Abstract
The DNA damage response (DDR) and the spindle assembly checkpoint (SAC) are two critical mechanisms by which mammalian cells maintain genome stability. There is a growing body of evidence that DDR elements and SAC components crosstalk. Here we report that Bub1 (Budding Uninhibited by Benzimidazoles 1), one of the critical kinetochore proteins essential for SAC, is required for optimal DDRs. We found that knocking-down Bub1 resulted in prolonged H2AX foci and comet tail formation as well as hypersensitivity in response to ionizing radiation (IR). Further, we found that Bub1-mediated Histone H2A Threonine 121 phosphorylation was induced after IR in an ATM-dependent manner. We demonstrated that ATM phosphorylated Bub1 on serine 314 in response to DNA damage in vivo. Finally, we showed that ATM-mediated Bub1 serine 314 phosphorylation was required for IR-induced Bub1 activation and for the optimal DDR. Together, we elucidate the molecular mechanism of DNA damage-induced Bub1 activation and highlight a critical role of Bub1 in DDR.
Keywords: ATM, Bub1, DNA damage response
1. Introduction
Eukaryotic genomic DNA is constantly insulted by various endogenous or exogenous factors, such as free radicals, UV radiation and ionizing radiation (IR). Once DNA is damaged, optimal DNA damage responses (DDRs) are initiated in order to sense and repair the lesions [1]. The failure of DNA damage repair can lead to cell death and/or tumorigenesis [2]. Among the diverse forms of DNA damage, an IR-induced DNA double strand break (DSB) is the most lethal and has been studied extensively for its potential significance in tumor treatment and anti-cancer drug development. The DDR is regulated by a comprehensive signal transduction network which includes damage sensors, transducers, adaptors and effectors [3]. Primarily, the function of the signal transduction network is to coordinate the cell cycle control system with the DNA repair process. The serine/threonine protein kinase ATM (Ataxia Telangiectasia Mutated) is the primary kinase in cellular responses to IR [4]. ATM is activated after IR and sends signals to downstream targets by phosphorylation [5]. Proteomic studies have identified more than 700 proteins that are direct targets of ATM-mediated phosphorylation [6]. Functional significance of these phosphorylation events remains to be fully studied.
In addition to DNA damage checkpoints, the spindle assembly checkpoint (SAC) also functions in the maintenance of genome stability by monitoring the attachment of kinetochores and microtubules to ensure a faithful segregation of duplicated chromosomes [7]. The proteins responsible for SAC signaling pathways include Mad1, Mad2/Mad3, Bub1, Bub3, Cdc20, Mps1, Aurora B and etc [7]. Several SAC proteins, including Bub1, Mad1, Mad2BP and Sgo1, were identified by a proteomic study as ATM substrates [6], indicating that DDR and SAC pathways crosstalk. However, it is not known whether these proteins are directly involved in DDR, and the functional significance of the ATM-mediated phosphorylation events has yet to be elucidated.
Bub1 (budding uninhibited by benomyl 1) is a critical protein with multiple roles in mitosis as evidenced by loss-of-function mutations or absence of Bub1 being linked to aneuploidy, chromosomal instability (CIN), premature senescence and cancer [8, 9]. Bub1 is a protein kinase which can phosphorylate substrates including Mad1, Cdc20, INCENP, CENP-F, MCAK, and Sgo1 in vitro [10]. In vivo evidence has shown that Bub1 phosphorylates Histone H2A to activate SAC [11]. During the early stages of mitosis, Bub1 recruits several SAC substrates to unattached kinetochores to facilitate the formation of the mitotic checkpoint complex (MCC), which is required for the block of the metaphase-anaphase transition [8]. Meanwhile, Bub1 mediates the phosphorylation of Cdc20 which leads to inhibition of the ligase activity of APC/C [12]. Aside from the finding that Bub1 is among the mitotic proteins identified as a potential ATM target in response to IR, little is known about the role Bub1 plays in DDR signaling pathways.
In this study, we report that depletion of Bub1 leads to delayed DNA repair and hypersensitivity to IR. We also show that IR activates Bub1 to phosphorylate Histone H2A on threonine 121. Further, we demonstrate that ATM-mediated Bub1 serine 314 phosphorylation is required for IR-induced Bub1 activation and for efficient DNA repair. Our findings demonstrate the dual function of Bub1 in the DDR and mitotic pathways.
2. Materials and methods
2.1 Cell lines and cell culture
The human cervical cancer cell line HeLa (obtained from The American Type Culture Collection, Manassas, VA) and the SV-40 transformed human fibroblast cell lines GM00637 and GM09607 (both were obtained from the NIGMS Human Mutant Cell Repository, Camden, NJ) were used in this study. HeLa was cultured in Dulbecco's modified Eagle's medium (DMEM) (Hyclone) with 10% fetal bovine serum (Hyclone, Logan, UT). Fibroblast cell line were cultured in RPMI-1640 medium (Hyclone) with 15% fetal bovine serum (Hyclone). All the cell lines were grown in a 5%, CO2 incubator at 37°C.
