Abstract
Ocean acidification (OA), caused by the dissolution of increasing concentrations of atmospheric carbon dioxide (CO2) in seawater, is projected to cause significant changes to marine ecology and biogeochemistry. Potential impacts on the microbially driven cycling of nitrogen are of particular concern. Specifically, under seawater pH levels approximating future OA scenarios, rates of ammonia oxidation (the rate-limiting first step of the nitrification pathway) have been shown to dramatically decrease in seawater, but not in underlying sediments. However, no prior study has considered the interactive effects of microbial ammonia oxidation and macrofaunal bioturbation activity, which can enhance nitrogen transformation rates. Using experimental mesocosms, we investigated the responses to OA of ammonia oxidizing microorganisms inhabiting surface sediments and sediments within burrow walls of the mud shrimp Upogebia deltaura. Seawater was acidified to one of four target pH values (pHT 7.90, 7.70, 7.35 and 6.80) in comparison with a control (pHT 8.10). At pHT 8.10, ammonia oxidation rates in burrow wall sediments were, on average, fivefold greater than in surface sediments. However, at all acidified pH values (pH ≤ 7.90), ammonia oxidation rates in burrow sediments were significantly inhibited (by 79–97%; p < 0.01), whereas rates in surface sediments were unaffected. Both bacterial and archaeal abundances increased significantly as pHT declined; by contrast, relative abundances of bacterial and archaeal ammonia oxidation (amoA) genes did not vary. This research suggests that OA could cause substantial reductions in total benthic ammonia oxidation rates in coastal bioturbated sediments, leading to corresponding changes in coupled nitrogen cycling between the benthic and pelagic realms.
Keywords: ocean acidification, ammonia oxidation, marine sediments, bioturbation, Upogebia deltaura
1. Introduction
In the past 200 years, increasing levels of atmospheric CO2 dissolving in the world's oceans have caused the pH of surface waters to decrease by 0.1 units to an average of pH 8.10, a process termed ‘ocean acidification’ (OA). By the end of this century, global ocean surface pH could decrease by a further 0.3–0.5 units [1,2]. Varying responses to OA, both positive and negative, have been observed in marine organisms. However, there have been relatively few studies that have investigated the impacts of OA on microbial processes, particularly those that affect the major biogeochemical cycles within the ocean [3,4]. Of these, it is generally assumed that the marine nitrogen cycle will be especially susceptible to disruption [5]. In particular, ammonia oxidation rates in seawater have been shown to decline by 3–44% as a linear function of decreasing pH, in both in situ and experimental studies covering a range of pH values (from 6.0 to approx. 8.10) [6–9].
Ammonia oxidation is generally considered to be the critical rate-limiting step of the nitrification process, which converts ammonium to nitrate via ammonia oxidation (NH4+ → NO2−) and nitrite oxidation (NO2− → NO3−). As such, ammonia oxidation rates are often measured as an indication of overall nitrification rates. The significant inhibition of ammonia oxidation rates within the water column is widely thought to be due to the kinetics equilibrium of the NH3/NH4+ redox-pair, which predicts a reduced availability of NH3, the substrate for ammonia oxidation, at pH levels below pH 8.0 [10,11].
Sediments are particularly important sites for microbially driven transformations of nitrogen, where nitrification provides an essential link between organic matter degradation and nutrient regeneration, thus supporting primary productivity in marine ecosystems [12,13]. Potential nitrification rates can be 10–1000-fold higher in sediments than in the overlying water [14]. Recently, we have shown [8] that for several different sediment types a reduction in seawater pH had no effect upon ammonia oxidation rates in surface sediments, in contrast to the reduction in rates observed within the corresponding overlying water samples. We suggested two reasons for this discrepancy: firstly, that the dissolution of carbonate minerals within the sediment may act to buffer the pore water pH, minimizing the effect of OA, and/or, secondly, that a change in the structure of microbial nitrifying communities occured within the sediment, favouring bacteria or archaea that are more tolerant of low-pH conditions [8].
A further consideration highlighted by Blackford & Gilbert [9] is the specific effects of OA on benthic macrofauna, which can enhance the fluxes of dissolved inorganic nitrogen across the sediment–water interface, as well as the rates of denitrification and coupled nitrification–denitrification [15]. Current models for predicting future nitrification rates in North Sea coastal zones do not consider such interactions [9]; however, studies involving macrofaunal bioturbators reveal that significant changes to nutrient fluxes across the sediment–water interface can occur at reduced seawater pH [16–19]. In order to understand the impacts of OA on marine ecosystems, it is therefore important to consider benthic, as well as pelagic, systems.
Thalassinidean shrimp (mud or ghost shrimp) are one of the most active groups of burrowing macrofauna in coastal and estuarine sediments, globally [20,21]. Of these, the species Upogebia deltaura (Leach, 1815) [22] is distributed between Norway and the Mediterranean Sea and is highly abundant in the Irish Sea, the Black Sea, the North Sea and the Baltic Sea, where it has been found at densities of up to 50 individuals per m2 [23,24]. U. deltaura is one of two species of thalassinidean shrimp which dominate the soft sediments of Plymouth Sound (UK) [25]. We have previously shown that the large, semi-permanent burrows of this species support bacterial communities that are distinct from, and more diverse than, the surrounding surface and subsurface ambient sediment [26]. Additionally, the presence of U. deltaura is known to increase threefold the rates of denitrification and coupled nitrification–denitrification within the sediment [27]. Therefore, we have used U. deltaura as a model bioturbator whose activities significantly enhance the microbially driven cycling of nitrogen within coastal sediments. A previous study [28] of the effects of OA on this species indicates that it is physiologically and behaviourally affected by reduced seawater pH. However, thus far, these effects have only been demonstrated at very low pH (less than pH 7.00) in ‘mimic’ burrows composed of transparent plastic tubing housed in experimental aquaria [28].
In this study, we present ammonia oxidation rate data from a 14-week mesocosm study of coastal muddy-sand sediment inhabited by individual U. deltaura and acidified (by CO2 addition) to one of four pH treatments (7.90, 7.70, 7.35, 6.80), compared with an ambient control treatment (8.10). Ammonia oxidation rates were measured at all pH levels in sediment collected from the shrimp burrow walls and sediment surface, as well as in the overlying water. Ammonia-oxidizing bacteria (AOB) and ammonia-oxidizing archaea (AOA) were enumerated using quantitative polymerase chain reaction (q-PCR). In order to assess the physiological responses of the shrimp to decreasing pH, we also measured changes in shrimp haemolymph pH, as an indication of extracellular acidosis. The results presented here enhance our understanding of how ecosystem-level responses to OA are both complex and likely to be dependent upon interactions between multiple life forms.
2. Material and methods
(a). Benthic mesocosm system and sampling
Manipulative experiments designed to assess the medium-term (14-week) impacts of OA upon benthic ammonia oxidation rates and carbon chemistry were carried out within the benthic mesocosm of the Plymouth Marine Laboratory. Sandy-mud sediment [40% sand (2–0.063 mm); 60% mud (less than 0.063 mm)] was collected on 29 September 2009 using a box corer from a site in Jennycliff Bay, Plymouth Sound (50.346 N, 04.127 W; 10 m water depth). This sediment was defaunated of large macrofauna by hand as previously described [26] and used to fill 20 sediment cores (65 cm deep, internal diameter 30 cm). Any U. deltaura recovered were placed in individual plastic mesh containers and immersed in continuously flowing seawater. Sediment cores were randomly distributed between 10 tanks (see the electronic supplementary material), topped up with seawater and allowed to settle overnight. Each tank was filled with seawater on 1 October, 2009 before shrimp were added. Ten individuals of U. deltaura were collected from the Jennycliff Bay site on 29 September, and on 1 October a single U. deltaura (equivalent to a population density of 14 individuals m−2) was introduced into each of 10 of the sediment cores to be used in this experiment (see electronic supplementary material, figure S1). As the first sampling trip did not yield enough shrimp for the study, on 7 October a second group of shrimp were collected from Jennycliff Bay and introduced into the remaining 10 cores (see electronic supplementary material, figure S1). All shrimp were weighed (blotted wet weight) prior to addition to the sediment and monitored to ensure that they successfully established sediment burrows.
Seawater was collected from the L4 site in the Western English Channel (www.westernchannelobservatory.org.uk) and filtered (1 µm) prior to addition to the tanks. Ambient air (at approximately 380 ppm CO2) was bubbled through each tank from an external air supply. For each row, water was allowed to circulate freely between tanks for approximately nine weeks before acidification treatments were started. This settling period was to allow shrimp to establish their burrows and to allow microbial communities to regain their spatial structure after sediment disturbance during collection. At the end of this period, between-tank circulation was stopped, and each tank thereafter treated separately.
