Abstract
Neuroprotection in glaucoma as a curative strategy complementary to current therapies to lower intraocular pressure (IOP) is highly desirable. This study was designed to investigate neuroprotection by 17β-estradiol (E2) to prevent retinal ganglion cell (RGC) death in a glaucoma model of surgically elevated IOP in rats. We found that daily treatment with E2 containing eye drops resulted in significant E2 concentration in the retina with concomitant profound neuroprotective therapeutic benefits, even in the presence of continually elevated IOP. The number of apoptotic cells in the RGC layer was significantly decreased in the E2-treated group, when compared to the vehicle-treated controls. Deterioration in visual acuity in these animals was also markedly prevented. Using mass spectrometry-based proteomics, beneficial changes in the expression of several proteins implicated in the maintenance of retinal health were also found in the retina of E2-treated animals. On the other hand, systemic side-effects could not be avoided with the eye drops, as confirmed by the measured high circulating estrogen levels and through the assessment of the uterus representing a typical hormone-sensitive peripheral organ. Collectively, the demonstrated significant neuroprotective effect of topical E2 in the selected animal model of glaucoma provides a clear rationale for further studies aiming at targeting E2 into the eye while avoiding systemic E2 exposure to diminish undesirable off target side-effects.
Keywords: 17β-estradiol, eye drop, glaucomatous retinopathy, rat model, estrogen quantitation, retina proteomics, neuroprotection, visual acuity, contrast sensitivity, LC–MS/MS
INTRODUCTION
Glaucoma, the second leading cause of blindness worldwide,1 is a group of complex and slow progressing neurodegenerative diseases characterized by the gradual loss of retinal ganglion cells (RGC) and their axons.2 While elevated intraocular pressure (IOP) is a major risk factor for developing the disease, visual field loss and RGC death also occur in cases where IOP is controlled or normal.3,4 Therefore, and since lowering elevated IOP is currently the only available treatment target for glaucoma, complementary strategies independent of IOP lowering are also urgently needed to manage the associated ocular pathology.
Due to many mechanistic properties qualifying glaucoma as a neurodegenerative disease and similarities to other chronic degenerative diseases affecting the brain,5-8 pursuing neuroprotection as a therapeutic target in glaucoma is a convincing and rational strategy.2-4,9,10 With this approach the goal is not only to prevent or slow down neuronal cell death, but also to preserve neuronal functions. In the context of glaucoma, neuroprotective approaches that are capable of successfully promoting RGC survival are needed. While our understanding of retinal pathophysiology involving glaucomatous neurodegeneration is incomplete due to the multi factorial nature of the disease, excitotoxicity, inflammation, oxidative stress and mitochondrial dysfunction have been implicated, among others, as potential factors contributing to the initiation and progression of the disease.11-14 Development of multifunctional neuroprotective agents to impact multiple mechanisms in a single drug delivery and therapy approach would, therefore, be of great clinical value.15 This type of agents would also decrease undesirable drug-drug interactions and lessen disease burden, thereby improving the well-known compliance issues associated with currently available glaucoma medications.16
Several lines of evidence have demonstrated that the most potent human estrogen, 17β-estradiol (E2, Figure 1), elicits broad spectrum neuroprotection in various in vitro and in vivo paradigms due to its pleiotropic genomic and non genomic actions.17,18 As such, E2 is capable of protecting neurons via a variety of mechanisms; e.g., by interfering with cellular death signaling cascades, altering neurotransmitters levels, scavenging free radicals, promoting synaptic plasticity as well as preventing axonal and dendritic pruning. Beside basic science studies, epidemiological observations also argue for the neuroprotective role of endogenous estrogens.19,20
Figure 1.
Chemical structure of 17β-estradiol (E2).
E2 has been shown to be beneficial for the health of various ocular structures.21-24 These findings are not surprising considering that estrogen receptors (ERα and β) are abundantly expressed throughout the eye, and in particular in the retina.25,26 In animal models of various retinal diseases, E2 treatments have elicited protection of RGCs, photoreceptor cells, optic nerve and retinal pigment epithelium.27-29 The attenuation of osmotic swelling of RGCs30 and of the breakdown of the blood-retina barrier31 by this steroid has also been reported. Additionally, a very recent clinical review specifically discusses detrimental consequences of estrogen deficiency after menopause as a causative factor for the increased susceptibility to glaucomatous damage with age.32
Treatment regimens using topical administration are the current standard of care in clinical glaucoma therapy.16 E2’s significant lipophilicity and, thus, extremely low water-solubility do not allow for its direct formulation in aqueous solution. Cyclodextrins have gained attention when difficulties in drug formulation of lipophilic agents arise.33,34 Complexation with a cyclodextrin not only allows for formulations of poorly water-soluble agents in aqueous vehicles, but also offers specific advantages in terms of improving transcorneal penetration and reducing corneal irritation seen with other excipients.34 In particular, a chemically modified cyclodextrin, hydroxypropyl-β-cyclodextrin (HPBCD), has frequently been used due to its favorable toxicity profile and enhanced water-solubility compared to the unmodified cyclic oligosaccharide.35
The goal of the present study was to determine for the first time the effectiveness of topical E2 for alleviating RGC death by measuring functional effects resulting from neuroprotection in a surgically induced in vivo model of glaucomatous retinopathy36. We specifically focused on monitoring the attenuation of losses in visual acuity and contrast sensitivity upon exposing the animals to a daily E2 eye drop dosing regimen. By using ovariectomized (OVX) rats lacking significant endogenous E2 sources, additional mechanistic and pharmacokinetic information were also derived; we measured E2 levels by a validated liquid chromatography–tandem mass spectrometry (LC-MS/MS) assay37 in the retina, blood and uterus. The latter represents a typical E2-sensitive peripheral organ that respond with rapid structural changes after exposure to E2. Changes in retinal protein expression were also recognized as a potential mechanistic outcome for E2’s neuroprotection in the retina using MS-based proteomics.38
EXPERIMENTAL SECTION
Materials
17β-estradiol (E2) was purchased from Steroids, Inc. (New Port, RI). 13C-labeled E2-(13C6 E2) with an isotopic purity of 99% was purchased from Cambridge Isotope Laboratories (Andover, MA, USA). All other chemicals were obtained from Sigma Chemical Company (St. Louis, MO, USA), unless otherwise noted. The solvents were of analytical grade. Formulation of E2 for eye drops was done in 20% w/v HPBCD in saline. E2 was applied in 0.05% (w/v) solution.
Animals
OVX adult Brown Norway rats weighing 200-250 g were obtained from Charles Rivers Laboratories (Wilmington, DE, USA). Animals were kept under standard 12-h light/12-h dark cycle and room temperature was maintained at 21 °C. Animals had full access to standard diet and water. Rats were treated according to institutional animal care and use guidelines.
