Human myosin 1c and its light chain calmodulin were co-expressed and purified to homogeneity from Sf9 cells and crystallized. The crystals belonged to space group P21 and diffracted to 3 Å resolution.
Keywords: GLUT4 exocytosis, hearing loss, Myo1c
Abstract
Myosin 1c (Myo1c) is implicated in several cellular processes such as vesicle transport and the mediation of adaptation in the inner ear. Consequently, mutations impairing Myo1c motor activity lead to hearing loss in humans. To understand the role of Myo1c in this process on a molecular level, its crystal structure in complex with the light chain calmodulin was determined. A human Myo1c construct encompassing the motor domain and the first IQ motif was co-expressed with calmodulin in Sf9 cells and purified to homogeneity. The protein complex crystallized readily, and the crystals belonged to space group P21 and diffracted to 3 Å resolution. Attempts to determine the structure by molecular replacement are currently under way.
1. Introduction
Myosins are actin-based molecular machines that couple the hydrolysis of ATP to the generation of force via a series of conformational changes across the molecule. In addition to the conventional myosins that mediate muscle contraction, several other myosins are known in humans, adding up to 38 heavy-chain genes in total. Based on sequence similarity they are grouped into 13 classes (I, II, III, V, VI, VII, IX, X, XV, XVI, XII, XIX and XXV; Odronitz & Kollmar, 2007 ▶). Unconventional myosins fulfil various functions within cells, e.g. exocytosis and endocytosis, vesicle transport and processes such as cell motility (Hartman et al., 2011 ▶).
Myosin 1c (Myo1c) is a short-tailed single-headed myosin that is implicated, for instance, in endocytosis (Sokac et al., 2006 ▶) and the exocytosis of GLUT4 storage vesicles in insulin-stimulated glucose uptake (Bose et al., 2002 ▶, 2004 ▶). Furthermore, an Myo1c splice isoform in the nucleus is implicated in DNA transcription (Pestic-Dragovich et al., 2000 ▶; Steinberg et al., 2011 ▶). Moreover, it could be shown that it links the actin network to cell membranes via a lipid-binding pleckstrin homology domain in its tail region (Hokanson & Ostap, 2006 ▶). Myo1c was shown to be a key player in the adaptation response in the stereocilia of the inner ear (Holt et al., 2002 ▶; Stauffer et al., 2005 ▶), and mutations in the gene coding for Myo1c result in deafness in humans (Zadro et al., 2009 ▶). Adaptation is strongly regulated by calcium ions and the kinetic properties of Myo1c are changed in such a way that the duty ratio is lowered with increasing calcium concentration. This means that the time spent in strongly actin-bound states per ATPase cycle decreases. This effect is probably regulated by calmodulin (CaM) bound to the first IQ motif of Myo1c (Adamek et al., 2008 ▶). However, the exact biochemical mechanism and the structural changes that occur upon Ca2+ binding are not known. A crystal structure of Myo1c in complex with calmodulin at the first IQ motif promises to provide crucial insights into myosin–calmodulin crosstalk and the Ca2+-dependent mechanisms that govern Myo1c regulation.
In this study, we present the expression, purification, crystallization and preliminary X-ray crystallographic analysis of human Myo1c in complex with its light chain calmodulin in the apo state.
2. Materials and methods
2.1. Cloning and expression
The truncated open reading frame (corresponding to amino acids 1–725) of myosin 1c was amplified by PCR from human cDNA (IMAGE ID 6144867). The gene with the addition of a C-terminal FLAG tag was cloned into a pFastBac Dual vector (Invitrogen, Carlsbad, California, USA) under the control of the polyhedrin promoter. Co-expression with human calmodulin (CALM1, amplified from cDNA; IMAGE ID 2821489) under control of the p10 promoter was used to ensure sufficient saturation of Myo1c with its light chain. The recombinant plasmid was transformed into competent Escherichia coli DH10Bac cells (Invitrogen) for transposition into the bacmid. Sf9 cells were transfected with recombinant bacmid using FuGENE HD transfection reagent (Promega, Fitchburg, Wisconsin, USA) followed by 5 d of growth at 300 K. After several rounds of virus amplification, recombinant protein (Myo1c1IQ) was expressed by infecting Sf9 cells for 3 d at 300 K with shaking at 90 rev min−1 in suspension culture. Cell pellets were either used directly for protein purification or flash-frozen in liquid nitrogen and stored at 193 K.