2.2 Plasmids and antibodies
To make Flag-tagged Bub1 plasmids, full length Bub1 coding sequences were obtained by RT-PCR and subcloned to the vector pCDNA3.1. The primers are: 5′-ACTGGATCC ATGGACACCCCGGAAAATGTCCTT-3′ and 5′-AGTCTCGAG TTTTC GTGAACGCTTACATTCTAAGAGC-3′. The S314A mutant was generated using the QuikChange II XL site-directed Mutagenesis kit. The primers are: 5′- GATCTGCCCGCTGCTCAGGAAAGGTCCGAGGTTAATCCAGCAC -3′ and 5′- GTG CTG GAT TAA CCT CGG ACC TTT CCT GAG CAG CGG GCA GAT C-3′. The rabbit phosphor-Bub-S314 antibody was raised against peptide KLHQVVESTSHEDLPA(pS)QERSNH2 by EzBioLab (Westfield, IN). Commercial antibodies were obtained as follows: Rabbit anti-H2A-T121 p antibody from Assay BioTech (Sunnyvale,CA), rabbit anti-H2A antibody from Cell Signaling (Danvers, MA) and mouse anti-Bub1 from Abcam (Cambridge, MA).
2.3 In vitro kinase assay
GST-ATM-N (N-terminal, a.a. 248-522) and GST-ATM-C (C-terminal, a.a. 2709-2964) purified from E.coli were incubated with un-phosphorylated Bub1 peptides in the kinase buffer (25 mM Tris-Hcl (pH7.5), 5 mM β-glycerophosphate, 2 mM dithiothreitol, 0.1 mM Na3VO4,10 mM MgCl2, 10 mM Mn2Cl2) with 5 mM ATP for 1 h at 30oC. The samples were boiled and fractionated on SDS-PAGE and subjected to Coomassie Blue staining and Western blot analysis using the anti-phospho-Serine 314 Bub1 antibody.
2.4 Western blot analysis
Cell lysates were obtained by treatment with the lysis buffer (Fisher, Pittsburgh, PA) containing the protease inhibitor cocktail (Roche, Indianapolis, IN), and the protein concentration was determined using DC kit (Bio-Rad, Hercules, CA). Equal volumes of cell lysates were loaded into 4-12% Bis-Tris precast gels (Bio-Rad) for electrophoresis. Proteins were then transferred from gels to the nitrocellulose membrane. Following incubation with 5% of non-fat milk (LabScientific, Livingston, NJ) for 30 minutes, the membrane was incubated with primary and horseradish-peroxidase conjugated secondary antibodies for overnight or 2h. Signals were detected by adding chemiluminescence reagents.
2.5 siRNA and plasmid transfection
The control siRNA for Bub1 knock-down was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and the two Bub1 siRNAs (Bub1-5si,CGAAGAGUGAUCACGAUUU; Bub1-6si, CAAAGAAGGGUGUAAACA) were purchased from Thermo Scientific (Rockford, IL). siRNAs were transfected into cells by oligofectamine reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instruction and 20nM siRNAs were used in each experiment. For flag-tagged Bub1 transfection, plasmids were transfected into cells by FuGENE® HD Transfection Reagent (Roche) according to the manufacturer's instruction and 1.5μg/well of plasmid was used per 6-well plate.
2.6 Cell proliferation assay and cell surviving assay
For the cell proliferation assay: following RNA interference, HeLa cells were seeded into 96-well plates a day before treatment. Cells were then treated with mock of IR (4Gy). Cell proliferation was detected by CellTiter 96@ AQueous One Solution Cell Proliferation Assay system (Promega, Madison, WI) at indicated time points. According to the manufacturer's instructions, 20μl of One Solution reagent per well was loaded in a 96-well plate and incubated for 2 hours. Cell proliferation was measured using absorbance at 490 nm of microplate reader (BioRad Model 680). For the colony formation assay, exponentially growing cells (control cells and Bub1 depleted cells) were plated into 6-well plates in quadruplicates. The plates were subjected to irradiation at indicated doses. 10-14 days later, cells were fixed for 30 min in formaldehyde (3.7%) and stained with crystal violet (0.1%) for 30 min. Colonies were then scored. Only colonies with 50 or more cells were counted as surviving colonies. The plating efficiency was calculated by dividing the average number of colonies per dish by the amount of cell plated. Survival fractions were calculated by normalization to the plating efficiency of appropriate control groups.
2.7 Immunofluorescence
Cells grown on microscope cover glasses (Fisher) were fixed with 4% paraformaldehyde for 15 min, followed by permeabilization in 0.5% Triton X-100 for 15 min. After blocking with 5% BSA for 30min, cells were incubated with primary antibodies and fluorescent labeled secondary antibodies for 1h each,. Cells were washed with 0.1% PBS-T three times between incubations. For nuclear visibility, cellular DNA was stained with DAPI (1mg/ml) diluted in 1×PBS with a ratio of 1:2000 for 2 min. Dilutions used for primary antibodies were 1:200 for rabbit phosphor-Bub1 S314 and 1:500 for mouse gamma-H2AX.
2.8 Single-cell gel electrophoresis assay (Comet assay)
Neutral Comet assays were performed using the Comet Assay kits purchased from Trevigen, Inc (Gaithersburg, MD). Each kit includes Lysis Solution, Comet LMAgarose,CometSlide and SYBR®Green. Mock-treated and 6Gy IR-treated cells were harvested by trypsin and washed with 1×PBS at indicated time points after IR. 500 cells from each time point were mixed with low-melting-point agarose and dropped into CometSlides. Slides were incubated with the lysis solution for 1h, washed twice in the electrophoresis buffer and electrophoresed at 25V for 45min. The DNA was then stained with SYBR®Green and visualized using a fluorescent microscope with 20×lens. Olive Tail Moment was quantified using the CASP software.
2.9 Statistics
Data were analyzed by Student's t test and p values ≤ 0.05 were considered significant.