Topical E2 Treatment Protocol
For E2 quantitation and retina proteomics studies, nine animals per group received 10 μL of E2 eye drops once daily (q.d.) in both eyes for 19 days. Dosing started after IOP was stably elevated at 10 days; 24 h after the last topical treatment concluding a 30 day observational period, in vivo studies specified below were conducted. The control group received the vehicle (20% w/v HPBCD in saline) only. After 24 h of the last treatment animals were euthanized by CO2 overexposure. Blood was drawn through intracardiac puncture into heparinized tubes; clotted on ice and spun at 3000 rpm for 20 min to obtain serum samples. The eyes were immediately enucleated and processed; cornea, lens and retina were isolated, rinsed with saline, blotted dry and weighed. The brain and uterus were also collected. For E2 quantitation by LC–MS/MS, tissues from six animals were used. One retina per animal was used for the proteomic survey and three retina samples were processed from each treatment group. For neuroprotection studies using a rat model of surgically elevated intraocular pressure (IOP),36 after stabilization of the elevated IOP in twelve rats, animals were randomly divided into 3 groups (surgery control, vehicle control and E2-treatment, n=4). The control group was treated with the vehicle only and the treatment group received 10 μL of E2 eye drops into the ipsilateral eye, q.d., for 19 days, analogously to those of drug distribution/proteomics studies.
Sample Preparation for E2 Quantitation
Tissues were frozen by a BioSqueezer (Biospec Products, Bartlesville, OK) and, then, ground up in a Cryo-Cup Grinder (Biospec Products, Bartlesville, OK, USA). From the collected frozen powders, 20% (w/v) homogenates were made in pH 7.4 phosphate buffer. The homogenates were spiked with 100 pg internal standard (IS, 13C6-E2), diluted two fold with saline and extracted twice with 2 volumes of methyl tert butyl ether. The organic layers obtained from the liquid liquid extraction were removed, transferred to reacti-vials (Supelco, Bellefonte, PA, USA) and evaporated under a nitrogen stream to yield samples for derivatization for subsequent LC-MS/MS analysis. Derivatization was done by dansyl chloride (Dns-Cl, 30 μL of 1 mg/mL in acetonitrile) and 20 μL of sodium bicarbonate buffer (100 mM, pH 10.5) according to our recently reported method.37 The samples were vortexed and then incubated in a heating block at 60 °C for 10 min. Thereafter, the samples were centrifuged at 14,500 rpm for 3 min, transferred to autosampler vials, sealed, and assayed by LC-MS/MS. Serum (100 μL) obtained from blood was extracted analogously.
LC–MS/MS Analysis
E2 levels in sera and tissues were measured by a validated LC–MS/MS assay applying isotope-dilution quantification.37 The assay was performed on a TSQ Quantum Ultra triple-quadrupole instrument operated in positive ion mode and interfaced via a heated electrospray ionization (H-ESI) probe to a Surveyor LC System (Thermo Electron Corporation, Trace Chemical Analysis, Austin, TX, USA). Separations were carried out on a Phenomenex (Torrance, CA, USA) Kinetex Phenyl Hexyl column (50 × 2.1 mm i.d., 2.6 μm “core shell” particles) using gradient elution with a flow rate of 0.4 mL/min. The eluent system consisted of (A) 0.1% (v/v) formic acid in water and (B) 0.1% (v/v) formic acid in acetonitrile. The eluent composition was initially set at 50% B and was linearly increased after sample injection to 87% B in 6 min. Then the column was flushed with 100% B for 1.8 min; and finally, was equilibrated with 50% B for 3 min. The autosampler injection volume was 5 μL. H-ESI spray voltage, H-ESI temperature, and capillary temperature were maintained at 3.5 kV, 350 °C, and 325 °C, respectively. Selected reaction monitoring (SRM) with unit mass resolution for the precursor and product ions was used for quantification. SRM transitions of m/z 506 → 171, and 512 → 171 were set up for Dns-E2, and Dns-13C6-E2, respectively. Data acquisition and processing were controlled by the XCalibur software (version 2.1) of the instrument.
Mass Spectrometry-Based Retina Proteomics
The extracted proteins were digested with trypsin and analyzed by data-dependent nanoflow liquid chromatography coupled with electrospray ionization mass spectrometry and tandem mass spectrometry (LC-ESI-MS/MS) according to a previously published protocol.38 Separation was performed on an Eksigent nano LC-2D system (Dublin, CA, USA) using a 2.5 cm × 75 Ym i.d. IntegraFrit™ sample trap (New Objective, Woburn, MA, USA) and a 15 cm × 75 Ym i.d. PepMap C18 column (LC Packings, Dionex, San Francisco, CA, USA). The mobile phase for gradient elution was mixed from solvent A [0.1% acetic acid and 99.9% water (v/v)] and solvent B [0.1% acetic acid and 99.9% acetonitrile (v/v)] raising solvent B from 5% to 40% in 90 min after keeping the initial composition for 5 min during sample injection. Injection volume and flow rates were 5 μL and 250 nL/min, respectively. The effluent from the PepMap column was analyzed by data-dependent ESI MS/MS on a hybrid linear ion trap–Fourier transform ion cyclotron resonance mass spectrometer (LTQ-FT, Thermo Fisher, San Jose, CA, USA) equipped with the manufacturer’s nanoelectrospray ionization source and operated with the Xcalibur (version 2.0) and Tune Plus (version 2.2) data acquisition programs. After searching a protein database (SwissProt IPI, Rattus norvegicus) using the Mascot software (Matrix Science, Boston, MA), differentially expressed proteins were identified via spectral counting option of Scaffold (Proteome Software, Portland, OR, USA) followed by summation-based G-tests, as described earlier.38
Rat Model of Glaucoma and IOP Measurements
The procedure that was used to elevate the rat IOP was described previously.36,39 Briefly, surgery for IOP elevation was performed on anesthetized animals (1 mL/kg body weight i.p. injection of a standard rat anesthesia cocktail: mixture of ketamine at 5 mL of 100 mg/mL, xylazine at 0.5 mL of 100 mg/mL, acepromazine at 1 mL of 10 mg/mL, and 0.5 mL of water). One eye (left side) of each animal was used for the IOP elevation. 50 μL of a sterile 1.8 M hypertonic saline solution was injected into the episcleral vein with a micro glass needle connected to an injection pump. After stabilization of IOP elevation to 25% above that of the contralateral control eye, IOP measurements were taken with a Tono-Pen XL tonometer (Mentor, Norwell, MA, USA) on conscious animals in the presence of topical anesthesia with eye drops containing proparacaine (0.1%, w/v) twice a week for up to 30 days post-surgery. Following the experimentation period, rats were euthanized by an i.p. injection of pentobarbital (120 mg/kg body weight).
