Abstract
Climate change may cause ecosystems to become trophically restructured as a result of primary producers and consumers responding differently to increasing CO2 and temperature. This study used an integrative approach using a controlled microcosm experiment to investigate the combined effects of CO2 and temperature on key components of the intertidal system in the UK, biofilms and their consumers (Littorina littorea). In addition, to identify whether pre-exposure to experimental conditions can alter experimental outcomes we explicitly tested for differential effects on L. littorea pre-exposed to experimental conditions for two weeks and five months. In contrast to predictions based on metabolic theory, the combination of elevated temperature and CO2 over a five-week period caused a decrease in the amount of primary productivity consumed by grazers, while the abundance of biofilms increased. However, long-term pre-exposure to experimental conditions (five months) altered this effect, with grazing rates in these animals being greater than in animals exposed only for two weeks. We suggest that the structure of future ecosystems may not be predictable using short-term laboratory experiments alone owing to potentially confounding effects of exposure time and effects of being held in an artificial environment over prolonged time periods. A combination of laboratory (physiology responses) and large, long-term experiments (ecosystem responses) may therefore be necessary to adequately predict the complex and interactive effects of climate change as organisms may acclimate to conditions over the longer term.
Keywords: primary productivity, biofilm, grazing, climate change, ocean acidification, physiological performance
1. Introduction
The structure and function of coastal marine ecosystems reflect the interaction between a range of anthropogenic and natural drivers that emanate from marine, terrestrial and atmospheric origins. Although there is broad recognition that changes to the physical environment can drive the biology of individual species (e.g. see review by [1]), there is less recognition of how multiple stressors may alter biological interactions [2]. For example, increasing temperatures are predicted to increase consumption of plant biomass by herbivores [3] but multiple stressors often alter this relationship [4]. Therefore, while the physico-chemical environment can be highly variable over short time-scales (e.g. tidal cycles and sudden weather events [1]), long-term alteration in conditions, such as with steadily increasing temperatures and ocean acidification, may exert strong influence on the interactions between coastal species and, subsequently, the structure and function of communities and ecosystems [5,6].
Global sea surface temperature (SST) has warmed by 0.4–0.8°C during the last century, although regional differences are evident [7,8]. The Northeast Atlantic is warming faster than the global average, by up to 1°C between the mid-1980s and 2000s with further warming of between 1.5°C and 4°C by 2100 predicted [9–11]. It is no surprise therefore that coastal marine species have shown some of the fastest responses to climate change in any system, with species-specific responses to thermal stress causing poleward shifts in biogeographic distributions towards cooler environments, as well as changes in phenology and regime shifts. Such alterations in the spatial and temporal distribution of marine organisms ultimately drive subsequent changes in ecosystem structure and functioning [1,12–15].
In addition to rising temperatures, another impact of rising anthropogenic CO2 concentrations is ocean acidification. This phenomenon is caused by the rapid uptake of atmospheric CO2 into the surface oceans, where the CO2 causes a series of changes in seawater carbonate chemistry. These changes include a lowering of pH and carbonate saturation state as well as an increase in dissolved CO2 and bicarbonate ions. These changes have already proved to be significant with a lowering of the average surface ocean pH by 0.1 unit observed since the onset of the Industrial Revolution [16]: a shift in pH that equates to the oceans having become 30% more acidic. Model forecasts suggest that average pH may decrease by as much as 0.5 pH units in total by 2100 [17]. Coastal benthic invertebrates and algae have shown a range of responses to elevated CO2 in a number of short-term experiments and natural observational studies. These responses include impacts on calcification rates [18,19], immune function [20], reproduction and carryover effects in larval and juvenile stages of invertebrates [21], enhanced productivity in phytobenthos [22–25] but reduced calcification and growth in calcareous algae [26–28].
It has been suggested that species inhabiting these coastal ecosystems may be evolutionarily more robust with respect to changes in environmental pH [29]. However, extensive literature on the response of intertidal species to observed warming suggests that intertidal species may already exist close to their tolerance limits throughout large sections of their biogeographic distributions, and further warming may cause rapid range shifts [30]. Therefore, small changes in other environmental stressors, such as ocean acidification, may drive significant physiological and ecological responses [14,29,31,32]. The same may also be the case for tolerance to external pH conditions for coastal species, although intertidal species show varied and sometimes positive responses to reduced pH [33,34].
Evidence for the impacts of changes to single environmental conditions on individual species is rapidly growing [14,34], whereas data on the synergistic impacts of multiple stressors and how species interactions may moderate these effects across trophic levels are still lacking ([35,36], but see also [37]). It is acknowledged that these knowledge gaps are limiting the use of current ecosystem models that are otherwise well developed with respect to coastal biogeochemistry. In particular, current models lack sufficient biological detail at higher resolution than functional group level and do not incorporate trophic interactions other than via nutrient fluxes and pathways [38]. One major pathway to be addressed is the interaction between changing environmental temperature and concentration of CO2 (and therefore reduced pH), the resultant composition and primary production within microbial communities and the implications of altered food resources for grazing species. Simultaneously, changes in energy partitioning and energy budgets of invertebrates caused by chronic exposure to altered environmental conditions may affect rates of food consumption [39], and also the resultant ‘farming’ feedbacks of grazers on biofilms.
Benthic biofilms are composed of a community of photoautotrophic organisms, predominantly diatoms and cyanobacteria, together with heterotrophic microbes, bound together by an extracellular polymeric substance (EPS). Their formation starts with the development of a polysaccharide and protein conditioning layer, followed by bacterial adhesion, growth and expansion to form either a single or multispecies community [40]. Biofilm formation can occur on many natural and artificial surfaces and can form a major source of primary productivity in benthic systems [41,42]. Biofilms rapidly reorganize in response to environmental disturbance and are likely to be both indicators and drivers of community level change in response to ocean acidification. Photoautotrophic biofilms contribute significantly to intertidal primary productivity [43] and are the main food resource for many benthic grazers [44]. Biofilms also influence the structure and functioning of marine benthic communities by promoting or inhibiting the settlement of algal and invertebrate propagules [45–47].
This paper uses an integrative approach to examine the direct influence of elevated temperature and reduced pH on the biomass and composition of benthic biofilm, and the relative primary impacts of these drivers versus secondary effects via altered biofilm food supply on the grazing activity of Littorina littorea (Linnaeus 1758), a key grazing species of periwinkle present on rocky intertidal habitat across the northeast Atlantic. In addition, we test how different acclimation times (weeks versus months) before commencement of experiments may alter the experimental outcomes. These data provide new information on the relative roles of direct and indirect perturbations of warming and ocean acidification on primary productivity, trophic interactions and grazing activity in rocky intertidal systems.
2. Material and methods
(a). Experimental design and set-up
Adult L. littorea and biofilms were maintained under experimental conditions in 9 l (l × w × h: 30 × 20 × 15 cm) transparent plastic microcosms with lids. The microcosms were housed in the mesocosm facility located at the Plymouth Marine Laboratory, Plymouth, UK. The study was conducted over five weeks during July and August 2011. Experimental conditions were crossed combinations of temperature (ambient local August SST 14°C versus 18°C) and CO2 (380 versus 750 versus 1000 ppm), with four replicate microcosms per treatment combination (total of 24 microcosms). Each microcosm was divided into three equal sections by mesh barriers, of which two sections contained a single L. littorea each (hereafter termed ‘grazed sections’) and one section which did not contain any L. littorea (hereafter termed ‘ungrazed sections’) (see below). The two L. littorea in each microcosm had experienced different conditions prior to the experiment: one L. littorea had been kept under the experimental conditions for five months (hereafter termed ‘5-month’), and one had been collected from the low intertidal at the same time as the settlement panels and exposed to laboratory conditions for two weeks prior to the start of the experiment (hereafter termed ‘2-week’).
Two weeks prior to the start of the experiment, six 5 × 5 cm PVC settlement panels not containing any biofilm were placed into each microcosm (two panels per section). In addition, three settlement panels with natural biofilm communities were placed in the ‘ungrazed’ section of each microcosm, thus allowing a biofilm community to establish on the blank panels under the relevant experimental treatment. The panels with natural biofilm had previously been inoculated with biofilm in the low intertidal for six weeks from May to June 2011 and were only used to provide a seed for biofilm communities under experimental conditions and are not subsequently discussed here.
