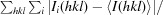The crystallization and preliminary X-ray analysis of the full-length amylomaltase from C. glutamicum are reported.
Keywords: amylomaltase, Corynebacterium glutamicum
Abstract
Amylomaltase (AM; EC 2.4.1.25) belongs to the 4-α-glucanotransferase group of the α-amylase family. The enzyme can produce cycloamylose or large-ring cyclodextrin through intramolecular transglycosylation or cyclization reactions of α-1,4-glucan. Amylomaltase from the mesophilic bacterium Corynebacterium glutamicum (CgAM) contains extra residues at the N-terminus for which the three-dimensional structure is not yet known. In this study, CgAM was overexpressed and purified to homogeneity using DEAE FF and Phenyl FF columns. The purified CgAM was crystallized by the vapour-diffusion method. Preliminary X-ray data showed that the CgAM crystal diffracted to 1.7 Å resolution and belonged to space group P212121, with unit-cell parameters a = 73.28, b = 82.61, c = 118.64 Å. To obtain the initial phases, crystals of selenomethionyl-substituted amylomaltase were produced, and multiple-wavelength anomalous dispersion phasing and structure refinement are now in progress.
1. Introduction
Amylomaltase (EC 2.4.1.25) is a member of the 4-α-glucanotransferase (4αGTase) group of the α-amylase family. Amylomaltase catalyses the hydrolysis and transfer of α-1,4-glucan units to another linear oligosaccharide: the so-called intermolecular transglycosylation or disproportionation reaction. The enzyme also catalyses a unique intramolecular transglycosylation reaction or cyclization reaction within a single α-d-glucan molecule, producing cycloamylose (CA) or large-ring cyclodextrin (LR-CD), a cyclic α-1,4-glucan with a degree of polymerization (DP) of 16 or higher (Takaha & Smith, 1999 ▶). LR-CDs can form inclusion complexes with various guest molecules and potentially improve the properties of guest molecules such as their solubility, stability and reactivity (Zheng et al., 2002 ▶; Tomono et al., 2002 ▶).
Amylomaltases have been found in microorganisms as well as in plants, where they are known as disproportionating enzymes (D-enzymes; Takaha et al., 1996 ▶; Kakefuda & Duke, 1989 ▶; Lin & Preiss, 1988 ▶). In microorganisms, amylomaltase was first identified in Escherichia coli as a maltose-inducible enzyme (Monod & Torriani, 1950 ▶). Later, amylomaltases from many archaea and bacterial strains, including from Corynebacterium glutamicum, were characterized (Kaper et al., 2005 ▶, 2007 ▶; Terada et al., 1999 ▶; Godány et al., 2008 ▶; Srisimarat et al., 2011 ▶). To date, crystal structures of amylomaltase from Thermus aquaticus (PDB entry 1cwy; Przylas, Tomoo et al., 2000 ▶), T. thermophilus (PDB entry 2owx; Barends et al., 2007 ▶), T. brockianus (PDB entry 2x1i; Jung et al., 2011 ▶), Aquifex aeolicus (PDB entry 1tz7; T. R. M. Barends, H. Korf, T. Kaper, M. J. E. C. van der Maarel, L. Dijkhuizen & B. W. Dijkstra, unpublished work), Thermotoga maritima (PDB entry 1lwh; Roujeinikova et al., 2002 ▶) and Solanum tuberosum (PDB entry 1x1n; Imamura et al., 2005 ▶) have been determined. However, the amylomaltase from C. glutamicum (CgAM) showed low amino-acid sequence similarity (28–32%) to these amylomaltases. CgAM showed a different LR-CD production profile from that of the well characterized T. aquaticus enzyme (Terada et al., 1999 ▶; Srisimarat et al., 2011 ▶). CgAM gave CA with a DP of 19 and higher, while T. aquaticus amylomaltase produced CD22 as the smallest product. In addition, the LR-CD production profile of CgAM depended on the incubation time and the enzyme concentration. The X-ray crystal structure of T. aquaticus amylomaltase illustrated the well conserved catalytic site along with another substrate-binding site, the so-called ‘second binding site’. This second binding site was proposed to play a role in LR-CD formation through the hydrophobic interaction of Tyr54 and Tyr101 with substrate. The mutation of these residues altered the hydrolysis activity of T. aquaticus amylomaltase, resulting in changes in the cyclization activity of the enzyme (Fujii et al., 2005 ▶, 2007 ▶). In addition, a recent study of the Y172A mutant, CgAM with an alanine substitution at Tyr172, which corresponds to Tyr54 in T. aquaticus amylomaltase, also suggested that Tyr172 of CgAM plays an important role in determination of the LR-CD production profile (Srisimarat et al., 2012 ▶).