Behavioral Testing of Rat Vision and Visual Acuity
Visual performance of rats was measured non-invasively by trained observers blinded to the identity of the individual experimental groups and using a behavioral assay determining the animal’s optomotor reflex response to a moving visual stimulus.40 Animals exhibit tracking behavior by following the rotating visual stimulus with small head movements in the direction of the rotation. This behavior was quantified with a computerized system that collects visual acuity and contrast sensitivity data (OptoMotry; CerebralMechanics, Lethbride, Alberta, Canada) as described previously.41-44 Ipsi- and contralateral eyes were assessed independently by means of changing the direction of the rotating stimulus pattern while the contralateral eye served as an intrinsic control.41,42
TUNEL Assay for Detection of Apoptosis in Ex Vivo Retina Material
After the behavioral testing of visual performance, animals were euthanized. Eyes were dissected and immersion-fixed in 4% paraformaldehyde overnight at 4 °C. Subsequently, after three washes in PBS, eyes were embedded in OCT compound (Sakura Finetek USA Inc., Torrance, CA, USA) and were sectioned vertically at 12 μm thickness on a cryostat microtome.45 Apoptotic cells in eye sections were visualized with the DeadEnd Fluorometric TUNEL System (Promega, Madison, WI, USA) following the manufacturer’s instructions. Sections were mounted on cover slips with Prolong Gold antifade reagent mounting medium containing 1.5 μg/mL DAPI (Molecular Probes, Eugene, OR, USA). Negative controls included omission of the TdT enzyme incubation, while positive controls were performed using DNase I incubation on the sections. Fluorescently labeled, TUNEL-positive cells were identified using standard epi-fluorescence microscopy, digital microphotography and quantitative stereotaxis to determine the number of TUNEL-positive cells in the ganglion cell layer (GCL) as stained profile counts (SimplePCI, Compix Inc., Image Systems, Sewickley, PA, USA). The TUNEL assay was chosen to identify late-stage apoptotic cells in the GCL as the most conservative assessment of cell death with apoptosis being the predominant form of cell death in the RGC.46 Specifically, this enzyme-based assessment of cellular commitment to the final stage of programmed cell death generates an extremely reliable assay for complex tissues when combined with markers for nucleic acids as shown by Kelly et al.47
Statistical Analysis
Descriptive statistics were calculated for each group and for all outcomes. Unless otherwise noted, determination of the difference between mean values for each experimental group was assessed by Student’s t-test or with standard one-way ANOVA followed by post hoc Dunnett’s test for significant differences among groups, with p < 0.05 considered statistically significant.
RESULTS AND DISCUSSION
Topical Ocular Delivery of E2 into the Retina of OVX Rats
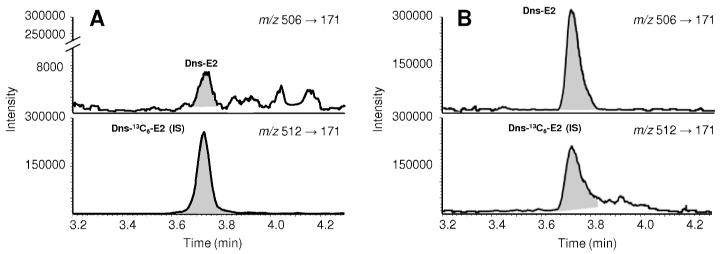
E2 quantifications with isotope dilutions and SRM were performed using a validated LC-MS/MS assay after derivatization with dansyl chloride. We used 13C6-labeled E2 as the internal standard (IS)37 to avoid a potential matrix effect arising from the differential suppression or enhancement of ionization upon the use of a traditionally applied deuterium-labeled E2-IS.48 The analytical method employed also permitted measuring the very low E2 contents in the corresponding OVX control eye tissues. E2 formation in the eye, and prominently in the retina, has been proposed previously,49 but to our knowledge, our study is the first to report the actual endogenous E2 contents in the OVX rat eye that were obtained via a reliable and sensitive LC-MS/MS assay.37 In Figure 2, we show representative SRM chromatograms of retina extracts from the OVX control (Figure 2A) and the E2-treated (Figure 2B) groups. In animals exposed to daily E2 eye drop treatments, we measured high E2 concentrations (ng/g range), as shown in Table 1. In particular, we detected about 20 ng/g E2 in the retina and, expectedly, a significant quantity of E2 was also present in other selected eye segments, such as the lens and the cornea.
Figure 2.
Representative LC-MS/MS selected reaction monitoring (SRM) traces of retinal extracts obtained from A) OVX rats after vehicle (20% w/v HPBCD in saline); and B) E2-eye drops (0.05% w/v) treatments, respectively (10μL, q.d., for 19 days, n=6). Quantitation was done by isotope dilution using 13C6-E2 as internal standard (IS) and SRM (with transitions indicated) of the dansyl- (Dns-) derivatized analyte and IS.
Table 1. E2 concentrations in selected eye compartments in OVX rats after E2 (0.05% w/v) or vehicle eye drop treatment (10 μL, q.d. for 19 days).
| Tissue | E2 | |
|---|---|---|
| Vehicle (control) (ng/g) |
E2-treated (ng/g) |
|
| Retina | 0.13 ± 0.02 | 22 ± 2* |
| Cornea | 0.40 ± 0.01 | 78 ± 12* |
| Lens | 0.03 ± 0.003 | 3 ± 1* |
Data are expressed as mean ± SEM for n=6.
Significant difference compared to control, p< 0.05, n=6).
Mass Spectrometry-Based Retina Proteomics
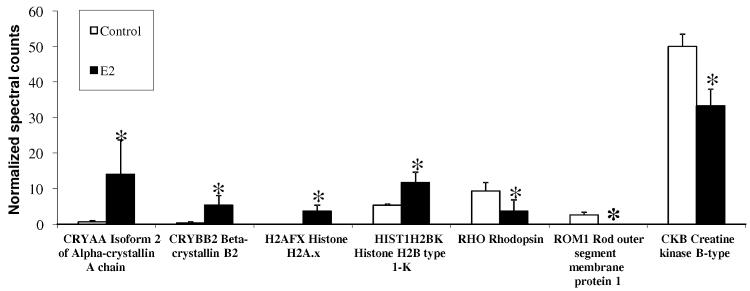
As shown in Figure 3, proteomic analyses not only supported data shown in Table 1 for retinal delivery of E2, but they also demonstrated that E2 affected the expression of various retinal proteins that could be linked to important retinal functions with potential implications to neuroprotective ocular drug therapy. In particular, αA- and βB-crystallins50 have been linked to the survival of RGCs after optic nerve axotomy51, a model of glaucoma-mediated injury. In addition, increased oxidative stress-induced retinal degeneration has been associated with decreased levels of α-crystallin expression.52 Upregulation of a specific isoform of this protein (αA crystallin (Figure 3), has also been obtained upon intravitreal injection of E2,53 which further supports distribution of the hormone into the retina after topical application as eye drops in our study. CRYAA is a small heat shock protein with chaperone-like activity that prevents the aggregation of denatured or unfolded proteins.50 CRYAA is involved in cytoskeleton remodeling, which inhibits apoptosis and enhances cellular resistance to stress. Altogether, upregulation of α-crystallins by E2 treatment should have RGC-protective effects. We also obtained an upregulation of β-crystallin b2(CRYBB2), which has been proposed as a novel class of neurite-promoting factors likely to operate through an autocrine mechanism and involved in the elongation of axons during retinal regeneration.54
Figure 3.
