Abstract
Warming of sea surface temperatures and alteration of ocean chemistry associated with anthropogenic increases in atmospheric carbon dioxide will have profound consequences for a broad range of species, but the potential for seasonal variation to modify species and ecosystem responses to these stressors has received little attention. Here, using the longest experiment to date (542 days), we investigate how the interactive effects of warming and ocean acidification affect the growth, behaviour and associated levels of ecosystem functioning (nutrient release) for a functionally important non-calcifying intertidal polychaete (Alitta virens) under seasonally changing conditions. We find that the effects of warming, ocean acidification and their interactions are not detectable in the short term, but manifest over time through changes in growth, bioturbation and bioirrigation behaviour that, in turn, affect nutrient generation. These changes are intimately linked to species responses to seasonal variations in environmental conditions (temperature and photoperiod) that, depending upon timing, can either exacerbate or buffer the long-term directional effects of climatic forcing. Taken together, our observations caution against over emphasizing the conclusions from short-term experiments and highlight the necessity to consider the temporal expression of complex system dynamics established over appropriate timescales when forecasting the likely ecological consequences of climatic forcing.
Keywords: acclimation, adaptation, long term, seasonal, phenology, multiple stressors
1. Introduction
Since the industrial revolution, CO2 emissions from the burning of fossil fuels and changes in land use have steadily increased atmospheric CO2 concentration from preindustrial levels of 280 ppm to currently approximately 385 ppm; these levels are projected to increase to 700–1000 ppm by the end of the twenty-first century [1], causing concomitant changes in global sea surface temperature (2–4.5°C rise [2]) and chemistry (e.g. 0.4–0.5 pH reduction [2]). Although historical records indicate that atmospheric CO2 concentrations and sea surface temperatures have undergone significant oscillations and have exceeded present-day levels in the past [3,4], it is the unprecedented rates of change that are fuelling concerns over whether organisms will retain the capacity to mediate vital ecosystem functions and services [5,6]. A key component in answering this question will be a need to establish the likelihood, and realized extent, of species acclimation (or adaptation) to environmental change [7,8] and, if common across functionally important taxa, how such coping and adaptive strategies will alter species–environment interactions in the long term [9].
Over the last decade, the impacts of warming and ocean acidification have received considerable attention, and there is a clear consensus that a continued upward trend in these stressors will have far-reaching consequences for the structure and functioning of food webs [10–13]. However, much of the evidence in support of this view stems from experiments that have focused on a restricted number of calcifying (shell-forming) organisms exposed to non-varying conditions [14,15]. These species, including molluscs, echinoderms, corals and coccolithophores, do not necessarily provide a representative indication of the broader ecological impacts of ocean acidification (for counter argument based on the variability of crustacean responses, see [16]), because the declining saturation level for calcium carbonate minerals required to maintain calcite- and aragonite-based shells and skeletons means that these species may be disproportionately negatively affected relative to non-calcifying species [14,15,17,18]. Such a process-orientated focus [19] understates the importance of species that do not have calcified shells or skeletons and ignores other critical responses to climatic forcing, such as change in behaviour or ecological role [20], that can influence the mediation of many ecosystem functions [21,22] or, indeed, the structure of entire ecosystems [23,24]. Establishing an overview, however, has been complicated by the variability in documented responses, not least because of differences in timing (life stage, season) and context (habitat condition, exposure history) between investigations, but the influence of these aspects has not been considered. Without a more holistic consideration of the variety and timing of responses to climate change [25,26], summarizing the most likely net response across multiple habitats will remain challenging [27] and subject to a great deal of uncertainty [12], and could lead to misleading conclusions.
Provision of more accurate projections of the ecological consequences of warming and ocean acidification requires an improved understanding of longer term processes that moderate the susceptibility of species to a changing environment [28,29]. Much of the currently available evidence stems from short-term experimental manipulations, typically performed over days to weeks [30], that do not allow the development of diachronic response mechanisms, such as acclimation, adaptation [29,31,32], physiological or behavioural compensatory mechanisms linked to biodiversity–environmental context and/or seasonal timing [16,33,34]. These emergent properties of ecological communities can radically alter (perceived or realized) species vulnerabilities and ecosystem responses to climatic forcing and, when considered collectively, have the potential to lead to scenarios that differ from those suggested by current projections [35]. Form & Riebesell [29], for example, show that calcification rates in the cold-water coral Lophelia pertusa decrease after 8 days of exposure to high CO2 but increase over the medium term (6 months), because compensatory metabolic pathways may take extended periods of time to become established, depending on other factors such as life cycle phase [28] or exposure history [36]. These compensatory mechanisms are only identifiable during prolonged observations, although they may not be sustainable without significant cost to other physiological (e.g. muscle depletion [37], intestinal base loss [38]) or behavioural functions (e.g. predator avoidance [39]). One way to account for such feedback mechanisms is to extend the duration of experiments or observations, but doing so raises the probability of including other processes that operate over longer timescales and have the potential to modify the outcome. For example, at different times of the year or life cycle [40], species sensitivities to warming and CO2 may integrate in such a way that elevated CO2 levels may enhance the sensitivity of organisms to their thermal extremes or vice versa [41,42], changing the way in which species respond to a given level of climatic forcing. Indeed, the synergistic effects of warming and ocean acidification on biological process rates, species behaviour and ecosystem functioning [43–48] demonstrate strong interdependencies between climatic forcing and a range of variables that are known to vary over time, including differences in species physiology [49–51], behaviour [52], process rates [51,53], community composition [44], ecosystem structure [54] and other ecosystem properties (e.g. food availability [55], irradiance [56]) that influence species performance. How spatio-temporal variability in local environmental conditions and species composition will affect the response of ecosystems represents a significant knowledge gap; the paucity of longer term experiments and lack of consideration of such response modifiers are likely to go some way in explaining apparently divergent results between studies [16].
Previous work has shown that individual drivers in isolation, including biodiversity loss [57] and changes in temperature and ocean acidification, affect species behaviour and, subsequently, nutrient turnover and primary production at the ecosystem level [44,47]. It is also known that species are not necessarily sedentary and move between habitat patches that differ in terms of resource availability, thereby altering species composition and density distributions which, in turn, can affect ecosystem processes at the local scale and ecosystem functioning at larger scales [16,58]. These context-dependent changes in behaviour and species–environment interactions mean that the functional role of an individual species may fundamentally change in response to sources of temporal (diurnal, seasonal and interannual) and/or spatial (vertical and horizontal) variation [26,51,59–61], yet these factors have not been included in investigations of the ecological impacts of warming and ocean acidification. This is surprising, given that considerable differences in both the nature and magnitude of responses to warming and ocean acidification have been documented when behavioural dynamics have been assessed over time and/or space [54,62,63], even for species considered to have high acclimation potential [64,65]. To reliably predict the ecosystem consequences of climatic forcing in natural systems, it is critical to perform experiments that incorporate, or at least acknowledge, the sources and mechanisms of temporal variability in order to improve summaries and assessments of the long-term effects of climatic forcing [66].