Water temperature was maintained to an accuracy of ±0.5°C using water baths surrounding the microcosms, with the bath water being maintained by bar heaters and pumps to circulate the water. CO2 concentrations were manipulated by bubbling pre-mixed gas at the experimental concentrations at equal flow rates and pressures through all microcosms, which had lids to maintain a stable internal atmosphere. The pre-mixed gas was created following methods described by Findlay et al. [48]. Gas concentration was monitored using an infrared gas analyser (Licor LI-820, Nebraska, USA). Salinity, temperature (LF187, WTW, Weilheim, Germany) and pH (826 pH mobile with a glass electrode, Metrohm, Cheshire, UK) were monitored three times a week and total alkalinity (AT) was measured weekly using standard methods proposed by Dickson & Millero [49] and then analysed by pentiometric titration using an Alkalinity Titrator (Model AS-ALK2, Apollo SciTech, Bogart, USA) and Dickson certified reference materials (Batch 100). Concentrations of bicarbonate (HCO3−), carbonate (CO32−) and seawater pCO2 were then calculated from measured AT, pH, salinity and temperature using the CO2SYS program for Excel [50] with constants from Mehrbach et al. [51], as adjusted by Dickson & Millero [49] (table 1). A 4.5 l water change was carried out every other day in each microcosm with fresh seawater at close to experimental temperatures to ensure water quality, while maintaining a constant treatment temperature and pH.
Table 1.
Seawater measurements from experimental aquaria: measured values of temperature (T), salinity (S), pH (NBS scale), total alkalinity (TA); and calculated values (using CO2SYS) of dissolved inorganic carbon (DIC), seawater pCO2, saturation states for calcite (Ωcalcite) and aragonite (Ωaragonite), bicarbonate (HCO3−) and carbonate concentration (CO32−).
| target CO2 (ppm) | 380 | 750 | 1000 | 380 | 750 | 1000 |
|---|---|---|---|---|---|---|
| target T (°C) | 14 | 14 | 14 | 18 | 18 | 18 |
| T (°C) | 14.36 ± 1.05 | 14.45 ± 0.98 | 14.03 ± 0.93 | 19.09 ± 0.93 | 18.60 ± 1.24 | 18.19 ± 0.91 |
| S | 34.17 ± 0.33 | 34.09 ± 0.24 | 34.04 ± 0.21 | 34.41 ± 0.60 | 34.43 ± 0.57 | 34.52 ± 0.92 |
| pH | 8.25 ± 0.041 | 7.97 ± 0.076 | 7.79 ± 0.063 | 8.28 ± 0.064 | 7.98 ± 0.087 | 7.86 ± 0.065 |
| TA (µmol kg−1) | 2440 ± 18 | 2452 ± 39 | 2437 ± 29 | 2451 ± 37 | 2441 ± 36 | 2442 ± 34 |
| DIC (µmol kg−1) | 2168 ± 24 | 2300 ± 34 | 2351 ± 35 | 2115 ± 38 | 2248 ± 50 | 2314 ± 45 |
| pCO2 (µatm) | 333 ± 32 | 663 ± 141 | 969 ± 82 | 296 ± 24 | 602 ± 104 | 867 ± 141 |
| Ωcalcite | 4.71 ± 0.26 | 2.94 ± 0.51 | 2.05 ± 0.13 | 5.82 ± 0.37 | 3.57 ± 0.51 | 2.64 ± 0.31 |
| Ωaragonite | 3.02 ± 0.17 | 1.89 ± 0.32 | 1.31 ± 0.08 | 3.77 ± 0.24 | 2.31 ± 0.33 | 1.71 ± 0.20 |
| HCO3− (µmol kg−1) | 1959 ± 32 | 2153 ± 41 | 2228 ± 36 | 1863 ± 43 | 2079 ± 63 | 2175 ± 49 |
| CO32− (µmol kg−1) | 196 ± 11 | 123 ± 21 | 86 ± 5 | 242 ± 15 | 149 ± 21 | 110 ± 13 |
(b). Consumption of biofilm by gastropods
The amount of biofilm consumed by grazers was quantified by subtracting the final percentage cover of biofilm on grazed plates from percentage cover of ungrazed plates in the same treatment (i.e. ungrazed – grazed = consumption). As none of the ungrazed plates attained a 100% cover of biofilm, this measure inherently takes growth of the biofilm into account, and so is a time-integrated measure of consumption. Percentage cover was quantified by placing a quadrat containing 25 random dots over a digital photograph of each panel and scoring as either presence or absence of biofilm with each dot equating to 4% cover. All panels were weighed before the start of the experiment. At the end of the experiment all settlement panels were dried in an oven at 60°C for 48 h and then re-weighed, with biofilm weight being end dry weight—plate weight.
(c). Gastropod resting metabolic rates
The resting metabolic rate of experimental gastropods was quantified at the end of the experiment during closed incubations during which changes in the oxygen saturation of seawater were measured using an oxygen meter (781, Strathkelvin Instruments, Glasgow, UK). The oxygen meter was calibrated using a two-point calibration prior to every incubation using aerated distilled water as 100% saturation and de-oxygenated distilled water prepared using sodium sulfate as 0% saturation. Individual snails were placed in small (69 ml) blacked-out glass jars which were sealed while submerged in the experimental microcosms to prevent air bubbles, and put into water baths set to representative treatment water temperature to maintain the experimental thermal regime during incubations. The L. littorea were allowed for a 15 min acclimation period in the jars with the lids on loosely before three replicate water samples were taken with a syringe and run through the oxygen meter to determine the starting saturation. Bottles were sealed and left for 30 min along with a ‘blank’ bottle to control for any microbial respiration in the seawater. At completion of the incubation, the lid was partially removed and three 5 ml samples were immediately taken using syringes to measure the final oxygen concentration of the water.
Temperature, salinity and atmospheric pressure were also recorded for each incubation chamber (using the equipment detailed above), and Weiss Table [52] correction factors for oxygen saturation were used to calculate change in oxygen concentration per millilitre chamber volume per min. Following incubations, gastropods were euthanized and removed from their shells, and their soft tissue was dried on a paper towel and then weighed on an electronic balance so that final oxygen consumption could be calculated per gram of dry flesh weight (μmol O2 g−1 dry weight (DW) h−1 STP).
(d). Biofilm communities
(i). Clone library construction and sequence analysis
Biofilm material was scraped from the tiles with a sterile scalpel, placed into a 2 ml microtube and the DNA extracted using the PowerBiofilm DNA Isolation Kit (MO BIO) according to the manufacturer's instructions. Possible changes to the cyanobacterial and micro-algal community was first analysed using PCR–denaturing gradient gel electrophoresis (DGGE) using the PCR primer pair CYA-359F (5′-GGGGAATYTTCCGCAATGGG-3′) (containing a GC-clamp) and CYA-781R (5′-GACTACWGGGGTATCTAATCCCW-3′) following the methodology of Nubel et al. [46]. This primer pair amplifies 16S rRNA gene fragments which are specific for cyanobacteria and micro-algae chloroplast [53]. Each 25 µl PCR mixture contained 1 µl biofilm DNA, 5 µl PCR buffer (Promega), 2 mM MgCl2, 0.2 mM dNTPs, 2.5 U of GoTaq Flexi DNA polymerase (Promega) and 1 mM of each primer. PCR products were amplified using the following conditions: 95°C for 5 min, followed by 35 cycles of 95°C for 1 min, 58°C for 1 min and 72°C for 1 min with a final extension time of 5 min at 72°C. PCR products from each biofilm sample were run on polyacrylamide gradient gels with denaturing gradients of 20–60%. Gels were electrophoresed in 1 × TAE at a constant temperature of 60°C for 16 h at 60 V. DGGE fingerprinting patterns were converted to presence/absence data using Gel Compar II software (Applied Maths, The Netherlands) and the data imported into PRIMER v. 6.1 multivariate analysis software [54] for statistical analysis. The four replicates from each treatment were also pooled and six clone libraries constructed. PCR products were cloned using the pGEM-T Easy Vector System I cloning kit (Promega) according to the manufacturer's instructions. Transformants were selected on Luria-Bertani agar plates containing ampicillin (50 µg ml−1), X-gal (40 µg ml−1) and 0.1 M isopropyl-β-d-thiogalactopyranoside. White colonies were screened by PCR using the vector primers M13F (5′-GTAAAACGACGGCCAG-3′) and M13R (5′-CAGGAAACAGCTATGAC-3′) and the resulting PCR products were sequenced using an ABI 3730 XL (LGC Genomics). Thirty two clones were sequenced from each clone library, providing 192 sequences in total. Sequences were processed using the QIIME pipeline (Quantitative Insights into Microbial Ecology v. 1.2) [55]. All 196 sequences were clustered into operational taxonomic units (OTUs) based on 97% sequence similarity using Uclust. This 97% sequence similarity cut-off was chosen as it is typically considered to be representative of a species. To assign taxonomy to each OTU, a representative sequence from each OTU cluster was chosen, the representative sequences aligned using PyNAST, and taxonomy assigned by comparison with the Greengenes database (version February 2011) [56] which uses cyanoDB taxonomy for classification of the Cyanobacteria [57]. The Greengenes classification was also compared with the NCBI database. The QIIME pipeline and PRIMER v. 6.1 multivariate analyses software [54] were used to calculate alpha diversity for each clone library. Sequences obtained in this study have been deposited in GenBank under the following accession nos. KC895985–KC896007.