3. Results
3.1 Bub1 depletion results in suboptimal DNA damage responses and hyperradiosensitivity
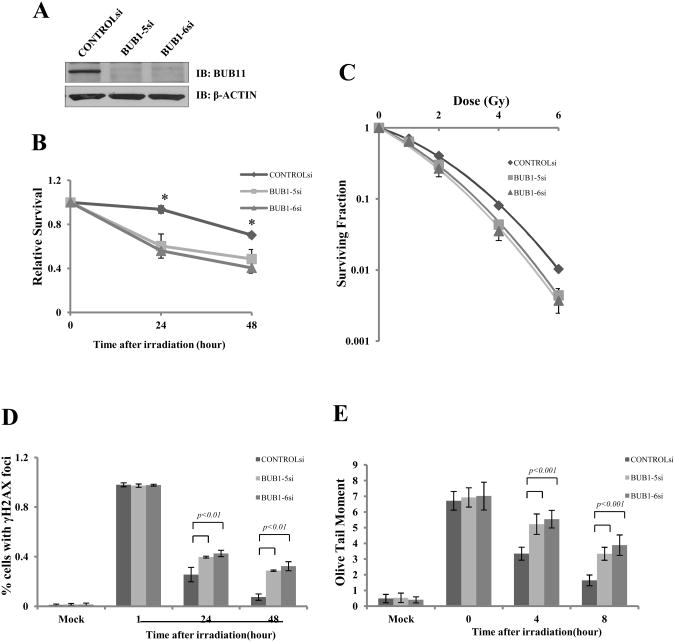
To explore the role of Bub1 in the DNA damage response, we knocked down Bub1 in HeLa cells by two independent siRNA oligos (Fig. 1A). We first examined the sensitivity of Bub1-depleted cells to IR treatment. Initial experiments were conducted using the MTT-based cell proliferation assay. We found that, compared with control siRNA transfected cells, Bub1 knock-down cells showed a significantly decreased proliferation rate 24 and 48h after IR (Fig. 1B, P=0.001), indicating that Bub1-depleted cells are more sensitive to IR treatment. This phenotype was confirmed by the colony formation assay (Fig. 1C). We found that Bub1 knock-down cells showed statistically significant lowered surviving fractions (Supplemental Table 1). The mean lethal dose (D0) for Bub1-si5 and Bub1-si6 is 1.7Gy and 1.6Gy, respectively, as compared to 2.1Gy for the control cells. These results indicate that Bub1 plays an important role in the cellular response to IR-induced DNA damage.
Fig.1. Bub1 siRNA knock-down resulted in defects in the DNA damage response.
HeLa cells were transiently transfected with a control siRNA or two independent siRNA oligos (Bub1 si5 and si6) against Bub1 and the following experiments were conducted: (A) Total cell lysates were harvested 36 hours after transfection followed by immunoblotting with the indicated antibodies. (B) Cells were treated with 4Gy of IR. At 24 or 48 hours after IR, cells were stained with the MTT-based proliferation assay. Shown are the averages of relative survival rates, and * indicates statistical significance (P=0.001, T-test). (C) Cells were treated with indicated doses of IR and colony formation assays were conducted. Shown are the averages of at least triplicate samples. Standard errors are shown by error bars. (D) Cells were plated onto cover-slips and after IR cells were fixed and stained with the anti-Histone λ-H2AXantibody followed by immunofluorescence microscopy. Nuclear H2AX foci were counted and shown are the averages of 100 cells. Standard errors are shown by error bars. Statistical analyses were conducted using T-test. (E) The Single Cell Gel Electrophoresis assay was conducted and Olive Tail Moment was recorded. Shown are the averages of 100 cells. Standard errors are shown by error bars.
To investigate how Bub1 was involved in DDR, we analyzed the level of γ-H2AX focus formation (a molecular marker of DNA DSBs) in Bub1 knock-down cells by immunofluorescence microscopy. We observed a prolonged existence of H2AX foci in Bub1-depleted cells 48 hours after IR (Fig. 1D and Supplemental Fig. S1, p<0.01) while the foci had already returned to normal levels in the control cells. The retention of γ-H2AX foci in Bub1-depleted cells suggests a delay of DNA damage repair in the absence of Bub1. To further investigate this, we performed the single cell gel electrophoresis assay (the Comet assay) in cells transfected with control or Bub1 siRNAs (Supplemental Fig. S2). We found that the Olive tail moment was significantly higher in Bub1-knockdown cells at 4 and 8 hours after IR (Fig. 1E, P<0.001), indicating a significant delay of IR-induced DNA damage repair. Together, our data demonstrate a critical role of Bub1 in regulation of efficient DNA repair and radiosensitivity.
