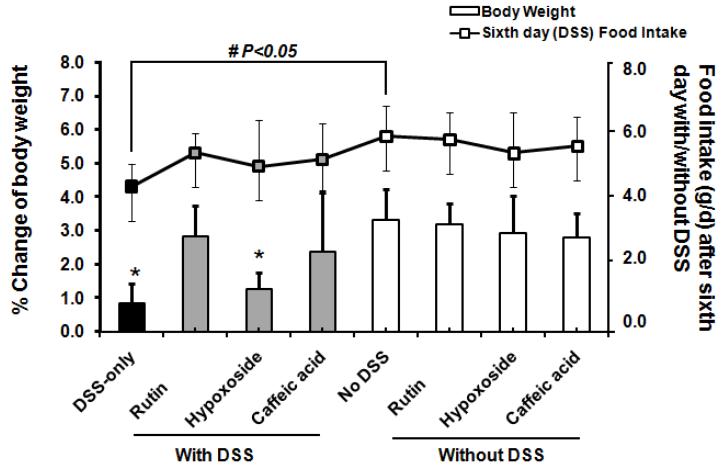
Regardless of the treatment group, food intakes measured on the day before drinking DSS-supplemented water and second day of the DSS treatment period did not differ (Table 2). Food intake was decreased at final day after DSS-treated alone compared with control not treated with DSS. Food intakes in the phenolic-treated groups not treated with DSS did not differ from control not treated with DSS (Table 2). Mice given rutin, hypoxoside, and caffeic acid had normalized food intake at the sixth day DSS-treated compared to animals not given DSS control (Figure 3). Body weight change ( the percentage of body weight gained in DSS period over the body weight before DSS-treated ) of 0.8% in DSS-positive control was significantly less than in DSS-negative control by fourfold (P < 0.01). DSS-treated mice given diets supplemented with either rutin or caffeic acid showed improved body weight whereas mice administered hypoxoside with DSS did not significantly increase their body weight (Figure 3). The mice fed rutin, hypoxoside, or caffeic acid not treated DSS were similar to controls without DSS in body weight increase (Figure 3). The cecal macroscopic scores did not differ among treatments with or without DSS. No diarrhea and rectal bleeding were observed during the period of DSS treatment. The colon length of the mice given DSS or DSS plus rutin was significantly shortened by ~8% (P < 0.01) as compared with all treatment groups not given DSS (Table 2). The colon lengths of DSS-treated mice fed caffeic acid or hypoxoside were significantly longer than those from the DSS-only controls (P < 0.01) (Table 2). Within treatment groups, no differences were found between males and females for colon length, food intake or body weight change (data not shown).
Table 2.
Colon Lengths Were Increased by Caffeic Acid or Hypoxoside and Food Intakes Were Improved by Each Phenolic in C3H/HeOuJ Mouse Model of Colitis1
| Colon lenth (mm) |
Food intake (g/d) |
|||
|---|---|---|---|---|
|
|
||||
| First day before DSS |
Second day after DSS |
Final day after DSS |
||
| DSS-only | 52.5±5.0* | 5.7±1.2 # | 5.8±0.9 # | 4.5±0.7‡ |
| Rutin and DSS | 54.8±5.7* | 5.3±0.9 | 5.3±0.7 | 5.1±0.6 |
| Hypoxoside and DSS | 57.1±5.5 | 5.2±1.3 | 5.1±1.7 | 4.9±1.4 |
| Caffeic acid and DSS | 58.8±3.2 | 5.5±0.7 | 5.6±1.3 | 5.1±1.1 |
| Control diet (no DSS) | 57.2±5.6 | 5.8±0.8 | 6.0±1.4 | 5.8±0.9 |
| Rutin (no DSS) | 57.6±4.4 | 5.3±1.1 | 5.5±0.9 | 5.7±0.8 |
| Hypoxoside (no DSS) | 59.6±4.9 | 5.2±1.3 | 5.1±1.3 | 5.3±1.2 |
| Caffeic acid (no DSS) | 60.0±4.3 | 5.6±0.8 | 5.3±0.6 | 5.5±0.9 |
P < 0.01 significantly shorter compared to the control diet (no DSS) with other treatment groups in colon length
P < 0.01 significantly greater compared with food intake on final day after DSS, within DSS-only group
P < 0.05 significantly less than the control diet (no DSS) in final day food intake after DSS
In the experimental period, C3H/HeOuJ mice were fed AIN 93G supplemented with rutin, caffeic acid, or hypoxoside.
Figure 3.
Rutin and caffeic acid normalized body weight in DSS-treated mice. *P < 0.01 significantly less compared with control (no DSS) group; mice given rutin, hypoxoside, and caffeic acid had normalized food intake at the sixth day DSS-treated compared with animals not given DSS control; #P < 0.05 significantly less in DSS-only group on food intake after the sixth day DSS-treated than the control diet (no DSS).
Colonic Myeloperoxidase (MPO) Activity of Phenolic Treatments and DSS Controls
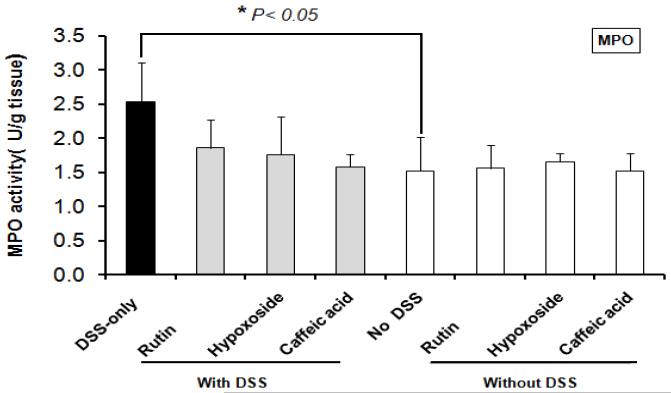
Colonic MPO activity in DSS-only controls was significantly greater than in non-treated control mice (P < 0.01). MPO activities were significantly decreased in the colonic extracts from DSS-treated mice fed each of the supplemented diets (rutin, hypoxoside, or caffeic acid) compared with the colonic extracts from the DSS-only control mice (P < 0.01). MPO activities in mice fed phenolics without DSS exposure did not differ from untreated control mice (Figure 4). Within treatments, no difference was found between males and females for MPO activity (data not shown).
