Abstract
The chemopreventive properties of edible berries have been demonstrated both in vitro and in vivo, however, the specific molecular mechanisms underlying their anti-cancer effects are largely unknown. Our previous studies have shown that a methanol extract fraction of freeze-dried black raspberries inhibits benzoapyrene (BaP)-induced transformation of Syrian hamster embryo cells. This fraction also blocks activation of activator protein-1 (AP-1) and nuclear factor κB (NF-κB) induced by benzoapyrene diol-epoxide (BaPDE) in mouse epidermal JB6 Cl 41 cells. To determine if different berry types exhibit specific mechanisms for their anti-cancer effects, we compared the effects of extract fractions from both black raspberries and strawberries on BaPDE-induced activation of various signaling pathways in Cl 41 cells. Black raspberry fractions inhibited the activation of AP-1, NF-κB, and nuclear factor of activated T cells (NFAT) by BaPDE as well as their upstream PI-3K/Akt-p70S6K and mitogen-activated protein kinase pathways. In contrast, strawberry fractions inhibited NFAT activation, but did not inhibit the activation of AP-1, NF-κB or the PI-3K/Akt-p70S6K and mitogen-activated protein kinase pathways. Consistent with the effects on NFAT activation, tumor necrosis factor-α (TNF-α) induction by BaPDE was blocked by extract fractions of both black raspberries and strawberries, whereas vascular endothelial growth factor (VEGF) expression, which depends on AP-1 activation, was suppressed by black raspberry fractions but not strawberry fractions. These results suggest that black raspberry and strawberry components may target different signaling pathways in exerting their anti-carcinogenic effects.
Keywords: transcription factors, BaPDE, berry, gene expression, chemoprevention
INTRODUCTION
A growing number of investigations have indicated that a consistently higher intake of fruits, vegetables, whole grains and plants is associated with a markedly reduced risk of cancer, heart disease, and some chronic diseases of aging [1–3]. Both epidemiological studies and experimental research indicate that high berry consumption can reduce cancer risk [4,5]. Berries are rich in polyphenolic compounds, including the anthocyanins, flavonoid glycosides, and simple polyphenols, which are thought to contribute to their ability to prevent disease [6]. The inhibitory activity of berries on tumor development seems to be linked, at least in part, to the anti-mutagenic [7], anti-tumor promotion [5,8], and anti-angiogenic [9,10] properties of their bioactive components. In vivo studies have shown that dietary consumption of freeze-dried black raspberries and strawberries inhibit events associated with the initiation, promotion, and progression of aerodigestive tract carcinogenesis in F344 rats [5,11]. Both berry types were equally active in preventing esophageal tumorigenesis in rats induced by the nitrosamine carcinogen, N-nitrosomethylbenzylamine (NMBA) [11]. In cultured Syrian hamster embryo cells, methanol extract fractions from both strawberries (FA-ME) and black raspberries (RO-ME) inhibited benzoapyrene (BaP)-induced cellular transformation [4].
In a previous report, we found that the RO-ME extract fraction of black raspberries inhibits benzoa pyrene-7,8-diol-9,10-epoxide (BaPDE)-induced activation of activator protein-1 (AP-1) and nuclear factor-κB (NFκB), and their associated kinases, in mouse epidermal JB6 Cl 41 cells [12]. More recently, we found that RO-ME inhibits vascular endothelial growth factor (VEGF) induction by BaPDE in Cl 41 cells, and this effect is mediated by inhibition of the phosphotidylinositol 3-kinase (PI-3K)/Akt/AP-1 pathway [13]. In the present study, we investigated the effects of both black raspberry and strawberry extract fractions on BaPDE-induced activation of multiple genes including AP-1, NF-κB, nuclear factor of activated T cell (NFAT), tumor necrosis factor-α (TNF-α) and vascular endothelial growth factor (VEGF) in Cl 41 cells to determine if there are differences between the two berry types in their molecular mechanisms of action. Our data suggest that the components of black raspberries and strawberries target different cell signaling pathways, which may provide implications for their cancer inhibitory effects.
MATERIALS AND METHODS
Cell Culture and Reagents
The mouse epidermal JB6 Cl 41 cell line and its stable transfectants containing AP-1-, NFκB-, NFAT-, TNF-α-, and VEGF-luciferase reporter genes respectively were cultured as monolayers in Eagle’s minimal essential medium (MEM) containing 5% fetal bovine serum (FBS), 2 mM L-glutamine, and 25 μg gentamicin/ml. The cultures were detached with trypsin and transferred to new 75-cm2 tissue culture flasks (Fisher, Pittsburgh, PA) every 2 or 3 d. MEM was purchased from Calbiochem (San Diego, CA); FBS from Life Technologies, Inc. (Gaithersburg, MD); luciferase assay substrate from Promega (Madison, WI); and the non-phosphorylated and phospho-specific antibodies against Akt, p70 S6 kinase (p70S6K), p38K, extracellular signal-regulated kinases (ERKs) and c-Jun NH2-terminal kinases (JNKs) were from Cell Signaling Technology (Beverly, MA). BaPDE was a kind gift from Dr. Shantu Amin, Department of Pharmacology, School of Medicine, the Pennsylvania State University (Hershey, PA) and was dissolved in DMSO at a stock concentration of 2 mM.