(e). Statistical analyses
Changes in the performance (percentage cover of biofilm consumed and metabolic rate) of the two sample groups of grazers (5-month and 2-week) in response to the different experimental treatments were first analysed using three-factor analysis of variances (ANOVAs). Grazer sample group, temperature and CO2 were treated as fixed and orthogonal factors, with two levels in the factors of grazer sample group (5-month versus 2-week) and temperature (14°C versus 18°C) and three levels in the factor CO2 (380 versus 750 versus 1000 ppm). Changes in percentage cover for the two settlement panels within each grazer treatment (5-month, 2-week or ungrazed) within each microcosm treatment were averaged and analysed with microcosms as replicates (n = 4). Following these analyses, the responses of grazers and biofilm to experimental treatments were analysed separately using two-factor ANOVAs, with the factors of temperature and CO2 (treated as above). Data were checked for heterogeneity and ln(X + 1) transformed prior to analysis where necessary. Where significant treatment effects were detected, Student–Newman–Keuls (SNK) post hoc comparisons of means were run. All analyses were done with PERMANOVA for PRIMER v. 6.
3. Results
(a). Consumption of biofilm and gastropod metabolism
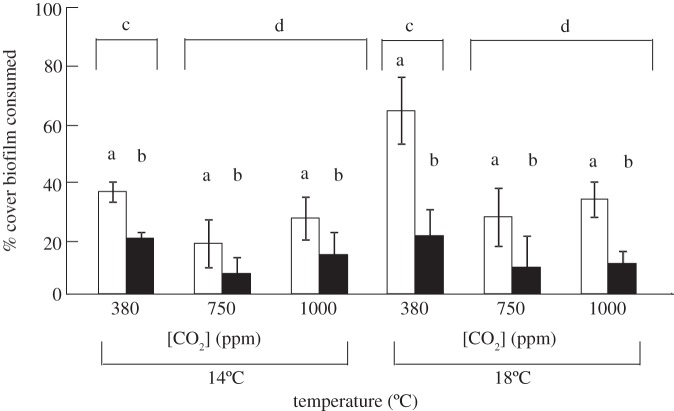
Overall, grazers that were exposed to altered CO2 and temperature conditions for 5 months consumed more biofilm than the 2-week exposed grazers, with both CO2 and temperature altering the consumption rate. The percentage cover of biofilm consumed differed significantly between groups of grazers, with consumption by 2-week grazers being reduced relative to the 5-month grazers (figure 1; F1,36 = 20.76, p < 0.001, electronic supplementary material, table S1). When both sets of grazers are taken into account, the biggest driver of consumption was CO2 concentration, with consumption of biofilm being less at 750 and 1000 ppm than that at 380 ppm (figure 1 and electronic supplementary material, table S1). For grazers exposed to experimental conditions for five months, both temperature and CO2 had an effect on the amount of biofilm grazed but there was no significant interaction between these two factors (figure 1; F2,18 = 1.01, p = 0.38, electronic supplementary material, table S2a). Temperature increased grazing (figure 1; F1,18 = 4.68, p = 0.04, electronic supplementary material, table S2a) on average by 79%, 53% and 25%, at 380, 750 and 1000 ppm, respectively. In contrast, elevated concentrations of CO2 reduced grazing (figure 1; F1,18 = 5.92, p = 0.01, electronic supplementary material, table S2a) by between 25 and 58%. Even though there appeared to be a similar trend in the effects of temperature and CO2 on grazing by the 2-week grazers, no effect of temperature or CO2 was statistically detected (figure 1; all F1,18 < 1.34, all p > 0.2, electronic supplementary material, table S2b). Therefore, the amount of biofilm consumed by L. littorea that were exposed to experimental conditions for 5 months was greater than that consumed by the 2-week individuals.
Figure 1.
The effect of different combinations of temperature (14°C versus 18°C) and concentrations of CO2 (380 versus 750 versus 1000 ppm) on the mean (±s.e.) percentage cover of biofilm consumed by gastropods pre-exposed to experimental conditions for five months (open bars) and two weeks (filled bars) prior to the experiment. SNK tests are presented on the figure, where different letters show significant differences. The amount of biofilm consumed differed between grazers (5-month > 2-week) and was affected by CO2 (380 > 750 = 1000 ppm). There were no significant interactions between treatments (all interaction term p > 0.1).
In contrast to grazing rates, resting metabolic rates did not differ between 5-month and 2-week grazers (figure 2; F1,28 = 0.0034, p = 0.84, electronic supplementary material, table S3). Temperature caused an overall increase in the metabolic rate of both groups of grazer (figure 2; F1,28 = 4.25, p = 0.04, electronic supplementary material, table S3), while CO2 did not affect metabolism (figure 2; F1,28 = 0.19, p = 0.82, electronic supplementary material, table S3).
Figure 2.
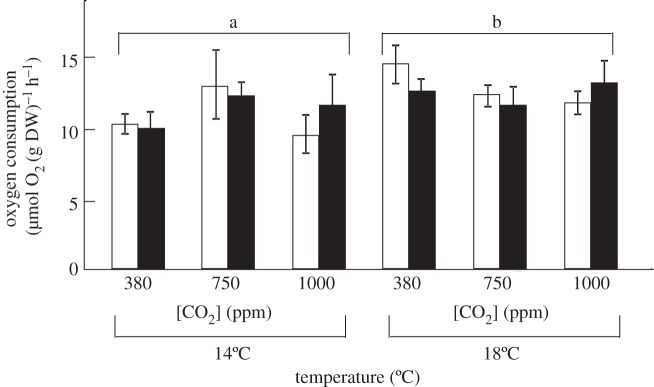
The effect of temperature (14°C versus 18°C) and CO2 (380 versus 750 versus 1000 ppm) on the mean (±s.e.) metabolic rates (μmol O2 (g DW)−1 h−1) of 5-month (open bars) and 2-week (filled bars) gastropods. SNK tests are presented on the figure, where different letters show significant differences.
(b). Biofilm community
Both temperature and CO2 affected the composition of the biofilms that grew on settlement panels. Elevated temperature but not CO2 caused an increase in the percentage cover of biofilm present on ungrazed settlement panels (figure 3, electronic supplementary material, table S4). During the course of the experiment, visible differences in the colour of the biofilm communities that formed in different treatments became evident. The biofilms incubated at 14°C and 380 and 750 ppm were mostly green in colour, whereas the majority of those within the other treatments contained patches of pink colouration (figure 4). This was an indication that the composition of the biofilm cyanobacterial community differed between treatments.
Figure 3.
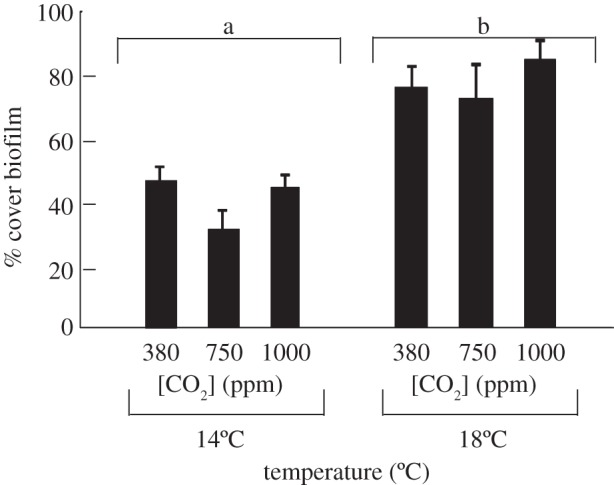
The effect of temperature (14 versus 18°C) and CO2 (380 versus 750 versus 1000 ppm) on the percentage cover (±s.e.) of biofilm on ungrazed settlement panels. SNK tests are presented on the figure, where different letters show significant differences.
Figure 4.

Photographs of the biofilms on settlement panels from different experimental treatments; (a) 14°C, 380 ppm; (b) 18°C, 1000 ppm. Note the relatively large patches of different biofilm in (b) (white boxes). (Online version in colour.)