Figure 4.
Rutin, hypoxoside and caffeic acid normalized colonic myeloperoxidase (MPO) activity in DSS-treated mice. *P < 0.05 greater compared with control (no DSS) group.
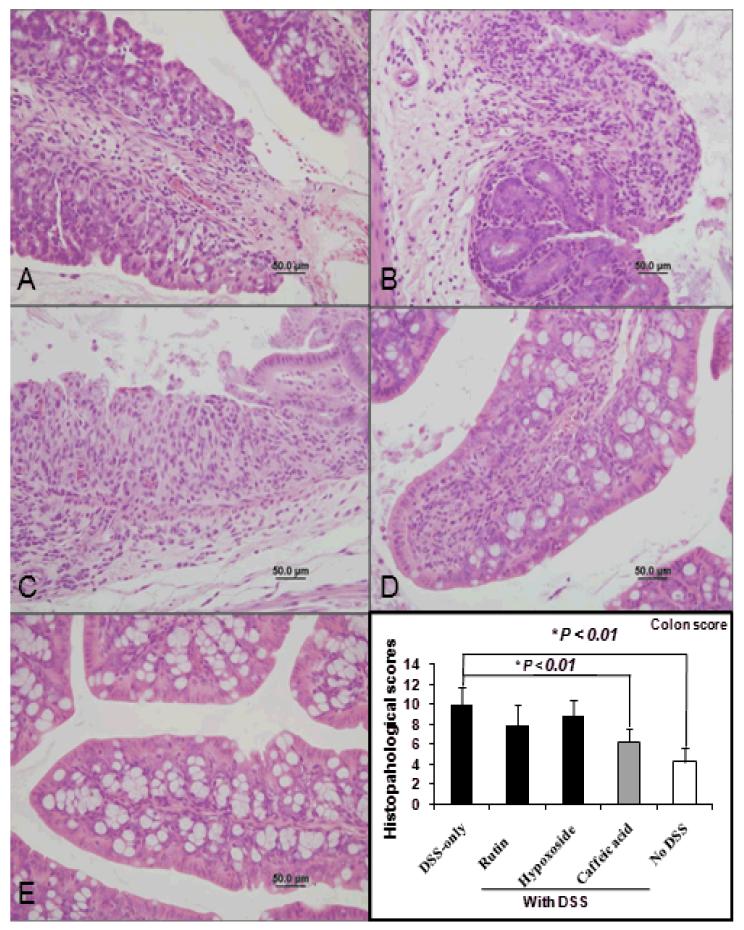
Colonic and Cecal Histopathology Changes in C3H Mice
Colonic and cecal histopathology scores of the DSS-treated mice were significantly more severe compared with control mice not given DSS, respectively (P < 0.01, Table 3). The group fed caffeic acid with DSS had significantly less severe colonic and cecal microscopic lesion scores than mice treated with DSS alone. Representative histological images from each group are shown (Figure 7). The mean colonic and cecal histopathology scores were both significantly decreased by 37% in the group fed caffeic acid with DSS compared to DSS-only controls (P < 0.01, Figure 7), whereas the histopathology scores in mice given rutin or hypoxoside along with DSS were not significantly attenuated in comparison to control mice not given DSS (P < 0.01,Table 3). Controls fed the non-supplemented AIN 93 G alone or diets containing rutin, caffeic acid or hypoxoside had significantly lesser histopathology scores than the DSS treated controls (P < 0.01) (Table 3). Within treatments, no difference was found between males and females for histopathology score (data not shown).
Table 3.
Histopathology Scores Were Decreased by Caffeic Acid treatment in C3H/HeOuJ Mouse Model of Colitis
| Treatment | Colon histopathology score | Cecal histopathology score |
|---|---|---|
| DSS-only | 10.0±1.8* | 10.9±2.5# |
| Rutin and DSS | 8.0±2.0* | 9.2±4.7# |
| Hypoxoside and DSS | 8.8±1.7* | 9.0±2.8# |
| Caffeic acid and DSS | 6.3±1.2 | 6.9±2.8 |
| Control (no DSS) | 4.3±1.4 | 7.1±4.8 |
| Rutin (no DSS) | 4.8±1.0 | 5.1±1.4 |
| Hypoxoside (no DSS) | 5.0±0.9 | 5.7±1.6 |
| Caffeic acid (no DSS) | 4.8±1.5 | 4.8±1.2 |
Scoring for the colonic and cecal histopathology evaluation of gastrointestinal inflammation included mucosal height, inflammatory cells, erosions, inflammatory score, and edema score in C3H/HeOuJ mouse treated with rutin, hypoxoside and caffeic acid with or without DSS.
P < 0.01 significantly greater than control (no DSS) in colon histopathology score
P < 0.01 significantly greater than control (no DSS) in cecal histopathology score.
Figure 7.
Colon histopathology (A-E, magnification = 400X, Scale bar = 50um). Colon from mouse given DSS only (A), Rutin + DSS (B) Hypoxicide + DSS (C), Caffeic acid + DSS (D), and control mouse given neither DSS or dietary supplement (E). Note that caffeic acid treatment leads to reduced inflammatory cell infiltration within the lamina propria and prevents epithelial ulceration. * P < 0.01less in caffeic acid + DSS and no DSS as compared with DSS-only control.