To date, no crystal structure of CgAM has been reported. Multiple amino-acid sequence alignment of CgAM and other thermostable amylomaltases showed that CgAM contains an extra N-terminal region of approximately 240 amino acids that does not share sequence similarity with other enzymes (Srisimarat et al., 2011 ▶). In order to identify the function of this region and understand the basis of the cyclization mechanism of CgAM, the crystallization and preliminary X-ray analysis of full-length CgAM (residues 1–706) are reported in this study.
2. Materials and methods
2.1. Expression and purification
The ORF of CgAM (GenBank BAB99690.1) has 2121 bp and was deduced to encode 706 amino-acid residues (Fig. 1 ▶). Full-length CgAM was overexpressed and purified as described previously (Srisimarat et al., 2012 ▶). In brief, E. coli BL21 (DE3) cells harbouring the pET-CgAM plasmid were cultured in Luria–Bertani medium containing 100 µg ml−1 ampicillin at 310 K and expression of CgAM was induced by the addition of β-d-1-thiogalactopyranoside (IPTG) to a final concentration of 0.4 mM. After 2 h, the cultured cells were harvested and disrupted by sonication (Bandelin, Germany). Cell debris was removed by centrifugation and the supernatant was applied onto DEAE FF and Phenyl FF (GE Healthcare, UK). The protein fractions containing amylomaltase activity were then collected. The purity of the protein was determined by 7.5% SDS–PAGE.
Figure 1.
The deduced amino-acid sequence of amylomaltase from C. glutamicum ATCC 13032.
2.2. Crystallization
The purified protein was dialysed in 10 mM phosphate buffer pH 6.0 and concentrated to 5 mg ml−1. The concentration of protein was measured using the BCA Protein Assay Kit (Pierce, USA). Crystallization experiments were carried out at 291 K using the vapour-diffusion method. Crystallization conditions were screened using the Index screen (Hampton Research, USA) in sitting droplets consisting of 0.5 µl protein solution and 0.5 µl precipitant solution in a 96-well plate. After a week, small crystals appeared in a droplet containing precipitant solution consisting of 0.1 M bis-tris pH 5.5, 2.0 M ammonium sulfate. Diffraction-quality crystals were produced in a VDX plate (Hampton Research, USA) using the hanging-drop vapour-diffusion method.
2.3. Data collection and processing
Prior to data collection, the crystal was immersed in reservoir solution supplemented with 30%(v/v) xylitol for a few seconds and was then flash-cooled in liquid nitrogen. X-ray diffraction data were collected from the crystal using an ADSC Quantum 315r CCD area detector on BL13B1 at the National Synchrotron Radiation Research Center (NSRRC), Taiwan. Data were processed using the HKL-2000 software package (Otwinowski & Minor, 1997 ▶).
3. Results and discussion
Recombinant CgAM was overexpressed and purified using DEAE and Phenyl FF column chromatography. Purified CgAM shows a single band on SDS–PAGE with an apparent molecular mass of 81 kDa. Crystals suitable for data collection were produced as described in §2. The crystal grew to maximum dimensions of 0.2 × 0.4 × 0.1 mm (Fig. 2 ▶) and diffracted to 1.7 Å resolution at a synchrotron source. The crystal belonged to space group P212121, with unit-cell parameters a = 73.28, b = 82.61, c = 118.64 Å. The diffraction data set has a resolution range of 50–1.7 Å with 97.3% completeness and an R merge of 5.5%. The Matthews coefficient was calculated to be about 2.26 Å3 Da−1, corresponding to 46% solvent content (Matthews, 1968 ▶; Kantardjieff & Rupp, 2003 ▶). Diffraction statistics are summarized in Table 1 ▶.
Figure 2.