Coastal species in intertidal habitats are regarded as being particularly tolerant to changes in temperature and pH because they are subjected to tidal and seasonal-induced environmental fluctuations [61,67]. However, many intertidal species may already be living at their upper tolerance limits, such that further increases in environmental stress imposed by climatic forcing are likely to have significant physiological and ecological impacts [68]. Coastal and shelf sediments support critical biogeochemical processes that are important at a global scale [69] as they supply nutrients vital for primary production [70]. These biogeochemical processes are significantly influenced by infaunal burrowing and irrigation activities, which enhance microbial regeneration of organic matter [71,72]. As microbial–faunal activities can be strongly mediated by temperature [53,65,73], warming has the potential to significantly affect global balances of carbon and nutrients [74]. The effects of ocean acidification on infaunal behaviour and the associated effects on biogeochemical processes, however, have received comparatively little attention. Limited evidence suggests that although ocean acidification may not directly affect the behaviour of infaunal organisms over the short term (e.g. Nereis virens, five weeks [21] and Amphiura filiformis, 40 days [75]), nutrient generation is affected, probably in response to compositional changes in the microbial communities responsible for nutrient transformations [62,71]. Here, we investigate how long-term (542 days) exposure, incorporating seasonally changing environmental conditions (temperature and photoperiod), to warming and ocean acidification affects the growth, behaviour and contribution to ecosystem functioning (nutrient release) of a functionally important intertidal species (Alitta virens). We hypothesize that the effects of warming and acidification will develop over an extended period of time (months) but will be more pronounced as species respond to seasonal changes in temperature and photoperiod. If emergent response mechanisms are present, establishment of the importance of environmental variability and context will have significant implications for determining ecosystem responses to climate change.
2. Material and methods
(a). Faunal and sediment collection
Individuals of the infaunal polychaete A. virens (Sars (1835); Polychaeta, Nereididae) were obtained from Dragon Baits Ltd. (Port Talbot, UK) in October 2010 and maintained at ambient temperature and photoperiod at the University of Aberdeen (Aberdeen, UK). Surficial sediment (less than 3 cm depth, total organic carbon content approx. 4% and mean particle size approx. 50 μm [71]) was collected from the Ythan Estuary (Aberdeen, UK) (57°20.085′ N, 02°0.206′ W) in early December 2010, sieved (500 µm mesh) in a seawater (10 µm filtered, UV sterilized and salinity 33) bath to remove macrofauna, allowed to settle for 24 h to retain the fine fraction (less than 63 µm) and homogenized.
(b). Experimental set-up and design
Aquaria (total = 104) consisted of transparent Perspex square cores (internal dimensions, LWH: 12 × 12 × 33 cm) containing sieved sediment (12 cm depth) overlain by 20 cm of seawater (UV sterilized, 5 μm pre-filtered, salinity 32.4 ± 1; see electronic supplementary material, figures S1 and S2). Aquaria were continually aerated by bubbling a treatment-specific air–CO2 gas mixture (see below) through a glass pipette and maintained under a natural day–night cycle (Aqualine T5 Reef White 10 K fluorescent light tubes, Aqua Medic). The number of daylight hours (UK Hydrographic Office; see electronic supplementary material, table S1) and water temperature (daily monitoring records, Oceanlab, University of Aberdeen) were adjusted to reflect the historic (5 year archive) monthly mean level on the first day of each month. Two experiments were conducted to enable the separation of short- (7 days) versus long-term (3, 6, 12 and 18 months) effects. In each experiment, three individuals (i.e. 208 individuals m−2) of A. virens were added (total biomass: short term, 4.3380 ± 1.2116 g aquarium−1; long term, 4.2411 ± 0.6179 g aquarium−1) and were supplied with approximately 0.03 g of tropical fish flake food (Aquarian Tropical Flake) every 2 days.
Aquaria in the short-term experiment were held in a seawater bath at a constant temperature (Titan 1500 chiller unit, Aqua Medic) to reflect either the annual mean temperature of the study site (10.24 ± 0.02°C; see electronic supplementary material, figure S2) or a warming scenario of +4°C (14.36 ± 0.12°C; see electronic supplementary material, figure S2). Levels of atmospheric [CO2] were maintained at present-day level (380 ppm) and indicative future projections (750 and 1000 ppm). Each temperature × CO2 treatment was replicated four times (n = 24; table 1). For the long-term experiment, the constant temperature regimes of the seawater bath were replaced with a seasonal cycle (adjusted monthly; see electronic supplementary material, figure S2) to match either historical mean monthly temperatures at the study site (ambient) or a warming scenario (ambient cycle+4°C). Levels of atmospheric [CO2] were 380, 750 and 1000 ppm for the ambient treatment, and 380 and 750 ppm for the ambient+4°C treatment. To avoid the excessive accumulation of nutrients and metabolites, we performed a partial (80%) seawater change on each aquarium once a week. Alitta virens growth, behaviour and ecosystem function were examined after 3, 6, 12 and 18 months of exposure (table 1). Each CO2 × time point treatment was replicated four times (ambient, n = 48; ambient+4°C, n = 32; table 1).
Table 1.
Summary of experimental design for investigating the short- and long-term effects of enhanced temperature and atmospheric [CO2] on the behaviour of A. virens and changes in ecosystem functioning.
| experiment | total n | time point | temperature regime (°C) | atmospheric [CO2] ppm |
||
|---|---|---|---|---|---|---|
| short-term | 24 | 7 days | 10 or 14 | 380 | 750 | 1000 |
| long-term | 48 | 3, 6, 12, 18 months | ambient | 380 | 750 | 1000 |
| long-term | 32 | 3, 6, 12, 18 months | ambient+4 | 380 | 750 | |
(c). Measurements of growth and species behaviour
The growth (biomass, g) and behaviour (sediment particle reworking, hereafter bioturbation; ventilation activity, hereafter bioirrigation) of A. virens were determined at each time point. Bioturbation was estimated from sediment profile images (Canon 400D, 3900 × 2600 pixels, i.e. 10.1 megapixels, effective resolution 67 × 67 μm per pixel) of the redistribution of particulate tracers (dyed sediment that fluoresces under ultraviolet light; 15 g luminophores aquarium−1, 125–250 μm diameter) over a period of 6 days [76,77]). Bioirrigation was estimated from absolute changes in the concentration (mean starting concentration = 1081.13 ± 44.28 mg l−1) of the inert tracer sodium bromide (Δ[Br−], mg l−1; negative values indicate bioirrigation activity; [78]) over an 8 h period, determined using a Tecator flow injection auto-analyser (FIA Star 5010 series).