Reverse Transcription–Polymerase Chain Reaction Analysis of Cytokine mRNA
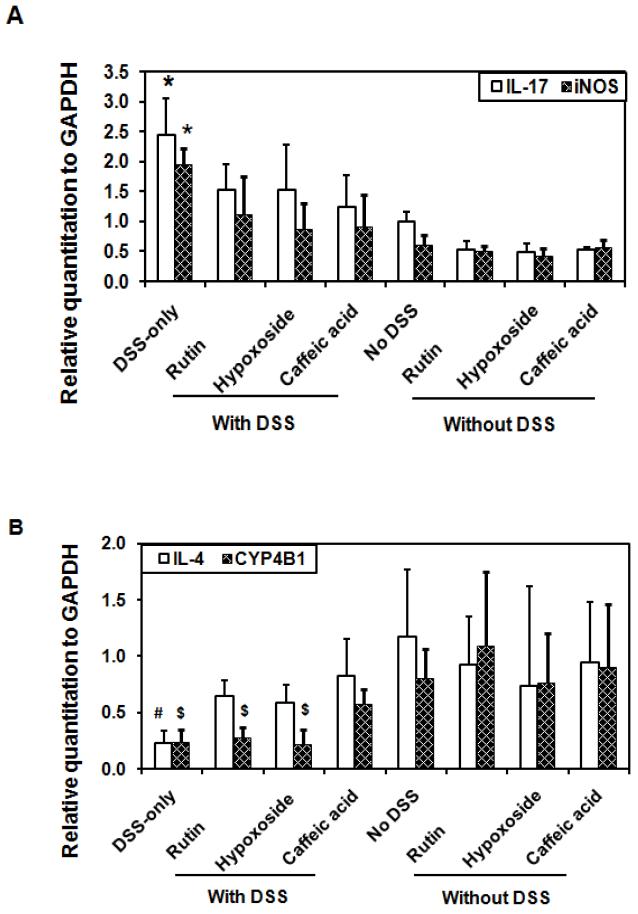
Some genes have been implicated in the pathogenesis of colitis, including IL-1β, IL-4, IL-6, IL-10, IL-17, iNOS, IFN-γ, TNF-α, ICAM and CYP4B1 (9, 13, 40, 41, 51, Table 1). To delve into the molecular mechanism underlying the suppression of colitis by phenolics in DSS-induced treatments, mRNA expression levels of possible pro-inflammatory mediators in colonic tissue were measured by RT-PCR. Preliminary evaluations demonstrated that the expression levels of IL-17-, iNOS-, IL-4-, and CYP4B1-specific mRNA differed among the various treatments. There was significant up-regulation of colonic tissue mRNA expression of IL-17 and iNOS (P < 0.01, Figure 5A), and significant down-regulation of IL-4 and CYP4B1 mRNA expression in DSS-treated controls compared to controls not given DSS (P < 0.01, Figure 5B). Colonic tissue mRNA expression of IL-17 and iNOS were significantly inhibited by each phenolic treatment (rutin, hypoxoside, and caffeic acid) when given with DSS, compared with DSS-only controls (P < 0.05, Figure 5A) whereas IL-4 –specific mRNA was significantly increased by each phenolic treatment (rutin, hypoxoside and caffeic acid) in groups treated with DSS compared with DSS-only controls (P < 0.05, Figure 5B). However, only mice fed caffeic acid and treated with DSS had significantly increased CYP4B1-specific mRNA levels compared with DSS-only controls (P < 0.05).
Figure 5.
Pro-inflammatory gene expressions in rutin, hypoxoside and caffeic acid with or without DSS-treated mice (n=6). (A) Rutin, hypoxoside and caffeic acid normalized IL-17 and iNOS gene expressions in DSS-treated mice. *P < 0.05 greater in DSS-only group compared with control (no DSS) group. (B) Rutin, hypoxoside or caffeic acid normalized IL-4 gene expressions and caffeic acid normalized CYP4B1 gene expression in DSS-treated mice. #P < 0.05 less in DSS-only group compared with control (no DSS) group; $P < 0.05 less compared with control (no DSS) group.
Relationship between the Colonic Myeloperoxidase Activity/Cytokine Gene Expression and Colonic Histopathology Score Evaluation
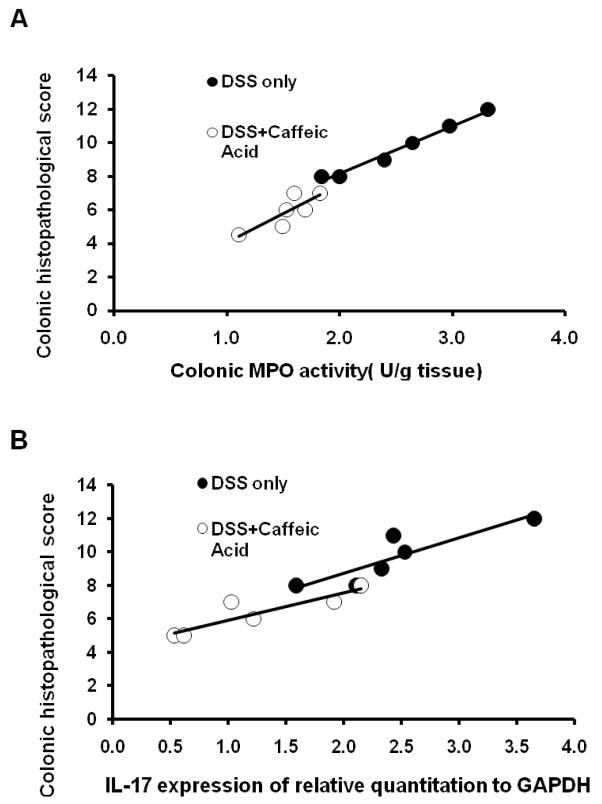
Relationships between colonic MPO activity/IL-17 gene expression and histopathology score in DSS-treated control and caffeic acid with DSS were shown (Figure 6). There was a significant association with colonic histopathology score on colonic level of IL-17 gene 8 expression and colonic MPO activity in DSS-induced with or without caffeic acid treated mice. In both DSS-treated control and caffeic acid with DSS mice, colonic MPO activity (Figure 6A) and colonic level of IL-17 gene expression (Figure 6B) were significantly related to colonic histopathology score, with less MPO activity and greater decrease in colonic IL-17 gene expression associated with decreased histopathology score.