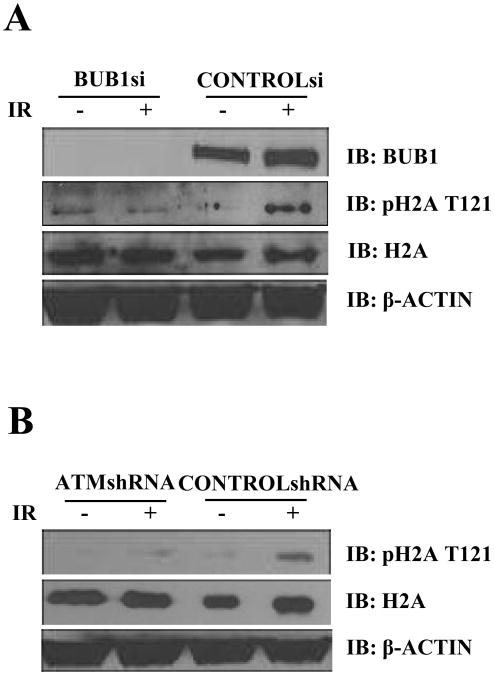
3.2 IR induces Bub1-mediated Histone H2A threonine 121 phosphorylation in an ATM-dependent manner
Bub1 is a kinetochore kinase required for SAC. However, we found that the localization of Bub1 was not changed after IR (Supplemental Fig. S3). Although there has been in vitro evidence that Bub1 phosphorylates a number of targets in the mitotic spindle checkpoint, it was not until recently that a bona fide substrate was identified in vivo as Histone H2A threonine 120 (in our system, the Threonine is numbered as 121 to include the first methionine in order to be consistent with other amino acid numbering in this study). With a phospho-specific antibody, we observed that IR induced Histone H2A Thr121 phosphorylation (Fig. 2A). Further, we showed that this IR-induced Thr121 phosphorylation was mediated by Bub1 as Bub1 knock-down cells displayed a defective Thr121 phosphorylation in response to IR (Fig. 2A). To test whether this IR-induced and Bub1-mediated Histone H2A modification was regulated by ATM, we utilized a pair of isogenic cell lines with stable expression of control shRNA or ATM shRNA. These cell lines have been well characterized in our recent studies [13]. We found that in the absence of ATM, Bub1-mediated H2A Thr121 phosphorylation became defective (Fig. 2B), indicating that ATM is required for Bub1 phosphorylation of H2A Thr120.
Fig.2. Histone H2A threonine 121 phosphorylation is induced in response to IR-induced DNA damage.
(A) HeLa cells transfected with control or Bub1 siRNA were treated with mock or IR (4Gy). Total cell lysates were collected followed by immunoblotting using indicated antibodies. (B) HeLa cells stably transfected with control shRNA or ATM shRNA were treated with mock or IR (4Gy). Total cell lysates were collected followed by immunoblotting using indicated antibodies.
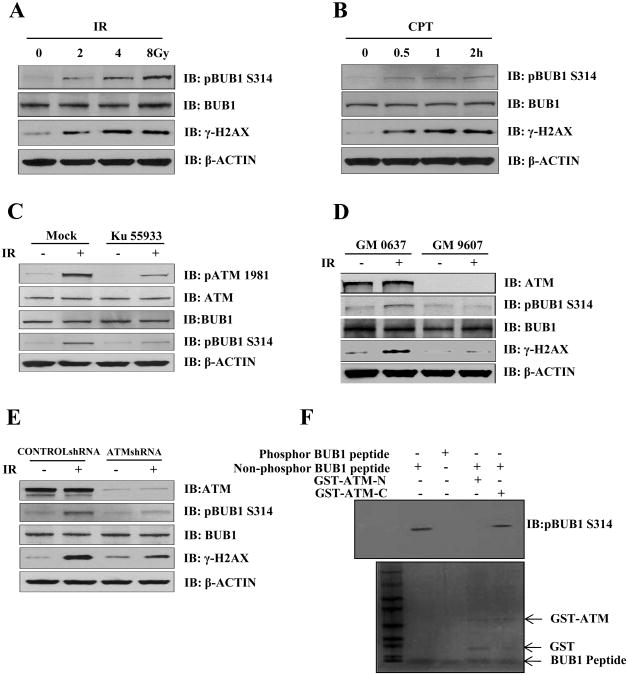
3.3 ATM phosphorylates Bub1 on serine 314 in response to IR
Bub1 was among a few mitotic checkpoint proteins identified as ATM/ATR substrates in response to IR by a large scale proteomic study [6]. To validate ATM-mediated Bub1 phosphorylation (on serine 314) in response to DNA damage, we utilized a phospho-specific antibody we developed in our laboratory to investigate Bub1 phosphorylation in mitosis [13] in cells treated with IR or the chemotherapeutic drug camptothecin (CPT). We found that IR induced Bub1 Ser314 phosphorylation in a dose-dependent manner (Fig. 3A). A similar phenotype was observed in cells treated with CPT (Fig. 3B). Additionally, Bub1 Ser314 phosphorylation in response to IR was abrogated when cells were treated with Ku55933, a specific inhibitor of ATM kinase activity [14] (Fig. 3C). Using a pair of SV-40 transformed human fibroblast cell lines with proficient (GM0637) or deficient ATM (GM9607), we showed that Bub1 Ser314 phosphorylation in response to IR is defective in GM9607 cells (Fig. 3D). To further study the role of ATM on Bub1 Ser314 phosphorylation, we transiently knocked down ATM in HeLa cells by siRNA and found that Bub1 Ser314 phosphorylation was diminished when ATM expression was depleted (Fig. 3E). Taken together, we conclude that ATM is required for IR-induced Bub1 serine 314 phosphorylation. To test the possibility of that ATM may directly phosphorylate Bub1 on serine314, we conducted an in vitro kinase assay using a C-terminal ATM fragment which has kinase activity (an N-terminal ATM fragment was used as a negative control). When a non-phospho-peptide corresponding to the Bub1 serine 314 sequence was used as substrate in the kinase assay, we observed a strong phosphorylation signal detected by the anti-phospho-Bub1 Ser314 antibody (Fig. 3F). Therefore we demonstrate in vitro evidence that ATM phosphorylates Bub1 on serine 314. Together with the in vivo phosphorylation data, we conclude that ATM is the primary kinase responsible for IR-induced Bub1 Ser314 phosphorylation.