(d). Measurements of ecosystem function
Water column concentration (mg l−1) of NH4-N, NOx-N (i.e. NO3-N + NO2-N) and PO4-P were determined over a 6-day incubation period at each time point from standardized water samples (approx. 10 cm depth, 0.45 μm NALGENE filtered) following standard protocols using a Tecator flow injection auto-analyser (FIA Star 5010 series).
(e). Controlling and monitoring seawater carbonate chemistry
Levels of [CO2] were controlled using a CO2–air mixing system, adapted from Findlay et al. [79], which continually maintained and monitored [CO2] in the air mixture supplied to each aquarium using infrared gas analysers (Licor LI-840A, 1 per [CO2] treatment). To minimize ambient air exchange and to ensure that the seawater carbonate system within each aquarium developed naturally and equilibrated with the head space conditions, each aquarium was covered with a clear Perspex lid. pH (NBS scale, Mettler-Toledo InLab Expert Pro temperature–pH combination electrode), temperature and salinity (Mettler-Toledo InLab 737 IP67 temperature–conductivity combination electrode) were measured every 7 days and total alkalinity (AT) was measured once a month. Total alkalinity was analysed by titration (Apollo SciTech Alkalinity Titrator AS-ALK2) following standard protocols at Plymouth Marine Laboratory, Plymouth, UK and the Carbonate Facility at the National Oceanography Centre, Southampton, UK. Concentrations of bicarbonate (HCO3−), carbonate (CO32−) and pCO2 were calculated from measured pH, AT, temperature and salinity [80,81] using CO2calc [82] with appropriate solubility constants ([83], refit by [84]) and KSO4 [85] (see electronic supplementary material, figure S3).
(f). Statistical analyses
Analysis of variance (ANOVA) models were developed for the dependent variables (growth, bioturbation, bioirrigation, NH4-N, NOx-N and PO4-P) with the independent nominal variables CO2 and temperature (short-term experiment), CO2 and time point (long-term experiment, elevated CO2 under ambient temperature conditions) or CO2, temperature regime and time point (long-term experiment, elevated CO2 under warming conditions). Where there was evidence of a violation of homogeneity of variance (long-term experiments), the data were analysed using a VarIdent variance–covariate structure and a generalized least-squares (GLS) estimation procedure (following [86,87]) to allow the residual spread to vary with individual explanatory variables. For the analyses of the long-term effects of elevated CO2 under ambient temperature conditions, the residual spread varied with time point for growth, bioirrigation and NH4-N concentration or CO2 × time point for NOx-N concentration. For the analyses of the long-term effects of elevated CO2 and warming, the residual spread varied with time point for growth and PO4-P concentration, time point × temperature regime for NH4-N concentration and CO2 × temperature regime for NOx-N concentration. We determined the optimal fixed-effects structure for each ANOVA model using backward selection informed by Akaike Information Criteria (AIC) and inspection of model residual patterns. For the GLS analyses, the optimal variance–covariate structure was determined using restricted maximum-likelihood (REML) estimation; the initial ANOVA model without variance structure is compared with the equivalent GLS model incorporating specific variance structures using AIC and visualization of model residuals. The optimal fixed structure is then determined by applying backward selection using the likelihood ratio test obtained by ML estimation [87,88].
All analyses were performed in R [89]. GLS analyses were conducted using the nlme package [90] and figures were constructed using the packages sciplot [91] and ggplot2 [92].
3. Results
(a). Short-term effects of warming and enhanced CO2
All individuals of A. virens survived. Short-term exposure (6 days) to enhanced CO2 did not significantly impact A. virens bioirrigation activity (F2,21 = 2.502, p = 0.106) or water column [NOx-N] (F2,21 = 2.304, p = 0.125). However, there was some weak evidence that bioirrigation activity was highest at 1000 ppm CO2 and [NOx-N] was highest at 750 ppm CO2 (see electronic supplementary material, figure S4). Bioturbation activity and [NH4-N] were not affected by either warming or enhanced CO2 (both ANOVA intercept only models).
(b). Long-term effects of enhanced CO2 under ambient temperature conditions
It was possible to relate our response variables to species behaviour in all but four aquaria (100% mortality: one aquarium after 12 months in the ambient+4°C 380 ppm [CO2] treatment; two aquaria after 18 months in the ambient +4°C 380 ppm [CO2] treatment and one aquarium after 18 months in the ambient+4°C 750 ppm [CO2] treatment), so these replicates were removed from the statistical analysis. Based on the remaining aquaria, we found that A. virens significantly increased in biomass over the 18 months exposure period (L. ratio = 20.780, p < 0.0001, d.f. = 3) from 6.64 ± 0.29 g in month 3 to 10.42 ± 1.16 g after 18 months (figure 1a). Irrespective of the length of exposure, growth was significantly reduced under enhanced CO2 conditions (L. ratio = 14.044, p < 0.0001, d.f. = 2; figure 1b). Comparison of model residuals for growth revealed that the increase in growth over the term of the experiment did not influence any of the response variables under investigation, providing confidence that any observed effects were due to our explanatory variables (see electronic supplementary material, figure S5). The mean maximum depth of bioturbation varied with length of exposure (F3,44 = 8.842, p < 0.001; figure 2a), but there was no evidence of a directional change with increasing exposure time. Instead, an increase in bioturbation depth in the warmer months (3.21 ± 0.31 and 1.90 ± 0.17 cm after 6 and 18 months) relative to colder months (2.03 ± 0.21 and 1.43 ± 0.25 cm after 3 and 12 months) suggests seasonal differences in species behaviour, although this winter–summer shift is much weaker in the second half of the experiment. Bioirrigation activity also varied with length of exposure, but these effects were dependent on the level of CO2 (time point × CO2, L. ratio = 21.208, p < 0.01, d.f. = 6). In the medium term (3 and 6 months), enhanced CO2 led to an increase in bioirrigation activity, whereas over the longer term (12 and 18 months) there was a reduction in bioirrigation activity with increasing CO2 (figure 2b).
Figure 1.