For 16 of the sediment cores, the overlying seawater was acidified to one of four target pH treatments (pHT 7.90, 7.70, 7.35 and 6.80) using CP grade 99.95% CO2 gas (BOC, Manchester, UK). Four cores were kept at control pH (pH 8.10). Each treatment comprised four replicate sediment cores with an individual burrow in each core, with the exception of pHT 6.80, in which one shrimp failed to burrow. Temperature, pH, salinity and alkalinity in the overlying water were monitored throughout the 14-week exposure period. Detailed descriptions of the acidification system and sediment sampling strategy are provided in the electronic supplementary material. Briefly, at the end of the exposure, the cores were drained and ammonia oxidation rate measurements made within overlying water samples as well as in surface sediment samples. The sediment cores were then extruded using a hydraulic pump to expose sediment horizons at 5 cm intervals, as previously described [26]. Shrimp burrows were broken apart by hand, and sediment samples for ammonia oxidation rates were taken from the burrow wall. Additional samples for elemental (CHN) and molecular analyses were taken from the burrow wall at the depths indicated in the electronic supplementary material, frozen immediately and stored until analysis.
(b). Ammonia oxidation rate measurements
Ammonia (NH3) oxidation rates were determined from sediment collected from a range of depths within the shrimp burrow walls (see electronic supplementary material for sampling strategy), from the surface sediments from each core and in the overlying water as previously described [8]. Briefly, for each sample, 24-h slurry type incubations were set up in three sets of triplicate bottles. For sediment slurries, vials were filled with approximately one-third sediment and approximately two-thirds seawater from the corresponding pH, while seawater alone was used only for overlying water incubations. Following incubations, the vials were dried and weighed to determine the dry weight of sediment in each bottle, which was converted into wet sediment weight using the sediment porosity determined separately. Two of these sets of vials were treated with allylthiourea (ATU) in order to inhibit NH3 oxidation, and the remaining set was treated with sodium chlorate (NaClO3) to inhibit NO2− oxidation. NH3 oxidation rates were calculated as accumulation of NO2− in the NaClO3 treatment compared with the ATU treatments from T0 and T24, with NO2− accumulation being determined by colorimetric assay [8,29]. We observed no additional NO2− production in ATU-treated vials at T24 in comparison to the start of the experiment (T0), indicating that ammonia oxidation was completely inhibited by ATU. Parallel incubations completed over 12, 24 and 48 h indicated that oxygen was not limited within the slurry incubations performed here.
(c). Microbial gene abundances
DNA was extracted from 0.5 g sediment samples using a bead-beating technique with phenol–chloroform, as previously described [26]. q-PCR assays were performed in order to quantify bacterial and archaeal 16S rRNA and amoA genes, using the primers and conditions stated in the electronic supplementary material.
(d). Carbon system measurements
Alkalinity samples (250 ml) were poisoned with 50 µl of a saturated solution of mercuric chloride (HgCl2) and analysed using potentiometric open-cell titration [30]. Elemental (CHN) analyses were performed according to Head et al. [31]. Carbonate system parameters (pCO2, dissolved inorganic carbon (DIC) and calcite and aragonite saturation states) for the overlying water in each tank for the experiment duration were calculated in CO2SYS [32], using measured temperature, salinity, alkalinity and pH values as well as silicate and phosphate concentrations.
(e). Shrimp haemolymph pH
As soon as each shrimp was exposed within its burrow during sampling, it was removed from the sediment, dried gently by dabbing with a paper towel and weighed. Haemolymph was then extracted and its pH measured as previously described [28]. Typically, between 0.5 and 1.5 ml of haemolymph was collected, depending on the size of the shrimp. In some cases, it was impossible to collect enough haemolymph for accurate pH measurement.
(f). Statistical analyses
Statistical analyses for rate measurements and gene abundance were performed in PRIMER v. 6.1 [33]. Data were transformed as stated subsequently in order to approximate normal distribution. The appropriate similarity matrices were then created from transformed data in PRIMER v. 6.1. Bray–Curtis resemblance matrices were used for gene data throughout this study, whereas Euclidean Distance similarity matrices were used for all process rate and environmental data. Significant differences between treatment groups were tested for using the PERMANOVA+ add-on [34]. For all tests, fixed factors were used (i.e. sediment category or pH treatment) in a crossed design, and 95% CIs (p < 0.05) were used as cut-off levels for significance. The degrees of freedom for individual tests are reported (Fd.f.1,d.f.2).
3. Results
(a). Ammonia oxidation response to pH
Ammonia oxidation rates in the overlying water decreased with reduced pH (figure 1). From the best linear fit to the data (R2 = 0.72), the ammonia oxidation rate in seawater (normalized to the concentration of ammonium in the water) can be calculated as
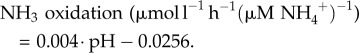 |
3.1 |
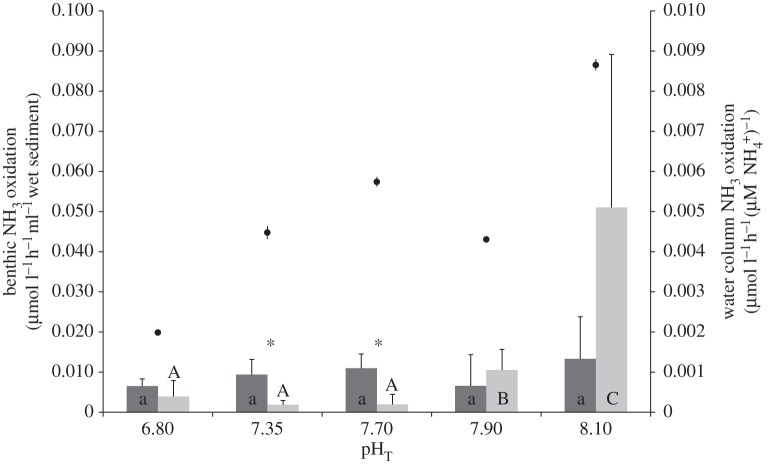
Figure 1.
Ammonia oxidation rates in sediments (left-hand axis) and overlying water column (right-hand axis). Rates were determined for different pH treatments after a 14-week exposure period. Surface sediment (dark grey bars); burrow sediment (light grey bars). Water-column ammonia oxidation rates (filled circles) are normalized to water column [NH4+]. Error bars represent standard deviation; for water column, n = 3; for surface sediments, n = 4; for burrow sediments, n = 8 except for pHT 6.80, where n = 5 (see electronic supplementary material). Significant differences in ammonia oxidation rates between different pH treatments are indicated by different lower case letters for surface sediment and different capital letters for burrow sediment. Within individual pH treatments, an asterisk (*) indicates a significant difference in ammonia oxidation rates between surface and burrow sediments.
Using this equation, the ammonia oxidation rates observed here decreased by 23% between the mean pre-exposure pH (8.06; table 1) at the beginning of this experiment and a year-2100 ‘worst-case scenario’ pH of 7.67 [9].
Table 1.
Physico-chemical variation within the carbonate system pre-exposure (PE) and at the start (s) and end (e) of the 14-week pH-exposure period. Mean values (M) ± standard deviation (s.d.) are shown (n = 19). pH, alkalinity, temperaturea and salinity were measured within mesocosm tanks as described in the electronic supplementary material. Values for pCO2, dissolved inorganic carbon (DIC), calcite saturation state (Ωcal) and aragonite saturation state (Ωarag) were calculated using CO2SYS [32].
| pH (NBS scale) |
alkalinity (µmol kg−1) |
temperature (°C) |
salinity |
pCO2 (µatm) |
DIC (µmol kg−1) |
Ωcal |
Ωarag |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| target pH (pHT) | M | s.d. | M | s.d. | M | s.d. | M | s.d. | M | s.d. | M | s.d. | M | s.d. | M | s.d. | ||
| PE | – | 8.06 | 0.02 | 2479 | 43 | 14.2 | 0.6 | 36.5 | 0.2 | 549 | 22 | 2292 | 39 | 3.4 | 0.2 | 2.2 | 0.1 | |
| 8.10 | s | 8.12 | 0.01 | 2398 | 60 | 16.3 | 0 | 35.1 | 0.3 | 475 | 20 | 2182 | 60 | 3.8 | 0.1 | 2.4 | 0 | |
| e | 8 | 0.06 | 2557 | 4 | 10.8 | 0.3 | 35.4 | 0.1 | 675 | 95 | 2421 | 22 | 2.7 | 0.3 | 1.7 | 0.2 | ||
| 7.90 | s | 7.89 | 0.06 | 2408 | 4 | 16.3 | 0 | 35.1 | 0.3 | 872 | 140 | 2290 | 29 | 2.4 | 0.3 | 1.5 | 0.2 | |
| e | 7.93 | 0.11 | 2573 | 39 | 10.9 | 0.1 | 35.3 | 0 | 802 | 212 | 2458 | 6 | 2.4 | 0.6 | 1.5 | 0.4 | ||
| 7.70 | s | 7.67 | 0.06 | 2468 | 83 | 16.4 | 0.3 | 35.2 | 0.1 | 1523 | 154 | 2425 | 61 | 1.6 | 0.3 | 1 | 0.2 | |
| e | 7.69 | 0 | 2637 | 6 | 11.6 | 0.8 | 35.2 | 0.1 | 1475 | 12 | 2606 | 1 | 1.5 | 0 | 0.9 | 0 | ||
| 7.35 | s | 7.49 | 0.05 | 2440 | 34 | 16.3 | 0 | 35.3 | 0 | 2359 | 313 | 2459 | 52 | 1 | 0.1 | 0.7 | 0.1 | |
| e | 7.27 | 0.08 | 3078 | 141 | 11.1 | 0.1 | 35.4 | 0.1 | 4693 | 725 | 3235 | 102 | 0.7 | 0.2 | 0.4 | 0.1 | ||
| 6.80 | s | 7.24 | 0.60 | 2453 | 16 | 16.5 | 0.2 | 35.1 | 0.3 | 6494 | 6955 | 2629 | 315 | 0.9 | 0.9 | 0.6 | 0.6 | |
| e | 6.76 | 0.20 | 3587 | 231 | 11.1 | 0.8 | 35.3 | 0 | 18081 | 445 | 4335 | 229 | 0.2 | 0 | 0.2 | 0 | ||
aTemperature was reduced gradually at the beginning of the exposure to match the average in situ temperatures within Jennycliff Bay between December and March (target temperature: 11°C).