Berry Extract Fractions
Fractions of black raspberries and strawberries were extracted according to previously published procedures [4]. Briefly, ripe black raspberries (Rubus occidentalis, RO) and strawberries (Fragara ananassa, FA) were washed immediately after picking and frozen at −20°C. Approximately 1 pound of freeze-dried berries of each type was extracted in three volumes of methanol overnight for three nights. The extract was filtered and then dried under vacuum at 60°C to produce fraction F001. A portion of F001 was partitioned with water: dichloromethane (1:1). The aqueous layer was concentrated under vacuum and dried (F003). The organic (dichloromethane) layer was dried under vacuum at 60°C, resulting in a water-insoluble fraction (F004). Additional F001 was dissolved in methanol and allowed to evaporate. The resulting precipitate was chromatographed on a silica gel column and eluted by dichloromethane/methanol (1:1). The resulting nonpolar elute (DM) and polar fraction (ME) were obtained. All extracts were stored at −20°C in the dark. For cell treatment, each extract was dissolved in DMSO to a stock concentration of 50 mg/ml and frozen at −70°C.
Luciferase Reporter Assay for Activation of AP-1, NFκB and NFAT, and for Induction of VEGF and TNF-α
Confluent monolayers of mouse epidermal Cl 41 cells transfected with either AP-1-, NFκB-, NFAT-, VEGF-, or TNF-α-luciferase reporter genes were trypsinized. Viable cells (8 × 103) of each type were suspended in 100 μl of MEM containing 5% FBS and added to each well of 96-well plates. Plates were incubated at 37°C in a humidified atmosphere of 5% CO2. After the cell density reached 80–90%, the cells were treated with the different berry extracts at concentrations ranging from 1 to 100 μg/ml for 30 min and then exposed to BaPDE for luciferase induction in MEM containing 0.1% FBS (total volume 200 μl). After incubation for 12 or 24 h, the cells were extracted with 50 μl of lysis buffer for 30 min at 4°C and luciferase activity was measured using Promega luciferase assay reagent with a luminometer (Wallac 1420 Victor2 multilabel counter system). The results are expressed as transcription factor activity or gene transcriptional induction relative to control medium containing DMSO (0.1% v/v).
PI-3K Assay
PI-3K activities were assayed as previously described [14]. In brief, the cells were cultured in monolayers in 100-mm tissue culture dishes using MEM containing 5% FBS. The medium was replaced with MEM supplemented with 0.1% FBS after the cell density reached 70–80%. After 45 h, the cells were incubated with fresh serum-free MEM medium for 3–4 h at 37°C. After pre-incubation with different berry extracts, BaPDE was added to cell cultures for PI-3K induction. Cells were washed once with ice-cold PBS and lysed in 400 μl lysis buffer per plate (20 mM Tris pH 8.0, 137 mM NaCl, 1 mM MgCl2, 10% glycerol, 1% Nonidet P-40, 1 nM dithiothreitol, 0.4 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride). The lysates were centrifuged and the supernatants incubated at 4°C with 40 μl agarose beads (previously conjugated with the monoclonal antiphosphotyrosine antibody Py20 overnight). Beads were washed twice with each of the following buffers: (1) PBS with 1% Nonidet P-40, 1 mM dithiothreitol; (2) 0.1 M Tris (pH 7.6), 0.5 M LiCl, l mM dithiothreitol; and (3) 10 mM Tris (pH 7.6), 0.1 M NaCl, 1 mM dithiothreitol. Beads were incubated for 5 min on ice in 20 μl of buffer 3, and then 20 μl of 0.5 mg/ml phosphatidylinositol (previously sonicated in 50 mM HEPES pH 7.6, 1 mM EGTA, 1 mM NaH2PO4) was added. After 5 min at room temperature, 10 μl of reaction buffer (50 mM MgCl2, 100 mM HEPES pH 7.6, 250 mM ATP containing 5 mCi of γ-32P ATP) was added, and beads were incubated for an additional 15 min. Reactions were stopped by the addition of 15 μl of 4 N HCl and 130 μl of chloroform/methanol (1:1). After being vortex mixed for 30 s, 30 μl of the phospholipid-containing chloroform phase was spotted onto thin-layer chromatography plates coated with silica gel H containing 1.3% potassium oxalate and 2 mM EDTA applied in H2O/methanol (3:2). Plates were heated at 110°C for at least 3 h before use. Plates were then placed in tanks containing chloroform/methanol/NH4OH/H2O (600:470:20:113) for 40–50 min so that the solvent reached the top of the plates. Plates were dried at room temperature and autoradiographed.
Kinase Phosphorylation Assay
Cl 41 cells (5 × 104) were cultured in each well of 6-well plates to 70–80% confluence with 5% FBS MEM. The medium was replaced with MEM supplemented with 0.1% FBS and cultured for 45 h. Cells were then incubated in 0.1% FBS MEM for 3–4 h at 37°C. Cells were pretreated with berry extract fractions for 30 min, exposed to BaPDE for 270 min, washed once with ice-cold PBS, and extracted with an SDS-sample buffer. Western blots were performed with either phospho-specific or non-phosphorylated antibodies against various proteins, including Akt, p70S6K, ERKs, JNKs, and p38K. The protein band specifically bound to the primary antibody was detected with an anti-rabbit immunoglobin-AP-linked secondary antibody and ECF Western blotting system (Amersham Biosciences, Piscataway, NJ).