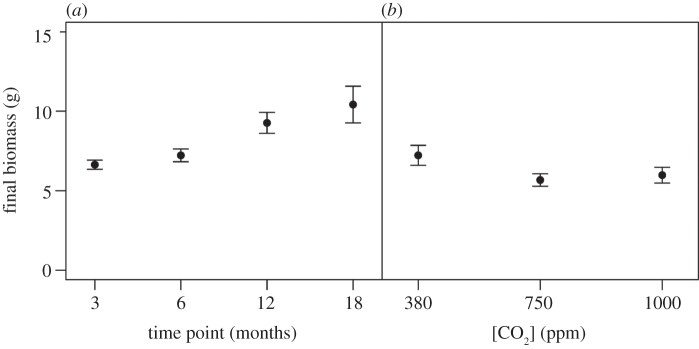
The independent effects of (a) the length of exposure (time point (months)) and (b) [CO2] (ppm) on the biomass (g, mean±s.e.) of A. virens under ambient temperature conditions.
Figure 2.
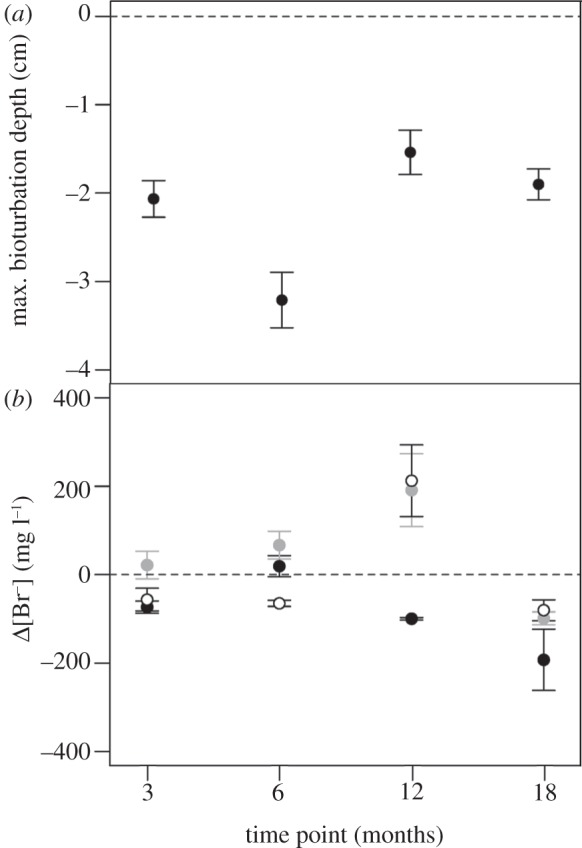
The independent effect of (a) the length of exposure (time point (months)) on the mean maximum bioturbation depth (cm, mean ± s.e.) and (b) the interactive effects of time point (months) × [CO2] (ppm) on bioirrigation activity (Δ[Br−], mg l−1, mean ± s.e.), with [CO2] indicated by: open circles = 380 ppm, grey closed circles = 750 ppm and black closed circles = 1000 ppm, for A. virens.
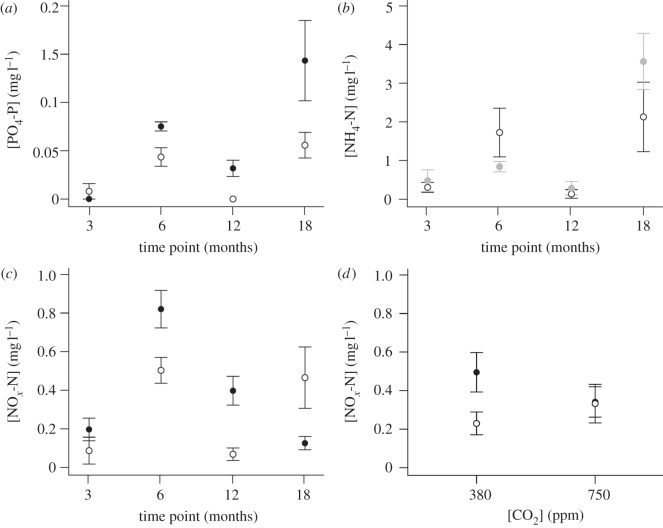
Irrespective of identity, nutrient concentrations varied significantly with length of exposure and were highest in the summer (i.e. after 6 and 18 months exposure: PO4-P, F3,42 = 11.724, p < 0.0001; NOx-N, L. ratio = 46.453, d.f. = 3, p < 0.0001; figure 3a,b). Concentrations of NH4-N, however, were dependent on an interaction between length of exposure and CO2 (CO2 × time point, L. ratio: 16.649, d.f. = 6, p < 0.05). After 6 months of exposure, [NH4-N] was significantly lower in the 750 ppm treatment (0.76 ± 0.24 mg l−1), relative to the 380 ppm treatment (2.42 ± 1.22 mg l−1), but this effect was reversed after 18 months of exposure (1.64 ± 0.65 mg l−1 at 380 ppm and 4.86 mg l−1 at 750 ppm; figure 3c).
Figure 3.
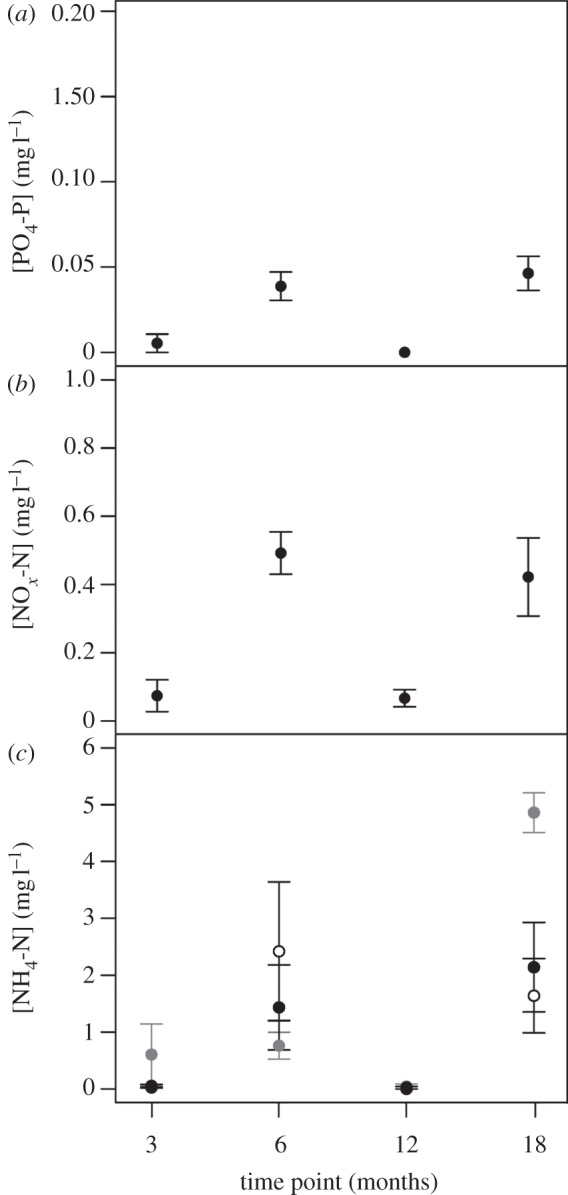
The effects of the length of exposure (time point (months)) on water column nutrient concentration (mg l−1, mean ± s.e.), (a) [PO4-P], (b) [NOx-N] and (c) [NH4-N]. In (c) [NH4-N] was dependent on the interaction time point (months) × [CO2] (ppm), with [CO2] indicated by: open circles = 380 ppm, grey closed circles = 750 ppm and black closed circles = 1000 ppm.