Within the sediment, there was a significant interaction between pH and sediment category (surface or burrow wall sediment; F4,47 = 5.08, p < 0.01). Within surface sediments, there was no significant effect of pH on ammonia oxidation rates (F4,15 = 1.09, p > 0.05). However, within burrow wall sediments, ammonia oxidation rates decreased by 80% from pH 8.10 to 7.90 (F1,14 = 2.64, p < 0.03) and by a further 83% between pH 7.90 and 7.70 (F1,14 = 5.10, p < 0.01; figure 1). There were no further significant differences between ammonia oxidation rates in burrow sediments at pH 7.70, 7.35 and 6.80. However, at pHT 7.35 and 7.70, the burrow wall ammonia oxidation rates were lower than those in surface sediments at these pH levels (F1,7 = 5.84 and 4.85, respectively; p < 0.01; figure 1). At pH 7.90 and 8.10, there was no significant difference between the ammonia oxidation rates in burrow sediment and surface sediment, although at pHT 8.10 burrow wall rates were, on average, five times higher than surface rates (figure 1). Furthermore, it is clear that at pH 8.10 there was more variability in burrow wall rates (NH3 oxidation (µmol l−1 h−1 ml−1 wet sediment) range: 0.004–0.028 in surface sediments; 0.001–0.100 in burrow sediments).
(b). Changes to the carbon system
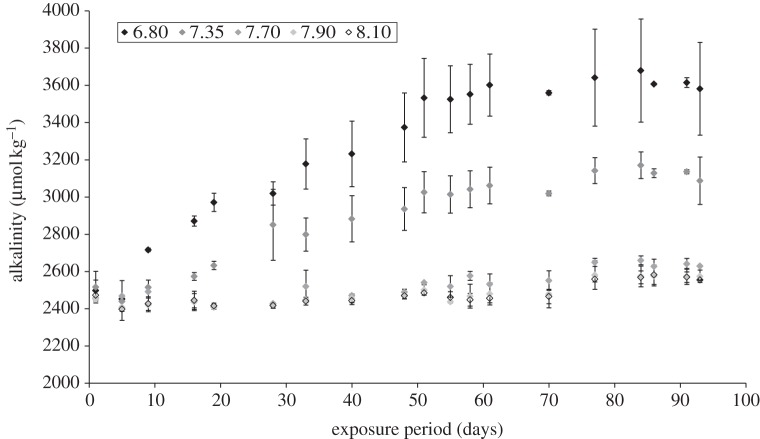
As these experiments were carried out using pH-controlled systems, the pH remained stable at the target treatment values (pHT) over the exposure period. Measured pH and calculated pCO2 levels were significantly different between different treatments (pH: F4,94 = 474, p < 0.01; pCO2: F4,94 = 343, p < 0.01; table 1). In all pH treatments, total alkalinity (figure 2) increased significantly after 14 weeks (F72,94 = 5.64, p < 0.01; table 1). In particular, alkalinity increased overall in the pH 7.35 and pH 6.80 treatments by 26% and 46%, respectively, compared with an average 6.8% increase for other treatments. In all treatments, pCO2 increased by the end of the 14-week exposure (table 1) as a result of the increase in total alkalinity, which altered the carbonate chemistry of the seawater. Calculated DIC (using CO2SYS) therefore also increased significantly throughout the exposure period (F72,94 = 2.99, p < 0.01; table 1). For pH 7.70, 7.90 and 8.10, DIC increased by an average 9%. For pH 7.35 and 6.80, the increase in DIC was 32% and 65%, respectively. The observed increases in DIC and alkalinity were of an asymptotic function, showing that the carbonate system was adjusting to a new state over time (ca 50 days).
Figure 2.
Water column total alkalinity for different pH treatments over a 14-week exposure period, beginning on 2 December 2009 and ending on 5 March 2010. pH treatments are as indicated. Error bars represent standard deviation for duplicate tanks within each pH treatment (n = 2).
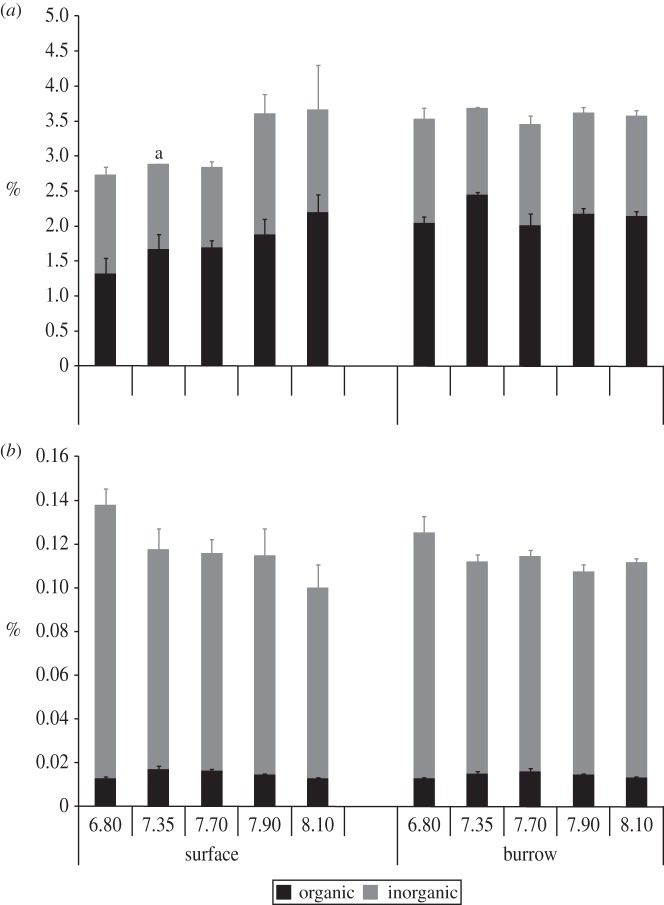
There was a significant, negative overall effect of reduced pH on sediment inorganic carbon content (F4,49 = 3.67, p < 0.05; figure 3a). The % inorganic carbon was significantly lower at pHT 6.80 than at pHT 8.10, 7.90 and 7.35 (F1,17 = 3.04, F1,22 = 2.94 and F1,15 = 2.54, respectively; p < 0.05). This effect was seemingly greatest within surface sediments (figure 3a); however, post hoc tests revealed no significant differences between pH levels within individual sediment categories. Overall, the inorganic carbon (CaCO3) content in both surface and burrow sediments was low (below 2.5%). Organic carbon content did not differ significantly with either sediment category or pH (figure 3a). In general, total nitrogen did not vary between surface or burrow sediments, but organic nitrogen content (%) was significantly higher in burrow and surface sediments at pH 6.80 (F4,50 = 5.29, p < 0.05; figure 3b), when compared with higher pH treatments.
Figure 3.
Inorganic and organic (a) carbon and (b) nitrogen content in surface and burrow wall sediments with varying pH, measured after a 14-week period. Error bars represent standard error; for surface sediment, n = 4; for burrow sediment, n = 5 at pHT 8.10; n = 8 at pHT 7.90; n = 7 at pHT 7.70; n = 4 at pHT 7.35; n = 3 at pHT 6.80. *Standard error not determined because n = 2 (see the electronic supplementary material).