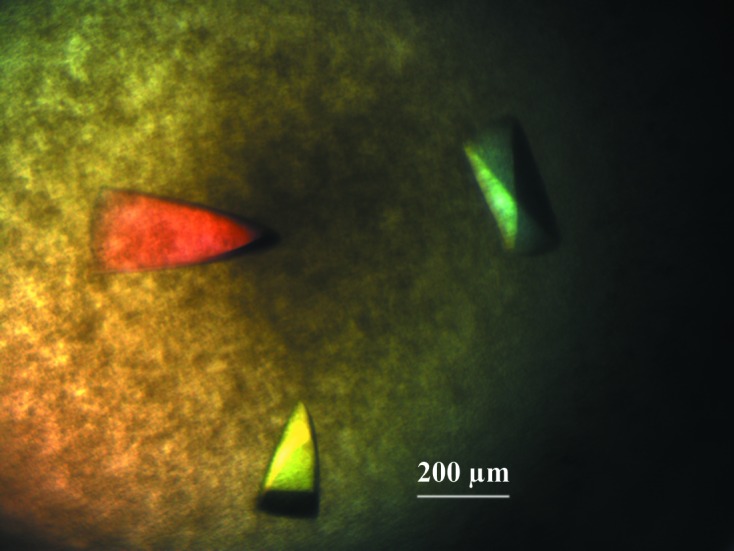
Crystals of CgAM with approximate dimensions of 0.2 × 0.4 × 0.1 mm.
Table 1. X-ray diffraction data and processing statistics.
Values in parentheses are for the outermost resolution shell.
| Space group | P212121 |
| Unit-cell parameters (Å, °) | a = 73.28, b = 82.61, c = 118.64, α = 90.0, β = 90.0, γ = 90.0 |
| Volume of the unit cell (Å3) | 718272.3 |
| Solvent content (%) | 46 |
| Matthews coefficient† (Å3 Da−1) | 2.26 |
| Resolution range (Å) | 50.0–1.70 (1.76–1.70) |
| Total No. of reflections | 869461 |
| No. of unique reflections | 77144 |
| Completeness (%) | 97.3 (94.2) |
| Average I/σ(I) | 36.0 (6.2) |
| R merge ‡ (%) | 5.5 (31.2) |
| Average multiplicity | 11.3 (11.5) |
Attempts were made to solve the crystal structure of CgAM using the molecular-replacement method. The structures of amylomaltases from PDB entries 1esw (28% sequence similarity; Przylas, Terada et al., 2000 ▶) and 1tz7 (32% sequence similarity; T. R. M. Barends, H. Korf, T. Kaper, M. J. E. C. van der Maarel, L. Dijkhuizen & B. W. Dijkstra, unpublished work) were used as search models in MOLREP (Winn et al., 2011 ▶). However, this method was not successful. This is probably because of conformational differences or low amino-acid sequence similarity. Since CgAM has a high methionine frequency (15 methionine residues in 706 residues), it should be possible to obtain experimental phases by the multi-wavelength anomalous dispersion (MAD) method using selenomethionine-incorporated crystals. As a result, selenomethionine-incorporated CgAM crystals were grown and used for MAD data collection. At present, phase determination and structure refinement of CgAM are in progress.
Acknowledgments
This work was supported by the Integrated Innovation Academic Center: Chulalongkorn University Centenary Academic Development Project and the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission (FW650B to PP and FW657B to KK). WS received a Postdoctoral Fellowship under the Ratchadaphiseksomphot Endowment Fund of Chulalongkorn University. The authors acknowledge the instrument support from the Thai Government Stimulus Package 2 (TKK 2555) under the Project PERFECTA. We are grateful to the supporting staff at NSRRC (Taiwan) for technical assistance in X-ray data collection at BL13B1. We thank Dr Krupa O’Neill for editing the manuscript.