(c). Long-term effects of enhanced CO2 under warming conditions
Growth of A. virens was positively influenced by the independent effects of warming (from 6.42 ± 0.66 g at ambient to 8.60 ± 0.52 g at ambient+4°C; L. ratio = 23.361, d.f. = 1, p < 0.0001; figure 4a) and length of exposure (from 6.33 ± 0.28 g after 3 months to 9.69 ± 1.51 g after 18 months; L. ratio = 16.335, d.f. = 3, p < 0.01; figure 4b), but was negatively affected, albeit marginally, by an independent effect of enhanced CO2 (7.99 ± 0.61 g at 380 ppm, 7.25 ± 0.62 g at 750 ppm; L. ratio = 5.747, d.f. = 1, p < 0.05; figure 4c). The mean maximum depth of bioturbation depended on the interactive effects of length of exposure and temperature regime (time point × temperature regime, F3,52 = 3.734, p < 0.05), with the shallowest (1.36 ± 0.28 cm) bioturbation occurring after 12 months under the warming regime and the deepest (3.23 ± 0.37 cm) bioturbation occurring after 6 months under ambient conditions. The seasonal increase in maximum bioturbation depth observed under ambient temperature conditions did not hold under the ambient+4°C temperature regime; the maximum bioturbation depth was consistently less at ambient temperature during the first 12 months of exposure (figure 5a). Bioirrigation activity was affected by the interactive effects of CO2 × temperature regime (F1,50 = 4.338, p < 0.05) and length of exposure × temperature regime (F3,50 = 2.905, p < 0.05). Hence, after an initial reduction in activity after 6 months of exposure, bioirrigation is most pronounced after longer exposures under a warming regime (figure 5b). At the same time, there is also evidence that enhanced CO2 reduces bioirrigation, although this effect is less marked under a warming regime (figure 5c). Overall bioirrigation activity was higher under the ambient+4°C regime and followed a seasonal pattern of activity, increasing during the winter (after 3 and 12 months exposure) and decreasing during the summer (after 6 and 18 months exposure) (figure 5c).
Figure 4.
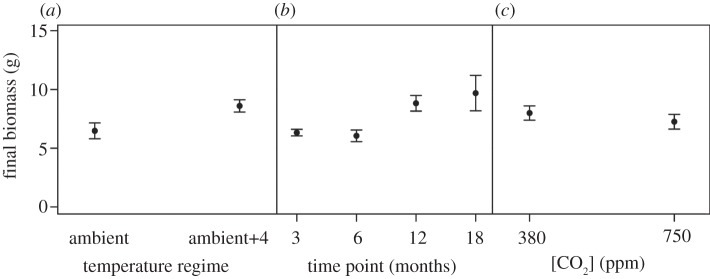
The independent effects of (a) temperature regime (ambient versus ambient+4°C), (b) length of exposure (time point (months)) and (c) [CO2] (ppm) on the biomass (g, mean ± s.e.) of A. virens.
Figure 5.
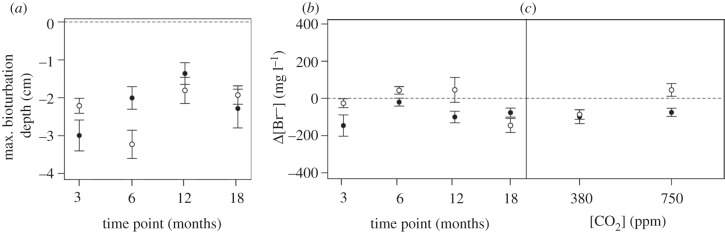
The interactive effects of (a) the length of exposure (time point (months)) × temperature regime on mean maximum bioturbation depth (cm, mean ± s.e.), (b) the length of exposure (time point (months)) × temperature regime and (c) [CO2] (ppm) × temperature regime on bioirrigation activity (Δ[Br−], mg l−1, mean ± s.e.) of A. virens. In a–c, temperature regimes are ambient (open circles) or ambient+4°C (filled circles).
Nutrient concentrations, in all cases, were highest after 6 and 12 months but depended upon complex interactions between the length of exposure time and other variables (figure 6). These were temperature regime for [PO4-P], which were highest under the warming regime (time point × temperature regime, L. ratio = 17.248, d.f. = 3, p < 0.0001; figure 6a), and enhanced CO2 for [NH4-N], which were higher with increasing CO2 (time point × CO2, L. ratio = 8.729, d.f. = 3, p < 0.05; figure 6b). For the latter, differences in [NH4-N] were greatest after 6 and 18 months of exposure (figure 6b). [NOx-N] were dependent on the interactive effects of length of exposure time and temperature regime (time point × temperature regime, L. ratio = 18.878, d.f. = 3, p < 0.0001; figure 6c) as well as the interactive effects of enhanced CO2 and temperature regime (CO2 × temperature regime, L. ratio = 4.205, d.f. = 1, p < 0.05; figure 6d). These analyses indicate that [NOx-N] are higher during the summer (months 6 and 18) relative to the winter (months 3 and 12), are augmented under a warming scenario (figure 6c), and are either further increased (ambient temperature regime) or reduced (ambient+4°C temperature regime) by enhanced CO2 (figure 6d).
Figure 6.
The interactive effects of (a) length of exposure (time point (months)) × temperature regime on [PO4-P], (b) length of exposure (time point (months)) × [CO2] (ppm) on [NH4-N], (c) length of exposure (time point (months)) × temperature regime on [NOx-N] and (d) CO2 (ppm) × temperature regime on [NOx-N]. In a,c and d temperature regimes are ambient (open circles) or ambient+4°C (filled circles). In b, [CO2] is 380 ppm (open circles) or 750 ppm (grey circles). All error bars represent s.e.