(c). 16S rRNA and amoA gene abundances
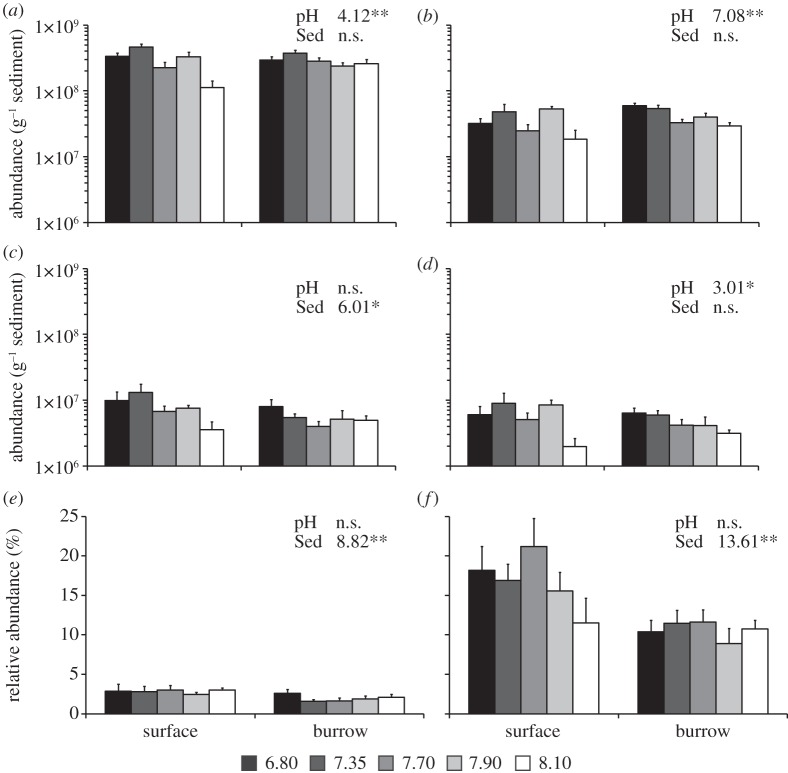
Bacterial and archaeal 16S rRNA gene abundances increased significantly with a reduction in pH (figure 4a,b). Bacterial and archaeal amoA gene abundances also increased at low pH; however, this effect was only significant for archaeal amoA (figure 4d). Bacterial amoA genes were on average 1.5 times more abundant in surface sediments than in burrow sediments (figure 4c). There were no significant interactions (p > 0.05) between sediment category (surface versus burrow) and pH for any of the genes studied herein. Bacterial amoA and 16S rRNA genes were on average 1.3 and eight times more abundant than the corresponding archaeal genes, respectively; however, there was no distinct trend in the ratio of bacterial : archaeal genes with pH or between sediment categories.
Figure 4.
Variation in abundance of bacterial and archaeal 16SrRNA and amoA genes with pH in surface and burrow sediments. (a) Bacterial 16S rRNA; (b) archaeal 16S rRNA; (c) bacterial amoA; (d) archaeal amoA; and variation in amoA relative abundance (normalized to the appropriate 16S rRNA gene abundance) in burrow and surface sediments for (e) bacterial amoA; (f) archaeal amoA. (a–d) are shown on the same logarithmic scale, for comparison. Error bars represent standard error based on n = 4 per pH treatment for surface sediment and n = 12 for burrow sediment, except for burrow samples from pHT 6.80, where n = 9. For each panel, statistical differences in gene abundances are shown between sediment category (surface versus burrow; Sed) and pH. PERMANOVA tests were performed on Bray–Curtis resemblance matrices derived from log (x + 1)-transformed q-PCR abundance data. F values significant at p < 0.05 (*) and p < 0.01 (**) are shown. n.s., not significant.
In order to assess the impacts of pH on functional genes relative to the changes to the total microbial community, amoA gene abundances were normalized to the abundance of the appropriate 16S rRNA gene and plotted as % relative abundance (figure 4e,f). While the relative abundance of bacterial amoA genes was typically 1.5–3%, the relative abundance of archaeal amoA was between 10 and 20%. Both bacterial and archaeal amoA had a higher relative abundance within surface sediments than within burrow sediments; however, there were no significant differences in relative abundance with pH (figure 4e,f). There was no significant correlation (p > 0.05) between either bacterial or archaeal amoA gene numbers and rates of ammonia oxidation.
(d). Shrimp haemolymph pH and mortality
Out of 20 shrimp, a total of four shrimp did not survive the exposure period. Two shrimp from pH 6.80 and one shrimp from pH 7.35 were found dead within their burrows on the sampling date indicated (table 2); a third shrimp from pH 6.80 had already been found dead outside of its burrow prior to the end of the exposure. This burrow was then considered to be a ‘relict’ burrow, which may be subject to passive flushing [35]. Shrimp haemolymph pH (table 2) was significantly lower at pHT 6.80 and 7.35 than at pHT ≥ 7.70 (F1,12 = 6.80, p < 0.05), indicating the occurrence of extracellular acidosis below pHT 7.70.
Table 2.
Distribution of shrimp weights across treatments, indicating the change in weight as a % of initial weight and the final haemolymph pH of the shrimp. Core and tank numbers refer to those depicted in the electronic supplementary material, figure S1. The mean (±s.d.) haemolymph pH for each treatment is shown in italic, except for pHT 6.80, for which only one observation was made. n.d., not determined.
| core no. | tank no. | treatment pH | date collected | end date | change in weight, % of initial weight | haemolymph pH |
|---|---|---|---|---|---|---|
| 6 | 1.3 | 8.10 | 29.09.09 | 08.03.10 | +8.62 | n.d. |
| 24 | 29.09.09 | 08.03.10 | −12.52 | 7.78 | ||
| 25 | 3.3 | 07.10.09 | 08.03.10 | +4.63 | n.d. | |
| 14 | 07.10.09 | 08.03.10 | +13.66 | 7.79 | ||
| 7.79 ± 0.01 | ||||||
| 9 | 1.4 | 7.90 | 29.09.09 | 09.03.10 | +2.52 | 8.02 |
| 8 | 29.09.09 | 09.03.10 | +51.15 | 7.92 | ||
| 5 | 2.3 | 07.10.09 | 09.03.10 | +10.85 | 7.72 | |
| 11 | 07.10.09 | 09.03.10 | +0.13 | 7.72 | ||
| 7.85 ± 0.15 | ||||||
| 13 | 1.1 | 7.70 | 29.09.09 | 10.03.10 | +17.54 | 8.04 |
| 15 | 29.09.09 | 10.03.10 | +14.51 | 7.83 | ||
| 12 | 2.2 | 07.10.09 | 10.03.10 | +7.85 | 7.86 | |
| 19 | 07.10.09 | 10.03.10 | +5.24 | 7.94 | ||
| 7.92 ± 0.09 | ||||||
| 17 | 2.4 | 7.35 | 29.09.09 | 11.03.10 | +14.48 | 7.25 |
| 1 | 29.09.09 | 11.03.10 | +22.65 | 7.64 | ||
| 22 | 3.2 | 07.10.09 | 11.03.10 | n.d.a | n.d.a | |
| 10 | 07.10.09 | 11.03.10 | −5.05 | 7.37 | ||
| 7.42 ± 0.20 | ||||||
| 16 | 1.2 | 6.80 | 29.09.09 | 12.03.10 | n.d.a | n.d.a |
| 7 | 29.09.09 | 12.03.10 | +43.70 | 7.53 | ||
| 3 | 3.4 | 07.10.09 | 12.03.10 | n.d.b | n.d.b | |
| 21 | 07.10.09 | 12.03.10 | n.d.c | n.d.c | ||
aFound dead (within burrow) on end date indicated.
bFound dead (outside burrow) on 22 January 2010.
cFound dead on date indicated; no burrow established.
4. Discussion
The current study has revealed considerable changes to the sediment system with declining pH. In particular, low-pH conditions in the overlying water caused: firstly, a significant reduction in ammonia oxidation rates within burrow wall sediments in contrast to surface sediments; secondly, increased alkalinity within the overlying waters; and thirdly, a notable decrease in inorganic carbon content within surface sediments. These changes were concurrent with an apparent decline in shrimp physiological function, indicated here by extracellular acidosis and mortality below pHT 7.70. We propose that our current understanding of the impacts of OA on coastal ecology and biogeochemistry is limited by studying its effects on individual species or systems. Specifically, the relationship between benthic macrofaunal activity and sediment microbial processes is likely to produce a complex ecosystem response to OA which may depend upon multiple species interactions.
(a). Changes to the carbon system may favour heterotrophs and buffer decreased pH in surface sediments
Sediment microbial communities already experience considerably lower pH conditions than those in the water column, owing to the production of CO2 during remineralization reactions that consume organic matter [36]. Experimental evidence so far has indicated the important role of metabolically derived CO2 in reducing the carbonate saturation state of the sediment, and therefore driving dissolution [37–41]. Within this current study, the fraction of inorganic carbon within the sediment decreased at low pH, concurrently with an increase in alkalinity in the overlying water. By controlling pH in our experimental system, we induced large increases in the seawater pCO2 as the carbon chemistry adapted over time. While the pCO2 levels reported here therefore exceed those for the corresponding future OA scenarios, it is interesting to note that the maintenance of constant pH within each treatment caused such a shift in the carbon system. It is predicted that the rates of pH-induced dissolution of carbonate minerals in sediments will significantly increase under OA conditions [42]. The pH buffering that occurs within sediment pore waters as a result of carbonate dissolution is one possible explanation for why decreasing seawater pH had no significant effect upon ammonia oxidation rates in surface sediments within this study. However, previous studies using bioturbating heart urchins in both sandy and muddy sediments have shown that after long-term (greater than 20 weeks) exposures, the sediment pH profiles are significantly altered by reduced pH in the overlying water, irrespective of sediment type [18,43]. It therefore seems likely that pore water pH buffering does not fully compensate for the effects of OA. Other changes may occur within the benthic environment that may also impact upon sediment pH and metabolic CO2 production.