Statistical Analysis
The significance of the difference between treated and untreated groups was determined with the Student’s t test. Results are expressed as mean ±SE. Differences were considered significant at a P <0.05.
RESULTS
Inhibition of BaPDE-Induced Activation of AP-1 and NFκB by Extracts From Black Raspberries but not Strawberries
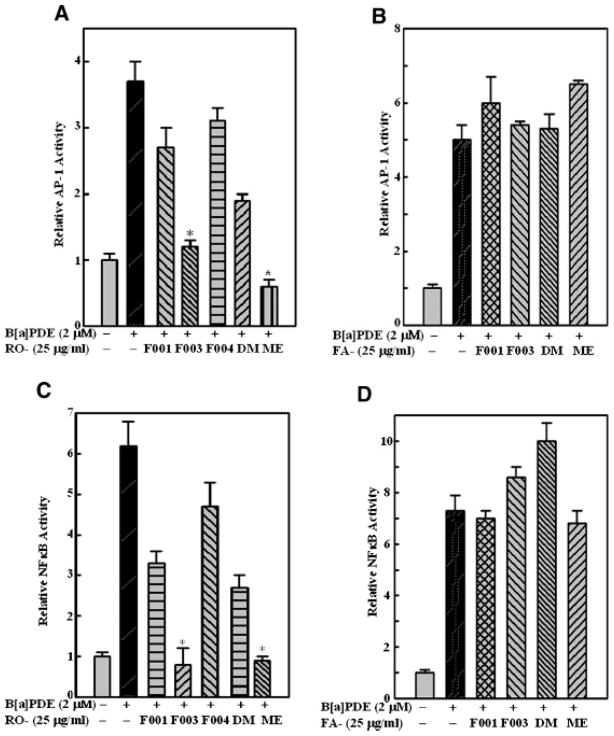
Transcription factors AP-1 and NFκB play a critical role in carcinogenic processes both in vitro and in vivo [15], so it was anticipated that both transcription factors could serve as prime molecular targets for chemoprevention [16]. Our previous studies found that among freeze-dried black raspberry extract fractions, RO-ME and RO-F003 exhibited the most potent inhibitory effect on BaPDE-induced transactivation of AP-1 and NFκB [12]. To determine whether strawberry extract fractions could also inhibit BaPDE-induced activation of AP-1 and NFκB, we used Cl41 stable transfectants containing luciferase reporter for AP-1 or NFκB. Pretreatment of cells with black raspberry fractions RO-F003 or RO-ME resulted in a remarkable inhibition of BaPDE-induced activation of AP-1 (Figure 1A) and NFκB (Figure 1C), which was consistent with our previous findings [12]. In contrast, activation of neither transcription factor was inhibited by pre-treatment of the cells with any of the strawberry fractions (Figure 1B and D). These results suggest that extract fractions from strawberries exhibit anti-cancer effects via mechanisms other than inhibition of AP-1 and NFκB.
Figure 1.
Inhibition of BaPDE-induced activation of AP-1 and NFκB by fractions from black raspberries but not strawberries. Mouse epidermal Cl 41 cells stably transfected with AP-1 luciferase (A and B) or with NFκB luciferase (C and D) were seeded into each well of 96-well plates at 8 × 103 cells/well and cultured in 5% FBS MEM at 37°C for 12 h. The cells were pretreated with various fractions of black raspberry extracts (A and C) or strawberry extracts (B and D) for 30 min and then exposed to BaPDE (2 μM) for AP-1 and NFκB induction for 12 h. The cells were extracted with lysis buffer, and luciferase activity was measured using Promega luciferase assay reagent with a luminometer after the addition of 50 μl of lysis buffer for 30 min at 4°C. Results are presented as AP-1- or NFκB-dependent transcriptional activity relative to medium control (relative AP-1 or NFκB activity). Each bar indicates the mean and standard error of four repeat assay wells. The asterisk (*) indicates a significant decrease from BaPDE treatment alone (P <0.05). DM and ME refer to non-polar and polar fractions of a silica gel column fractionation.
BaPDE-Induced VEGF Expression is Inhibited by Fractions From Black Raspberries but not Strawberries
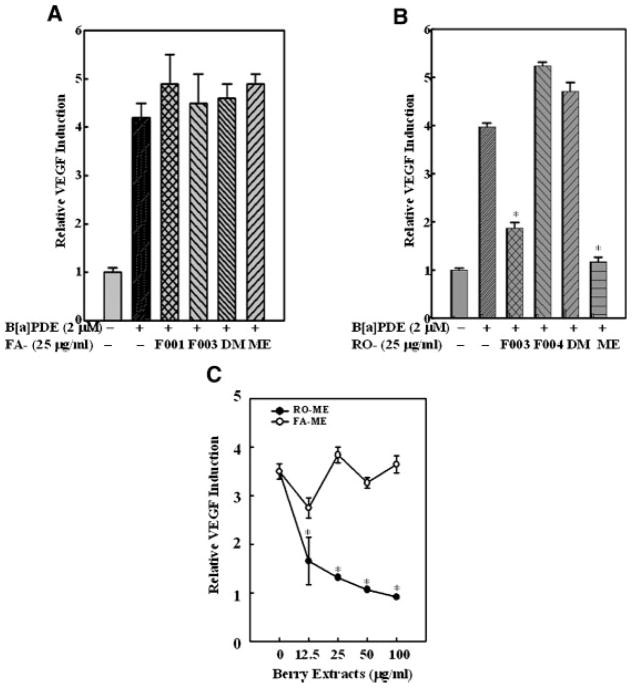
Expression of VEGF is often obligatory for tumor angiogenesis and promotion, thus inhibition of VEGF expression or function has been fervently pursued as a strategy for cancer treatment [17]. Recently, we demonstrated that the RO-ME fraction from black raspberries markedly inhibits BaPDE-induced VEGF expression through suppression of the PI-3K/Akt/AP-1–dependent pathway [13]. In the present study, we investigated the effects of extract fractions from strawberries on VEGF expression following BaPDE induction. Consistent with effects on AP-1 and NFκB activation, all strawberry fractions failed to inhibit VEGF expression (Figure 2A), whereas both RO-ME and RO-F003 reduced VEGF induction by BaPDE (Figure 2B). These results were further confirmed by a dose-response study (Figure 2C). Our data suggest that the anti-carcinogenic effects of strawberries in vitro is not via inhibition of VEGF expression.