2.2. Purification
The cells were suspended in lysis buffer (50 mM HEPES pH 7.5, 200 mM NaCl, 5 mM DTT, 4 mM MgCl2, 4 mM ATP, 1 mM EGTA and protease-inhibitor cocktail) and sonicated for 2 × 60 s. The lysate was centrifuged at 100 000g for 1 h at 277 K and the supernatant was incubated for 1 h with Anti-FLAG M2 affinity gel (Sigma–Aldrich, St Louis, Missouri, USA) equilibrated with lysis buffer. The column material was washed with 10 CV (column volumes) of lysis buffer and 10 CV lysis buffer without MgCl2 and ATP. The protein was eluted with 5 CV elution buffer (10 mM HEPES pH 7.5, 50 mM KCl, 1 mM EGTA, 1 mM DTT, 0.15 mg ml−1 FLAG peptide, 5 µM calmodulin). The elution fractions were concentrated and applied onto a Superdex 200 10/300 gel-filtration column (GE Healthcare) equilibrated and run in gel-filtration buffer (10 mM HEPES pH 7.5, 50 mM KCl, 1 mM EGTA, 1 mM DTT).
Pooled peak fractions from the gel-filtration run were concentrated to a final concentration of 6 mg ml−1 and either directly used for crystallization or supplemented with 3%(w/v) sucrose, flash-frozen in liquid nitrogen and stored at 193 K.
Calmodulin was expressed and purified from E. coli Rosetta pLySs (DE3) cells with a pET-3a vector, which was kindly provided by Professor Bähler, University of Münster. Cells were grown in LB medium to an OD600 of 0.8 at 310 K. Expression was induced by adding 1 mM IPTG and the cells were grown for an additional 4 h at 310 K. The cells were suspended in 50 mM HEPES pH 7.5, 5 mM CaCl2, lysed by sonication and centrifuged for 30 min at 37 000g and 277 K. By heating the supernatant to 343 K for 10 min, contaminating proteins were precipitated, whereas calmodulin remained stable. After centrifugation for 30 min at 100 000g and 277 K the supernatant contained 90% pure calmodulin. The protein was further purified by loading it onto a phenyl Sepharose column and washing with lysis buffer. Pure calmodulin was eluted with 50 mM HEPES pH 7.5, 1 mM EGTA.
2.3. Crystallization and X-ray data collection
Prior to crystallization, Myo1c1IQ–CaM was supplemented with 2 mM NaVO4, 2 mM ADP and 2 mM MgCl2, incubated for 1 h on ice and centrifuged at 18 000g and 277 K for 30 min. The complex was crystallized at 291 K using the sitting-drop vapour-diffusion method by mixing equal amounts (200 nl) of concentrated protein solution and reservoir solution. Commercially available screens were used for initial screening (Jena Bioscience, Jena, Germany). Diffraction-quality crystals appeared overnight with dimensions of approximately 400 × 200 × 20 µm in the condition 20%(w/v) PEG 3350, 0.2 M sodium malonate pH 7. Single crystals were transferred to cryoprotectant solution (mother liquor containing 10% glycerol) and flash-cooled in liquid nitrogen. X-ray diffraction data were collected at 100 K on beamline P13 of PETRA III at the EMBL Outstation, DESY, Hamburg. A complete data set was collected and data-collection statistics are summarized in Table 1 ▶. Data were indexed, integrated and scaled using XDS (Kabsch, 2010 ▶).