Delille et al. [44] suggested that under high-CO2 conditions, the flux of particulate organic carbon to the seafloor may increase. This is probably owing to an over-production of carbon by primary producers under high-CO2 conditions, causing increased exudation of polysaccharides which act as stimuli for particle aggregation [45]. The increased sedimentation of organic matter could lead to greater microbial production rates in surface sediments [46,47]. Within this current study, it was assumed that the input of new CaCO3 and organic matter to the sediments was minimal, owing to the closed-system nature of the mesocosm facility. However, under low-pH conditions, there was an increase in the abundance of both bacterial and archaeal 16S rRNA genes, indicating that at least some microbial groups responded positively to increased pCO2. A recent study by Krause et al. [48] suggests that the Gammaproteobacteria may be among those most affected by reduced seawater pH. However, their acidification method using hydrogen chloride overlooks the responses of microbial communities to changes in pCO2. Grossart et al. [49] showed that within pelagic bacterial communities associated with a phytoplankton bloom, high-pCO2 conditions led to increased growth rates, increased bacterial protein production and increased protease activity, suggesting enhanced heterotrophy. This was particularly evident within the bacteria that were attached to sediment particles; it is possible that a similar response occurred within the sediment microbial communities observed in this current study.
(b). Shrimp behaviour may determine the response of ammonia oxidizers to reduced pH within the burrow
Ammonia oxidation rates within burrow wall sediment decreased drastically at reduced pH; however, it is important to note that this was not simply an inhibition of the ‘normal’ rates. Instead, at the control pH (pHT 8.10), the burrow wall sediments supported, on average, fivefold greater rates compared with surface sediments, indicating active stimulation of ammonia oxidation. Conversely, at pH levels below 7.90, ammonia oxidation rates within burrow sediments were inhibited in comparison to rates in surface sediments. Evidently, within sediments heavily populated by U. deltaura (or similar bioturbators), this could have a significant impact upon the exchange of nutrients within the system. For example, the presence of U. deltaura has been shown to cause a threefold increase in coupled nitrification–denitrification rates within the bulk sediment [27]. Any decrease in nitrification activity could therefore have impacts on other key ecosystem functions, such as denitrification. Interestingly, the burrow wall sediments within the uninhabited ‘relict’ burrow at pHT 6.80 showed the highest ammonia oxidation rates within this treatment (equal to the rates observed within surface sediments). This supports the notion that these relict structures continue to act as important sites for biogeochemical cycling via passive flushing of the burrow [50–52].
The shrimp within this current study were apparently unable to compensate for external changes in pH below pHT 7.70. In agreement with this finding, Donohue et al. [28] studied the effects of reduced seawater pH on U. deltaura kept in individual ‘mimic’ burrows constructed from plastic tubing and found that the shrimp experienced extracellular acidosis at pHT ≤ 7.35. While the authors of this previous study found no other indications of physiological stress in the shrimp, they observed behavioural differences at reduced pH. For example, prior to 100% mortality by the end of the 35-day exposure period, shrimp kept within pHT 6.71 spent a higher proportion of time beating their pleopods (walking legs) [28]. The authors suggested that this behaviour was an attempt by the shrimp to increase their oxygen supply in response to metabolic stress [53]. However, it could also be interpreted as an attempt at ‘flushing’ the burrow of the build-up of metabolic waste products; such as hydrogen sulfide (H2S or HS−), nitrous acid (HNO2; at low pH) or NH3 (at high pH). Hansen et al. [54] showed that nitrifiers that have undergone periods of inactivity owing to anoxia are able to recover almost instantly following re-exposure to O2. By contrast, nitrifying microorganisms may take weeks to recover from inhibition by free sulfide [55]. It therefore seems probable that, in this current study, the decline in ammonia oxidation rates within burrows inhabited by a living shrimp may be due to prolonged alteration of the physico-chemical conditions within the burrow, possibly resulting from pH-induced changes in shrimp behaviour.
Microbial communities inhabiting bioturbated sediments are presumably well adapted to take advantage of fluctuating physico-chemical conditions within the burrow. Within a thalassinidean shrimp burrow, pH can oscillate between 8.0 and 7.6 as the redox chemistry of the burrow changes with ventilation events [53]. How can ammonia oxidation activity occur at all under these conditions? In some acidic soils, it is thought that nitrification activity is able to continue when microbial cells are present in high density as aggregates [56] or as biofilms [57]. It is possible that ammonia oxidizers inhabiting burrow wall sediments use similar strategies. Extracellular polymeric substances are produced directly by microorganisms and serve as an important stabilizing component of coastal sediments as well as providing a source of labile organic carbon and a reactive surface for microbial growth [14,58–60]. Additionally, burrowing macrofauna including thalassinidean shrimp use the addition of biopolymers to the burrow sediment to aid compaction and smoothness of the burrow wall [15,61–65]. This burrow lining has been associated with greater numbers of sediment-attached bacteria within burrows of the polychaete worm Nereis diversicolor compared with the surrounding sediment [66]. If we assume that this behaviour occurred within this study, the cohesive nature of the burrow lining could also explain why burrow wall sediment was apparently resistant to carbonate dissolution, even at very low pH levels. If this is the case, it emphasizes the important role of the shrimp in controlling the burrow's physico-chemical environment.
(c). Cell-specific ammonia oxidation rates may decline at low pH
Gene abundance data must always be interpreted with the caveat that not all of the target genes and/or phylotypes will always be detected, and so any discrepancies between gene abundance data and process rate measurements may merely be reflective of this gap in the data. For example, the PCR primers used in this study targeted only betaproteobacterial ammonia oxidizers. Gammaproteobacterial AOB have been detected in low abundance within the water column [67,68] but may be widespread in marine sediments [69]. It is also probable that some members of the community, for both AOB and AOA, are missed by the use of single amoA primer sets. Sintes et al. [70] recently identified different AOA ‘ecotypes’ corresponding to low and high ammonium concentrations (HAC) in the water column, of which the PCR primers used here may only target the HAC groups. However, it is also worth noting that microbial metabolic activity (i.e. ammonia oxidation rates) may be dependent upon a number of factors, including gene transcription rates, protein synthesis rates or enzyme activity, and may therefore be more sensitive to a change in one of these processes than to the absolute abundance of amoA genes within the environment [71–73]. Furthermore, the ammonia-oxidizing archaea are a relatively new addition to our understanding of the marine nitrogen cycle [74], and it is not yet clear whether all AOA are obligately involved in the nitrification process [75].
Within this context, the data presented here for burrow sediment (whereby the abundance of AOA genes increased at low pH, whereas ammonia oxidation rates decreased) might be cautiously interpreted to suggest that archaeal cell-specific ammonia oxidation rates decreased drastically at low pH. This would agree with previous studies investigating both bacterial and archaeal amoA gene transcript (mRNA) abundances, which increased at reduced pH in temperate soils [76] and Arctic fjord sediments [77]. In both studies, transcript abundance was always lower than gene abundance, seemingly indicating a certain level of inactivity within the ammonia oxidizing communities, particularly at low pH [76,77]. Our understanding of the kinetics and cell transport mechanisms involved in ammonia oxidation is far from definitive. It has been previously suggested that pH directly affects enzyme activity as well as substrate availability [71,78,79]. Chemolithoautotrophic nitrification is an energetically expensive process, and growth yields for nitrifying bacteria are consequently relatively low [14,80]. If there is a requirement for active ammonium uptake at low pH, it may be that the upkeep of a proton motive force is too energetically demanding. Moreover, the need to maintain a near-neutral internal pH may place too much stress on the cell's physiology under low-pH conditions [79,81]. Clearly, further investigation is required in order to confirm whether the cell-specific activities of AOA do indeed decline in response to reduced pH, or whether these observations are due instead to shifts within the active members of the community.
(d). Coastal sediment–water dynamics in a high-CO2 world
Several studies have suggested that under future OA scenarios, the balance of the entire marine nitrogen cycle might be altered. Using a linear interpolation of the data from Huesemann et al. [6], Blackford & Gilbert [9] projected an approximately 20% decrease in pelagic nitrification rates at an atmospheric CO2 concentration of 1000 ppm (the ‘worst-case scenario’ for 2100), corresponding in their model to a pH of approximately 7.67. Under their projected pH values for current-day and year-2100 pH, the linear equation derived from water-column nitrification rates in this study (equation (3.1)) gives a similar decrease in pelagic nitrification of 23%. In agreement with these, Beman et al. [7] also found a 3–44% decrease in ammonia oxidation rates at various pH levels within in situ incubations. It has been suggested that decreased water-column nitrification rates could cause a shift in the structure of phytoplankton communities, as well as altering the fluxes of dissolved inorganic nitrogen species (NO3−, NO2−, NH4+) available for primary production or microbial reduction reactions (such as denitrification) [7,9]. This effect could be further compounded by the increased production of NH4+ by nitrogen-fixing photoautotrophs [82]. Evidently, this would alter the dynamics of the marine carbon cycle as well as the nitrogen cycle. Importantly, any direct impacts upon the community-level microbial responses to OA are also likely to be seasonally contingent, as indicated by Krause et al. [48].
Our experiments effectively acted to isolate the sediment from any additional sources of organic or inorganic carbon by preventing photosynthesis. Therefore, we were able to consider the benthic system independently from the influence of primary production within the overlying water. However, we have demonstrated that changes to the nitrogen cycle at reduced pH do not occur in isolation: the impacts of OA on nitrification rates may be ameliorated (CaCO3 buffering) or potentially compounded (metabolic CO2 production inferred from increased microbial abundance) by changes to the carbon chemistry. Any changes to the flux of organic carbon reaching the seafloor will therefore affect the interactions between the sediment's microbial and chemical processes [83].