Figure 2.
Effects of black raspberry and strawberry fractions on VEGF induction by BaPDE. Mouse epidermal Cl 41 cells stably transfected with VEGF luciferase were seeded into each well of 96-well plates at 8 × 103 cells/well and cultured in 5% FBS MEM at 37°C for 12 h. The cells were pretreated with various fractions of strawberry extracts (A and C) or black raspberry extracts (B and C) as indicated for 30 min and then exposed to BaPDE (2 μM) for VEGF induction. After 24 h incubation, cells were extracted with lysis buffer, and luciferase activity was measured. Results are presented as VEGF induction relative to medium control (relative VEGF induction). Each bar indicates the mean and standard error of four repeat assay wells. The asterisk (*) indicates a significant decrease from BaPDE treatment alone (P <0.05).
BaPDE-Induced PI-3K/Akt-p70S6K/Mitogen-Activated Protein Kinase (MAPK) Activation is not Affected by Strawberry Fractions
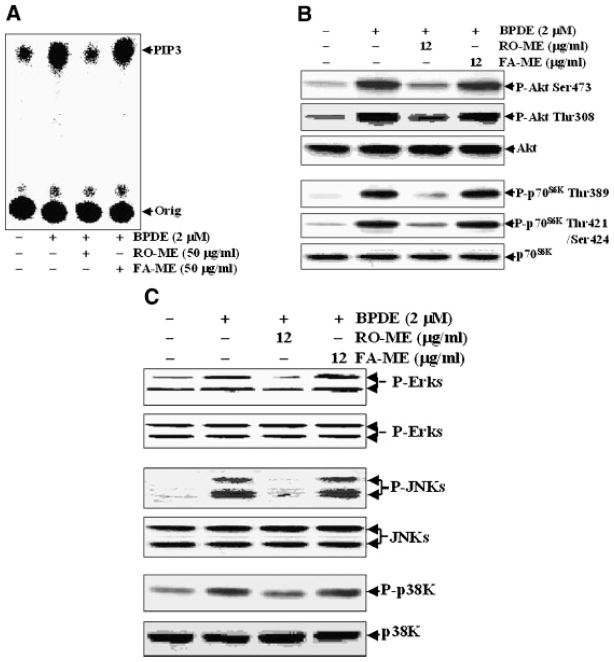
In our recent studies, BaPDE treatment resulted in activation of the PI-3K/Akt pathway in Cl 41 cells, which accounted for AP-1 transactivation [14,18]. Moreover, the inhibitory effect of RO-ME on BaPDE-induced AP-1 activation and VEGF expression in these cells was mediated by inhibition of the PI-3K/Akt pathway [13]. In the present study, we investigated whether fractions from strawberries affect the PI-3K/Akt-p70S6K/MAPKs signal pathway. As indicated in Figure 3A, pretreatment of cells with the methanol extract fraction of strawberries (FA-ME) did not reduce BaPDE-induced PI-3K activation whereas RO-ME effectively blocked BaPDE-induced PI-3K activity. Since Akt is a PI-3K downstream kinase responsible for mediation of carcinogen-induced AP-1 activation [14,18], it was interesting to determine the effect of FA-ME on BaPDE-induced Akt activation. As expected, FA-ME failed to inhibit Akt activation whereas RO-ME blocked BaPDE-induced Akt phophorylation at Thr308 and Ser473 (Figure 3B). p70S6K is another PI-3K downstream target [14], thus we compared the effects of RO-ME and FA-ME on BaPDE-induced p70S6K activation. As shown in Figure 3B, the effects of RO-ME and FA-ME on p70S6K were similar to those on Akt activation. Finally, RO-ME and FA-ME inhibited MAPK activation differently as well; that is, FA-ME did not affect MAPK activation and RO-ME inhibited BaPDE-induced activation of ERKs, JNKs, and p38K (Figure 3C). These results indicate that RO-ME inhibits PI-3K/Akt-p70S6K-MAPKs-AP-1/NFκB pathways, whereas FA-ME does not inhibit any of these pathways.
Figure 3.