Figure 6.
Relationship between colonic MPO activity/ IL-17 expression and histopathology score in DSS-only control and caffeic acid-treated with DSS mice. (A) Colonic histopathological score was significantly associated with colonic MPO activity in DSS-only control (r = 0.83, P =0.04); and in caffeic acid-treated with DSS mice (r = 0.92,P = 0.009). (B) Colonic histopathological score was significantly associated with IL-17 expression in DSS-only control (r = 0.88, P =0.02); and in caffeic acid-treated with DSS mice (r = 0.89, P =0.02).
Discussion
The study of dietary phenolics that may prevent diseases such as colitis is made more relevant to humans by considering the likelihood of ingestion of effective doses of these phenolics from common foods or dietary supplements. Regular coffee consumers generally ingest 0.5–1 g chlorogenic acid/d, which may be converted to 250–500 mg caffeic acid/d (24). From a German survey, daily intake of caffeic acid was 206 mg/d, and the principal sources were coffee (92% of caffeic acid) and fruit and fruit juices combined (25). In our study, based on mouse body weight and food intake, the daily caffeic acid intake was 45 mg/kg BW/day, which was approximately 7-fold higher than the dose of 500 mg/d (7 mg/ kg BW). Thus, the dose used in these studies was relevant to that typically consumed by humans, because mice have a tenfold greater surface area than do humans proportionate to body weight. The human equivalent dose of a compound given to mice would therefore be about 10-fold greater than the human dose per kg BW. This assumes similar absorbability of caffeic acid in mice and humans. From an estimate of flavonol intake in Finland of ~ 20 mg/person per day or ~0.3 mg/kg BW (26), the current dose of the flavonol glucoside, rutin (~45 mg/kg BW), was approximately an order of magnitude greater than a human dietary equivalent dose (by the same logic as above), but this might be a feasible human intake if supplements were included. Additional studies of human bioavailability of phenolics are needed for the development of colitis preventive diets.
DSS-induced colitis is partially triggered by aberrant or exaggerated immune responses to bacterial antigens derived from the intestinal lumen (27, 28). Shortening of the large intestine is thought to be induced by the thickening of colon caused by edema and muscular hypertrophy, as observed in ulcerative colitis. Diarrhea may be due to loss of absorptive epithelium that results in the shortening of the colon (29). Damage to the epithelium is a key feature of acute DSS-induced colitis (30), characterized by multi-focal areas of mucosal erosion, colonic epithelial cell injury, and significant mucosal infiltration of neutrophils, key immune cells during inflammatory responses. Increased IL-17, a pro-inflammatory cytokine and decreased IL-4, an anti-inflammatory cytokine accompany DSS-induced colitis, with these changes hypothesized to be prevented by proposed anti-inflammatory dietary components, caffeic acid and rutin. In our study, expression of several other genes possibly associated with inflammatory responses including cytochrome p450 (CYP4B1) were evaluated in response to prophylactic treatment with phenolic compounds during DSS-induced colitis.
Herbs and plant foods contain a variety of phenolic compounds that may modulate immune function. Several caffeic acid derivatives, major phenolic acids in plants, have been identified from herbs such as Echinacea (e.g., caftaric acid, echinacoside, cichoric acid). The flavonoid, rutin is found in many herbs including St. John’s wort (Hypericum perforatum). In vivo administration of extracts from H. perforatum and E. purpurea in carrageenan-induced paw edema in mice showed that H. perforatum at 100 mg/kg inhibited both iNOS and COX-2 expression, two pro-inflammatory genes, whereas treatment with 100 mg/kg E. purpurea decreased COX-2 expression only (31). In the present study, colonic gene expression of iNOS was inhibited by rutin, hypoxoside, and caffeic acid in DSS-treated mice in association with decreased MPO (neutrophil activity). Caffeic acid has antioxidant, anti-inflammatory, and antibacterial properties. Ovariectomized rats subjected to trinitrobenzene sulfonic acid (TNBS)-induced colitis and treated with a caffeic acid derivative (CAPE, 30 mg/kg) had decreased malondialdehyde (MDA), increased superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH) associated with decreased colitis (32). E. coli-induced pyelonephritis was also decreased in rats given CAPE, along with decreased MDA and nitric oxide and increased GSH and SOD activity (33) suggesting that anti-inflammatory effects of caffeic acid might occur through antioxidant mechanisms (32, 33). Rutin had protective effects in a Wistar rat model of gastric lesions induced by 50% ethanol (34). The gastroprotection of 200 mg rutin/kg diet given before ethanol treatment was thought to be due to anti-lipoperoxidative and antioxidant enzyme activity based on decreased MDA and increased glutathione peroxidase, compared with ethanol-treated controls. However, the anti-inflammatory marker, ethanol-induced neutrophil infiltration expressed as myeloperoxidase activity was not decreased by rutin in gastric lesions, compared with our study in which rutin diminished MPO in DSS-induced colitis (34). Anti-inflammatory and antioxidant effects of dietary phenolics generally occur together, but which effects or mechanisms are most crucial are not clear as of yet. Additional factors in inflammatory responses are under investigation as well.