Fig.3. ATM phosphorylates Bub1 on serine 314 in response to IR.
Total cell lysates were harvested from: (A) HeLa cells treated with mock or IR (4Gy); (B) Camptothecin (CPT); (C) HeLa cells treated with mock or IR (4Gy) in the presence or absence of KU55933; (D) SV-40 transformed fibroblast cells lines GM0637 and GM9607 treated with mock or IR (4Gy); (E) HeLa cells transiently transfected with either control siRNA or ATM siRNA followed by mock or IR treatment. Western blot analyses were conducted using indicated antibodies. and (F) The in vitro kinase assay using ATM fragments (either GST-tagged N-terminal a.a. 248-522 or C-terminal fragments of ATM a.a. 2709-2964) in the presence of non-phosphorylated form of Bub1 peptides. The blot was stained by coomassie blue and immunoblotted with the anti-phospho-Ser314 Bub1 antibody.
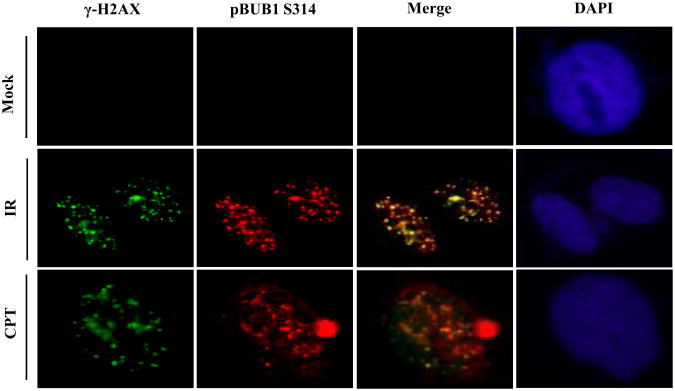
3.4 Bub1 serine 314 phosphorylation forms nuclear foci in response to IR
We further examined the localization of Bub1 Ser314 phosphorylation by immunofluorescence microscopy. We found that unlike total Bub1, Ser314 phosphorylated Bub1 formed nuclear foci in response to DNA damage induced by IR and CPT (Fig. 4). It is interesting to observe that Bub1 Ser314 foci colocalized with λ-H2AX foci in response to DNA damage, indicating a critical role of Bub1 Ser314 phosphorylation in DNA damage recognition and DNA repair. It is noted that the specificity of the antibody was demonstrated that Bub1 knock-down cells showed no Ser314 phosphorylation signals (Supplemental Fig. S4).
Fig.4. Bub1 Serine 314 phosphorylation forms nuclear foci that are colocalized with H2AX foci in response to DNA damage.
Immunofluorescence microcopy was conducted using indicated antibodies in HeLa cells treated with mock, IR or CPT.
3.5 ATM-mediated Bub1 serine 314 phosphorylation is required for Bub1 activation and optimal DNA damage responses
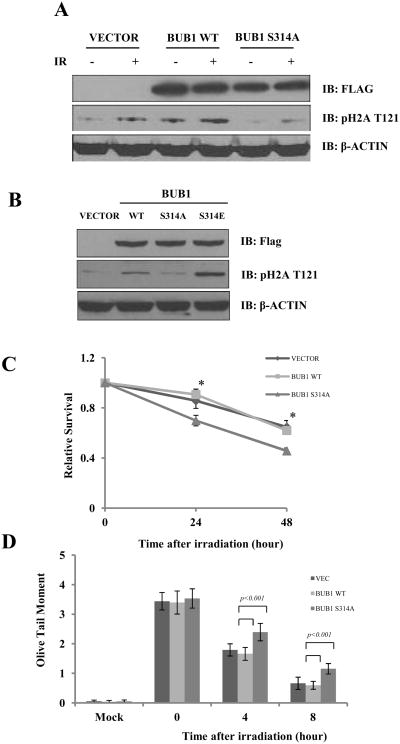
To investigate the functional significance of ATM-mediated Bub1 Ser314 phosphorylation in response to DNA damage, we constructed flag-tagged, wild-type or the Serine 314 to Alanine mutant form of Bub1. Transient expression of the exogenous proteins (Fig. 5A) was achieved. We observed no change of pattern on cellular localization with the S314A mutation (Supplemental Fig. S5). We found that expressing wild-type Bub1 resulted in H2A Thr121 phosphorylation even in the absence of DNA damage and IR further enhanced phosphorylation. However, expression of the S314A mutant Bub1 failed to induce Thr121 phosphorylation in un-irradiated cells and IR-induced H2A Thr121 phosphorylation was significantly reduced, indicating that ATM-mediated Bub1 phosphorylation is essential for Bub1 activation in the DNA damage response. To further study the functional significance of serine 314 phosphorylation, we generated the serine 314 to glutamic acid mutant Bub1 (S314E) which mimics phosphorylation. Interestingly we found that, unlike S314A which abrogated IR-induced Thr121 phosphorylation, S314E increased H2A Thr121 phosphorylation (Fig. 5B). Further, we found that expression of the S314A mutant Bub1 resulted in an increased level of comet tail formation as compared to that of wild-type Bub1 (Fig. 5B, P<0.001). In addition, we found that cells expressing the S314A mutant are hypersensitive to IR (Fig. 5C, P<0.001). Since the phenotypes we observed in cells expressing S314A Bub1 recapitulate the defects in Bub1 knock-down cells, we conclude that ATM-mediated Bub1 serine 314 phosphorylation is critical for the function of Bub1 in the DNA damage response.