Comparison of effects of RO-ME and FA-ME on BaPDE-induced activation of PI-3K, Akt, p70S6K, and MAPKs. Cl 41 cells were seeded into 100-mm tissue culture dishes (A) or each well of 6-well plates (B and C), and cultured in 5% FBS MEM at 37°C until cell density reached 70–80%. The cell culture medium was replaced with 0.1% FBS MEM. Forty-five hours later, cells were incubated with fresh serum-free MEM for 3–4 h at 37°C. Cells (A) were then pretreated with the indicated berry fractions at 50 μg/ml for 30 min, exposed to BaPDE (2 μM) for 30 min, and lysed in 400 μl lysis buffer. PI-3K activity was measured as described in Materials and Methods; or (B and C) were pretreated with indicated berry fractions at 12 μg/ml for 30 min, exposed to BaPDE (2 μM) for 270 min, and extracted with an SDS-sample buffer and Western blot analysis carried out as described in Materials and Methods.
Extracts From Strawberries Specifically Inhibit BaPDE-Induced NFAT Activation and TNF-α Transcription
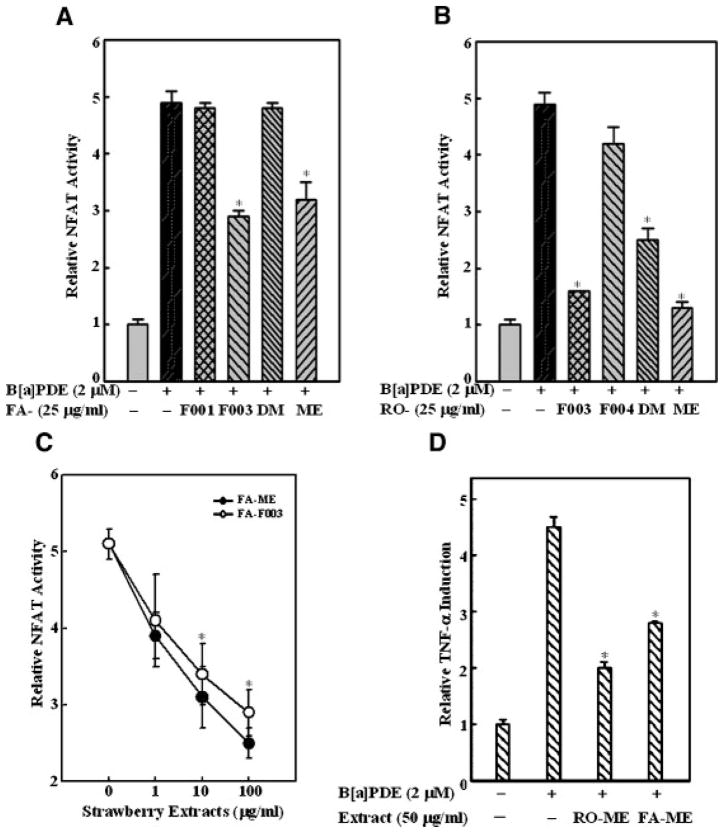
Our most recent studies show that NFAT is another important transcription factor involved in cell responses to environmental carcinogens, such as nickel subsulfide [19], vanadium [20], asbestos [21], as well as BaPDE-induced Cl 41 cell transformation (Huang et al., unpublished work). To test the potential involvement of NFAT in the anti-cancer effects of berry fractions, Cl 41 cells stably transfected with the NFAT-luciferase reporter gene were pre-treated with different berry extract fractions for 30 min, and then exposed to BaPDE for NFAT induction. FA-ME and FA-F003 significantly inhibited BaPDE-induced NFAT activation (Figure 4A) as did RO-ME, RO-F003, and RO-DM (Figure 4B). The inhibition by FA-ME and FA-F003 appeared to be dose dependent (Figure 4C). We also demonstrated that NFAT activation is critical for BaPDE-induced TNF-α expression and cell transformation (Li and Huang, et al., unpublished work). Thus, we tested whether strawberry and black raspberry extract fractions could inhibit BaPDE-induced TNF-α induction in Cl 41 cells. Consistent with their effects on NFAT activity, both RO-ME and FA-ME reduced BaPDE-induced TNF-α transcription (Figure 2D), suggesting that inhibition of BaPDE-induced NFAT activation and TNF-α expression by both strawberries and black raspberries may be one mechanism for their anti-cancer effects. Meanwhile, we need to acknowledge that the extent of inhibition seen with the strawberry extracts is considerably less than that seen with the raspberry extracts. These data suggest that inhibition of NFAT activation by strawberry fractions may be important for their anti-carcinogenic effects in vitro, whereas the anti-cancer effects of black raspberries are through multiple signaling pathways, including inhibition of AP-1, NFκB, and NFAT.
Figure 4.
Effects of berry fractions on BaPDE-induced NFAT transactivation and TNF-α expression. Mouse epidermal Cl 41 cells stably transfected with NFAT luciferase or TNF-α luciferase were seeded into each well of 96-well plates at 8 × 103 cells/well and cultured in 5% FBS MEM at 37°C for 12 h. The cells were pretreated with various fractions of strawberry extracts (A, C, and D) or black raspberry extracts (B and D) as indicated for 30 min at the concentrations indicated, and then exposed to BaPDE (2 μM) for and luciferase activity was measured using Promega luciferase assay reagent with a luminometer. Results are presented as NFAT activity or TNF-α induction relative to medium control (relative NFAT activity or relative TNF-α induction). Each bar indicates the mean and standard error of four repeat assay wells. The asterisk (*) indicates a significant decrease from BaPDE treatment alone (P <0.05).