Mass spectrometry-based retina proteomics. Normalized spectral counts obtained from data-dependent LC-MS/MS analyses are measures of protein abundance. Asterisks indicate statistically significant difference from control (summation-based G-test, p<0.05; three biological replicates per group and two analytical replicates per sample). Several differentially expressed proteins (up- and downregulated compared to vehicle control) were found in the retina of OVX rats after 19-day q.d. treatments with E2 (0.05%, w/v in 20% w/v HPCD-containing saline vehicle).
This identification of potential RGC-protective effects mediated by an upregulation of crystallines was mirrored by the upregulation of another group of proteins: E2 dosing also resulted in the upregulation of histones (Figure 3). An increased availability of histones for histone-dependent processes such as eu- and heterochromatin remodeling has been implicated in the proper function of diurnally regulated retinal activity and anti-apoptotic processes.55,57 Therefore, E2 may also exert positive effects by this mechanism, which is different from but potentially synergistic with the crystalline-mediated protection. The same analyses also identified critical molecules in the outer retina being modulated by the delivery of E2 to the retina. Specifically, we observed a hitherto not reported E2-induced downregulation of rhodopsin (RHO) and of a rod outer segment membrane protein (ROM1) (Figure 3). RHO expression levels affect rod outer segment morphology and the kinetics of the light response,58 and lower concentrations of RHO may lead to expanded disk membrane incisures that promote the longitudinal diffusion of soluble substances such as cGMP and Ca2+ and thereby disturb the gain and reproducibility of the single photon response.59 ROM1 may function as an adhesion molecule involved in stabilization and compaction of outer segment disks and, therefore, it is essential for rod photoreceptor viability and disk morphogenesis.60 On the other hand, a reduction in creatine kinase levels (Figure 3) may be an indicator of improved normal metabolic function and a decreased need for supplementary metabolic pathways typically activated by cellular and oxidative stress.61 Altogether, tissue proteomics provided additional evidence that a physiologically and potentially also therapeutically relevant amount of E2 reached the back of the eye after administration in eye drops thereby inducing significant changes in retinal protein expression with relevance to viability of retinal neurons.
Topical E2 Treatment in the Morrison Rat Model of Glaucoma
After establishing that the above treatment regimen allowed the generation of a significant E2 concentration in the retina (Table 1) with a concomitant profound change in retinal protein expression linked to signaling pathways relevant for neuroprotection (Figure 3), the functional consequences of these findings on RGC viability were investigated in a widely accepted rat model of glaucoma in which aqueous humor outflow is partially inhibited by surgical means to produce a gradually elevated IOP.36
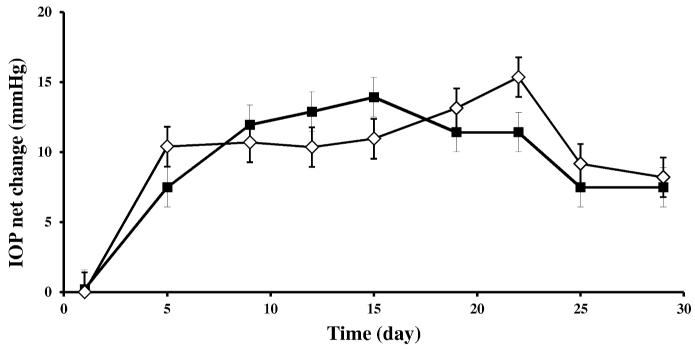
As shown in Figure 4, a consistent IOP elevation was seen in the ipsilateral eyes when compared to contralateral eyes that served as untreated controls and it remained elevated ipsilaterally for up to 30 days. Average baseline IOP (2 days before induction of IOP elevation) was 19 ± 3 mmHg, however, post surgery, average IOP in the injected ipsilateral eyes was 29 ± 4 mmHg for the duration of up to 19 days (Figure 4). Neither E2 nor vehicle treatment significantly altered IOP in comparison to the untreated contralateral eye when the animals received 10 μL of E2 or vehicle eye drops, q.d. for 19 days, analogous to the experiments on E2 quantitation in ocular tissues and retina proteomics (Table 1 and Figure 3, respectively). Altogether, the expected consistent IOP elevation in the ipsilateral eye in the rat model,36 was not significantly influenced by topical E2-treatment. Since our study aimed at investigating the neuroprotective potential of topical E2 in this glaucoma model, the finding that E2 does not ameliorate IOP is not a contraindication in the present context. It has been strongly indicated, that preservation of neuronal viability in the glaucomatous retina is needed independently from the management of the IOP, as neurodegeneration may also be significant when IOP is normal or controlled.3,4,7,9
Figure 4.
IOP changes in a rat model of glaucoma after vehicle (20% w/v, HPBCD in saline, ◇) and E2 (0.05%, w/v in vehicle, ■) eye drop treatments (10 μL, q.d., for 19 days) in the ipsilateral eye of OVX rats. The contralateral eye served as untreated control. Each graph represents mean IOP values in mmHg ± S.D. for net changes between the experimental (ipsilateral) eye and the control (contralateral) eye for four individual animals per group maintained for 30 days. Day zero was the day of initiation of IOP elevation.