Fig. 5. ATM-mediated Bub1 serine 314 phosphorylation is required for optimal DNA damage responses.
(A) HeLa cells were transiently transfected with vector-only, wild-type, or S314A mutant of Bub1 followed by experiments Western blot analysis to detect Histone H2A Thr121 phosphorylation in response IR; (B) HeLa cells transiently transfected with vector-only, wild-type, S314A or S314E mutant of Bub1 followed by immunoblotting with indicated antibodies. (C) The cell survival assay after IR in HeLa cells transfected with vector, wild-type or S314A Bub1; and (D) the single cell gel electrophoresis assay to detect comet tail formation in HeLa cells transfected with vector, wild-type or S314A Bub1.
4. Discussion
Bub1 functions as a tumor suppressor by playing a critical role in proper chromosomal segregation [15]. In this report, we demonstrate that Bub1 is also a critical component for the DNA damage response. We show that Bub1-mediated Histone H2A threonine 121 phosphorylation is induced by IR and this requires ATM-mediated Bub1 serine 314 phosphorylation. There is growing evidence that signaling pathways regulating the DDR intersect with the mitotic spindle checkpoint pathways. In budding yeast, Pds1 is a central protein essential for both the spindle checkpoint and the DNA damage checkpoint [16]. In mammalian cells, a number of DNA damage responsive elements, including Chk1, Chk2, Brca1-Bard1 and Brca2, have been reported to play a role in spindle checkpoint activation [17-21]. We have recently demonstrated that ATM is activated in mitosis and required for the spindle checkpoint [13]. On the other hand, SAC proteins such as Aurora-B [22, 23], BubR1 [24] and Mad2 [25-27] were previously shown to be critical parts of the DNA damage response. For example, Aurora-B is inactivated in response to IR and this inactivation process contributes to the activation of the G2/M checkpoint [22]. Meanwhile, BubR1 interacts with Poly ADP-ribose (PARP1) to regulate the DNA damage induced G2/M checkpoint [24]. Our study presented here adds Bub1 to the growing list of proteins that have dual functions in DNA damage repair and mitosis.
The functional role of Bub1 in DDR is apparently different from the other two mitotic kinases BubR1 and Aurora-B. BubR1 is rapidly degraded upon DNA damage [24], and Aurora-B kinase activity is inhibited in response to IR [22]. On the contrary, Bub1 activity is enhanced in response to IR as shown by the increase of Histone H2A threonine 121 phosphorylation. However, the protein level, as well as the cellular localization, of Bub1 is not altered in response to DNA damage. Therefore, we reason that enzymatic activation of the kinase is the major mechanism regulating Bub1 function in the DNA damage response. In addition, it is expected that a critical role of Bub1 is recruiting DNA repair proteins at the sites of damage, given the fact that Bub1 Ser314 phosphorylation foci co-localize with γ-H2AX foci,
H2A threonine 121 phosphorylation mediated by Bub1 is required for localization of shugoshin to activate the spindle checkpoint [11]. However, the IR-induced H2A Thr121 phosphorylation observed in our study is somehow an unexpected result, because if this is a mitotic-dependent event then we would not have observed an increase of phosphorylation since IR can cause cells to arrest at the G2 phase before mitosis. Therefore, it is likely that the phosphorylation induced by IR has other functions beyond mitosis. Since we have shown that Bub1 is critical for DNA damage repair, the notion that Histone H2A modification by activated Bub1 in response to IR might lead to the recruitment of DNA repair proteins is further supported.
The functional domains of Bub1 include an N-terminal tetratricopeptide repeat (TPR) domain which is required for its kinetochore localization, two docking motifs (KEN boxes) functioning to recruit Cdc20, an N-terminal extension to organize the ATP-binding pocket and activation segment of Bub1, and a C-terminal serine/threonine (S/T) kinase domain required for its enzymatic activity [28]. The ATM-mediated Bub1 phosphorylation happens on serine 314 which is near the N-terminal domain. It has previously been shown that Cdc2-dependent Bub1 phosphorylation is required for mitotic Bub1 activation and the spindle checkpoint [29, 30]. Therefore it is likely that ATM-mediated Bub1 phosphorylation leads to Bub1 activation in response to DNA damage. We have observed that this phosphorylation event is both mitotic [13] and DDR dependent (Fig. 3 and 4). We observed that the Serine314 to Alanine mutation had a dominant negative effect against endogenous Bub1, and the Serine 314 to Glutamic Acid substitution increased H2A Thr121 phosphorylation, indicating that ATM-mediated Bub1 serine 314 phosphorylation regulates Bub1 activity.
The role of Bub1 is not limited to the spindle assembly checkpoint in mitosis [31]. For example, Bub1 is required for accurate chromosome segregation [32, 33]. In addition, there is evidence that Bub1 plays a role in chromosome congression in mammalian cells [34, 35]. Bub1 is also reported as a negative regulator of caspase-independent mitotic death (CIMD), which is a mechanism to prevent aneuploidy induced cell death [36]. The role of Bub1 in DNA damage repair extends its function beyond mitosis.
5. Conclusions
In summary, our study reveals a novel function of Bub1 in the DNA damage response. Bub1 is phosphorylated by ATM on serine 314 and the phosphorylation is essential for IR-induced Bub1 phosphorylation of H2AX Thr121 and for optimal DNA repair.