Possible changes to the community composition of the cyanobacterial community was first analysed using DGGE of cyanobacteria and micro-algae chloroplast 16S rRNA gene PCR products (figure 5). Although there was minor variability in DGGE banding pattern between replicates, particularly for the samples incubated at 1000 ppm CO2, clear distinction in the microbial community between CO2 (F = 4.55; p = 0.003) and temperature (F = 4.68; p = 0.02) was apparent, and so we analysed the community composition from each temperature/CO2 treatment in detail using clone libraries. Analysis of sequence data obtained from clone libraries of PCR-amplified cyanobacteria and micro-algae chloroplast 16S rRNA gene sequences revealed that at 97% nucleotide identity 23 distinct OTUs were present. Species richness decreased at both elevated temperature and CO2 (Margalef and ChaoI species richness, table 2), indicating a possible shift of the community composition within the biofilms exposed to elevated temperature and CO2. To compare biofilm community composition within the different treatments, taxonomy was assigned by comparison of sequences to the Greengenes database [56]. The sequences were first grouped at class and order level taxonomies (table 2). This indicated that only three taxonomic orders were evident within the clone libraries: the Pseudanabaenales of the class Synechococcophycideae (60%), the Chroococcales of the class Oscillatoriophycideae (36%) and the eukaryote Stramenopiles (4%). Although the biofilms incubated at 14°C and 380 and 750 ppm were dominated by the order Pseudanabaenales, all other treatments contained equal numbers of Pseudanabaenales and Chroococcales. When examined in detail, this shift in community composition could be explained by the four most dominant OTUs, three Pseudanabaenales, two of which were most closely related to Leptolyngbya sp. (OTUs nos 10 and 20), and one which most closely related to Halomicronema sp. (OTU no. 4), and one Chroococcales which most closely related to Dermocarpella sp. (OTU no. 13) (table 2). The response of these OTUs were opposite, with OTUs nos 10 and 20 decreasing and OTUs nos 4 and 13 increasing in abundance.
Figure 5.
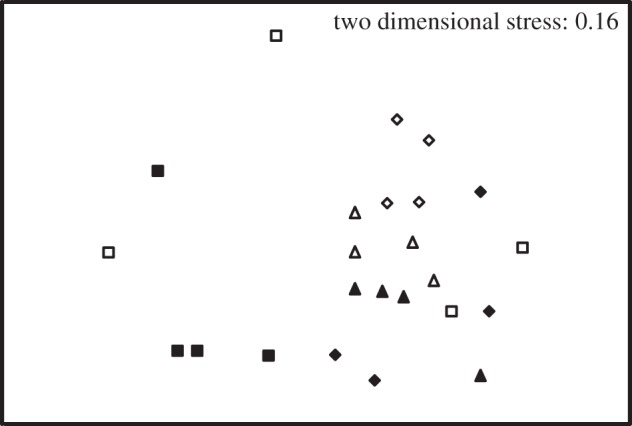
Effect of CO2 and temperature on cyanobacterial community structure as assessed by PCR-DGGE. The plot shows a non-metric multi-dimensional scaling (MDS) ordination of a Bray–Curtis resemblance matrix, generated from presence/absence of DGGE bands. Open symbols represent samples incubated at 14°C and closed symbols are from 18°C incubations. Triangles, 380 ppm CO2; diamonds, 750 ppm CO2; squares, 1000 ppm CO2.
Table 2.
Diversity and species richness indices estimates and taxonomic classification for biofilm communities that developed on settlement panels exposed to different combinations of temperature (14°C versus 18°C) and concentrations of CO2 (380 versus 750 versus 1000 ppm). Numbers of operational taxonomic units (OTUs) per clone library and the Chao1 species richness indices were identified at 97% sequence similarity using the QIIME pipeline. Margalef species richness, Shannon diversity and Pielou's evenness were calculated from OTU abundance matrices using the Diverse test in PRIMER v. 6.1 [54]. Also shown are the number of sequences assigned to each taxonomic order within each treatment and the number of sequences within the four most dominant OTUs within the biofilm clone libraries.
| temperature (°C) | 14°C |
18°C |
|||||
|---|---|---|---|---|---|---|---|
| CO2 (ppm) | 380 | 750 | 1000 | 380 | 750 | 1000 | |
| diversity metrics | no. OTUs (97%) | 9 | 10 | 8 | 9 | 7 | 7 |
| Margalef species richness | 2.31 | 2.60 | 2.02 | 2.31 | 1.73 | 1.73 | |
| ChaoI species richness | 14.08 | 14.3 | 10.87 | 11.83 | 7 | 7 | |
| Shannon diversity | 1.68 | 1.64 | 1.58 | 1.67 | 1.45 | 1.4 | |
| Pielou evenness | 0.77 | 0.71 | 0.76 | 0.76 | 0.75 | 0.72 | |
| taxonomic order | Chroococcales | 6 | 7 | 15 | 14 | 13 | 16 |
| Pseudanabaenales | 24 | 25 | 16 | 15 | 18 | 15 | |
| Stramenopiles | 2 | 0 | 1 | 3 | 1 | 1 | |
| dominant OTUs | no. 13 (Dermocarpella sp.) | 4 | 4 | 15 | 14 | 13 | 15 |
| no. 10 (Leptolyngbya sp.) | 15 | 17 | 6 | 7 | 10 | 9 | |
| no. 20 (Leptolyngbya sp.) | 5 | 3 | 4 | 1 | 0 | 0 | |
| no. 4 (Halomicronema sp.) | 0 | 0 | 0 | 4 | 5 | 4 | |
4. Discussion
Abiotic conditions in marine systems will continue to change substantially over the coming century, in particular temperature and CO2 concentration, leading to warmer, lower pH oceans [8,17]. In an effort to predict the probable effects of these altered conditions on primary producers and primary consumers, and thereby on marine ecosystems, there has been a rapid increase in laboratory-based experimental studies [35]. Yet, for logistical reasons, a minimal acclimation period is used in many manipulative experiments and is assumed to be sufficient to provide results that could be indicative of real impacts in nature. Some recent studies have shown, however, that this assumption is not correct and that longer experimental periods may be necessary [58–60]. We show here that it is probable that responses to shifting abiotic conditions may be altered if a longer acclimation period is used as opposed to a shorter one (but see [61]). While the metabolic responses of L. littorea in our experiment did not differ according to time of exposure to treatments before the experiment, the amount of primary productivity that was consumed did differ; therefore, there was no difference from an individual perspective, but there was an ‘ecological difference’, with a greater rate of biofilm consumption observed for grazers exposed to experimental conditions for a shorter period of weeks compared to months.
Ectotherms are in general adapted to, and depend upon maintenance of the characteristic temperature window of their natural environment [1], the location and width of which determines the sensitivity of fauna to environmental temperature extremes, and can alter with lifestage or acclimation to differing environmental conditions [28,62]. Metabolic rate should increase as temperature increases above an optimum within this thermal tolerance range due to oxygen and capacity-limited thermal tolerance until the upper pejus (meaning getting worse) limit is reached, characterized by the onset of anaerobic metabolism [37]. At this juncture, the organism moves to a passive state characterized by the onset of a decrease in arterial pO2 due to reduced ventilatory and cardiac performance [63], which can be characterized by alterations in feeding, growth and reproduction. The onset of this transition is the upper pejus temperature, Tp, defined as the temperature at which aerobic scope begins to decline [64]. Beyond upper pejus limits, oxygen supply capacity becomes limiting, maintenance demand rises, aerobic scope starts to decrease and hypoxaemia develops, resulting in a decline in whole organism functional capacity (e.g. exercise or growth performance). We found that increased metabolic rate in response to a 4°C warming (the 18°C treatment) was evident within individuals held in ambient CO2 conditions, suggesting that these individuals shifted to be closer to the upper tolerance limits of the performance curve, but did not exceed the upper pejus limits for aerobic respiration. Recent investigations on numerous invertebrates have shown that organisms respond to a change in their environmental conditions by shifting their physiological and energetic states. Different responses appear between individuals and species as a result of their flexibility to make these alterations, i.e. their plasticity. A recent short-term (10 days acclimation + 30 days exposure) study specifically on L. littorea showed that elevated temperature and CO2 concentration reduced metabolic rate and caused a shift in energetic status such that there was an increased reliance on anaerobic metabolism [65] with shifts in energetic partitioning contributing to decreases in shell growth (see discussion in [65,66]), which is a common response to elevated CO2 in this species [67,68].