E2 Treatment Significantly Reduces Apoptotic Cell Death in the Ganglion Cell Layer
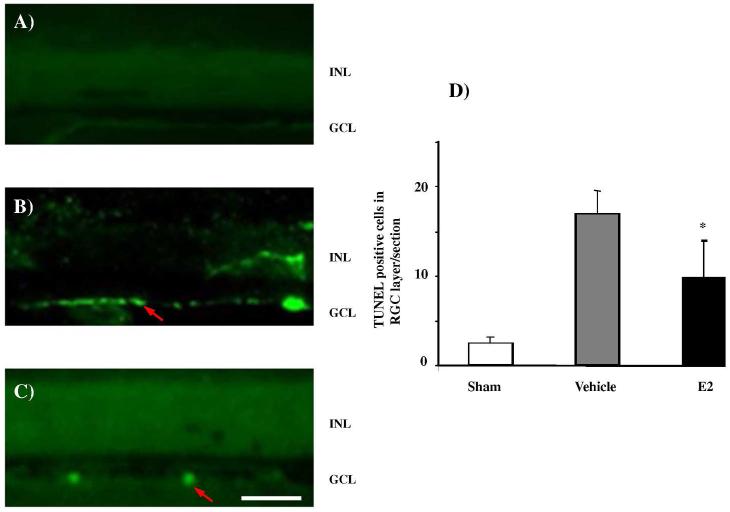
Apoptotic cells in sections from eyes with elevated IOP and treated with either the vehicle or E2 eye drops were detected by TUNEL staining. TUNEL histochemistry shows that in normotensive, contralateral controls the number of apoptotic cells in the ganglion cell layer (GCL) is extremely low (Figure 5A), was elevated in the vehicle-treated ipsilateral group that experienced elevated IOP (Figure 5B) and was significantly reduced in the E2-treated group when compared to vehicle (Figure 5C). Quantification of TUNEL-positive cells in the GCL (Figure 5D) confirmed significantly reduced apoptosis in the GCL of the E2-treated groups compared to the vehicle treated group. While RGC loss in the vehicle treated group was 34 ± 5%, in the E2-treated animals only 19 ± 6% of RGCs underwent cell death. Accordingly, approximately 50% less RGC death was detected in the E2 tretament group, than without the neuroprotective intervention (i.e., with vehicle only). Interestingly, the present study also indicates that the number of apoptotic cells in the GCL was small even in the vehicle treated group. This is potentially caused by the fact that the TUNEL assay used to detect apoptosis assayed only one time point at the end of the experimental regimen and the likely possibility exists that RGC undergoing cell death might have already passed or not reached detectability by TUNEL labeling system.
Figure 5.
Fluorescence micrographs of 12 μm thick vertical cryo sections of rat eyes stained for TUNEL-positive cells. Arrows identify some representative TUNEL-positive cells in ganglion cell layer. (GCL). INL denotes inner nuclear layer. (A) No specific cellular staining and only fluorescence background was detected in the contralateral control (B) The number of TUNEL-positive cells in GCL (bright green fluorescence) increased in the vehicle treated rat eye, while fewer TUNEL-positive cells were found in the GCL of the E2-treated group (C); scale bar in (C) for (A) through (C), 50μm. (D) Summary diagram of analyses of TUNEL-positive, apoptotic cells in the GCL per section of glaucomatous retina with and without E2 eye drop treatments (regimen specified in legends of Figures 2 – 4). Asterisk (*) indicates statistically significant difference from the vehicle treated group, p<0.05, n=4.
Behavioral Testing of Rat Visual Performance
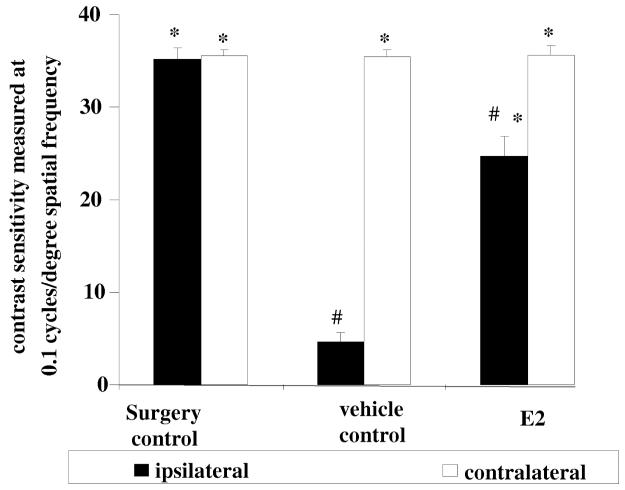
In the animal model of glaucoma generated by surgically induced elevated IOP,36 we also measured the functional consequences of the neuroprotective effect on RGC degeneration elicited by topical E2 treatments (Figure 5). Using the OptoMotry system developed by Prusky et al.,42 we determined the animals’ visual performance by testing their optomotor response to moving visual stimuli. Figure 6 shows, that topical delivery of E2 via eye drops significantly preserved visual function and prevented loss of vision, measured as contrast sensitivity at a given spatial frequency when compared to vehicle control. This E2-mediated profound preservation of contrast sensitivity, leading to better visual function when compared to vehicle controls mirrors our histological quantitative measurements of RGC degeneration (Figure 5).
Figure 6.
Protective effect of E2 on visual function measured as contrast sensitivity at a given spatial frequency in the rat model of glaucoma. Either vehicle (20% w/v HPCD in saline) or E2 (0.5% w/v in vehicle) were administered topically as eye-drops for intervention treatments (same regimen as for Figures 2-5). Animals were tested prior to euthanasia and histological assessment. Contrast sensitivity was measured at a given spatial frequency for each eye individually and compared to the ipsilateral vehicle treated eye (*; p<0.05, n=3 per group) or to the sham surgery control (#; p<0.05; n=3 per group).
Off-Target Peripheral Effects of Topical E2 Treatments
Even subchronic systemic administration of E2 produces undesirable and harmful peripheral hormonal exposure.62 Therefore, it is imperative to address this issue when E2 is used as eye drops, considering that a significant portion of drug will end up in the circulatory and gastrointestinal system and therefore the periphery.63 In Table 2 we show that the circulating E2 level in the hormone treated group was significantly higher than in the OVX control group (463±29 versus 9±3 pg/mL). Due to this substantial peripheral hormonal burden, we also observed a significant uterotrophic effect in the treatment group. The uterus is one of the most estrogen-sensitive organs in the periphery; therefore, its weight gain due to fluid imbibition triggered by exogenously administrated estrogenic compounds to OVX animals has been a frequently used marker for the presence of estrogens in the circulation.64 Indeed, we found that the wet uterus weight in the E2 group was approximately five-times higher than in the control group, indicating that a significant uterine stimulation was brought about by E2 eye drop treatments. Previously we have shown65 that the uterotrophic effect triggered by high levels of circulating E2 also results in significantly increased E2 content in the uterus that may be directly responsible for the increased risk for developing endometrial cancer.
Table 2. Serum E2 concentration and uterotrophic effect in OVX rats after E2 (0.05% w/v) or vehicle eye drop treatments (10 μL, q.d. for 19 days).
| Treatment | Serum E2 (pg/mL)a | Uterus wet weight (mg)a |
|---|---|---|
| Vehicle (control) | 9 ± 3 | 83 ± 2 |
| E2 | 463 ± 29* | 449 ± 13* |
Data are expressed as mean ± SEM for n=6.
Significant difference compared to control, p<0.05, n=6).