Following DSS treatment, colonic mRNA expression of IL-17 was inhibited by each phenolic treatment compared with DSS-only controls. IL-17+ cells, IL-17 mRNA expression, and IL-17 protein levels were detectable and significantly elevated in inflamed mucosa and in the serum of patients with IBD (35). IL-17 family members (IL-17A/F) have been associated with inflammatory diseases, autoimmune diseases and cancer. Induction of IL-17A/F induced chemokines (CXCL8, CXCL6, CXCL1), growth factors (G-CSF, GM-CSF, IL-6), and adhesion molecules (ICAM-1) have been shown to augment neutrophil accumulation (36). Increased IL-17 gene expression was detected in a mouse colitis model in the acute phase at day 7 after exposure to 5% DSS in drinking water, but the DSS concentration was much higher than our study (37). Upregulation of IL-17, IL-1β, and IL-12 p70 was found during chronic colitis in C57BL/6 mice whereas production of IL-1β, IL-6, IL-18, and G-CSF was elevated in BALB/c mice. Chronic production of IL-17 and IL-12 p70 has been correlated with extensive inflammatory cell infiltration as DSS-induced colitis progressed from the acute to chronic stages of inflammation in C57BL/6 mice (37). In the present studies, IL-17 gene expression was elevated in DSS-induced colitis in C3H/HeOuJ mice (Fig.5A). Colonic IL-17 gene expression was also associated with colonic histopathological score in DSS-induced with or without caffeic acid treated mice (Fig.6B). IL-17 gene expression may be related to histopathological change to evaluate the anti-inflammatory mechanisms. In contrast, dietary supplementation with each of the three phenolic compounds attenuated IL-17 gene expression that was also associated with other anti-inflammatory effects (decreased iNOS, increased IL-4) but diminished histopathological scores were only associated with caffeic acid treatment.
Colonic mRNA expression of IL-4 was increased by each phenolic treatment compared with DSS-only controls. In a previously reported study, CD4+ T cells, IL-4, IL-2, and IFN-γ mRNA expression were significantly increased in female BALB/c mice by supplementation with 20 mg/kg CAPE (38). In contrast, Ansorge et al. (39) showed that propolis as well as its constituent (CAPE) suppressed Th2 type (IL-4) and Th1 type (IFN-γ) lymphocytes. Based on the results of the present study, upregulation of IL-4 gene expression may not be strongly associated with attenuated colitic lesions because only caffeic acid suppressed the severity of histopathological lesion scores, even though all three phenolics increased IL-4 (Fig.5B).
A key finding in the present study was that the colonic tissue mRNA expression of CYP4B1was increased in DSS-treated mice fed caffeic acid compared with DSS treated controls. CYP4B1 has attracted interest due to its possible ability to oxygenate fatty acids and form some eicosanoids (40) that suggest a function in inflammatory processes. In our study, increased CYP4B1 occurred in association with decreased colitic pathology after caffeic acid ingestion by DSS-treated mice. The CYP4B1 gene encodes a cytochrome P450 monooxygenase that catalyzes many xenobiotic and endogenous reactions, both detoxifying and activating (41). P450 gene expression is altered according to gender, microsomal enzyme inducers, age, diet, and hormones (42). Species-specific CYP4B1 mRNA have been reported to be mostly distributed in heart, brain, spleen, testis, lung, liver, skeletal muscle, and kidney in mice and humans (43). CYP4B1 mRNA has also been measured throughout small intestine and colon in rabbit and at low levels in human colon using in situ hybridization (44). Although induced further by 2-aminofluorene, CYP4B1 was an abundantly expressed isoform of P450 in rabbit gastrointestinal tract. Our finding is the first evidence that CYP4B1 is found in mouse colon, and may be metabolically important in colitis. In addition to the intestinal tract, P450 expression and activity has been previously reported to be decreased in lung, liver and extrahepatic tissues during inflammation and infection (42, 45). The mRNA level of CYP4B1 was increased after resolution of the allergic pulmonary inflammation (45). Although CYP4B1 is responsible for bioactivation of many toxicants, a CYP4B1 transgene may provide some benefits of gene therapy for cancer or replacement studies using 2-aminoanthracene/4-ipomeanol (46, 47). The CYP4B1/4-1M system efficiently and rapidly killed hepatocellular carcinoma cells (48). Thus, the role of CYP4B1 in colitis and its putative regulation by caffeic acid are interesting targets for further study.
Some mechanisms reported to regulate certain members of P450 may be related to elucidate CYP4B1 gene regulation. CYP2C11 gene contains a binding site for the transcriptional factor NF-κB (49). The inhibition of NF-κB binding also improved the CYP2C11 promoter-reporter gene. Down-regulation of CYP2C11 and CYP3A mediated by IL-2 in combination with cytokine-induced activation of NF-κB was reported in rat hepatocytes and may relate to IL-2 induction of the proto-oncogene transcription factor c-myc (50). However, little is known about the relationship between the CYP2C11 and CYP4B1 gene. It was recently reported that CYP3A4 expression was suppressed following NF-κB activation by lipopolysaccharide (LPS) and TNF-α through interactions between NF-κB and pregnane X receptor (PXR) and retinoid X receptor (RXR) complex. NF-κB p65 disassociated the PXR/RXR complex from DNA sequences as determined by electrophoretic mobility shift assay and chromatin immunoprecipitation assays (51). Based on the above evidence, the effect of caffeic acid phenethyl ester (CAPE) to decrease NF-κB shown in bacterial peptidoglycan polysaccharide-induced colitis in rats (12) suggests that caffeic acid might modify CYP4B1-specific mRNA expression through effects on NF-κB as well, which deserves further study.