An alternative explanation to the different grazing activity among experimental treatments could be associated with the shift in community composition of the biofilm. In addition to greater primary productivity, shifts in biofilm community structure have been observed along pH gradients at natural CO2 seeps [69]. Littorina littorea are Taenioglassan grazers that predominantly graze on micro-algae (diatoms and cyanobacteria) within the biofilm [70]. Biofilm communities dominated by cyanobacteria have high nutritional quality, and this, together with their high turnover rates, can support high level of secondary production [71]. But, it is not known whether certain cyanobacteria are more nutritious than others. In the study of Witt et al. [72], biofilms incubated at 305 ppm contained diverse cyanobacterial species whereas only Chroococcales were present at 1140 ppm. This shift in biofilm community suggests that this group of predominantly unicellular cyanobacteria may have a selective advantage over other cyanobacteria at elevated CO2, possibly owing to a higher growth rate. Not only did biofilm productivity in our experiment increase under elevated CO2 (as seen in the percentage cover of biofilm on ungrazed plates; figure 2 [57]), but we observed a shift in biofilm community from one dominated more by cyanobacteria of the order Chroococcales to the order Pseudanabaenales at higher CO2. If there was a corresponding shift in the nutritional value of the biofilms available to grazers (e.g. increases in algae [73] but cf. [74] in diatoms), then they would need to consume less to meet their nutritional needs (e.g. [75], but cf. [76]). Alternatively, the choice of food by gastropods, and the amount consumed, can be affected not only by the quality of the food but also by the feeding history of gastropods and their subsequent nutritional requirements [76,77]. Ultimately, it seems probable that the impact of multiple stressors on these organisms and their consumption of resources will be a combination of shifting energetics (i.e. a direct effect on the animal [78]) together with food availability and quality (i.e. an indirect effect through the food [73]).
In the short-to-medium term (present to 2050), temperature is predicted to exert a greater effect on performance and survival of temperate marine ectotherms than the decline in oceanic pH given the relative rates of change in these drivers compared to physiological performance and thermal tolerance windows [79]. In rocky intertidal systems, it is probable that biofilm biomass will increase and undergo alterations in species diversity and potentially nutritional value under warming environmental conditions [69]. Temperature-driven increases in metabolic rate of boreal grazers, such as L. littorea, are likely to promote increased grazing rates to maintain ‘normal’ biological functions in the face of greater metabolic demands. Warming environmental conditions will also continue to directly negatively impact reproduction and recruitment of boreal species, with impacts at the population and species distributional range levels [13,80]. In the longer term, CO2 emissions are predicted to rise in the range of 750–1000 ppm between 2050 and 2100 [17]. Our results add to the emerging body of evidence that CO2-driven reductions in oceanic pH of up to 1 unit will exacerbate the effects of approximately 4°C warming ([16,23,81] but see also [82]). Metabolic depression driven by a shift of, and reduction in, the physiological performance window of L. littorea may reduce fitness of individuals within populations close to southern (higher latitude) range limits. Increased maintenance costs could decrease energy available for somatic and reproductive activity unless adaptation can keep pace with the shifting performance window [83,84]. Therefore, the direct effects of temperature and CO2 on primary productivity within biofilms and primary consumption by grazers, coupled with altered interactions arising from changing quantity and/or quality of food resources available to grazers may thus have important implications for lower trophic levels and cascade up the food web.
Top-down control of primary productivity can be important in structuring marine ecosystems. The selective removal of filamentous micro- and macroalgae spores by benthic grazers provides habitat space that facilitates the settlement of habitat forming species (coral reefs: [85,86], temperate reefs: [87–89]) essential for the maintenance of a diverse benthic ecosystem. Thus, estimates of the amount of food consumed by grazers under experimentally manipulated environmental conditions can improve quantitative predictions of trophic interactions and increase understanding of the relative roles of species and processes in the maintenance of healthy, functioning ecosystems. In our experiment, grazers that were pre-exposed to the laboratory conditions only for two weeks consumed approximately 50% less cover of biofilm than grazers that had been pre-exposed to experimental conditions for five months. As the results from the control conditions also differed between grazers, this suggests that the individuals with short pre-exposure period were still acclimating to altered conditions within the experimental system and may have displayed an acute response to altered levels of temperature and pH. In contrast, individuals exposed to altered temperature and pH water conditions for prolonged periods showed a different response, likely to reflect chronic responses and, importantly, reflect different physiological mechanisms being employed to tackle short versus long-term exposure to deleterious environmental conditions. Indeed, it is being increasingly recognized that the combined effects of elevated temperature and CO2 may not be of the magnitude suggested by early work involving short-term experiments using unrealistic pH levels, and that longer term experiments are required (fish: [90]; urchins: [58]; corals: [91]). The potential for confounding influences on biofilm composition and grazing rates of L. littorea from exposure to a stressor and from being held for long time periods in artificial tank environments cannot be resolved here. Ongoing studies recognize this and incorporate sampling and measurement of the biotic parameters under study in the source field population at the same time as these are carried out for the mesocosm individuals. Comparisons of the control treatment animals with the source field population animals are able to show whether there has been an impact from individuals being held in artificial conditions during the experiment. It seems, therefore, that although short-term laboratory-based experiments provide value in identifying the potential mechanisms which underpin the response of physiological, behavioural and ecosystem-based processes to altered abiotic conditions, they are likely to overestimate the impact of novel conditions on individuals and underestimate the strength of grazers in structuring their environment as conditions change.
Longer term experiments are likely to be more representative of potential future changes to systems because they will not assess acclimation in individuals per se, which is only expressed in the system for the lifespan of the individual, but rather selection or other population level effects [92]. In our experiment, the 5-month gastropods that were used in the experiment were all individuals that had survived the 5-month exposure period and, as such, the different responses between 5-month and 2-week grazers could represent a combination of both acclimation and selection for stronger individuals. While we cannot distinguish between these two options in our experiment, if selection had occurred in the 5-month grazers it could be expected that the 2-week grazers would have greater variation in responses, which we did not see in our data. Experiments in other systems have shown that between five weeks (oysters [93]) and 16 months (sea urchins [58]) may be required for organisms to replenish energy stores and become fully acclimated to elevated CO2 conditions. Increasingly, it seems that the acute effects of ocean acidification are not expressed in the second generation, possibly meaning that the effects of ocean acidification will be overestimated unless epigenetic expression is taken into account. For example, juvenile mortality may not be elevated under high CO2, as previously thought, if parents are acclimated to high CO2 conditions [58,93]. However, there may still be ecosystem-level effects as energetic resources may be reassigned to produce fewer offspring to maintain quality, meaning that population size will decrease [94]. In the case of keystone species such as sea urchins, this will have large ramifications for ecosystem structure and function. For example, long-term exposure to high CO2 at natural CO2 seeps may cause multi-generational declines in populations of herbivorous sea urchins, which could allow macroalgae to increase in abundance, though this relationship is not simple and may partly depend on other factors such as the tolerance of different species to high CO2/low pH or abundance of preferred food [95]. While the use of small-scale laboratory experiments is informative with respect to the physiological responses of individuals to a range of environmental conditions, larger, long-term experiments will provide information on the ecosystem-level responses to these conditions.
5. Summary and conclusions
In summary, grazing rates in animals pre-exposed to experimental treatments for 5 months prior to the experiment were greater, and they were more sensitive to changes in temperature and CO2, than grazing rates in 2-week animals. Metabolic rate was affected only by temperature, and to maintain equivalent metabolic rates 5-month grazers needed more food than 2-week animals. Such experimental observations forecast a growing need to estimate energy budgets to identify whether such depressed consumption can be maintained in the longer term or whether animals are consuming metabolic reserves. Regardless, based on our experimental outcomes it appears that the metabolic rate of these grazers would need to be maintained at above present day levels to maintain energy budgets in response to forecast conditions of temperature and CO2. In the absence of long-term acclimation of animals to the new environmental conditions, ecosystems are likely to become trophically restructured unless the community shift in biofilms increases nutritional quality and quantity of the primary consumers' food source, allowing more energy to be consumed to counter detrimental shifts in the environment. In future, a combination of laboratory (physiology responses) and large, long-term experiments (ecosystem responses) may therefore be necessary to adequately predict the complex and interactive effects of climate change as organisms may acclimate to conditions over the longer term.
Acknowledgements
Thanks to R. Kleinjans for laboratory assistance, P. Calosi for use of his Strathkelvin O2 system and for the aid of S. Rastrick for assistance in setting it up.