Supplementary Material
Supplemental Figure S1. HeLa cells transfected with control or Bub1 siRNA were irradiated with 0 (Mock) or 4 Gy (IR), and harvested at indicated points before immunofluorescence microscopy was used to detect radiation-induced γ-H2AX foci.
Supplemental Figure S2. The single cell electrophoresis assay in HeLa cells transfected with control or Bub1 siRNAs.
Supplemental Figure S3. Localization of Bub1 in the absence or presence of IR-induced DNA damage. HeLa cells were treated with mock or IR (4Gy). 2h after treatment cells were stained with indicated antibodies and images from immunofluorescence microscopy are shown.
Supplemental Figure S4. HeLa cells transfected with siRNA-Bub1 were treated with mock, IR or CPT. 2h after treatment cells were stained with indicated antibodies and images from immunofluorescence microscopy are shown.
Supplemental Figure S5. Localization of S314A Bub1 in the absence and presence of IR-induced DNA damage. HeLa cells transfected with vector or flag-tagged wild-type or S314A Bub1 were treated with mock or IR (4Gy). 2h after treatment cells were stained with indicated antibodies and images from immunofluorescence microscopy are shown.
Supplemental Table S1. Statistical analysis of surviving fractions
Highlights.
Bub1 is required for the DNA damage response;
ATM phosphorylates Bub1 on Serine 214 in response to ionizing irradiation;
ATM-mediated Bub1 phosphorylation is essential for optimal DNA damage responses.
Acknowledgments
We thank everybody in the Xu laboratory for their help. The immunofluorescence microscopy was performed at the TMHRI Advanced Cellular and Tissue Microscope Core Facility. This work is supported by NIH grants R01CA133093 and R01ES016354.
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bartek J, Bartkova J, Lukas J. DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene. 2007;26:7773–7779. doi: 10.1038/sj.onc.1210881. [DOI] [PubMed] [Google Scholar]
- 2.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iliakis G, Wang Y, Guan J, Wang H. DNA damage checkpoint control in cells exposed to ionizing radiation. Oncogene. 2003;22:5834–5847. doi: 10.1038/sj.onc.1206682. [DOI] [PubMed] [Google Scholar]
- 4.Shiloh Y. ATM and ATR: networking cellular responses to DNA damage. Curr Opin Genet Dev. 2001;11:71–77. doi: 10.1016/s0959-437x(00)00159-3. [DOI] [PubMed] [Google Scholar]
- 5.Kastan MB, Lim DS. The many substrates and functions of ATM. Nat Rev Mol Cell Biol. 2000;1:179–186. doi: 10.1038/35043058. [DOI] [PubMed] [Google Scholar]
- 6.Matsuoka S, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 7.Wassmann K, Benezra R. Mitotic checkpoints: from yeast to cancer. Curr Opin Genet Dev. 2001;11:83–90. doi: 10.1016/s0959-437x(00)00161-1. [DOI] [PubMed] [Google Scholar]
- 8.Williams GL, Roberts TM, Gjoerup OV. Bub1: escapades in a cellular world. Cell Cycle. 2007;6:1699–1704. doi: 10.4161/cc.6.14.4493. [DOI] [PubMed] [Google Scholar]
- 9.Marchetti F, Venkatachalam S. The multiple roles of Bub1 in chromosome segregation during mitosis and meiosis. Cell Cycle. 2010;9:58–63. doi: 10.4161/cc.9.1.10348. [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Tang Z. Bub1 multitasking in mitosis. Cell Cycle. 2005;4:262–265. [PubMed] [Google Scholar]
- 11.Kawashima SA, Yamagishi Y, Honda T, Ishiguro K, Watanabe Y. Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science. 2010;327:172–177. doi: 10.1126/science.1180189. [DOI] [PubMed] [Google Scholar]
- 12.Tang Z, Shu H, Oncel D, Chen S, Yu H. Phosphorylation of Cdc20 by Bub1 provides a catalytic mechanism for APC/C inhibition by the spindle checkpoint. Mol Cell. 2004;16:387–397. doi: 10.1016/j.molcel.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 13.Yang C, Tang X, Guo X, Niikura Y, Kitagawa K, Cui K, Wong STC, Fu L, Xu B. Aurora-B Mediated ATM Serine 1403 Phosphorylation Is Required For Mitotic ATM Activation and the Spindle Checkpoint. Molecular Cell. 2011 Nov 18; doi: 10.1016/j.molcel.2011.09.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hickson I, et al. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 2004;64:9152–9159. doi: 10.1158/0008-5472.CAN-04-2727. [DOI] [PubMed] [Google Scholar]
- 15.Malumbres M, Barbacid M. Cell cycle kinases in cancer. Curr Opin Genet Dev. 2007;17:60–65. doi: 10.1016/j.gde.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Fasullo M, Sun M. The Saccharomyces cerevisiae checkpoint genes RAD9, CHK1 and PDS1 are required for elevated homologous recombination in a mec1 (ATR) hypomorphic mutant. Cell Cycle. 2008;7:2418–2426. doi: 10.4161/cc.6411. [DOI] [PubMed] [Google Scholar]
- 17.Zachos G, et al. Chk1 is required for spindle checkpoint function. Dev Cell. 2007;12:247–260. doi: 10.1016/j.devcel.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stolz A, et al. The CHK2-BRCA1 tumour suppressor pathway ensures chromosomal stability in human somatic cells. Nat Cell Biol. 2010;12:492–499. doi: 10.1038/ncb2051. [DOI] [PubMed] [Google Scholar]