DISCUSSION
Transcription factors such as AP-1, NF-κB, and NFAT are crucial for the regulation of cell proliferation, cell differentiation, and other cell functions [22–24]. Inappropriate activation of these transcription factors may result in the development of various diseases, including cancer [16]. Inhibition of these transcription factors appears to contribute to the chemopreventive effects of certain phytochemicals [15,25,26]. In the present study, we investigated the effects of different black raspberry and strawberry extract fractions on BaPDE-induced activation of AP-1, NF-κB, and NFAT in Cl 41 cells to determine whether they have similar or different mechanisms for their anti-carcinogenic effects. We found that the black raspberry fractions, RO-ME and RO-F003, are the most potent inhibitors of the three transcription factors. The strawberry fractions, FA-ME and FA-F003, targeted only the NFAT pathway. These differential effects on signaling pathways may provide insight into the mechanisms of anti-carcinogenesis of the two berry types [5,11].
TNF-α, the crucial pro-inflammatory cytokine [27], is reported to be essential in cancer development [28], and to act as a linker between chronic inflammation and cancer. Our studies have defined the crucial role of TNF-α in BaPDE-induced cell transformation (Li and Huang et al., unpublished work), and have shown that TNF-α expression is dependent upon activation of the NFAT pathway. Pretreatment with RO-ME and FA-ME reduced TNF-α induction, which is consistent with their inhibition of NFAT activation. These data add to our knowledge of the molecular mechanisms for the anti-carcinogenic activity of berry extracts in vitro.
VEGF is a key regulator of physiologic and pathologic changes associated with cancer development [29–31]. Liu et al. [32] reported that an extract of black raspberries inhibits the initiation and growth of new blood vessels (angiogenesis). Using bioassay-guided fractionation they found that gallic acid is one of the active compounds in the refined black raspberry extract. Sashwati et al. [10] investigated the expression of VEGF induced by both H2O2 and TNF-α in human HaCat keratinocytes pretreated with extracts prepared from six common edible berries, including wild blueberry, bilberry, cranberry, elderberry, raspberry seed, and strawberry. Their results showed that all berry types studied potently inhibit both H2O2- as well as TNF-α-induced VEGF expression, and these effects are not dependent on their antioxidant properties. We recently showed that VEGF is induced by BaPDE exposure and, more importantly, that RO-ME and RO-F003 reduced VEGF induction via blocking the PI-3K/Akt/AP-1 pathway [13]. In the present study, however, all strawberry fractions were found to be ineffective in inhibiting BaPDE-induced VEGF expression. The difference between our results and those of Sashwati et al. about the effects of strawberry fractions on VEGF induction may be due to the use of different agents to induce VEGF expression. These agents may induce VEGF expression through different signaling pathways. This notion is supported by our most recent findings that RO-ME inhibits UV-induced activation of NFκB but not AP-1 (Huang et al., unpublished work), whereas it inhibits both AP-1 and NFκB activation induced by BaPDE [12]. Considering the key role of VEGF in controlling tumor angiogenesis and metastasis, the inhibition of BaPDE-induced VEGF expression by black raspberries may be one molecular mechanism for the anti-angiogenic properties reported by Liu et al. [32].
The PI-3K signaling pathway is now accepted as being at least as important as the Ras/MAP kinase pathway in cell survival and proliferation and is, therefore, gaining interest as a target for chemo-prevention and anticancer therapy [25,33]. Akt is a major downstream target of PI3-K and its product, 3-phosphoinositide [14]. After activation, Akt translocates to the plasma membrane, where it is phosphorylated at Ser473 and Thr308, and then it translocates to the cytosol and nucleus to phosphorylate its substrates [34]. p70S6K is another downstream target of PI-3K, and its activation has been implicated in the regulation of the translation, cell cycle control, and neuronal cell differentiation [35]. Activation of p70S6K occurs through a complex series of phosphorylation events on eight or more serine or threonine residues, identified as S404, S411, S418, S424, and T421 on the C-terminal autoinhibitory domain and T229, S371, and T389, which are critical for catalytic activity. Our previous findings revealed that BaPDE exposure results in PI-3K/Akt-dependent and p70S6K-independent AP-1 transactivation [14]. In the present study, we observed that pretreatment with the most potent black raspberry fraction, RO-ME, blocked PI-3 kinase activity, Akt phosphorylations at Thr308 and Ser473, and p70S6K phosphorylations at Thr389 and Thr421/Ser424 induced in Cl 41 cells by BaPDE. The strawberry fractions however, had no effect on activation of the PI-3K-Akt/p70S6K pathways, which is consistent with their effects on AP-1 and NFκB. JNKs, a member of the MAPKs, were found to be the mediator of AP-1 activation when the PI-3K/Akt pathway is activated by BaPDE [14]. Therefore, we investigated the effects of berry extracts on MAPK pathway activation. RO-ME inhibited phosphorylation of the ERKs, JNKs, and p38K induced by BaPDE whereas the strawberry fraction (FA-ME) was ineffective. NFκB activation induced by TNF-α and platelet-derived growth factor also depends on Akt activity by phosphorylating and activating IκB kinase, which induces the degradation of IκB, the NFκB inhibitor [36–38]. The absence of inhibitory effects of strawberries on AP-1 and NFκB activation may be due to their failure to effect activation of the PI-3K/Akt pathway.