Funding statement
B.R. and S.C. were supported by ARC grants and S.C. by an ARC Future Fellowship. N.M. was supported by a Marine Biological Association Research Fellowship. S.W., H.F., K.T. and N.M. received financial support from the NERC UK Ocean Acidification Programme and the work is a contribution to the project ‘Impacts of ocean acidification on key benthic ecosystems, communities, habitats, species and life cycles’.
References
- 1.Helmuth B, Mieszkowska N, Moore P, Hawkins SJ. 2006. Living on the edge of two changing worlds: forecasting the responses of rocky intertidal ecosystems to climate change. Annu. Rev. Ecol. Evol. Syst. 37, 373–404 (doi:10.1146/annurev.ecolsys.37.091305.110149) [Google Scholar]
- 2.Crain CM, Kroeker K, Halpern BS. 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 11, 1304–1315 (doi:10.1111/j.1461-0248.2008.01253.x) [DOI] [PubMed] [Google Scholar]
- 3.O'Connor MI. 2009. Warming strengthens an herbivore-plant interaction. Ecology 90, 388–398 (doi:10.1890/08-0034.1) [DOI] [PubMed] [Google Scholar]
- 4.Alsterberg C, Eklöf JS, Gamfeldt L, Havenhand JN, Sundbäck K. 2013. Consumers mediate the effects of experimental ocean acidification and warming on primary producers. Proc. Natl Acad. Sci. USA 110, 8603–8608 (doi:10.1073/pnas.1303797110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harley CDG, Connell SD. 2009. Shifts in abiotic variables and consequences for diversity. In Marine hard bottom communities (ed. Wahl M.), pp. 257–268 Berlin, Germany: Springer [Google Scholar]
- 6.Harley CDG, Hughes AR, Hultgren KM, Miner BG, Sorte CJB, Thornber CS, Rodriguez LF, Tomanek L, Williams SL. 2006. The impacts of climate change in coastal marine systems. Ecol. Lett. 9, 228–241 (doi:10.1111/j.1461-0248.2005.00871.x) [DOI] [PubMed] [Google Scholar]
- 7.Lima FP, Wethey DS. 2012. Three decades of high-resolution coastal sea surface temperatures reveal more than warming. Nat. Commun. 3, 704 (doi:10.1038/ncomms1713) [DOI] [PubMed] [Google Scholar]
- 8.IPCC 2007. Intergovernmental Panel on Climate Change: The AR4 Synthesis Report. See http://wwwipccch/
- 9.Hawkins SJ, Southward AJ, Genner MJ. 2003. Detection of environmental change in a marine ecosystem—evidence from the western English Channel. Sci. Total Environ. 310, 245–256 (doi:10.1016/S0048-9697(02)00645-9) [DOI] [PubMed] [Google Scholar]
- 10.IPCC 2007. Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL.). Cambridge, UK: Cambridge University Press [Google Scholar]
- 11.UKCIP 2009. Potential changes in the hydrography and circulation of the northwest European continental shelf. In UK climate projections science report: marine and coastal projections (eds Lowe JA, Howard TP, Pardaens A, Tinker J, Holt J, Wakelin S, Milne G, Leake J, Wolf J, Horsburgh K, Reeder T, Jenkins G, Ridley J, Dye S, Bradley S.) pp. 67–84 Exeter, UK: Met Office Hadley Centre. [Google Scholar]
- 12.Helmuth B, Harley CDG, Halpin PM, O'Donnell M, Hofmann GE, Blanchette CA. 2002. Climate change and latitudinal patterns of intertidal thermal stress. Science 298, 1015–1017 (doi:10.1126/science.1076814) [DOI] [PubMed] [Google Scholar]
- 13.Mieszkowska N, Kendall M, Hawkins S, Leaper R, Williamson P, Hardman-Mountford N, Southward A. 2006. Changes in the range of some common rocky shore species in Britain—a response to climate change? Mar. Biodiv. 555, 241–251 (doi:10.1007/s10750-005-1120-6) [Google Scholar]
- 14.Sorte CJB, Williams SL, Carlton JT. 2010. Marine range shifts and species introductions: comparative spread rates and community impacts. Glob. Ecol. Biogeogr. 19, 303–316 (doi:10.1111/j.1466-8238.2009.00519.x) [Google Scholar]
- 15.Spencer M, et al. 2012. Region-wide changes in marine ecosystem dynamics: state-space models to distinguish trends from step changes. Glob. Change Biol. 18, 1270–1281 (doi:10.1111/j.1365-2486.2011.02620.x) [Google Scholar]
- 16.Doney SC, Fabry VJ, Feely RA, Kleypas JA. 2009. Ocean acidification: the other CO2 problem. Annu. Rev. Mar. Sci. 1, 169–192 (doi:10.1146/annurev.marine.010908.163834) [DOI] [PubMed] [Google Scholar]
- 17.Blackford JC, Gilbert FJ. 2007. pH variability and CO2 induced acidification in the North Sea. J. Mar. Syst. 64, 229–241 (doi:10.1016/j.jmarsys.2006.03.016) [Google Scholar]
- 18.Rodolfo-Metalpa R, et al. 2011. Coral and mollusc resistance to ocean acidification moderated by warming. Nat. Climate Change 1, 308–312 (doi:10.1038/nclimate1200) [Google Scholar]
- 19.Byrne M, Lamare M, Winter D, Dworjanyn SA, Uthicke S. 2013. The stunting effect of a high CO2 ocean on calcification and development in sea urchin larvae, a synthesis from the tropics to the poles. Phil. Trans. R. Soc. B 368, 20120439 (doi:10.1098/rstb.2012.0439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bibby R, Widdicombe S, Parry H, Spicer J, Pipe R. 2008. Effects of ocean acidification on the immune response of the blue mussel Mytilys edulis. Aquat. Biol. 2, 67–74 (doi:10.3354/ab00037) [Google Scholar]
- 21.Kurihara H, Ishimatsu A. 2008. Effects of high CO2 seawater on the copepod (Acartia tsuensis) through all life stages and subsequent generations. Mar. Pollut. Bull. 56, 1086–1090 (doi:10.1016/j.marpolbul.2008.03.023) [DOI] [PubMed] [Google Scholar]
- 22.Johnson VR, Brownlee C, Rickaby REM, Graziano M, Milazzo M, Hall-Spencer JM. In press Responses of marine benthic microalgae to elevated CO2. Mar. Biol. (doi:10.1007/s00227-011-1840-2) [Google Scholar]
- 23.Connell SD, Russell BD. 2010. The direct effects of increasing CO2 and temperature on non-calcifying organisms: increasing the potential for phase shifts in kelp forests. Proc. R. Soc. B 277, 1409–1415 (doi:10.1098/rspb.2009.2069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurd CL, Hepburn CD, Currie KI, Raven JA, Hunter KA. 2009. Testing the effects of ocean acidification on algal metabolism: considerations for experimental designs. J. Phycol. 45, 1236–1251 (doi:10.1111/j.1529-8817.2009.00768.x) [DOI] [PubMed] [Google Scholar]
- 25.Connell SD, Kroeker KJ, Fabricius KE, Kline DI, Russell BD. 2013. The other ocean acidification problem: CO2 as a resource among competitors for ecosystem dominance. Phil. Trans. R. Soc. B 368, 20120442 (doi:10.1098/rstb.2012.0442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuffner IB, Andersson AJ, Jokiel PL, Rodgers KS, Mackenzie FT. 2007. Decreased abundance of crustose coralline algae due to ocean acidification. Nat. Geosci. 1, 114–117 (doi:10.1038/ngeo100) [Google Scholar]
- 27.Russell BD, Thompson JI, Falkenberg LJ, Connell SD. 2009. Synergistic effects of climate change and local stressors: CO2 and nutrient driven change in subtidal rocky habitats. Glob. Change Biol. 15, 2153–2162 (doi:10.1111/j.1365-2486.2009.01886.x) [Google Scholar]
- 28.Martin S, Gattuso JP. 2009. Response of Mediterranean coralline algae to ocean acidification and elevated temperature. Glob. Change Biol. 15, 2089–2100 (doi:10.1111/j.1365-2486.2009.01874.x) [Google Scholar]
- 29.Widdicombe S, Spicer JI. 2008. Predicting the impact of ocean acidification on benthic biodiversity: what can animal physiology tell us? J. Exp. Mar. Biol. Ecol. 366, 187–197 (doi:10.1016/j.jembe.2008.07.024) [Google Scholar]
- 30.Stillman JH. 2003. Acclimation capacity underlies susceptibility to climate change. Science 301, 65–65 (doi:10.1126/science.1083073) [DOI] [PubMed] [Google Scholar]