Another mechanism related to P450 regulation was associated with the role of nitric oxide. Khatsenko et al. (52) indicated that down-regulation of CYP2B1/2 mRNA and protein induced by LPS was blocked with phenobarbital in rats, mediated at least in part by nitric oxide. Takemura et al. (53) reported that LPS-induced suppression of CYP2C11 and CYP3A2 gene expression was prevented by an inhibitor of iNOS, but they did not study regulation of CYP4B1. Expression of iNOS-specific mRNA was decreased in adult male Wistar rats treated with 10 μmol CAPE/kg prior to torsion/detorsion injury in the testis (54). iNOS expression and NF-κB binding activity were inhibited by CAPE in RAW 264.7 cells induced by LPS. The suppression of iNOS gene expression by CAPE may exert anti-inflammatory effects through NF-κB inactivation (55). Nitric oxide (NO) levels were reduced in CAPE-treated Wistar rats during Escherichia coli.-induced pyelonephritis (33). Decreased iNOS mRNA expression might play a role in normalizing CYP4B1 mRNA expression in the present study, but iNOS was decreased by all 3 phenolic treatments compared with DSS-only controls, and CYP4B1 was only normalized by caffeic acid compared with the no DSS group in the present study (Fig.5B). Thus the relation between the ability of phenolics to alter iNOS and CYP4B1 needs more study.
In conclusion, caffeic acid, rutin, and hypoxoside, decreased the gene expression of pro-inflammatory genes IL-17, iNOS, and increased IL-4 gene expression partially protecting from DSS-induced colitis, but CYP4B1 upregulated by caffeic acid was a key correlate of attenuated DSS-induced colitis in mice. Caffeic acid is a common phytochemical found widely in plant foods that may protect against IBD. Future studies should examine the extent to which various caffeic acid derivatives in plant foods and herbs might be metabolized to caffeic acid or other bioavailable metabolites related to caffeic acid (e.g., ferulic acid), and the function of CYP4B1 in colonic health and disease.
Acknowledgments
This research was made possible by Grant P01 ES012020 from the National Institute of Environmental Health Sciences (NIEHS) and the Office of Dietary Supplements (ODS), NIH and by grant 95P50AT004155 from the National Center of Complementary and Alternative Medicine (NCCAM) and ODS, NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the ODS, NIEHS, NCCAM, or NIH.
Footnotes
Parts of this manuscript were presented at the Experimental Biology Annual Meeting in Washington, DC, April 28 - May 2, 2007 and the Central States Society of Toxicology Meeting in Iowa City, Iowa, September 20-21, 2007.
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;47(6):417–29. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Shah S. Dietary factors in the modulation of inflammatory bowel disease activity. MedGenMed. 2007;9(1):60. [PMC free article] [PubMed] [Google Scholar]
- 3.Mellemkjaer L, Olsen JH, Frisch M, Johansen C, Gridley G, McLaughlin JK. Cancer in patients with ulcerative colitis. Int J Cancer. 1995;60:330–3. doi: 10.1002/ijc.2910600309. [DOI] [PubMed] [Google Scholar]
- 4.Hutto DL, Galvin JE, Wannemuehler MJ. Morphological and temporal characterisation of lesions in an enhanced model of Serpulina hyodysenteriae infection. J Med Microbiol. 1998;47:275–280. doi: 10.1099/00222615-47-3-275. [DOI] [PubMed] [Google Scholar]
- 5.Jergens AE, Wilson-Welder JH, Dorn A, Henderson A, Liu Z, Evans RB, Hostetter J, Wannemuehler MJ. Helicobacter bilis triggers persistent immune reactivity to antigens derived from the commensal bacteria in gnotobiotic C3H/HeN mice. Gut. 2007;56(7):934–40. doi: 10.1136/gut.2006.099242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–91. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevceva L, Pavli P, Buffinton G, Wozniak A, Doe WF. Dextran sodium sulphate-induced colitis activity varies with mouse strain but develops in lipopolysaccharide-unresponsive mice. J Gastroenterol Hepatol. 1999;14:54–60. doi: 10.1046/j.1440-1746.1999.01806.x. [DOI] [PubMed] [Google Scholar]
- 8.Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 9.Reed KL, Fruin AB, Gower AC, Gonzales KD, Stucchi AF, Andry CD, O’Brien M, Becker JM. NF-κB Activation Precedes Increases in mRNA Encoding Neurokinin-1 Receptor, Proinflammatory Cytokines, and Adhesion Molecules in DSS- Induced Colitis in Rats. Dig Dis Sci. 2005;50(12):2366–78. doi: 10.1007/s10620-005-3066-y. [DOI] [PubMed] [Google Scholar]
- 10.Podolsky DK. Inflammatory bowel disease (2) N Engl J Med. 1991;325:1008–1016. doi: 10.1056/NEJM199110033251406. [DOI] [PubMed] [Google Scholar]
- 11.Shanahan F. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology. 2001;120:622–635. doi: 10.1053/gast.2001.22122. [DOI] [PubMed] [Google Scholar]
- 12.Fitzpatrick LR, Wang J, Le T. Caffeic acid phenethyl ester, an inhibitor of nuclear factor-kappaB, attenuates bacterial peptidoglycan polysaccharide-induced colitis in rats. J Pharmacol Exp Ther. 2001;299:915–920. [PubMed] [Google Scholar]