4. Discussion
When the findings from studies that have experimentally manipulated climatic variables are combined with observations from natural systems that are experiencing localized enhancement of temperature and/or CO2 levels, such as volcanic vent sites [93] or regions of upwelling [94], the conclusion that the impacts of climate change on species, communities and ecosystems is likely to be extensive becomes compelling [15,95,96]. The vast majority of these experiments and observations, however, do not account for the influence of species and system responses that follow biological rhythms or take extended periods of time to develop; they are either too short in duration (median length of experiment for broad response categories in days: survival = 35(n = 68), growth = 25(n = 155), calcification = 21(n = 92), photosynthesis = 14(n = 76) and development = 5(n = 29) (K. J. Kroeker 2013, personal communication, based on [30]) or, in the case of field studies, are sufficiently small in scale that other small-scale processes (e.g. spatio-temporal relief from perturbation through avoidance behaviour [20], presence of collinear environmental factors [97]) may mask or subsidize the full extent of any response. Here, we find strong evidence for effects of warming and ocean acidification mediated over approximately 18 months through complex interactions on species growth, behaviour and related levels of ecosystem functioning. In the broadest terms, the relative importance and form of the interdependencies between warming and enhanced CO2 vary across response variables, but generally showed negative effects of enhanced CO2 and positive effects of increased temperature, consistent with expectations based on previous reports for A. virens and closely related species [44,73,98]. However, these responses were not present in our short-term experiment and did not appear until several months had elapsed in both of our longer term experiments. When they did emerge, they were highly influenced by attributes of the system that varied with season. These complex relationships, therefore, mean that the magnitude and direction of ecological response detected in laboratory and field investigations may be more dependent on seasonal and historical climatic context than on the absolute change in the level of forcing [99].
An important aspect in refining prediction uncertainty is whether weak or null responses are genuine. The lack of response following short-term exposure to enhanced levels of CO2 and temperature documented here confirms the findings of previous experiments with the same species [21] and lends support to the weak responses typically exhibited by other non-calcifying invertebrates [100,101]. Consequently, it is tempting to speculate that the concomitant effects of climatic forcing on ecosystem functioning, as mediated by the non-calcifying components of an ecological community, will most likely be negligible. Indeed, when considered in isolation, our short-term experiment supports this view because we did not find any effects of ocean acidification or warming on nutrient release, presumably because changes in microbial process rates are primarily linked to changes in, or contrasts between, infaunal activity and behaviour [44,71,72]; both effects were absent in our short-term experimental treatments. While such conclusions may be regarded as unsurprising and intuitive, because non-calcifying species lack the pertinent physiological traits that show the strongest response to climatic forcing, an alternative and often overlooked hypothesis is that the presence of weak or null responses reflects fundamental differences between calcifying and non-calcifying organisms in both the nature of response and the time required to elicit a response. Certainly, the effect sizes of processes that have less or no reliance on the process of calcification, such as growth, photosynthesis, development and species interactions [30], provide reason to expect that the contributions of non-calcifying species to ecosystem functioning will be compromised under climatic forcing. The capacity to repeatedly test the behaviour and contribution of A. virens to ecosystem functioning achieved here meant that it was possible to test this assertion. In so doing, we found temporal differences across all of our response variables that strongly suggest that the net susceptibility of species to climatic perturbation, and their associated effects on ecosystem properties, will reflect the summation of both trait-based responses that tend to be immediately expressed (positive [102,103] or negative [15]) and other responses that take extended periods of time to develop, such as physiological compensatory mechanisms, change in sensitivity levels and alteration of species behaviour and interactions [104]. In turn, these results imply that a priori arguments about the likely impact of climatic forcing on species or communities that are based on assumed differences in trait vulnerability (e.g. calcifying versus non-calcifying species) are unlikely to provide a useful means of estimating either the long-term survivorship or the per capita contributions of species to functioning, because the net effect of climatic forcing must balance both short- and long-term response mechanisms.
Although not previously an explicit feature of investigations of the effects of warming and ocean acidification, the extent and relative importance of physiological, behavioural and ecological response mechanisms have been recognized [29]. In the present investigation, we did not attempt to determine the precise physiological mechanism(s) underpinning the net observed responses, but the combined results from both short- and long-term experiments indicate that these do take several months to develop, expressed here through notable changes in growth, bioturbation and bioirrigation behaviour that, in turn, affected important ecosystem-level properties (nutrient concentrations). This most striking and consistent feature across all of our analyses returned distinct changes in the strength and, in some cases (bioirrigation), direction of response over the course of the experimental period (18 months). These patterns reflected, at least in part, a sustained response to prolonged exposure to climatic forcing, as both CO2 and temperature commonly featured in our statistical models. However, our analyses revealed a dominant effect of time point that tracked the experimentally imposed collinear seasonal cycles of temperature and photoperiod [98,105]. This was particularly prominent at the lower and higher ends of the seasonal range, where alterations in behaviour (type and magnitude of activity, alteration of the sediment mixing : bioirrigation ratio) led to detectable changes in the level of ecosystem functioning [73,106]. These effects were mostly driven by absolute temperature, rather than enhanced levels of CO2, and were exacerbated by supplementary warming. Consequently, the addition of the warming regime effectively shifted responses observed during the colder months (winter) towards those expected for the warmer months (summer), but as temperatures exceeded the bounds of the historic temperature range during the peak of the summer season, we observed more erratic responses (as in [73]) in species behaviour with corresponding effects on nutrient concentrations. Whilst one could speculate that these erratic effects may be expected (and may alter the seasonal boundary) at the northern and/or southern edge of a species distributional range, we wish to emphasize that the dominant effect of temperature observed here is consistent with findings elsewhere [44,107,108] and reveals that certain components of climatic forcing can play a fundamental and disproportional role in determining the overall system response [101,109].
We have observed changes in species activity and behaviour that affected important aspects of the ecosystem that were concurrent with both long-term forcing and short-term sources of natural variation (seasonal shifts in temperature and photoperiod). As we have shown, responses to short-term seasonal change (if taken in isolation) were sufficient to result in apparently divergent effects because of the synergistic interplay between multiple variables, phenology and natural sources of variation that operate over longer timescales. Indeed, the way in which climatic forcing is expressed in natural systems is not universal, with both spatial (local, regional and latitudinal) and temporal (periodic pulses and seasonal cycles) variations in pH and temperature that are sufficient to affect the direction (positive through to negative) and severity of effect depending on timing and context [110]. Although it is clear that further attention is required in defining and characterizing the aspects of this variation (e.g. frequency and amplitude [44,65]) that lead to differences in species responses, a more immediate empirical challenge is to characterize and separate the effects of periodic, stochastic and/or cyclical natural variations [61] from directional change imposed by long-term climatic forcing. If the effects of temporal variation exacerbate (or dampen) the effects of climatic forcing, then the consequences for the marine environment may exceed (or reduce) prior expectations, raising significant implications for the management and conservation of marine ecosystems. A first and necessary step is to reduce the systematic propagation of error in the direction and magnitude (imposed by assuming constancy in short-term experiments) of effect size by embracing the challenges of simultaneously incorporating both short- and long-term natural variability [111] in empirical investigations. Secondly, it needs to be recognized that management of non-climatic stressors that interact and reinforce the negative effects of warming and ocean acidification can go some way in mitigating the effects of climate change at the local level. Further research emphasis is needed in these areas if we are to reduce uncertainty in modelled forecasts of the ecological consequences of climate change.