Altogether, our data shown in Table 2 raise the concern that, even though significant RGC protection was provided against IOP elevation-induced neurodegeneration (Figure 5) with concomitantly high functional protection (Figure 6), undesirable hormonal peripheral side-effects could outweigh the benefits of topical E2 therapy. Therefore, appropriate strategies need to be developed for selective ocular delivery of the hormone to take advantage of its potent neuroprotective actions to protect the retina, while minimizing the observed and other potential side-effects. These efforts are especially relevant since a recent report66 further hypothesized that E2 has the potential to contribute to the prevention of glaucoma in menopausal women.
CONCLUSIONS
The main finding of this study showed that E2 formulated as eye drops generated significant E2 concentration in the retina and elicited profound, structurally and functionally measurable neuroprotective effects in an established in vivo rat model of glaucoma. Using mass spectrometry-based proteomics, several proteins involved in retinal health and potentially mediating E2 neuroprotection were identified. At the same time, our study also provides a clear rationale for the need to target E2 only to the retina to avoid systemic hormonal exposure and off-target side-effects.
ACKNOWLEDGMENT
The present study was supported by grants from the National Eye Institute (EY014227 and EY022774 to PK) and the National Institutes of Health (RR022570 and RR027093 to PK, AG031535 to LP, AG031421 to KP-T). Additional support by a Challenge Grant from Research to Prevent Blindness, the Felix and Carmen Sabates Missouri Endowed Chair in Vision Research, the Vision Research Foundation of Kansas City (to PK) and the Robert A. Welch Foundation (endowment BK-0031 to LP) are gratefully acknowledged. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The sponsors and funding organization had no role in the design or conduct of this research.
REFERENCES
- (1).Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Schmidt KG, Bergert H, Funk RH. Neurodegenerative diseases of the retina and potential for protection and recovery. Curr. Neuropharmacol. 2008;6:164–178. doi: 10.2174/157015908784533851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Vasudevan SK, Gupta V, Crowston JG. Neuroprotection in glaucoma. Ind. J. Ophthalmol. 2011;59:S102–S113. doi: 10.4103/0301-4738.73700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Chidlow G, Wood JP, Casson RJ. Pharmacological neuroprotection for glaucoma. Drugs. 2007;67:725–759. doi: 10.2165/00003495-200767050-00006. [DOI] [PubMed] [Google Scholar]
- (5).McKinnon SJ. Glaucoma: Ocular Alzheimer’s disease? Front. Biosci. 2003;8:S1140–S1156. doi: 10.2741/1172. [DOI] [PubMed] [Google Scholar]
- (6).Guo L, Duggan J, Cordeiro MF. Alzheimer’s disease and retinal neurodegeneration. Curr. Alzheimer Res. 2012;7:3–14. doi: 10.2174/156720510790274491. [DOI] [PubMed] [Google Scholar]
- (7).Gupta N, Yücel YH. Glaucoma as a neurodegenerative disease. Curr. Opin. Ophthalmol. 2007;18:110–114. doi: 10.1097/ICU.0b013e3280895aea. [DOI] [PubMed] [Google Scholar]
- (8).Chiu K, Chan TF, Wu A, Yan Pui Leung I, So KF, Chuen-Chung Chang R. Neurodegeneration of the retina in mouse models of Alzheimer’s disease: what can we learn from the retina? Age. 2012;34:633–649. doi: 10.1007/s11357-011-9260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Cordeiro MF, Levin LA. Clinical evidence for neuroprotection in glaucoma. Am. J. Ophthalmol. 2011;152:715–716. doi: 10.1016/j.ajo.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Levin LA. Retinal ganglion cells and neuroprotection for glaucoma. Surv. Ophthalmol. 2003;48:S21–S24. doi: 10.1016/s0039-6257(03)00007-9. [DOI] [PubMed] [Google Scholar]
- (11).Casson RJ. Possible role of excitotoxicity in the pathogenesis of glaucoma. Clin. Exp. Ophthalmol. 2006;34:54–63. doi: 10.1111/j.1442-9071.2006.01146.x. [DOI] [PubMed] [Google Scholar]
- (12).Zhou X, Li F, Kong L, Tomita H, Li C, Cao W. Involvement of inflammation, degradation, and apoptosis in a mouse model of glaucoma. J. Biol. Chem. 2005;280:31240–31248. doi: 10.1074/jbc.M502641200. [DOI] [PubMed] [Google Scholar]
- (13).Izzotti A, Bagnis A, Saccà SC. The role of oxidative stress in glaucoma. Mutat. Res. 2006;612:105–114. doi: 10.1016/j.mrrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- (14).Kong GY, Van Bergen NJ, Trounce IA, Crowston JG. Mitochondrial dysfunction and glaucoma. J. Glaucoma. 2009;18:93–100. doi: 10.1097/IJG.0b013e318181284f. [DOI] [PubMed] [Google Scholar]
- (15).Fang J, Jiang F, Li J, Zhu Y. Rationale for the use of multifunctional drugs as neuroprotective agents for glaucoma. Neural Regen. Res. 2011;6:313–318. doi: 10.3969/j.issn.1673-5374.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Tsai JC. Medication adherence in glaucoma: approaches for optimizing patient compliance. Current Opin. Ophthalmol. 2006;17:190–195. doi: 10.1097/01.icu.0000193078.47616.aa. [DOI] [PubMed] [Google Scholar]
- (17).Guo JB, Duckles SP, Weiss JH, Li XJ, Krause DN. beta-Estradiol prevents cell death and mitochondrial dysfunction by an estrogen receptor-dependent mechanism in astrocytes after oxygen-glucose deprivation/reperfusion. Free Rad. Biol. Med. 2012;52:2151–2160. doi: 10.1016/j.freeradbiomed.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Spence RD, Voskuhl RR. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front. Neuroendocrinol. 2012;33:105–115. doi: 10.1016/j.yfrne.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Ritzel RM, Capozzi LA, McCullough LD. Sex, stroke, and inflammation: The potential for estrogen-mediated immunoprotection in stroke. Hormones Behav. 2013;63:238–253. doi: 10.1016/j.yhbeh.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Deschenes MC, Descovich D, Moreau M. Postmenopausal hormone therapy increases retinal blood flow and protects the retinal nerve fiber layer. Invest. Ophthalmol. Vis. Sci. 2010;51:2587–2600. doi: 10.1167/iovs.09-3710. [DOI] [PubMed] [Google Scholar]