In addition, we have shown that macrofauna living within the sediment have the potential to significantly alter the impact of OA upon benthic microbial processes. Previous studies have indicated that changes to the benthic–pelagic flux of nutrients will probably depend upon both sediment type and the presence of bioturbation [16,18,84]. It is imperative also to remember that populations of macrofauna interact with each other in natural ecosystems, and while some members of the macrofaunal community may be at risk of loss of abundance or extinction in future oceans, others will benefit from these changing competitive dynamics [85]. Globally, the impacts of OA on bioturbated sediments may therefore be linked to seasonal and latitudinal differences in bioturbation activity [86] as well as potential interactions between multi-species assemblages of macrofauna [87]. Clearly, a full understanding of coastal nitrogen-cycling dynamics in future oceans requires an appreciation of multi-trophic ecosystem interactions as well as the interactions occurring between the carbon and nitrogen cycles in different sediment systems.
In conclusion, muddy coastal sediments inhabited by macrofaunal bioturbators may support enhanced microbial functions such as ammonia oxidation, an important link in the marine nitrogen cycle. We have shown that the relationship between macrofauna and microorganisms will have a significant impact upon the benthic ecosystem response to OA. Essentially, although ammonia oxidation rates in surface sediments are unaffected by reduced pH, OA may negate the positive impact of thalassinidean shrimp bioturbation upon microbial nitrogen cycling by (i) directly affecting the behaviour of the shrimp and therefore altering the relationship between macrofaunal and microbial activity and (ii) causing long-term changes in the abundance and success of the shrimp. In order to scale these results to a global level, future studies should consider the interactive effects of season, sediment type and macrofaunal species, as well as any potential synergistic effects of OA with increasing sea surface temperature and coastal eutrophication (e.g. see [88–91]).
Acknowledgements
We thank Spencer Burge for his help with sediment sampling; Dr Cindy Smith for her help with the molecular work; the crew of the RV Sepia for their help with sediment and animal collection; and three anonymous referees for their constructive input to the manuscript. This paper is a contribution to the NERC UKOA funded project ‘Impacts of ocean acidification on key benthic ecosystems, communities, habitats, species and life cycles’.
Data accessibility
All data that are not shown in full within this manuscript have been deposited in the Dryad Digital Repository (http://dx.doi.org/doi:10.5061/dryad.b98m6) [50].
Funding statement
B.L. acknowledges support from a NERC Algorithm PhD Studentship (NE/F008864/1) and from the NERC-funded programme Oceans 2025 (Theme 3: Coastal and shelf processes).
References
- 1.Caldeira K, Wickett ME. 2005. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J. Geophys. Res. 110, C09S04 (doi:10.1029/2004JC002671) [Google Scholar]
- 2.Meehl G, et al. 2007. Global climate projections. In Climate change 2007: The physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL.), pp. 747–845 Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Liu J, Weinbauer MG, Maier C, Dai M, Gattuso J-P. 2010. Effect of ocean acidification on microbial diversity and on microbe-driven biogeochemistry and ecosystem functioning. Aquat. Microb. Ecol. 61, 291–305 (doi:10.3354/ame01446) [Google Scholar]
- 4.Joint I, Doney SC, Karl DM. 2011. Will ocean acidification affect marine microbes? ISME J. 5, 1–7 (doi:10.1038/ismej.2010.79) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutchins DA, Mulholland MR, Fu F. 2009. Nutrient cycles and marine microbes in a CO2-enriched ocean. Oceanography 22, 128–145 (doi:10.5670/oceanog.2009.103) [Google Scholar]
- 6.Huesemann MH, Skillman AD, Crecelius EA. 2002. The inhibition of marine nitrification by ocean disposal of carbon dioxide. Mar. Poll. Bull. 44, 142–148 (doi:10.1016/S0025-326X(01)00194-1) [DOI] [PubMed] [Google Scholar]
- 7.Beman JM, Chow C-E, King AL, Feng Y, Fuhrman JA, Andersson A, Bates NR, Popp BP, Hutchins DA. 2011. Global declines in oceanic nitrification rates as a consequence of ocean acidification. Proc. Natl Acad. Sci. USA 108, 208–213 (doi:10.1073/pnas.1011053108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitidis V, Laverock B, McNeill LC, Beesley A, Cummings D, Tait K, Osborn AM, Widdicombe S. 2011. Impact of ocean acidification on benthic and water column ammonia oxidation. Geophys. Res. Lett. 38, L21603 (doi:10.1029/2011GL049095) [Google Scholar]
- 9.Blackford JC, Gilbert FJ. 2007. pH variability and CO2 induced acidification in the North Sea. J. Mar. Sys. 64, 229–241 (doi:10.1016/j.jmarsys.2006.03.016) [Google Scholar]
- 10.Suzuki I, Abee T, Kwok SC. 1974. Ammonia or ammonium ion as substrate for oxidation by Nitrosomonas europaea cells and extracts. J. Bacteriol. 120, 556–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frijlink MJ, Abee T, Laanbroek HJ, Boer WD, Konings WN. 1992. The bioenergetics of ammonia and hydroxylamine oxidation in Nitrosomonas europaea at acid and alkaline pH. Arch. Microbiol. 157, 194–199 [Google Scholar]
- 12.Laverock B, Gilbert JA, Tait K, Osborn AM, Widdicombe S. 2011. Bioturbation: impact on the marine nitrogen cycle. Biochem. Soc. Trans. 39, 315–320 (doi:10.1042/BST0390315) [DOI] [PubMed] [Google Scholar]
- 13.Herbert RA. 1999. Nitrogen cycling in coastal marine ecosystems. FEMS Microbiol. Rev. 23, 563–590 (doi:10.1111/j.1574-6976.1999.tb00414.x) [DOI] [PubMed] [Google Scholar]
- 14.Canfield DE, Thampdrup B, Kristensen E. 2005. Aquatic geomicrobiology. Advances in marine biology, vol. 48 London, UK: Elsevier Academic Press [Google Scholar]
- 15.Kristensen E. 2000. Organic matter diagenesis at the oxic/anoxic interface in coastal marine sediments, with emphasis on the role of burrowing animals. Hydrobiologia 426, 1–24 (doi:10.1023/A:1003980226194) [Google Scholar]
- 16.Widdicombe S, Needham HR. 2007. Impact of CO2-induced seawater acidification on the burrowing activity of Nereis virens and sediment nutrient flux. Mar. Ecol. Prog, Ser. 341, 111–122 (doi:10.3354/meps341111) [Google Scholar]
- 17.Wood HL, Spicer JI, Widdicombe S. 2008. Ocean acidification may increase calcification rates, but at a cost. Proc. R. Soc. B 275, 1767–1773 (doi:10.1098/rspb.2008.0343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Widdicombe S, Dashfield SL, McNeill CL, Needham HR, Beesley A, McEvoy A, Øxnevad S, Clarke KR, Berge JA. 2009. Effects of CO2 induced seawater acidification on infaunal diversity and sediment nutrient fluxes. Mar. Ecol. Prog. Ser. 379, 59–75 (doi:10.3354/meps07894) [Google Scholar]
- 19.Kroeker KJ, Micheli F, Gambi MC, Martz TR. 2011. Divergent ecosystem responses within a benthic marine community to ocean acidification. Proc. Natl Acad. Sci. USA 108, 14 515–14 520 (doi:10.1073/pnas.1107789108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffis RB, Suchanek TH. 1991. A model of burrow architecture and trophic modes in thalassinidean shrimp (Decapoda: Thalassinidea). Mar. Ecol. Prog. Ser. 79, 171–183 (doi:10.3354/meps079171) [Google Scholar]
- 21.Dworschak PC. 2000. Global diversity in the Thalassinidea (Decapoda). J. Crust. Biol. 20, 238–245 [Google Scholar]
- 22.Leach WE. 1815. A tabular view of the external characters of four classes of animals, which Linné arranged under Insecta: with the distribution of the genera composing three of these classes into 7 orders. Trans. Linn. Soc. Lond. Zool. 11, 306–400 [Google Scholar]
- 23.Tunberg B. 1986. Studies on the population ecology of Upogebia deltaura (Leach) (Crustacea, Thalassinidea). Estuar. Coast. Shelf Sci. 22, 753–765 (doi:10.1016/0272-7714(86)90097-1) [Google Scholar]