- 13.Kwon KH, Murakami A, Tanaka T. Dietary rutin, but not its aglycone quercetin, ameliorates dextran sulfate sodium-induced experimental colitis in mice: attenuation of pro-inflammatory gene expression. Biochem Pharmacol. 2005;69(3):395–406. doi: 10.1016/j.bcp.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Bereta J, Bereta M, Allison AC, Kruger PB, Koj A. Inhibitory effect of di-catechol rooperol on VCAM-1 and iNOS expression in cytokine-stimulated endothelium. Life Sci. 1997;60:325–34. doi: 10.1016/s0024-3205(96)00633-9. [DOI] [PubMed] [Google Scholar]
- 15.Dietzsch E, Albrecht CF, Parker MI. Effect of rooperol on collagen synthesis and cell growth. IUBMB Life. 1999;48:321–5. doi: 10.1080/713803515. [DOI] [PubMed] [Google Scholar]
- 16.Kitajima S, Takuma S, Morimoto M. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp Anim. 1999;48:137–143. doi: 10.1538/expanim.48.137. [DOI] [PubMed] [Google Scholar]
- 17.Murakami A, Hayashi R, Tanaka T, Kwon KH, Ohigashi H, Safitri R. Suppression of dextran sodium sulfate-induced colitis in mice by zerumbone, a subtropical ginger sesquiterpene, and nimesulide: separately and in combination. Biochem Pharmacol. 2003;66:1253–1261. doi: 10.1016/s0006-2952(03)00446-5. [DOI] [PubMed] [Google Scholar]
- 18.Nibbelink SK, Wannemuehler MJ. An enhanced murine model for studies of Serpulina (Treponema) hyodysenteriae pathogenesis. Infect Immun. 1992;60:3433–3436. doi: 10.1128/iai.60.8.3433-3436.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jergens AE, Dorn A, Wilson J, Dingbaum K, Henderson A, Liu Z, Hostetter J, Evans RB, Wannemuehler MJ. Induction of differential immune reactivity to members of the flora of gnotobiotic mice following colonization with Helicobacter bilis or Brachyspira hyodysenteriae. Microbes Infect. 2006;8(6):1602–10. doi: 10.1016/j.micinf.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Overbergh L, Giulietti A, Valckx D, Decallonne R, Bouillon R, Mathieu C. The use of real-time reverse transcriptase PCR for the quantification of cytokine gene expression. J Biomol Tech. 2003;14:33–43. [PMC free article] [PubMed] [Google Scholar]
- 21.Overbergh L, Valckx D, Waer M, Mathieu C. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine. 1999;11:305–12. doi: 10.1006/cyto.1998.0426. [DOI] [PubMed] [Google Scholar]
- 22.Xia Y, Zweier JL. Measurement of myeloperoxidase in leukocyte-containing tissues. Anal Biochem. 1997;245:93–96. doi: 10.1006/abio.1996.9940. [DOI] [PubMed] [Google Scholar]
- 23.Palic D, Andreasen CB, Menzel BW, Roth JA. A rapid, direct assay to measure degranulation of primary granules in neutrophils from kidney of fathead minnow (Pimephales promelas Rafinesque, 1820) Fish and Shellfish. Immunology. 2005;19(3):217–227. doi: 10.1016/j.fsi.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Clifford MN. Chlorogenic acids and other cinnamates--nature, occurence and dietary burden. J Sci Food Agric. 1999;79:362–72. [Google Scholar]
- 25.Radtke J, Linseisen J, Wolfram G. Phenolic acid intake of adults in a Bavarian subgroup of the national food composition survey. Z Ernahrungswiss. 1998;37:190–7. doi: 10.1007/s003940050016. [DOI] [PubMed] [Google Scholar]
- 26.Bobe G, Weinstein SJ, Albanes D, Hirvonen T, Ashby J, Taylor PR, Virtamo J, Stolzenberg-Solomon RZ. Flavonoid intake and risk of pancreatic cancer in male smokers (Finland) Cancer Epidemiol Biomarkers Prev. 2008;17:553–62. doi: 10.1158/1055-9965.EPI-07-2523. [DOI] [PubMed] [Google Scholar]
- 27.Mayer L. IBD: Immunological research at the Mount Sinai hospital. Mnt Sinai J Med. 2000;67:208–213. [PubMed] [Google Scholar]
- 28.Shanahan F. Crohn’s disease. The Lancet. 2002;359:62–69. doi: 10.1016/S0140-6736(02)07284-7. [DOI] [PubMed] [Google Scholar]
- 29.Ciancio MJ, Chang EB. Epithelial secretory response to inflammation. Ann NY Acad Sci. 1992;664:210–221. doi: 10.1111/j.1749-6632.1992.tb39762.x. [DOI] [PubMed] [Google Scholar]
- 30.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 31.Raso GM, Pacilio M, Di Carlo G, Esposito E, Pinto L, Meli R. In-vivo and in-vitro anti-inflammatory effect of Echinacea purpurea and Hypericum perforatum. Journal of Pharmacy and Pharmacology. 2002;10(5):1379–1383. doi: 10.1211/002235702760345464. [DOI] [PubMed] [Google Scholar]
- 32.Ek RO, Serter M, Ergin K, Yildiz Y, Cecen S, Kavak T, Yenisey C. The Effects of Caffeic Acid Phenethyl Ester (CAPE) on TNBS-induced Colitis in Ovariectomized Rats. Dig Dis Sci. 2008;53(6):1609–17. doi: 10.1007/s10620-007-0056-2. [DOI] [PubMed] [Google Scholar]
- 33.Celik S, Gorur S, Aslantas O, Erdogan S, Ocak S, Hakverdi S. Caffeic acid phenethyl ester suppresses oxidative stress in Escherichia coli-induced pyelonephritis in rats. Mol Cell Biochem. 2007;297(1-2):131–8. doi: 10.1007/s11010-006-9337-x. [DOI] [PubMed] [Google Scholar]
- 34.La Casa C, Villegas I, Alarcon de la Lastra C, Motilva V, Martin Calero MJ. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J Ethnopharmacol. 2000;71(1-2):45–53. doi: 10.1016/s0378-8741(99)00174-9. [DOI] [PubMed] [Google Scholar]