Acknowledgements
We thank H. Findlay and S. Widdicombe (Plymouth Marine Laboratory) for technical advice, J. Polanski and the late O. McPherson (University of Aberdeen) for building the infrastructure, and V. Johnston (University of Aberdeen) for assisting with the metrology and maintenance of the long-term experiments.
Funding statement
Supported by the Natural Environment Research Council (NERC) through the UK Ocean Acidification Research Programme (UKOARP, grant no. NE/H017445/1). All data are archived with the British Oceanographic Data Centre, http://www.bodc.ac.uk.
References
- 1.Caldeira K, Wickett ME. 2003. Anthropogenic carbon and ocean pH. Nature 425, 365 (doi:10.1038/425365a) [DOI] [PubMed] [Google Scholar]
- 2.Meehl GA, et al. 2007. Global climate projections. In Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL.), pp. 745–845 Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Thornalley DJR, Elderfield H, McCave IN. 2009. Holocene oscillations in temperature and salinity of the surface subpolar North Atlantic. Nature 457, 711–714 (doi:10.1038/nature07717) [DOI] [PubMed] [Google Scholar]
- 4.Lüthi D, et al. 2008. High-resolution carbon dioxide concentration record 650,000–800,000 years before present. Nature 453, 379–382 (doi:10.1038/nature06949) [DOI] [PubMed] [Google Scholar]
- 5.Raven J, Caldeira K, Elderfield H, Hoegh-Guldberg O, Liss P, Riebesell U, Shepherd J, Turley C, Watson A. 2005. Ocean acidification due to increasing atmospheric carbon dioxide. Policy document 12/05 London, UK: The Royal Society [Google Scholar]
- 6.Khatiwala S, Primeau F, Hall T. 2009. Reconstruction of the history of anthropogenic CO2 concentrations in the ocean. Nature 462, 346–349 (doi:10.1038/nature08526) [DOI] [PubMed] [Google Scholar]
- 7.Lohbeck KT, Riebesell U, Reusch TBH. 2012. Adaptive evolution of a key phytoplankton species to ocean acidification. Nat. Geosci. 5, 346–351 (doi:10.1038/NGEO1441) [Google Scholar]
- 8.Madeira D, Narciso L, Cabral H, Vinagre C. 2012. Thermal tolerance and potential climate change impact in marine and estuarine organisms. J. Sea Res. 70, 32–41 (doi:10.1016/j.seares.2012.03.002) [Google Scholar]
- 9.Le Quesne WJF, Pinnegar JK. 2012. The potential impacts of ocean acidification: scaling from physiology to fisheries. Fish Fish. 13, 333–344 (doi:10.1111/j.1467-2979.2011.00423.x) [Google Scholar]
- 10.Sanford E. 1999. Regulation of keystone predation by small changes in ocean temperature. Science 283, 2095–2097 (doi:10.1126/science.283.5410.2095) [DOI] [PubMed] [Google Scholar]
- 11.Hughes TP, et al. 2003. Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933 (doi:10.1126/science.1085046) [DOI] [PubMed] [Google Scholar]
- 12.Hoegh-Guldberg O, Bruno JG. 2010. The impact of climate change on the world's marine ecosystems. Science 328, 9316–9321 (doi:10.1126/science.1189930) [DOI] [PubMed] [Google Scholar]
- 13.Brose U, Dunne JA, Montoya JM, Petchey OL, Schneider FD, Jacob U. 2012. Climate change in size-structured ecosystems. Phil. Trans. R. Soc. B 367, 2903–2912 (doi:10.1098/rstb.2012.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doney S, Fabry V, Feely R, Kleypas J. 2009. Ocean acidification: the other CO2 problem. Annu. Rev. Mar. Sci. 1, 169–192 (doi:10.1146/annurev.marine.010908.163834) [DOI] [PubMed] [Google Scholar]
- 15.Kroeker KJ, Kordas RL, Crim RN, Singh GG. 2010. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 13, 1419–1434 (doi:10.1111/j.1461-0248.2010.01518.x) [DOI] [PubMed] [Google Scholar]
- 16.Kroeker KJ, Kordas RL, Crim RN, Singh GG. 2011. Response to technical comment on 'meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms'. Ecol. Lett. 14, E1–E2 (doi:10.1111/j.1461-0248.2011.01665.x) [DOI] [PubMed] [Google Scholar]
- 17.Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, Fabry VJ, Millero FJ. 2004. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 305, 362–366 (doi:10.1126/science.1097329) [DOI] [PubMed] [Google Scholar]
- 18.Hannisdal B, Henderiks J, Liow LH. 2012. Long-term evolutionary and ecological responses of calcifying phytoplankton to changes in atmospheric CO2. Glob. Change Biol. 18, 3504–3516 (doi:10.1111/gcb.12007) [Google Scholar]
- 19.Orr JC, et al. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686 (doi:10.1038/nature04095) [DOI] [PubMed] [Google Scholar]
- 20.Briffa M, de la Haye K, Munday PL. 2012. High CO2 and marine animal behaviour: potential mechanisms and ecological consequences. Mar. Pollut. Bull. 64, 1519–1528 (doi:10.1016/j.marpolbul.2012.05.032) [DOI] [PubMed] [Google Scholar]
- 21.Widdicombe S, Needham HR. 2007. Impact of CO2-induced seawater acidification on the burrowing activity of Nereis virens and sediment nutrient flux. Mar. Ecol. Prog. Ser. 341, 111–122 (doi:10.3354/meps341111) [Google Scholar]
- 22.Gooding RA, Harley CDG, Tang E. 2009. Elevated water temperature and carbon dioxide concentration increase the growth of a keystone echinoderm. Proc. Natl Acad. Sci. USA 106, 9316–9321 (doi:10.1073/pnas.0811143106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connell SD, Russel BD. 2010. The direct effects of increasing CO2 and temperature on non-calcifying organisms: increasing the potential for phase shifts in kelp forests. Proc. R. Soc. B 277, 1409–1415 (doi:10.1098/rspb.2009.2069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Connell SD, Kroecker KJ, Fabricius KE, Kline DI, Russell BD. 2013. The other ocean acidification problem: CO2 as a resource among competitors for ecosystem dominance. Phil. Trans. R. Soc. B 368, 20120442 (doi:10.1098/rstb.2012.0442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warren R. 2011. The role of interactions in a world implementing adaptation and mitigation solutions to climate change. Phil. Trans. R. Soc. A 369, 217–241 (doi:10.1098/rsta.2010.0271) [DOI] [PubMed] [Google Scholar]