Table 1. Diffraction data-collection and processing statistics for human Myo1c1IQ–CaM.
Values in parentheses are for the outer shell.
| Beamline | P13, PETRA III, EMBL Hamburg |
| Detector | PILATUS 6M |
| Wavelength (Å) | 1.003 |
| Space group | P21 |
| Unit-cell parameters (Å, °) | a = 58.37, b = 161.14, c = 114.68, α = γ = 90, β = 91.64 |
| No. of molecules in unit cell Z | 2 |
| Resolution (Å) | 93–3.0 (3.18–3.00) |
| Completeness (%) | 99.5 (99.5) |
| Multiplicity | 3.8 (3.9) |
| 〈I/σ(I)〉 | 15.05 (2.65) |
| R merge (%) | 5.6 (54.2) |
3. Results and discussion
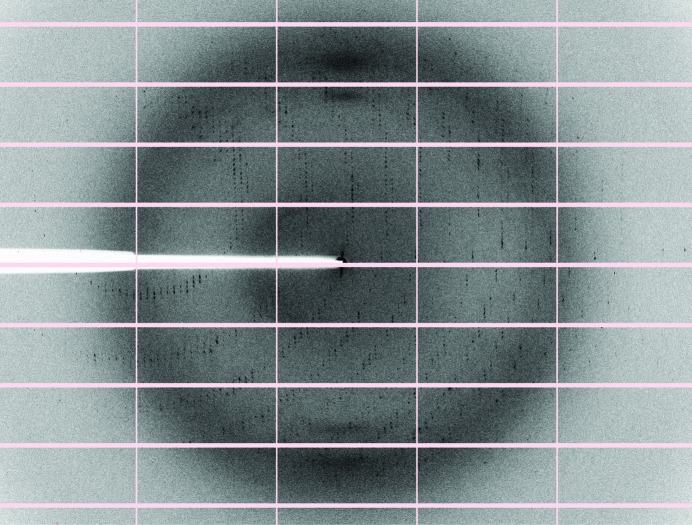
A truncated construct of human myosin 1c was co-expressed with calmodulin in insect cells. The construct encompasses the motor domain, the first IQ motif (amino acids 1–725) and a C-terminal FLAG tag to aid purification. The protein was successfully overexpressed and purified to homogeneity using FLAG affinity chromatography and gel filtration (Fig. 1 ▶). The yield was approximately 1 mg from 1 l of Sf9 cells grown to a cell density of 106 cells ml−1. Diffraction-quality crystals (Fig. 2 ▶ a) were grown overnight in a hanging-drop vapour-diffusion setup in 20%(w/v) PEG 3350, 0.2 M sodium malonate pH 7. A transition-state analogue (Mg–ADP–VO4) was used to trap the myosin in the ADP–Pi state (pre-power stroke state). The fact that a complex of Myo1c1IQ and calmodulin was indeed crystallized was verified by washing and dissolving several crystals and running them on SDS–PAGE (Fig. 2 ▶ b). A complete data set was collected to 3 Å resolution and the data were indexed in the monoclinic space group P21, with unit-cell parameters a = 58.37, b = 161.14, c = 114.68 Å, α = γ = 90, β = 91.64° (a representative diffraction image is shown in Fig. 3 ▶). The Matthews coefficient (Matthews, 1968 ▶) was 2.7 Å3 Da−1 and the solvent content was 54% assuming that the asymmetric unit contained two molecules. Further data-collection and processing statistics are summarized in Table 1 ▶. We are currently in the process of determining the structure by molecular replacement.
Figure 1.