- (21).Bigsby RM, Cardenas H, Caperell–Grant C, Grubbs CJ. Protective effects of estrogen in a rat model of age-related cataracts. Proc. Nat. Acad. Sci. USA. 1999;96:9328–9332. doi: 10.1073/pnas.96.16.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Yamashita H, Sugihara K, Yamada C, Tsutsumi S, Iwaki Y. Effect of estrogen on electroretinographic responses in streptozotocin-induced diabetic female rats. Exp. Eye Res. 2010;90:591–597. doi: 10.1016/j.exer.2010.02.003. [DOI] [PubMed] [Google Scholar]
- (23).Zhou X, Li F, Ge J, Sarkisian SR, Jr, Tomita H, Zaharia A, Chodosh C, Cao W. Retinal ganglion cell protection by 17-beta-estradiol in a mouse model of inherited glaucoma. Dev. Neurobiol. 2007;67:603–616. doi: 10.1002/dneu.20373. [DOI] [PubMed] [Google Scholar]
- (24).Nonaka A, Kiryu J, Tsujikawa A, Yamashiro K, Miyamoto K, Nishiwaki H, Mandai M, Honda Y, Ogura Y. Administration of 17beta-estradiol attenuates retinal ischemia-reperfusion injury in rats. Ophthalmol. Vis. Sci. 2000;41:2689–2696. [PubMed] [Google Scholar]
- (25).Wickham LA, Gao J, Toda I, Rocha EM, Ono M, Sullivan DA. Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta Ophthalmol. Scand. 2000;78:146–153. doi: 10.1034/j.1600-0420.2000.078002146.x. [DOI] [PubMed] [Google Scholar]
- (26).Kobayashi K, Kobayashi H, Ueda M, Honda Y. Estrogen receptor expression in bovine and rat retinas. Invest. Ophthalmol. Vis. Sci. 1998;39:2105–2510. [PubMed] [Google Scholar]
- (27).Kaja S, Yang SH, Wei J, Fujitani K, Liu R, Brun-Zinkernagel, A M, Simpkins JW, Inokuchi K, Koulen P. Estrogen protects retinal ganglion cells from ischemia-induced loss of Vesl-1L/Homer 1c immunoreactive synaptic connections and from apoptosis in vivo. Invest. Ophthal. Vis. Sci. 2003;44:3155–3162. doi: 10.1167/iovs.02-1204. [DOI] [PubMed] [Google Scholar]
- (28).Russo R, Cavaliere F, Watanabe C, Nucci C, Bagetta G, Corasaniti MT, Sakurada S, Morrone LA. 17β-Estradiol prevents retinal ganglion cell loss induced by acute rise of intraocular pressure in rat. Prog. Brain Res. 2008;173:583–590. doi: 10.1016/S0079-6123(08)01144-8. [DOI] [PubMed] [Google Scholar]
- (29).Giddabasappa A, Bauler M, Yepuru M, Chaum E, Dalton JT, Eswaraka J. 17-β estradiol protects ARPE-19 cells from oxidative stress through estrogen receptor-β. Invest. Ophthalmol. Vis. Sci. 2012;51:5278–5287. doi: 10.1167/iovs.10-5316. [DOI] [PubMed] [Google Scholar]
- (30).Neumann F, Wurm A, Linnertz R, Pannicke T, Iandiev I, Wiedemann P, Reichenbach A, Bringmann A. Sex steroids inhibit osmotic swelling of retinal glial cells. Neurochem. Res. 2010;35:522–530. doi: 10.1007/s11064-009-0092-8. [DOI] [PubMed] [Google Scholar]
- (31).Chen X-F, Zhang M-N, Jiang C-H, Guo W, Liu H-Z, Wei S-H. Estrogen attenuates VEGF-initiated blood-retina barrier breakdown in male rats. Horm. Metab. Res. 2011;43:614–618. doi: 10.1055/s-0031-1283149. [DOI] [PubMed] [Google Scholar]
- (32).Vajaranant TS, Pasquale LR. Estrogen deficiency accelerates aging of the optic nerve. Menopause-J. N. Am. Menop. Soc. 2012;19:942–947. doi: 10.1097/gme.0b013e3182443137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Dahan A, Miller JM, Hoffman A, Amidon GE, Amidon GL. The solubility–permeability interplay in using cyclodextrins as pharmaceutical solubilizers: Mechanistic modeling and application to progesterone. J. Pharm. Sci. 2010;99:2739–2749. doi: 10.1002/jps.22033. [DOI] [PubMed] [Google Scholar]
- (34).Wang S, Li D, Ito Y, Liu X, Zhang J, Wu C. An ocular drug delivery system containing zinc diethyldithiocarbamate and HPbetaCD inclusion complex-corneal permeability, anti-cataract effects and mechanism studies. J. Pharm. Pharmacol. 2004;56:1251–1257. doi: 10.1211/0022357044526. [DOI] [PubMed] [Google Scholar]
- (35).Gould S, Scott RC. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): A toxicology review. Food Chem. Toxicol. 2005;43:1451–1459. doi: 10.1016/j.fct.2005.03.007. [DOI] [PubMed] [Google Scholar]
- (36).Morrison JC, Moore CG, Deppmeier LM, Gold BG, Meshul CK, Johnson, E C. A rat model of chronic pressure-induced optic nerve damage. Exp. Eye Res. 1997;4:85–96. doi: 10.1006/exer.1996.0184. [DOI] [PubMed] [Google Scholar]
- (37).Szarka S, Nguyen V, Prokai L, Prokai-Tatrai K. Separation of dansylated 17β-estradiol, 17β-estradiol and estrone on a single HPLC column for simultaneous quantitation by LC-MS/MS. Anal. Bioanal. Acta. 2013;405:3399–3406. doi: 10.1007/s00216-013-6710-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Prokai L, Stevens SM, Jr, Rauniyar N, Nguyen V. Rapid label-free identification of estrogen-induced differential protein expression in vivo from mouse brain and uterine tissue. J. Proteome Res. 2009;8:3862–3871. doi: 10.1021/pr900083v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Morrison JC, Nylander KB, Lauer AK, Cepurna WO, Johnson E. Glaucoma drops control intraocular pressure and protect optic nerves in a rat model of glaucoma. Invest. Ophtalmol. Vis. Sci. 1998;39:526–531. [PubMed] [Google Scholar]
- (40).Cowley A, Franzini C. The retinal origin of uncrossed optic nerve fibers in rats and their role in visual discrimination. Exp. Brain Res. 1979;35:443–456. doi: 10.1007/BF00236763. [DOI] [PubMed] [Google Scholar]
- (41).Douglas RM, Alam NM, Silver BD, McGill TJ, Tschetter WW, Prusky GT. Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis. Neurosci. 2005;22:677–684. doi: 10.1017/S0952523805225166. [DOI] [PubMed] [Google Scholar]