In summary, strawberry extract fractions FA-ME and FA-FOO3 specifically blocked BaPDE-induced NFAT activation, and black raspberries extract fractions RO-ME and RO-F003 inhibited activation of AP-1, NFκB, and NFAT. RO-ME and FA-ME both reduced TNF-α induction. Consistent with their effects on signaling pathway activation, black raspberry extracts were able to block VEGF induction by BaPDE whereas strawberry extracts were not. These results suggest that absence of the inhibition of AP-1 and NFκB activation by strawberry fractions may be due to their inability to suppress the PI-3K/Akt/MAPK pathway. Inhibition of BaPDE-induced NFAT activation and consequently TNF-α expression may be involved in the anti-carcinogenic effects of strawberries. These results add to our knowledge of the molecular mechanisms underlying the anti-carcinogenic effects of black raspberries and strawberries in vitro.
Acknowledgments
This work was supported in part by grants from NIH/NCI (R01 CA103180, R01 CA094964, and R01 CA112557) and NIH/NIEHS (R01 ES012451 and ES000260).
Abbreviations
- FA
Fragara ananassa (strawberry)
- RO
Rubus occidentalis (black raspberry)
- BaP
benzoapyrene
- BaPDE
benzoa pyrene-7,8-diol-9,10-epoxide
- AP-1
activator protein-1
- NFκB
nuclear factor-κB
- VEGF
vascular endothelial growth factor
- PI-3K
phosphotidylinositol 3-kinase
- NFAT
nuclear factor of activated T cells
- TNF-α
tumor necrosis factor-alpha
- MEM
Eagle’s minimal essential medium
- FBS
fetal bovine serum
- p70S6K
p70 S6 kinase
- ERK
extracellular signal-regulated kinase
- JNK
c-Jun NH2-terminal kinase
- MAPK
mitogen-activated protein kinase
References
- 1.Heber D. Vegetables, fruits and phytoestrogens in the prevention of diseases. J Postgrad Med. 2004;50:145–149. [PubMed] [Google Scholar]
- 2.Heggie SJ, Wiseman MJ, Cannon GJ, et al. Defining the state of knowledge with respect to food, nutrition, physical activity, and the prevention of cancer. J Nutr. 2003:3837S–3842. doi: 10.1093/jn/133.11.3837S. [DOI] [PubMed] [Google Scholar]
- 3.Genkinger JM, Platz EA, Hoffman SC, Comstock GW, Helzlsouer KJ. Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington county, Maryland. Am J Epidemiol. 2004;160:1223–1233. doi: 10.1093/aje/kwh339. [DOI] [PubMed] [Google Scholar]
- 4.Xue H, Aziz RM, Sun N, et al. Inhibition of cellular transformation by berry extracts. Carcinogenesis. 2001;22:351–356. doi: 10.1093/carcin/22.2.351. [DOI] [PubMed] [Google Scholar]
- 5.Kresty LA, Morse MA, Morgan C, et al. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001;61:6112–6119. [PubMed] [Google Scholar]
- 6.Cooke D, Steward WP, Gescher AJ, Marczylo T. Anthocyans from fruits and vegetables—Does bright colour signal cancer chemopreventive activity? Eur J Cancer. 2005;41:1931–1940. doi: 10.1016/j.ejca.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Hope Smith S, Tate PL, Huang G, et al. Antimutagenic activity of berry extracts. J Med Food. 2004;7:450–455. doi: 10.1089/jmf.2004.7.450. [DOI] [PubMed] [Google Scholar]
- 8.Wedge D, Meepagala K, Magee J, Smith S, Huang G, Larcom L. Anticarcinogenic activity of strawberry, blueberry, and raspberry extracts to breast and cervical cancer cells. J Med Food. 2001;4:49–51. doi: 10.1089/10966200152053703. [DOI] [PubMed] [Google Scholar]
- 9.Bagchi D, Sen C, Bagchi M, Atalay M. Anti-angiogenic, antioxidant, and anti-carcinogenic properties of a novel anthocyanin-rich berry extract formula. Biochemistry (Mosc) 2004;69:75–80. doi: 10.1023/b:biry.0000016355.19999.93. [DOI] [PubMed] [Google Scholar]
- 10.Sashwati R, Savita K, Helaine MA, et al. Anti-angiogenic property of edible berries. Free Radic Res. 2002;36:1023–1032. doi: 10.1080/1071576021000006662. [DOI] [PubMed] [Google Scholar]
- 11.Carlton PS, Kresty LA, Siglin JC, et al. Inhibition of N-nitrosomethylbenzylamine-induced tumorigenesis in the rat esophagus by dietary freeze-dried strawberries. Carcino-genesis. 2001;22:441–446. doi: 10.1093/carcin/22.3.441. [DOI] [PubMed] [Google Scholar]
- 12.Huang C, Huang Y, Li J, et al. Inhibition of Benzo(a)pyrene diol-epoxide-induced transactivation of activated protein 1 and nuclear factor {kappa}B by black raspberry extracts. Cancer Res. 2002;62:6857–6863. [PubMed] [Google Scholar]
- 13.Huang C, Li J, Song L, et al. Black raspberry extracts inhibit benzo(a)pyrene diol-epoxide-induced activator protein 1 activation and VEGF transcription by targeting the phosphotidylinositol 3-kinase/akt pathway. Cancer Res. 2006;66:581–587. doi: 10.1158/0008-5472.CAN-05-1951. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Tang M-S, Liu B, Shi X, Huang C. A critical role of PI-3K/Akt/JNKs pathways in benzoapyrene diol-epoxide(BaPDE)-induced AP-1 transactivaiton in mouse epidermal Cl41 cells. Oncogene. 2004;23:3932–3944. doi: 10.1038/sj.onc.1207501. [DOI] [PubMed] [Google Scholar]