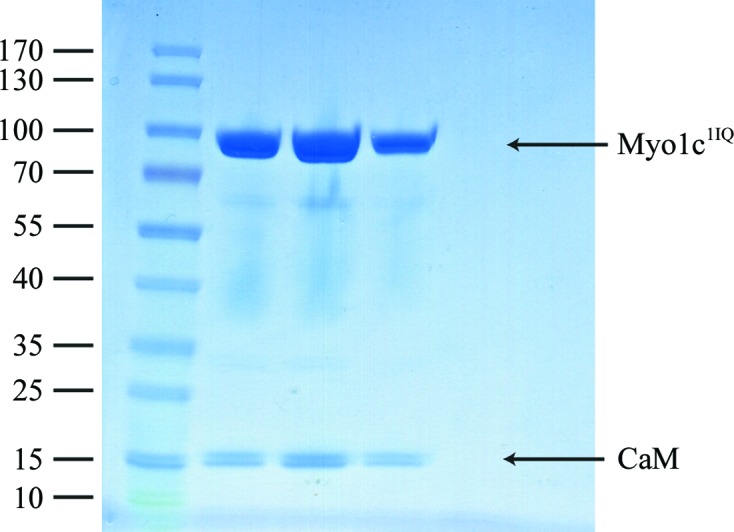
SDS–PAGE of the Myo1c1IQ–CaM fractions from the gel-filtration run. The left-hand lane contains molecular-mass markers (labelled in kDa).
Figure 2.
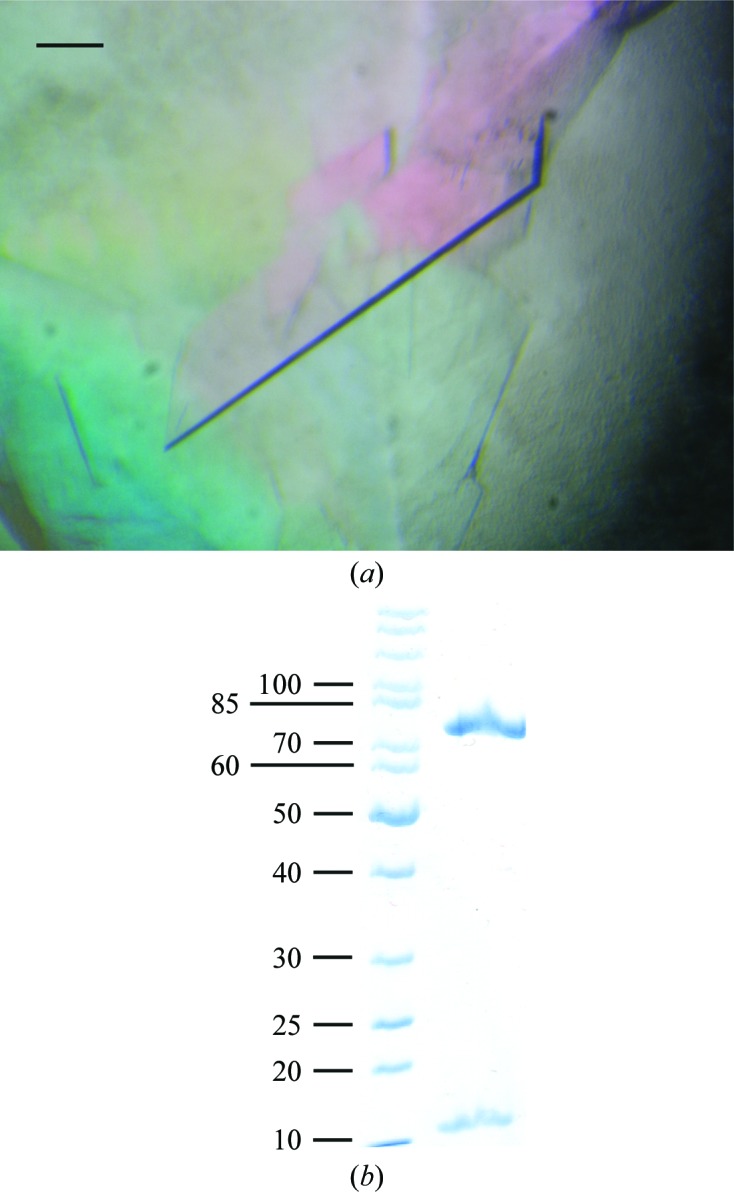
(a) Crystals of human Myo1c1IQ–CaM grown in 20%(w/v) PEG 3350, 0.2 M sodium malonate pH 7. The scale bar corresponds to 100 µm. (b) SDS–PAGE of washed and dissolved crystals shows the presence of both Myo1c1IQ and calmodulin in the crystals. The left lane contains molecular-mass markers (labelled in kDa).