- (42).Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest. Ophtalmol. Vis. Sci. 2004;45:4611–4616. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- (43).Burroughs SL, Kaja S, Koulen P. Quantification of deficits in spatial visual function of mouse models for glaucoma. Invest. Ophthalmol. Vis. Sci. 2011;52:3654–3659. doi: 10.1167/iovs.10-7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Jellali A, Meziane H, Ouagazzal AM, Rousseau S, Romand R, Auwerx J, Sahel J, Chambon P, Picaud S. The optomotor response: A robust first-line visual screening method for mice. Vision Res. 2005;45:1439–1446. doi: 10.1016/j.visres.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 45).Xin H, Yannazzo JA-A, Duncan RS, Gregg EV, Singh M, Koulen P. A novel organotypic culture model of the postnatal mouse retina allows the study of glutamate-mediated excitotoxicity. J. Neurosci. Methods. 2007;159:35–42. doi: 10.1016/j.jneumeth.2006.06.013. [DOI] [PubMed] [Google Scholar]
- (46).Osborne NN, Wood JPM, Chidlow G, Bae J-H, Melena J, Nash MS. Ganglion cell death in glaucoma: what do we really know? Br. J. Ophthalmol. 1999;83:980–986. doi: 10.1136/bjo.83.8.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Kelly KJ, Sandoval RM, Dunn KW, Molitoris BA, Dagher PC. A novel method to determine specificity and sensitivity of the TUNEL reaction in the quantitation of apoptosis. Am. J. Physiol. Cell. Physiol. 2003;284:C1309–1318. doi: 10.1152/ajpcell.00353.2002. [DOI] [PubMed] [Google Scholar]
- (48).Prokai L, Szarka S, Wang X, Prokai-Tatrai K. Capture of the volatile carbonyl metabolite of flecainide on 2,4-dinitrophenylhydrazine cartridge for quantitation by stable-isotope dilution mass spectrometry coupled with chromatography. J. Chromatogr. A. 2012;1232:281–287. doi: 10.1016/j.chroma.2012.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Cascioa C, Russoa D, Dragoa G, Galizzia G, Passantinoa R, Guarneria R, Guarneri P. 17β-Estradiol synthesis in the adult male rat retina. Exp. Eye Res. 2007;85:166–172. doi: 10.1016/j.exer.2007.02.008. [DOI] [PubMed] [Google Scholar]
- (50).Andley UP. Crystallins in the eye: function and pathology. Prog. Retin. Eye Res. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- (51).Munemasa Y, Kwong JM, Caprioli J, Piri N. The role of βA- and αB-crystallins in the survival of retinal ganglion cells after optic nerve axotomy. Invest. Ophthalmol. Vis. Sci. 2009;50:3869–3875. doi: 10.1167/iovs.08-3138. [DOI] [PubMed] [Google Scholar]
- (52).Yaung J, Kannan R, Wawrousek EF, Spee C, Sreckumar PG, Hinton DR. Exacerbation of retinal degeneration in the absence of alpha crystallins in an in vivo model of chemically induced hypoxia. Exp. Eye Res. 2008;86:355–365. doi: 10.1016/j.exer.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).D’Anna C, Cascio C, Cigna D, Galizzi G, Deidda I, Bianchi L, Russo D, Passantino R, Bini L, Guarneri P. A retinal proteomics-based study identifies βA-crystallin as a sex steroid-regulated protein. Proteomics. 2011;11:986–990. doi: 10.1002/pmic.201000561. [DOI] [PubMed] [Google Scholar]
- (54).Liedtke T, Schwamborn JC, Schroer U, Thanos S. Elongation of axons during regeneration involves retinal crystallin β-b2 (crybb2) Mol. Cell. Proteomics. 2007;6:895–907. doi: 10.1074/mcp.M600245-MCP200. [DOI] [PubMed] [Google Scholar]
- (55).Hoppe G, Rayborn ME, Sears JE. Diurnal rhythm of the chromatin protein Hmgb1 in rat photoreceptors is under circadian regulation. J. Comp. Neurol. 2007;501:219–230. doi: 10.1002/cne.21248. [DOI] [PubMed] [Google Scholar]
- (56).Franke AG, Gubbe C, Beier M, Duenker N. Transforming growth factor-beta and bone morphogenetic proteins: Cooperative players in chick and murine programmed retinal cell death. J. Comp. Neurol. 2006;495:263–278. doi: 10.1002/cne.20869. [DOI] [PubMed] [Google Scholar]
- (57).Perkins PS, Young RW. Histone proteins in fetal and adult human retinas. Jpn. J. Ophthalmol. 1987;31:590–597. [PubMed] [Google Scholar]
- (58).Makino CL, Wen X, Michaud NA, Covington HI, DiBenedetto E, Hamm HE, Lem J, Caruso G. Rhodopsin expression level affects rod outer segment morphology and photoresponse kinetics. PLoS ONE. 2012;7:e37832. doi: 10.1371/journal.pone.0037832. DOI: 10.1371/journal.pone.0037832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Caruso G, Bisegna P, Shen L, Andreucci D, Hamm HE, DiBenedetto E. Modeling the role of incisures in vertebrate phototransduction. Biophys. J. 2006;91:1192–1212. doi: 10.1529/biophysj.106.083618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Clarke G, Goldberg AF, Vidgen D, Collins L, Ploder L, Schwarz L, Molday LL, Rossant J, Szel A, Molday RS, Birch DG, McInnes RR. Rom-1 is required for rod photoreceptor viability and the regulation of disk morphogenesis. Nat. Genet. 2000;25:67–73. doi: 10.1038/75621. [DOI] [PubMed] [Google Scholar]
- (61).Hayashi K, Cheng HM, Xiong J, Xiong H, Kenyon KR. Metabolic changes in the cornea of vitamin A-deficient rats. Invest. Ophthalmol. Vis. Sci. 1989;30:769–772. [PubMed] [Google Scholar]
- (62).Prentice RL, Anderson GL. The women’s health initiative: Lessons learned. Ann. Rev. Public Health. 2008;29:131–150. doi: 10.1146/annurev.publhealth.29.020907.090947. [DOI] [PubMed] [Google Scholar]
- (63).Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J. 2010;12:348–360. doi: 10.1208/s12248-010-9183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Owens JW, Ashby J. Critical review and evaluation of the uterotrophic bioassay for the identification of possible estrogen agonists and antagonists: In support of the validation of the OECD uterotrophic protocols for the laboratory rodent. Crit. Rev. Toxicol. 2002;32:445–520. doi: 10.1080/20024091064291. [DOI] [PubMed] [Google Scholar]
- (65).Prokai-Tatrai K, Szarka S, Nguyen V, Sahyouni F, Walker C, White S, Talamantes T, Prokai L. “All in the mind”? Brain-targeting chemical delivery system of 17β-estradiol (Estredox) produces significant uterotrophic side effect. Pharm. Anal. Acta. 2012:S7. doi: 10.4172/2153-2435.S7-002. http://dx.doi.org/10.4172/2153-2435.S7-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Wei X, Cai S, Zhang X, Li X, Chen X, Liu L. Is low dose of estrogen beneficial for prevention of glaucoma? Med. Hypothesis. 2012;79:377–380. doi: 10.1016/j.mehy.2012.05.041. [DOI] [PubMed] [Google Scholar]