- 24.Christensen B, Vedel A, Kristensen E. 2000. Carbon and nitrogen fluxes in sediment inhabited by suspension-feeding (Nereis diversicolor) and non-suspension-feeding (N. virens) polychaetes. Mar. Ecol. Prog. Ser. 192, 203–217 (doi:10.3354/meps192203) [Google Scholar]
- 25.Parry D, Kendall M, Pilgrim D, Jones M. 2003. Identification of patch structure within marine benthic landscapes using a remotely operated vehicle. J. Exp. Mar. Biol. Ecol. 285–286, 497–511 (doi:10.1016/S0022-0981(02)00546-4) [Google Scholar]
- 26.Laverock B, Smith CJ, Tait K, Osborn AM, Widdicombe S, Gilbert JA. 2010. Bioturbating shrimp alter the structure and diversity of bacterial communities in coastal marine sediments. ISME J. 4, 1531–1544 (doi:10.1038/ismej.2010.86) [DOI] [PubMed] [Google Scholar]
- 27.Howe RL, Rees AP, Widdicombe S. 2004. The impact of two species of bioturbating shrimp (Callianassa subterranea and Upogebia deltaura) on sediment denitrification. J. Mar. Biol. Ass. UK 84, 629–632 (doi:10.1017/S002531540400966Xh) [Google Scholar]
- 28.Donohue PJC, Calosi P, Bates AH, Laverock B, Rastrick S, Mark FC, Strobel A, Widdicombe S. 2012. Impact of exposure to elevated pCO2 on the physiology and behaviour of an important ecosystem engineer, the burrowing shrimp Upogebia deltaura. Aquat. Biol. 15, 73–86 (doi:10.3354/ab00408) [Google Scholar]
- 29.Grasshoff K. 1983. Determination of nitrite. In Methods of seawater analysis (eds Grasshof K, Ehrhardt M, Kremling K.), pp. 139–142, 2nd edn Weinheim, Germany: Werlag Chemie [Google Scholar]
- 30.Dickson AG, Sabine CL, Christian JR. 2007. Guide to best practices for ocean CO2 measurements PICES Spec. Publ. 3, 191 [Google Scholar]
- 31.Head R, Medina G, Huskin I, Anadon R, Harris R. 2002. Phytoplankton and mesozooplankton distribution and composition during transects of the Azores Subtropical Front. Deep Sea Res. II 49, 4023–4034 (doi:10.1016/S0967-0645(02)00140-6) [Google Scholar]
- 32.Pierrot D, Lewis E, Wallace DWR. 2006. CO2SYS DOS program developed for CO2 system calculations. ORNL/CDIAC-105. Oak Ridge, TN: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory US Department of Energy [Google Scholar]
- 33.Clarke K, Gorley RN. 2006. PRIMER v6: user manual/tutorial. Plymouth, UK: PRIMER-E [Google Scholar]
- 34.Anderson M, Gorley R, Clarke K. 2008. PERMANOVA+ for PRIMER: guide to software and statistical methods. Plymouth, UK: PRIMER-E [Google Scholar]
- 35.Aller R. 1984. The importance of relict burrow structures and burrow irrigation in controlling sedimentary solute distributions. Geochim. Cosmochim. Acta 48, 1929–1934 (doi:10.1016/0016-7037(84)90375-2) [Google Scholar]
- 36.Soetaert K, Hofmann AF, Middelburg JJ, Meysman FJR, Greenwood J. 2007. The effect of biogeochemical processes on pH. Mar. Chem. 105, 30–51 (doi:10.1016/j.marchem.2006.12.012) [Google Scholar]
- 37.Jahnke R, Craven D, Gaillard J-F. 1994. The influence of organic matter diagenesis on CaCO3 dissolution at the deep-sea floor. Geochim. Cosmochim. Acta 58, 2799–2809 (doi:10.1016/0016-7037(94)90115-5) [Google Scholar]
- 38.Jahnke R, Craven D, McCorkle D, Reimers CE. 1997. CaCO3 dissolution in California continental margin sediments: the influence of organic matter remineralization. Geochim. Cosmochim. Acta 61, 3587–3604 (doi:10.1016/S0016-7037(97)00184-1) [Google Scholar]
- 39.Wenzhöfer F, Adler M, Kohls O, Hensen C, Strotmann B, Boehme S, Schulz HD. 2001. Calcite dissolution driven by benthic mineralization in the deep-sea: In situ measurements of Ca2+, pH, pCO2 and O2. Geochim. Cosmochim. Acta 65, 2677–2690 (doi:10.1016/S0016-7037(01)00620-2) [Google Scholar]
- 40.Jahnke R, Jahnke DB. 2004. Calcium carbonate dissolution in deep sea sediments: reconciling microelectrode, pore water and benthic flux chamber results. Geochim. Cosmochim. Acta 68, 47–59 (doi:10.1016/S0016-7037(03)00260-6) [Google Scholar]
- 41.Andersson A, Mackenzie F, Lerman B. 2006. Coastal ocean CO2–carbonic acid–carbonate sediment system of the Anthropocene. Glob. Biogeochem. Cycles 20, GB1S92 (doi:10.1029/2005GB002506) [Google Scholar]
- 42.Andersson A, Bates N, Mackenzie F. 2007. Dissolution of carbonate sediments under rising pCO2 and ocean acidification: observations from Devil's Hole, Bermuda. Aquat. Geochem. 13, 237–264 (doi:10.1007/s10498-007-9018-8) [Google Scholar]
- 43.Widdicombe S, Beesley A, Berge JA, Dashfield SL, McNeill CL, Needham HR, Øxnevad S. In press. Impact of elevated levels of CO2 on animal mediated ecosystem function: the modification of sediment nutrient fluxes by burrowing urchins. Mar. Poll. Bull. (doi:10.1016/j.marpolbul.2012.11.008) [DOI] [PubMed] [Google Scholar]
- 44.Delille B, et al. 2005. Response of primary production and calcification to changes of pCO2 during experimental blooms of the coccolithophorid Emiliania huxleyi. Glob. Biogeochem. Cycles 19, GB2023 (doi:10.1029/2004GB002318) [Google Scholar]
- 45.Engel A, Thoms U, Riebesell U, Rochelle-Newall E, Zondervan I. 2004. Polysaccharide aggregation as a potential sink of marine dissolved organic carbon. Nature 428, 929–932 (doi:10.1038/nature02453) [DOI] [PubMed] [Google Scholar]
- 46.Van Duyl F, Kop A, Kok A, Sandee A. 1992. The impact of organic matter and macrozoobenthos on bacterial and oxygen variables in marine sediment boxcosms. Neth. J. Sea Res. 29, 343–355 (doi:10.1016/0077-7579(92)90074-O) [Google Scholar]
- 47.Graf G, Schulz R, Peinart R, Meyer-Reil L-A. 1983. Benthic response to sedimentation events during autumn to spring at a shallow-water station in the Western Kiel Bight. Mar. Biol. 77, 235–246 (doi:10.1007/BF00395812) [Google Scholar]
- 48.Krause E, Wichels A, Giménez L, Lunau M, Schilhabel MB, Gerdts G. 2012. Small changes in pH have direct effects on marine bacterial community composition: a microcosm approach. PLoS ONE 7, e47035 (doi:10.1371/journal.pone.0047035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grossart H-P, Allgaier M, Passow U, Riebesell U. 2006. Testing the effect of CO2 concentration on the dynamics of marine heterotrophic bacterioplankton. Limnol. Oceanogr. 51, 1–11 (doi:10.4319/lo.2006.51.1.0001) [Google Scholar]
- 50.Laverock B, Kitidis V, Tait K, Gilbert JA, Osborn AM, Widdicombe S. 2013. Data from: bioturbation determines the response of benthic ammonia oxidising microorganisms to ocean acidification. Dryad Digital Repository. (doi:10.5061/dryad.b98m6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ray A, Aller R. 1985. Physical irrigation of relict burrows: implications for sediment chemistry. Mar. Geol. 62, 371–379 (doi:10.1016/0025-3227(85)90125-2) [Google Scholar]
- 52.Munksby N, Benthien M, Glud RN. 2002. Flow-induced flushing of relict tube structures in the central Skagerrak (Norway). Mar. Biol. 141, 939–945 (doi:10.1007/s00227-002-0874-x) [Google Scholar]
- 53.Astall CM, Taylor AC, Atkinson RJA. 1997. Behavioural and physiological implications of a burrow-dwelling lifestyle for two species of upogebiid mud-shrimp (Crustacea: Thalassinidea). Estuar. Coast. Shelf Sci. 44, 155–168 (doi:10.1006/ecss.1996.0207) [Google Scholar]
- 54.Hansen JI, Henriksen K, Blackburn TH. 1981. Seasonal distribution of nitrifying bacteria and rates of nitrification in coastal marine sediments. Microb. Ecol. 7, 297–304 (doi:10.1007/BF02341424) [DOI] [PubMed] [Google Scholar]
- 55.Joye SB, Hollibaugh JT. 1995. Influence of sulfide inhibition of nitrification on nitrogen regeneration in sediments. Science 270, 623–625 (doi:10.1126/science.270.5236.623) [Google Scholar]
- 56.De Boer W, Kowalchuk GA. 2001. Nitrification in acid soils: micro-organisms and mechanisms. Soil. Biol. Biochem. 33, 853–866 (doi:10.1016/S0038-0717(00)00247-9) [Google Scholar]
- 57.Allison SM, Prosser JI. 1993. Ammonia oxidation at low pH by attached populations of nitrifying bacteria. Soil. Biol. Biochem. 25, 935–941 (doi:10.1016/0038-0717(93)90096-T) [Google Scholar]
- 58.Meyer-Reil L-A. 1994. Microbial life in sedimentary biofilms: the challenge to microbial ecologists. Mar. Ecol. Prog. Ser. 112, 303–311 (doi:10.3354/meps112303) [Google Scholar]