References
- Barends, T. R. M., Bultema, J. B., Kaper, T., van der Maarel, M. J. E. C., Dijkhuizen, L. & Dijkstra, B. W. (2007). J. Biol. Chem. 282, 17242–17249. [DOI] [PubMed]
- Diederichs, K. & Karplus, P. A. (1997). Nature Struct. Biol. 4, 269–275. [DOI] [PubMed]
- Fujii, K., Minagawa, H., Terada, Y., Takaha, T., Kuriki, T., Shimada, J. & Kaneko, H. (2005). Appl. Environ. Microbiol. 71, 5823–5827. [DOI] [PMC free article] [PubMed]
- Fujii, K., Minagawa, H., Terada, Y., Takaha, T., Kuriki, T., Shimada, J. & Kaneko, H. (2007). J. Biosci. Bioeng. 103, 167–173. [DOI] [PubMed]
- Godány, A., Vidová, B. & Janecek, S. (2008). FEMS Microbiol. Lett. 284, 84–91. [DOI] [PubMed]
- Imamura, K., Matsuura, T., Ye, Z., Takaha, T., Fujii, K., Kusunoki, M. & Nitta, Y. (2005). Acta Cryst. F61, 109–111. [DOI] [PMC free article] [PubMed]
- Jung, J.-H., Jung, T.-Y., Seo, D.-H., Yoon, S.-M., Choi, H.-C., Park, B. C., Park, C.-S. & Woo, E.-J. (2011). Proteins, 79, 633–644. [DOI] [PubMed]
- Kakefuda, G. & Duke, S. H. (1989). Plant Physiol. 91, 136–143. [DOI] [PMC free article] [PubMed]
- Kantardjieff, K. A. & Rupp, B. (2003). Protein Sci. 12, 1865–1871. [DOI] [PMC free article] [PubMed]
- Kaper, T., Leemhuis, H., Uitdehaag, J. C. M., van der Veen, B. A., Dijkstra, B. W., van der Maarel, M. J. E. C. & Dijkhuizen, L. (2007). Biochemistry, 46, 5261–5269. [DOI] [PubMed]
- Kaper, T., Talik, B., Ettema, T. J., Bos, H., van der Maarel, M. J. E. C. & Dijkhuizen, L. (2005). Appl. Environ. Microbiol. 71, 5098–5106. [DOI] [PMC free article] [PubMed]
- Lin, T.-P. & Preiss, J. (1988). Plant Physiol. 86, 260–265. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Monod, J. & Torriani, A. M. (1950). Ann. Inst. Pasteur (Paris), 78, 65–77. [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol. 276, 307–326. [DOI] [PubMed]
- Przylas, I., Terada, Y., Fujii, K., Takaha, T., Saenger, W. & Strater, N. (2000). Eur. J. Biochem. 267, 6903–6913. [DOI] [PubMed]
- Przylas, I., Tomoo, K., Terada, Y., Takaha, T., Fujii, K., Saenger, W. & Strater, N. (2000). J. Mol. Biol. 296, 873–886. [DOI] [PubMed]
- Roujeinikova, A., Raasch, C., Sedelnikova, S., Liebl, W. & Rice, D. W. (2002). J. Mol. Biol. 321, 149–162. [DOI] [PubMed]
- Srisimarat, W., Kaulpiboon, J., Krusong, K., Zimmermann, W. & Pongsawasdi, P. (2012). Appl. Environ. Microbiol. 78, 7223–7228. [DOI] [PMC free article] [PubMed]
- Srisimarat, W., Powviriyakul, A., Kaulpiboon, J., Krusong, K., Zimmermann, W. & Pongsawasdi, P. (2011). J. Incl. Phenom. Macrocycl. Chem. 70, 369–375.
- Takaha, T. & Smith, S. M. (1999). Biotechnol. Genet. Eng. Rev. 16, 257–280. [DOI] [PubMed]
- Takaha, T., Yanase, M., Takata, H., Okada, S. & Smith, S. M. (1996). J. Biol. Chem. 271, 2902–2908. [DOI] [PubMed]
- Terada, Y., Fujii, K., Takaha, T. & Okada, S. (1999). Appl. Environ. Microbiol. 65, 910–915. [DOI] [PMC free article] [PubMed]
- Tomono, K., Mugishima, A., Suzuki, T., Goto, H., Ueda, H., Nagai, T. & Watanabe, J. (2002). J. Incl. Phenom. Macrocycl. Chem. 44, 267–270.
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
- Zheng, M., Endo, T. & Zimmermann, W. (2002). Aust. J. Chem. 55, 39–48.