Figure 3.
Diffraction image of a Myo1c1IQ–CaM crystal diffracting to 3 Å resolution. The edges of the detector are at 2.5 Å resolution.
The crystal structure of human Myo1c in complex with its regulating light chain calmodulin will provide essential insight into its molecular function and more specifically the exact regulatory mechanism by which the Ca2+ sensitivity of Myo1c is mediated. This will in turn shed light on its role in adaptation.
Acknowledgments
We thank the scientists of the PETRA III beamline P13 at the EMBL Outstation Hamburg (DESY) for their support during data collection. This work was supported by DFG (Deutsche Forschungsgemeinschaft) grant No. MA1081/19-1.
References
- Adamek, N., Coluccio, L. M. & Geeves, M. A. (2008). Proc. Natl Acad. Sci. USA, 105, 5710–5715. [DOI] [PMC free article] [PubMed]
- Bose, A., Guilherme, A., Robida, S. I., Nicoloro, S. M. C., Zhou, Q. L., Jiang, Z. Y., Pomerleau, D. P. & Czech, M. P. (2002). Nature (London), 420, 821–824. [DOI] [PubMed]
- Bose, A., Robida, S., Furcinitti, P. S., Chawla, A., Fogarty, K., Corvera, S. & Czech, M. P. (2004). Mol. Cell. Biol. 24, 5447–5458. [DOI] [PMC free article] [PubMed]
- Hartman, M. A., Finan, D., Sivaramakrishnan, S. & Spudich, J. A. (2011). Annu. Rev. Cell Dev. 27, 133–155. [DOI] [PMC free article] [PubMed]
- Hokanson, D. E. & Ostap, E. M. (2006). Proc. Natl Acad. Sci. USA, 103, 3118–3123. [DOI] [PMC free article] [PubMed]
- Holt, J. R., Gillespie, S. K., Provance, D. W., Shah, K., Shokat, K. M., Corey, D. P., Mercer, J. A. & Gillespie, P. G. (2002). Cell, 108, 371–381. [DOI] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 133–144. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Odronitz, F. & Kollmar, M. (2007). Genome Biol. 8, R196. [DOI] [PMC free article] [PubMed]
- Pestic-Dragovich, L., Stojiljkovic, L., Philimonenko, A. A., Nowak, G., Ke, Y., Settlage, R. E., Shabanowitz, J., Hunt, D. F., Hozak, P. & de Lanerolle, P. (2000). Science, 290, 337–341. [DOI] [PubMed]
- Sokac, A. M., Schietroma, C., Gundersen, C. B. & Bement, W. M. (2006). Dev. Cell, 11, 629–640. [DOI] [PMC free article] [PubMed]
- Stauffer, E. A., Scarborough, J. D., Hirono, M., Miller, E. D., Shah, K., Mercer, J. A., Holt, J. R. & Gillespie, P. G. (2005). Neuron, 47, 541–553. [DOI] [PMC free article] [PubMed]
- Steinberg, T., Ziegler, N., Alonso, A., Kohl, A., Müssig, E., Proksch, S., Schulz, S. & Tomakidi, P. (2011). Cell Calcium, 49, 259–271. [DOI] [PubMed]
- Zadro, C., Alemanno, M. S., Bellacchio, E., Ficarella, R., Donaudy, F., Melchionda, S., Zelante, L., Rabionet, R., Hilgert, N., Estivill, X., Van Camp, G., Gasparini, P. & Carella, M. (2009). Biochim. Biophys. Acta, 1792, 27–32. [DOI] [PubMed]