- 59.Solan M, Wigham B. 2005. Biogenic particle reworking and bacterial–invertebrate interactions in marine sediments. In Interactions between macro- and microorganisms in marine sediments (eds Kristensen E, Haese RR, Kostka JE.), pp. 105–124 Washington, DC: American Geophysical Union [Google Scholar]
- 60.Stal LJ. 2010. Microphytobenthos as a biogeomorphological force in intertidal sediment stabilization. Ecol. Eng. 36, 236–245 (doi:10.1016/j.ecoleng.2008.12.032) [Google Scholar]
- 61.Coelho RV, Cooper RA, Rodrigues SDA. 2000. Burrow morphology and behaviour of the mud shrimp Upogebia omissa (Decapoda: Thalassinidea: Upogebiidae). Mar. Ecol. Prog. Ser. 200, 229–240 (doi:10.3354/meps200229) [Google Scholar]
- 62.Kristensen E, Kostka JE. 2005. Macrofaunal burrows and irrigation in marine sediment: microbiological and biogeochemical interactions. In Interactions between macro- and microorganisms in marine sediments (eds Kristensen E, Haese RR, Kostka JE.), pp. 125–158 Washington, DC: American Geophysical Union [Google Scholar]
- 63.Steward CC, Nold SC, Ringelberg DB, White DC, Lovell CR. 1996. Microbial biomass and community structures in the burrows of bromophenol producing and non-producing marine worms and surrounding sediments. Mar. Ecol. Prog. Ser. 133, 149–165 (doi:10.3354/meps133149) [Google Scholar]
- 64.Papaspyrou S, Gregersen T, Cox RP, Thessalou-Legaki M, Kristensen E. 2005. Sediment properties and bacterial community in burrows of the ghost shrimp Pestarella tyrrhena (Decapoda: Thalassinidea). Aquat. Microb. Ecol. 38, 181–190 (doi:10.3354/ame038181) [Google Scholar]
- 65.Kinoshita K, Wada M, Kogure K, Furota T. 2008. Microbial activity and accumulation of organic matter in the burrow of the mud shrimp, Upogebia major (Crustacea: Thalassinidea). Mar. Biol. 153, 277–283 (doi:10.1007/s00227-007-0802-1) [Google Scholar]
- 66.Lucas FS, Bertru G, Höfle MG. 2003. Characterization of free-living and attached bacteria in sediments colonized by Hediste diversicolor. Aquat. Microb. Ecol. 32, 165–174 (doi:10.3354/ame032165) [Google Scholar]
- 67.Wuchter C, et al. 2006. Archaeal nitrification in the ocean. Proc. Natl Acad. Sci. USA 103, 12 317–12 322 (doi:10.1073/pnas.0600756103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lam P, Jensen MM, Lavik G, McGinnis DF, Müller B, Schubert CJ, Amann R, Thamdrup B, Kuypers MMM. 2007. Linking crenarchaeal and bacterial nitrification to anammox in the Black Sea. Proc. Natl Acad. Sci. USA 104, 7104–7109 (doi:10.1073/pnas.0611081104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ward BB, O'Mullan GD. 2002. Worldwide distribution of Nitrosococcus oceani, a marine ammonia-oxidizing γ-proteobacterium, detected by PCR and sequencing of 16S rRNA and amoA genes. Appl. Environ. Microbiol. 68, 4153–4157 (doi:10.1128/AEM.68.8.4153-4157.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sintes E, Bergauer K, De Corte D, Yokokawa T, Herndl GJ. 2013. Archaeal amoA gene diversity points to distinct biogeography of ammonia-oxidizing Crenarchaeota in the ocean. Environ. Microbiol. 15, 1647–1658 (doi:10.1111/j.1462-2920.2012.02801.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ward BB. 1987. Kinetic studies on ammonia and methane oxidation by Nitrosococcus oceanus. Arch. Microbiol. 147, 126–133 (doi:10.1007/BF00415273) [Google Scholar]
- 72.Röling WFM. 2007. Do microbial numbers count? Quantifying the regulation of biogeochemical fluxes by population size and cellular activity. FEMS Microbiol. Ecol. 62, 202–210 (doi:10.1111/j.1574-6941.2007.00350.x) [DOI] [PubMed] [Google Scholar]
- 73.Prosser JI, Nicol GW. 2008. Relative contributions of archaea and bacteria to aerobic ammonia oxidation in the environment. Environ. Microbiol. 10, 2931–2941 (doi:10.1111/j.1462-2920.2008.01775.x) [DOI] [PubMed] [Google Scholar]
- 74.Francis CA, Beman JM, Kuypers MMM. 2007. New processes and players in the nitrogen cycle: the microbial ecology of anaerobic and archaeal ammonia oxidation. ISME J. 1, 19–27 (doi:10.1038/ismej.2007.8) [DOI] [PubMed] [Google Scholar]
- 75.Santoro AE, Casciotti KL, Francis CA. 2010. Activity, abundance and diversity of nitrifying archaea and bacteria in the central California Current. Environ. Microbiol. 12, 1989–2006 (doi:10.1111/j.1462-2920.2010.02205.x) [DOI] [PubMed] [Google Scholar]
- 76.Nicol GW, Leininger S, Schleper C, Prosser JI. 2008. The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ. Microbiol. 10, 2966–2978 (doi:10.1111/j.1462-2920.2008.01701.x) [DOI] [PubMed] [Google Scholar]
- 77.Tait K, Laverock B, Widdicombe S. Submitted. Response of an Arctic sediment nitrogen cycling community to increased pCO2.
- 78.Jones RD, Morita RY. 1985. Low-temperature growth and whole-cell kinetics of a marine ammonium oxidizer. Mar. Ecol. Prog. Ser. 21, 239–243 (doi:10.3354/meps021239) [Google Scholar]
- 79.Prosser JI. 1986. Theoretical and experimental models of nitrification. Nitrification 20, 63–78 [Google Scholar]
- 80.Ward BB. 2000. Nitrification and the marine nitrogen cycle. In Microbial ecology of the oceans (ed. Kirchman DL.), pp. 427–454, 2nd edn New York, NY: Wiley-Liss [Google Scholar]
- 81.Groeneweg J, Sellner B, Tappe W. 1994. Ammonia oxidation in Nitrosomonas at NH3 concentrations near Km: effects of pH and temperature. Water Res. 28, 2561–2566 (doi:10.1016/0043-1354(94)90074-4) [Google Scholar]
- 82.Levitan O, Rosenberg G, Setlik I, Setlikova E, Grigel J, Klepetar J, Prasil O, Berman-Frank I. 2007. Elevated CO2 enhances nitrogen fixation and growth in the marine cyanobacterium Trichodesmium. Glob. Change Biol. 13, 1–6 (doi:10.1111/j.1365-2486.2006.01314.x) [Google Scholar]
- 83.Ridgwell A, Zeebe R. 2005. The role of the global carbonate cycle in the regulation and evolution of the Earth system. Earth Planet. Sci. Lett. 234, 299–315 (doi:10.1016/j.epsl.2005.03.006) [Google Scholar]
- 84.Wood HL, Widdicombe S, Spicer JI. 2009. The influence of hypercapnia and the infaunal brittlestar Amphiura filiformis on sediment nutrient flux: will ocean acidification affect nutrient exchange? Biogeosciences 6, 2015–2024 (doi:10.5194/bg-6-2015-2009) [Google Scholar]
- 85.Godbold JA, Solan M. 2009. Relative importance of biodiversity and the abiotic environment in mediating an ecosystem process. Mar. Ecol. Prog. Ser. 396, 273–282 (doi:10.3354/meps08401) [Google Scholar]
- 86.Teal LR, Bulling MT, Parker ER, Solan M. 2008. Global patterns of bioturbation intensity and mixed depth of marine soft sediments. Aquat. Biol. 2, 207–218 (doi:10.3354/ab00052) [Google Scholar]
- 87.Widdicombe S, Austen MC. 2005. Setting diversity and community structure in subtidal sediments: the importance of biological disturbance. In Interactions between macro- and microorganisms in marine sediments. (eds Kristensen E, Haese RR, Kostka JE.), pp. 217–232 Washington, DC: American Geophysical Union [Google Scholar]
- 88.Russell BD, Connell SD, Findlay HS, Tait K, Widdicombe S, Mieszkowska N. 2013. Ocean acidification and rising temperatures may increase biofilm primary productivity but decrease grazer consumption. Phil. Trans. R. Soc. B 368, 20120438 (doi:10.1098/rstb.2012.0438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Connell SD, Kroeker KJ, Fabricus KE, Kline DI, Russell BD. 2013. The other ocean acidification problem: CO2 as a resource among competitors for ecosystem dominance. Phil. Trans. R. Soc. B 368, 20120442 (doi:10.1098/rstb.2012.0442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tatters AO, et al. 2013. Short- and long-term conditioning of a temperate marine diatom community to acidification and warming. Phil. Trans. R. Soc. B 368, 20120437 (doi:10.1098/rstb.2012.0437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Godbold JA, Solan M. 2013. Long-term effects of warming and ocean acidification are modified by seasonal variation in species responses and environmental conditions. Phil. Trans. R. Soc. B 368, 20130186 (doi:10.1098/rstb.2013.0186) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that are not shown in full within this manuscript have been deposited in the Dryad Digital Repository (http://dx.doi.org/doi:10.5061/dryad.b98m6) [50].