- 31.Pörtner HO, Farrell AP. 2008. Physiology and climate change. Science 322, 690–692 (doi:10.1126/science.1163156) [DOI] [PubMed] [Google Scholar]
- 32.Melzner F, Gutowska MA, Langenbuch M, Dupont S, Lucassen M, Thorndyke MC, Bleich M, Portner HO. 2009. Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeoscience 6, 2313–2331 (doi:10.5194/bg-6-2313-2009) [Google Scholar]
- 33.Dupont S, Ortega-Martínez O, Thorndyke M. 2010. Impact of near-future ocean acidification on echinoderms. Ecotoxicology 19, 449–462 (doi:10.1007/s10646-010-0463-6) [DOI] [PubMed] [Google Scholar]
- 34.Wicks LC, Roberts JM. 2012. Benthic invertebrates in a high CO2 world. In Oceanography and marine biology: an annual review, vol. 50 (eds Gibson RN, Atkinson RJA, Gordon JDM, Hughes RN.), pp. 127–187 [Google Scholar]
- 35.Wernberg T, Smale DA, Thomsen MS. 2012. A decade of climate change experiments on marine organisms: procedures, patterns and problems. Glob. Change Biol. 18, 1491–1498 (doi:10.1111/j.1365-2486.2012.02656.x) [Google Scholar]
- 36.Connell SD, Russell BD, Irving AD. 2011. Can strong consumer and producer effects be reconciled to better forecast ‘catastrophic’ phase-shifts in marine ecosystems? J. Exp. Mar. Biol. Ecol. 400, 296–301 (doi:10.1016/j.jembe.2011.02.031) [Google Scholar]
- 37.Pörtner HO. 2010. Oxygen-and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol. 213, 881–893 (doi:10.1242/jeb.037523) [DOI] [PubMed] [Google Scholar]
- 38.Edwards K, Barciela R, Butenschön M. 2012. Validation of the NEMO-ERSEM operational ecosystem model for the North West European Continental Shelf. Ocean Sci. Discuss. 9, 745–786 (doi:10.5194/osd-9-745-2012) [Google Scholar]
- 39.Sokolova IM, Portner HO. 2003. Metabolic plasticity and critical temperatures for aerobic scope in a eurythermal marine invertebrate (Littorina saxatilis, Gastropoda: Littorinidae) from different latitudes. J. Exp. Biol. 206, 195–207 (doi:10.1242/jeb.00054). [DOI] [PubMed] [Google Scholar]
- 40.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49, 711–745 (doi:10.1146/annurev.mi.49.100195.003431) [DOI] [PubMed] [Google Scholar]
- 41.Hawkins S, Hartnoll R, Kain J, Norton T. 1992. Plant animal interactions on hard substrata in the north-east Atlantic. In Systematics association, Spec Vol 46 (eds John DM, Hawkins SJ, Price JH.), pp. 1–32 Oxford, UK: Clarendon Press [Google Scholar]
- 42.Thompson R, Norton T, Hawkins S. 2004. Physical stress and biological control regulate the producer–consumer balance in intertidal biofilms. Ecology 85, 1372–1382 (doi:10.1890/03-0279) [Google Scholar]
- 43.Bustamante RH, et al. 1995. Gradients of intertidal primary productivity around the coast of South Africa and their relationships with consumer biomass. Oecologia 102, 189–201 (doi:10.1007/BF00333251) [DOI] [PubMed] [Google Scholar]
- 44.Jenkins S, et al. 2001. European-scale analysis of seasonal variability in limpet grazing activity and microalgal abundance. Mar. Ecol. Prog. Ser. 211, 193–203 (doi:10.3354/meps211193) [Google Scholar]
- 45.Hadfield MG. 2011. Biofilms and marine invertebrate larvae: what bacteria produce that larvae use to choose settlement sites. Annu. Rev. Mar. Sci. 3, 453–470 (doi:10.1146/annurev-marine-120709-142753) [DOI] [PubMed] [Google Scholar]
- 46.Meadows P, Williams G. 1963. Settlement of Spirorbis borealis Daudin larvae on surfaces bearing films of micro-organisms. Nature 198, 610–611 (doi:10.1038/198610b0) [Google Scholar]
- 47.Thompson R, Norton T, Hawkins S. 1998. The influence of epilithic microbial films on the settlement of Semibalanus balanoides cyprids—a comparison between laboratory and field experiments. Hydrobiology 375, 203–216 (doi:10.1023/A:1017036301082) [Google Scholar]
- 48.Findlay HS, Kendall MA, Spicer JI, Turley C, Widdicombe S. 2008. Novel microcosm system for investigating the effects of elevated carbon dioxide and temperature on intertidal organisms. Aquat. Biol. 3, 51–62 (doi:10.3354/ab00061) [Google Scholar]
- 49.Dickson A, Millero F. 1987. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res. A Oceanogr. 34, 1733–1743 (doi:10.1016/0198-0149(87)90021-5) [Google Scholar]
- 50.Pierrot D, Lewis E, Wallace DWR. 2006. MS Excel program developed for CO2 system calculations: ORNL/CDIAC-105a. Oak Ridge, TN: Carbon Dioxide Information Analysis Center Oak Ridge National Laboratory, US Department of Energy [Google Scholar]
- 51.Mehrbach C, Culberson C, Hawley J, Pytkowicz R. 1973. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Ocean 18, 897–907 (doi:10.4319/lo.1973.18.6.0897) [Google Scholar]
- 52.Weiss RF. 1970. The solubility of nitrogen, oxygen, and argon in water and seawater. Deep Sea Res. 17, 721–735 (doi:10.1016/0011-7471(70)90037-9) [Google Scholar]
- 53.Nübel U, Garcia-Pichel F, Muyzer G. 1997. PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl. Environ. Microbiol. 63, 3327–3332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clarke K, Gorley R. 2006. PRIMER v6: user manual/tutorial. Plymouth, UK: PRIMER-E [Google Scholar]
- 55.Caporaso JG, et al. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336 (doi:10.1038/nmeth.f.303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeSantis T, Dubosarskiy I, Murray S, Andersen G. 2003. Comprehensive aligned sequence construction for automated design of effective probes (CASCADE-P) using 16S rDNA. Bioinformatics 19, 1461–1468 (doi:10.1093/bioinformatics/btg200) [DOI] [PubMed] [Google Scholar]
- 57.Komárek J, Hauer T. 2012. CyanoDB.cz—On-line database of cyanobacterial genera. Tr˘ebon˘, Czech Republic: University of South Bohemia & Inst. of Botany, Academy of Sciences of the Czech Republic [Google Scholar]
- 58.Dupont S, Dorey N, Stumpp M, Melzner F, Thorndyke M. In press Long-term and trans-life-cycle effects of exposure to ocean acidification in the green sea urchin Strongylocentrotus droebachiensis. Mar. Biol. (doi:10.1007/s00227-012-1921-x) [Google Scholar]
- 59.Munday PL, McCormick MI, Meekan M, Dixson DL, Watson S-A, Chivers DP, Ferrari MCO. 2012. Selective mortality associated with variation in CO2 tolerance in a marine fish. Ocean Acidification 1, 1–5 (doi:10.2478/oac-2012-0001) [Google Scholar]
- 60.Calosi P, et al. 2013. Adaptation and acclimatization to ocean acidification in marine ectotherms: an in situ transplant experiment with polychaetes at a shallow CO2 vent system. Phil. Trans. R. Soc. B 368, 20120444 (doi:10.1098/rstb.2012.0444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tatters AO, et al. 2013. Short- and long-term conditioning of a temperate marine diatom community to acidification and warming. Phil. Trans. R. Soc. B 368, 20120437 (doi:10.1098/rstb.2012.0437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marshall DJ, McQuaid CD, Williams GA. 2010. Non-climatic thermal adaptation: implications for species’ responses to climate warming. Biol. Lett. 6, 669–673 (doi:10.1098/rsbl.2010.0233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frederich M, Pörtner HO. 2000. Oxygen limitation of thermal tolerance defined by cardiac and ventilatory performance in spider crab, Maja squinado. Am. J. Physiol. 279, R1531–R1538 [DOI] [PubMed] [Google Scholar]
- 64.Pörtner HO, Peck MA. 2010. Climate change effects on fishes and fisheries: towards a cause-and-effect understanding. J. Fish Biol. 77, 1745–1779 (doi:10.1111/j.1095-8649.2010.02783.x) [DOI] [PubMed] [Google Scholar]