- 35.Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 37.Melgar S, Karlsson A, Michaëlsson E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol Gastrointest Liver Physiol. 2005;288(6):G1328–38. doi: 10.1152/ajpgi.00467.2004. [DOI] [PubMed] [Google Scholar]
- 38.Park JH, Lee JK, Kim HS, Chung ST, Eom JH, Kim KA, Chung SJ, Paik SY, Oh HY. Immunomodulatory effect of caffeic acid phenethyl ester in BALB/c mice. International Immunopharmacology. 2004;4:429–436. doi: 10.1016/j.intimp.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Ansorge S, Reinhold D, Lendeckel U. Propolis and some of its constituents down-regulate DNA synthesis and inflammatory cytokine production but induce TGF-beta1 production of human immune cells. Z Naturforsch [C] 2003;58(7-8):580–9. doi: 10.1515/znc-2003-7-823. [DOI] [PubMed] [Google Scholar]
- 40.Baer BR, Rettie AE. CYP4B1: an enigmatic P450 at the interface between xenobiotic and endobiotic metabolism. Drug Metab Rev. 2006;38(3):451–76. doi: 10.1080/03602530600688503. [DOI] [PubMed] [Google Scholar]
- 41.Ding X, Kaminsky LS. Human extrahepatic cytochromes P450: function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts. Annu Rev Pharmacol Toxicol. 2003;43:149–73. doi: 10.1146/annurev.pharmtox.43.100901.140251. [DOI] [PubMed] [Google Scholar]
- 42.Renton KW. Cytochrome P450 regulation and drug biotransformation during inflammation and infection. Curr Drug Metab. 2004;5:235–243. doi: 10.2174/1389200043335559. [DOI] [PubMed] [Google Scholar]
- 43.Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. Expression patterns of mouse and human CYP orthologs (families 1-4) during development and in different adult tissues. Arch Biochem Biophys. 2005;436(1):50–61. doi: 10.1016/j.abb.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 44.McKinnon RA, Burgess WM, Gonzalez FJ, Gasser R, McManus ME. Species-specific expression of CYP4B1 in rabbit and human gastrointestinal tissues. Pharmacogenetics. 1994;4(5):260–70. doi: 10.1097/00008571-199410000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Stoilov I, Krueger W, Mankowski D, Guernsey L, Kaur A, Glynn J, Thrall RS. The cytochromes P450 (CYP) response to allergic inflammation of the lung. Arch Biochem Biophys. 2006;456(1):30–8. doi: 10.1016/j.abb.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 46.Rainov NG, Dobberstein KU, Sena-Esteves M, Herrlinger U, Kramm CM, Philpot RM, Hilton J, Chiocca EA, Breakefield XO. New prodrug activation gene therapy for cancer using cytochrome P450 4B1 and 2-aminoanthracene/4-ipomeanol. Hum Gene Ther. 1998;9(9):1261–73. doi: 10.1089/hum.1998.9.9-1261. [DOI] [PubMed] [Google Scholar]
- 47.Frank S, Steffens S, Fischer U, Tlolko A, Rainov NG, Kramm CM. Differential cytotoxicity and bystander effect of the rabbit cytochrome P450 4B1 enzyme gene by two different prodrugs: implications for pharmacogene therapy. Cancer Gene Ther. 2002;9(2):178–88. doi: 10.1038/sj.cgt.7700422. [DOI] [PubMed] [Google Scholar]
- 48.Mohr L, Rainov NG, Mohr UG, Wands JR. Rabbit cytochrome P450 4B1: A novel prodrug activating gene for pharmacogene therapy of hepatocellular carcinoma. Cancer Gene Ther. 2000;7(7):1008–14. doi: 10.1038/sj.cgt.7700190. [DOI] [PubMed] [Google Scholar]
- 49.Iber H, Chen Q, Morgan ET. Suppression of CYP2C11 gene transcription by interleukin-1 mediated by NFκB binding at the transcription start site. Arch Biochem Biophys. 2000;377:187–194. doi: 10.1006/abbi.2000.1772. [DOI] [PubMed] [Google Scholar]
- 50.Tinel M, Elkahwaji J, Robin MA, Fardel N, Descatoire V, Haouzi D, Berson A, Pessayre D. Interleukin-2 overexpresses c-myc and down-regulates cytochrome P-450 in rat hepatocytes. J Pharmacol Exp Ther. 1999;289:649–655. [PubMed] [Google Scholar]
- 51.Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB, Gallo MA, Xie W, Tian Y. Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem. 2006;281(26):17882–9. doi: 10.1074/jbc.M601302200. [DOI] [PubMed] [Google Scholar]
- 52.Khatsenko OG, Boobis AR, Gross SS. Evidence for nitric oxide participation in down-regulation of CYP2B1/2 gene expression at the pretranslational level. Toxicol Lett. 1997;90:207–216. doi: 10.1016/s0378-4274(96)03857-x. [DOI] [PubMed] [Google Scholar]
- 53.Takemura S, Minamiyama Y, Imaoka S, Funae Y, Hirohashi K, Inoue M, Kinoshita H. Hepatic cytochrome P450 is directly inactivated by nitric oxide, not by inflammatory cytokines, in the early phase of endotoxemia. J Hepatol. 1999;30(6):1035–44. doi: 10.1016/s0168-8278(99)80257-8. [DOI] [PubMed] [Google Scholar]
- 54.Atik E, Gorur S, Kiper AN. The effect of caffeic acid phenethyl ester (CAPE) on histopathological changes in testicular ischemia-reperfusion injury. Pharmacol Res. 2006;54(4):293–7. doi: 10.1016/j.phrs.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 55.Song YS, Park EH, Hur GM, Ryu YS, Lee YS, Lee JY, Kim YM, Jin C. Caffeic acid phenethyl ester inhibits nitric oxide synthase gene expression and enzyme activity. Cancer Lett. 2002;175(1):53–61. doi: 10.1016/s0304-3835(01)00787-x. [DOI] [PubMed] [Google Scholar]