- 15.Kundu JK, Surh Y-J. Molecular basis of chemoprevention by resveratrol: NF-kappaB and AP-1 as potential targets. Mutat Res. 2004;555:65. doi: 10.1016/j.mrfmmm.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Young MR, Yang H-S, Colburn NH. Promising molecular targets for cancer prevention: AP-1, NF-kappaB and Pdcd4. Trends Mol Med. 2003;9:36–41. doi: 10.1016/s1471-4914(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 17.Tober KL, Cannon RE, Spalding JW, et al. Comparative expression of novel vascular endothelial growth factor/vascular permeability factor transcripts in skin, papillomas, and carcinomas of v-Ha-rasTg. AC transgenic mice and FVB/N mice. Biochem Biophys Res Commun. 1998;247:644–653. doi: 10.1006/bbrc.1998.8787. [DOI] [PubMed] [Google Scholar]
- 18.Ding J, Li J, Chen J, et al. Effects of polycyclic aromatic hydrocarbons (PAHs) on vascular endothelial growth factor induction through phosphatidylinositol 3-kinase/ap-1-dependent, HIF-1{alpha}-independent pathway. J Biol Chem. 2006;281:9093–9100. doi: 10.1074/jbc.M510537200. [DOI] [PubMed] [Google Scholar]
- 19.Huang C, Li J, Costa M, et al. Hydrogen peroxide mediates activation of nuclear factor of activated T cells (NFAT) by nickel subsulfide. Cancer Res. 2001;61:8051–8057. [PubMed] [Google Scholar]
- 20.Huang C, Ding M, Li J, et al. Vanadium-induced nuclear factor of activated T cells activation through hydrogen peroxide. J Biol Chem. 2001;276:22397–22403. doi: 10.1074/jbc.M010828200. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Huang B, Shi X, Castranova V, Vallyathan V, Huang C. Involvement of hydrogen peroxide in asbestos-induced NFAT activation. Mol Cell Biochem. 2002;161:234–235. [PubMed] [Google Scholar]
- 22.Horsley V, Pavlath GK. Nfat: Ubiquitous regulator of cell differentiation and adaptation. J Cell Biol. 2002;156:771–774. doi: 10.1083/jcb.200111073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palanki M. Inhibitors of AP-1 and NF-kappa B mediated transcriptional activation: Therapeutic potential in auto-immune diseases and structural diversity. Curr Med Chem. 2002;9:219–227. doi: 10.2174/0929867023371265. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-{{kappa}}B pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bode AM, Dong Z. Targeting signal transduction pathways by chemopreventive agents. Mutat Res. 2004;555:33–51. doi: 10.1016/j.mrfmmm.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Keum Y-S, Jeong W-S, Tony Kong AN. Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms. Mutat Res. 2004;555:191–202. doi: 10.1016/j.mrfmmm.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: Integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 28.Szlosarek P, Balkwill F. Tumour necrosis factor α: A potential target for the therapy of solid tumours. Lancet Oncol. 2003;4:565–573. doi: 10.1016/s1470-2045(03)01196-3. [DOI] [PubMed] [Google Scholar]
- 29.Bancroft CC, Chen Z, Yeh J, et al. Effects of pharmacologic antagonists of epidermal growth factor receptor, PI3K and MEK signal kinases on NF-κB and AP-1 activation and IL-8 and VEGF expression in human head and neck squamous cell carcinoma lines. Int J Cancer. 2002;99:538–548. doi: 10.1002/ijc.10398. [DOI] [PubMed] [Google Scholar]
- 30.McCarty MF, Liu W, Fan F, et al. Promises and pitfalls of anti-angiogenic therapy in clinical trials. Trends Mol Med. 2003;9:53–58. doi: 10.1016/s1471-4914(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 31.Bobrovnikova-Marjon EV, Marjon PL, Barbash O, Vander Jagt DL, Abcouwer SF. Expression of angiogenic factors vascular endothelial growth factor and interleukin-8/CXCL8 is highly responsive to ambient glutamine vailability: Role of nuclear factor-{kappa}B and activating protein-1. Cancer Res. 2004;64:4858–4869. doi: 10.1158/0008-5472.CAN-04-0682. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, Schwimer J, Liu D, Greenway FL, Anthony CT, Woltering EA. Black raspberry extract and fractions contain angiogenesis inhibitors. J Agric Food Chem. 2005;53:3909–3915. doi: 10.1021/jf048585u. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Law P, Loh H. Inhibition of PI3K/Akt signaling: An emerging paradigm for targeted cancer therapy. Curr Med Chem Anti Canc Agents. 2005;5:575–589. doi: 10.2174/156801105774574649. [DOI] [PubMed] [Google Scholar]
- 34.Lawlor MA, Alessi DR. PKB/Akt: A key mediator of cell proliferation, survival and insulin responses? Cell Sci. 2001;114:2903–2910. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- 35.Berven LA, Crouch MF. Cellular function of p70S6K: A role in regulating cell motility. Immunol Cell Biol. 2000;78:447–451. doi: 10.1046/j.1440-1711.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- 36.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 37.Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15:177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Nidai Ozes O, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]