- 65.Melatunan S, Calosi P, Rundle SD, Moody AJ, Widdicombe S. 2011. Exposure to elevated temperature and PCO2 reduces respiration rate and energy status in the periwinkle Littorina littorea. Physiol. Biochem. Zool. 84, 583–594 (doi:10.1086/662680) [DOI] [PubMed] [Google Scholar]
- 66.Findlay HS, Wood HL, Kendall MA, Spicer JI, Twitchett RJ, Widdicombe S. 2011. Comparing the impact of high CO2 on calcium carbonate structures in different marine organisms. Mar. Biol. Res. 7, 565–575 (doi:10.1080/17451000.2010.547200) [Google Scholar]
- 67.Bibby R, Cleall-Harding P, Rundle S, Widdicombe S, Spicer J. 2007. Ocean acidification disrupts induced defences in the intertidal gastropod Littorina littorea. Biol. Lett. 3, 699–701 (doi:10.1098/rsbl.2007.0457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Melatunan S, Calosi P, Rundle SD, Widdicombe S, Moody AJ. 2013. Effects of ocean acidification and elevated temperature on shell plasticity and its energetic basis in an intertidal gastropod. Mar. Ecol. Prog. Ser. 472, 155–168 (doi:10.3354/meps10046) [Google Scholar]
- 69.Lidbury I, Johnson V, Hall-Spencer JM, Munn CB, Cunliffe M. 2012. Community-level response of coastal microbial biofilms to ocean acidification in a natural carbon dioxide vent ecosystem. Mar. Pollut. Bull. 64, 1063–1066 (doi:10.1016/j.marpolbul.2012.02.011) [DOI] [PubMed] [Google Scholar]
- 70.Steneck R, Watling L. 1982. Feeding capabilities and limitation of herbivorous molluscs: a functional group approach. Mar. Biol. 68, 299–319 (doi:10.1007/BF00409596) [Google Scholar]
- 71.Nagarkar S, Williams GA, Subramanian G, Saha S. 2004. Cyanobacteria-dominated biofilms: a high quality food resource for intertidal grazers. Hydrobiology 512, 89–95 (doi:10.1023/B:HYDR.0000020313.09924.c1) [Google Scholar]
- 72.Witt V, Wild C, Anthony KRN, Diaz-Pulido G, Uthicke S. 2011. Effects of ocean acidification on microbial community composition of, and oxygen fluxes through, biofilms from the Great Barrier Reef. Environ. Microbiol. 13, 2976–2989 (doi:10.1111/j.1462-2920.2011.02571.x) [DOI] [PubMed] [Google Scholar]
- 73.Falkenberg LJ, Russell BD, Connell SD. 2013. Contrasting resource limitations between competing marine primary producers: implications for associated communities under enriched CO2 and nutrient regimes. Oecologia 172, 575–583 (doi:10.1007/s00442-012-2507-5) [DOI] [PubMed] [Google Scholar]
- 74.Rossoll D, Bermudez R, Hauss H, Schulz KG, Riebesell U, Sommer U, Winder M. 2012. Ocean acidification-induced food quality deterioration constrains trophic transfer. PLoS ONE 7, e34737 (doi:10.1371/journal.pone.0034737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fink P, Von Elert E. 2006. Physiological responses to stoichiometric constraints: nutrient limitation and compensatory feeding in a freshwater snail. Oikos 115, 484–494 (doi:10.1111/j.2006.0030-1299.14951.x) [Google Scholar]
- 76.Russell BD, Connell SD. 2007. Response of grazers to sudden nutrient pulses in oligotrophic versus eutrophic conditions. Mar. Ecol. Prog. Ser. 349, 73–80 (doi:10.3354/meps07097) [Google Scholar]
- 77.Imrie DW, McCrohan CR, Hawkins SJ. 1990. Feeding-behavior in Littorina littorea—a study of the effects of ingestive conditioning and previous dietary history on food preference and rates of consumption. Hydrobiologia 193, 191–198 (doi:10.1007/bf00028076) [Google Scholar]
- 78.Sokolova IM, Portner HO. 2001. Temperature effects on key metabolic enzymes in Littorina saxatilis and L. obtusata from different latitudes and shore levels. Mar. Biol. 139, 113–126 (doi:10.1007/s002270100557) [Google Scholar]
- 79.Pörtner HO. 2008. Ecosystem effects of ocean acidification in times of ocean warming: a physiologist's view. Mar. Ecol. Prog. Ser. 373, 203–217 (doi:10.3354/meps07768) [Google Scholar]
- 80.Mieszkowska N, Hawkins S, Burrows M, Kendall M. 2007. Long-term changes in the geographic distribution and population structures of Osilinus lineatus (Gastropoda: Trochidae) in Britain and Ireland. J. Mar. Biol. Assoc. UK 87, 537–545 (doi:10.1017/S0025315407053799) [Google Scholar]
- 81.Fabry VJ, Seibel BA, Feely RA, Orr JC. 2008. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 65, 414–432 (doi:10.1093/icesjms/fsn048) [Google Scholar]
- 82.Pistevos JCA, Calosi P, Widdicombe S, Bishop JDD. 2011. Will variation among genetic individuals influence species responses to global climate change? Oikos 120, 675–689 (doi:10.1111/j.1600-0706.2010.19470.x) [Google Scholar]
- 83.Miller GM, Watson S-A, Donelson JM, McCormick MI, Munday PL. 2012. Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nat. Clim. Change 2, 858–861 (doi:10.1038/nclimate1599) [Google Scholar]
- 84.Pespeni MH, et al. 2013. Evolutionary change during experimental ocean acidification. Proc. Natl Acad. Sci. USA 110, 6937–6942 (doi:10.1073/pnas.1220673110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hatcher BG, Larkum AWD. 1983. An experimental analysis of factors controlling the standing crop of the epilithic algal community on a coral reef. J. Exp. Mar. Biol. Ecol. 69, 61–84 (doi:10.1016/0022-0981(83)90172-7) [Google Scholar]
- 86.Nystrom M, Folke C, Moberg F. 2000. Coral reef disturbance and resilience in a human-dominated environment. Trends Ecol. Evol. 15, 413–417 (doi:10.1016/S0169-5347(00)01948-0) [DOI] [PubMed] [Google Scholar]
- 87.Hawkins S, et al. 2009. Consequences of climate-driven biodiversity changes for ecosystem functioning of North European rocky shores. Mar. Ecol. Prog. Ser. 396, 245–259 (doi:10.3354/meps08378) [Google Scholar]
- 88.Paine RT. 2002. Trophic control of production in a rocky intertidal community. Science 296, 736–739 (doi:10.1126/science.1069811) [DOI] [PubMed] [Google Scholar]
- 89.Russell BD, Connell SD. 2005. A novel interaction between nutrients and grazers alters relative dominance of marine habitats. Mar. Ecol. Prog. Ser. 289, 5–11 (doi:10.3354/meps289005) [Google Scholar]
- 90.Donelson JM, Munday PL, McCormick MI, Pitcher CR. 2012. Rapid transgenerational acclimation of a tropical reef fish to climate change. Nat. Clim. Change 2, 30–32 (doi:10.1038/nclimate1323) [Google Scholar]
- 91.Form AU, Riebesell U. 2012. Acclimation to ocean acidification during long-term CO2 exposure in the cold-water coral Lophelia pertusa. Glob. Change Biol. 18, 843–853 (doi:10.1111/j.1365-2486.2011.02583.x) [Google Scholar]
- 92.Russell BD, Harley CDG, Wernberg T, Mieszkowska N, Widdicombe S, Hall-Spencer JM, Connell SD. 2012. Predicting ecosystem shifts requires new approaches that integrate the effects of climate change across entire systems. Biol. Lett. 8, 164–166 (doi:10.1098/rsbl.2011.0779) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Parker LM, Ross PM, O'Connor WA, Borysko L, Raftos DA, Pörtner HO. 2012. Adult exposure influences offspring response to ocean acidification in oysters. Glob. Change Biol. 18, 82–92 (doi:10.1111/j.1365-2486.2011.02520.x) [Google Scholar]
- 94.Podolsky RD. 2006. Plasticity of embryo encapsulation and density in intertidal egg masses. Integr. Comp. Biol. 46, E112 [Google Scholar]
- 95.Calosi P, Rastrick SPS, Graziano M, Thomas SC, Baggini C, Carter HA, Hall-Spencer JM, Milazzo M, Spicer JI. In press Distribution of sea urchins living near shallow water CO2 vents is dependent upon species acid–base and ion-regulatory abilities. Mar. Pollut. Bull. (doi:10.1016/j.marpolbul.2012.11.040) [DOI] [PubMed] [Google Scholar]