- 19.Joukov V, et al. The BRCA1/BARD1 heterodimer modulates ran-dependent mitotic spindle assembly. Cell. 2006;127:539–552. doi: 10.1016/j.cell.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 20.Marmorstein LY, et al. A human BRCA2 complex containing a structural DNA binding component influences cell cycle progression. Cell. 2001;104:247–257. doi: 10.1016/s0092-8674(01)00209-4. [DOI] [PubMed] [Google Scholar]
- 21.Ayoub N, et al. The carboxyl terminus of Brca2 links the disassembly of Rad51 complexes to mitotic entry. Curr Biol. 2009;19:1075–1085. doi: 10.1016/j.cub.2009.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang X, et al. A novel ATM-dependent pathway regulates protein phosphatase 1 in response to DNA damage. Mol Cell Biol. 2008;28:2559–2566. doi: 10.1128/MCB.01711-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monaco L, et al. Inhibition of Aurora-B kinase activity by poly(ADP-ribosyl)ation in response to DNA damage. Proc Natl Acad Sci U S A. 2005;102:14244–14248. doi: 10.1073/pnas.0506252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang Y, et al. BubR1 is involved in regulation of DNA damage responses. Oncogene. 2006;25:3598–3605. doi: 10.1038/sj.onc.1209392. [DOI] [PubMed] [Google Scholar]
- 25.Mikhailov A, Cole RW, Rieder CL. DNA damage during mitosis in human cells delays the metaphase/anaphase transition via the spindle-assembly checkpoint. Curr Biol. 2002;12:1797–1806. doi: 10.1016/s0960-9822(02)01226-5. [DOI] [PubMed] [Google Scholar]
- 26.Garber PM, Rine J. Overlapping roles of the spindle assembly and DNA damage checkpoints in the cell-cycle response to altered chromosomes in Saccharomyces cerevisiae. Genetics. 2002;161:521–534. doi: 10.1093/genetics/161.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fung MK, et al. MAD2 interacts with DNA repair proteins and negatively regulates DNA damage repair. J Mol Biol. 2008;381:24–34. doi: 10.1016/j.jmb.2008.05.080. [DOI] [PubMed] [Google Scholar]
- 28.Kang J, et al. Structure and substrate recruitment of the human spindle checkpoint kinase Bub1. Mol Cell. 2008;32:394–405. doi: 10.1016/j.molcel.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen RH. Phosphorylation and activation of Bub1 on unattached chromosomes facilitate the spindle checkpoint. EMBO J. 2004;23:3113–3121. doi: 10.1038/sj.emboj.7600308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaguchi S, Decottignies A, Nurse P. Function of Cdc2p-dependent Bub1p phosphorylation and Bub1p kinase activity in the mitotic and meiotic spindle checkpoint. EMBO J. 2003;22:1075–1087. doi: 10.1093/emboj/cdg100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyarchuk Y, Salic A, Dasso M, Arnaoutov A. Bub1 is essential for assembly of the functional inner centromere. J Cell Biol. 2007;176:919–928. doi: 10.1083/jcb.200609044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warren CD, et al. Distinct chromosome segregation roles for spindle checkpoint proteins. Mol Biol Cell. 2002;13:3029–3041. doi: 10.1091/mbc.E02-04-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanoosthuyse V, Valsdottir R, Javerzat JP, Hardwick KG. Kinetochore targeting of fission yeast Mad and Bub proteins is essential for spindle checkpoint function but not for all chromosome segregation roles of Bub1p. Mol Cell Biol. 2004;24:9786–9801. doi: 10.1128/MCB.24.22.9786-9801.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson VL, Scott MI, Holt SV, Hussein D, Taylor SS. Bub1 is required for kinetochore localization of BubR1, Cenp-E, Cenp-F and Mad2, and chromosome congression. J Cell Sci. 2004;117:1577–1589. doi: 10.1242/jcs.01006. [DOI] [PubMed] [Google Scholar]
- 35.Meraldi P, Sorger PK. A dual role for Bub1 in the spindle checkpoint and chromosome congression. EMBO J. 2005;24:1621–1633. doi: 10.1038/sj.emboj.7600641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niikura Y, Dixit A, Scott R, Perkins G, Kitagawa K. BUB1 mediation of caspase-independent mitotic death determines cell fate. J Cell Biol. 2007;178:283–296. doi: 10.1083/jcb.200702134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. HeLa cells transfected with control or Bub1 siRNA were irradiated with 0 (Mock) or 4 Gy (IR), and harvested at indicated points before immunofluorescence microscopy was used to detect radiation-induced γ-H2AX foci.
Supplemental Figure S2. The single cell electrophoresis assay in HeLa cells transfected with control or Bub1 siRNAs.
Supplemental Figure S3. Localization of Bub1 in the absence or presence of IR-induced DNA damage. HeLa cells were treated with mock or IR (4Gy). 2h after treatment cells were stained with indicated antibodies and images from immunofluorescence microscopy are shown.
Supplemental Figure S4. HeLa cells transfected with siRNA-Bub1 were treated with mock, IR or CPT. 2h after treatment cells were stained with indicated antibodies and images from immunofluorescence microscopy are shown.
Supplemental Figure S5. Localization of S314A Bub1 in the absence and presence of IR-induced DNA damage. HeLa cells transfected with vector or flag-tagged wild-type or S314A Bub1 were treated with mock or IR (4Gy). 2h after treatment cells were stained with indicated antibodies and images from immunofluorescence microscopy are shown.
Supplemental Table S1. Statistical analysis of surviving fractions