- 26.Russell BD, Passarelli CA, Connell SD. 2011. Forecasted CO2 modifies the influence of light in shaping subtidal habitat. J. Phycol. 47, 744–752 (doi:10.1111/j.1529-8817.2011.01002.x) [DOI] [PubMed] [Google Scholar]
- 27.Malakoff D. 2012. Climate change. Researchers struggle to assess responses to ocean acidification. Science 338, 27–28 (doi:10.1126/science.338.6103.27) [DOI] [PubMed] [Google Scholar]
- 28.Hettinger A, Sanford E, Hill TM, Russell AD, Sato KNS, Hoey J, Forsch M, Page HN, Gaylord B. 2012. Persistent carry-over effects of planktonic exposure to ocean acidification in the Olympia oyster. Ecology 93, 2758–2768 (doi:10.1890/12-0567.1) [DOI] [PubMed] [Google Scholar]
- 29.Form AU, Riebesell U. 2012. Acclimation to ocean acidification during long-term CO2 exposure in the cold-water coral Lophelia pertusa. Glob. Change Biol. 18, 843–853 (doi:10.1111/j.1365-2486.2011.02583.x) [Google Scholar]
- 30.Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, Duarte CM, Gattuso J-P. 2013. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob. Change Biol. 19, 1884–1896 (doi:10.1111/gcb.12179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pörtner H-O. 2008. Ecosystem effects of ocean acidification in times of ocean warming: a physiologist's view. Mar. Ecol. Progr. Ser. 373, 203–217 (doi:10.3354/meps07768) [Google Scholar]
- 32.Hernroth B, Baden S, Thorndyke M, Dupont S. 2011. Immune suppression of the echinoderm Asterias rubens (L.) following long-term ocean acidification. Aquat. Toxicol. 103, 222–224 (doi:10.1016/j.aquatox.2011.03.001) [DOI] [PubMed] [Google Scholar]
- 33.Holcomb M, McCorkle DC, Cohen AL. 2010. Long-term effects of nutrient and CO2 enrichment on the temperate coral Astrangia poculata (Ellis and Solander, 1786). J. Exp. Mar. Biol. Ecol. 386, 27–33 (doi:10.1016/j.jembe.2010.02.007) [Google Scholar]
- 34.Seibel BA, Maas AE, Dierssen HM. 2012. Energetic plasticity underlies a variable response to ocean acidification in the Pteropod, Limacina helicina antarctica. PLOS ONE 7, e30464 (doi:10.1371/journal.pone.0030464) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL. 2011. Projecting coral reef futures under global warming and ocean acidification. Science 333, 418–422 (doi:10.1126/science.1204794) [DOI] [PubMed] [Google Scholar]
- 36.Calosi P, et al. 2013. Adaptation and acclimatization to ocean acidification in marine ectotherms: an in situ transplant experiment with polychaetes at a shallow CO2 vent system. Phil. Trans. R. Soc. B 368, 20120444 (doi:10.1098/rstb.2012.0444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood HL, Spicer JI, Widdicombe S. 2008. Ocean acidification may increase calcification rates, but at a cost. Proc. R. Soc. B 275, 1767–1773 (doi:10.1098/rspb.2008.0343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heuer RM, Esbaugh AJ, Grosell M. 2012. Ocean acidification leads to counterproductive intestinal base loss in the Gulf Toadfish (Opsanus beta). Physiol. Biochem. Zool. 85, 450–459 (doi:10.1086/667617) [DOI] [PubMed] [Google Scholar]
- 39.Bibby R, Cleall-Harding P, Rundle S, Widdicombe S, Spicer J. 2007. Ocean acidification disrupts induced defences in the intertidal gastropod Littorina littorea. Biol. Lett. 3, 699–701 (doi:10.1098/rsbl.2007.0457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Range P, Pilo D, Ben-Hamadou R, Chicharo MA, Matias D, Joaquim S, Oliveira AP, Chicharo L. 2012. Seawater acidification by CO2 in a coastal lagoon environment: effects on life history traits of juvenile mussels Mytilus galloprovincialis. J. Exp. Mar. Biol. Ecol. 424, 89–98 (doi:10.1016/j.jembe.2012.05.010) [Google Scholar]
- 41.Pörtner HO, Langenbuch M, Reipschläger A. 2004. Biological impact of elevated ocean CO2 concentrations: lessons from animal physiology and earth history. J. Oceanogr. 60, 705–718 (doi:10.1007/s10872-004-5763-0) [Google Scholar]
- 42.Munday PL, McCormick MI, Nilsson GE. 2012. Impact of global warming and rising CO2 levels on coral reef fishes: what hope for the future? J. Exp. Biol. 215, 3865–3873 (doi:10.1242/jeb.074765) [DOI] [PubMed] [Google Scholar]
- 43.Gooding RA, Harley CDG, Tang E. 2009. Elevated water temperature and carbon dioxide concentration increase the growth of a keystone echinoderm. Proc. Natl Acad. Sci. USA 106, 1266–1271 (doi:10.1073/pnas.0811143106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bulling MT, Hicks N, Murray L, Paterson DM, Raffaelli D, White PCL, Solan M. 2010. Marine biodiversity-ecosystem functions under uncertain environmental futures. Phil. Trans. R. Soc. B 365, 2107–2116 (doi:10.1098/rstb.2010.0022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Findlay HS, Kendall MA, Spicer JI, Widdicombe S. 2010. Post-larval development of two intertidal barnacles at elevated CO2 and temperature. Mar. Biol. 157, 725–735 (doi:10.1007/s00227-009-1356-1) [Google Scholar]
- 46.Wood HL, Spicer JI, Lowe DM, Widdicombe S. 2010. Interaction of ocean acidification and temperature; the high cost of survival in the brittlestar Ophiura ophiura. Mar. Biol. 157, 2001–2013 (doi:10.1007/s00227-010-1469-6) [Google Scholar]
- 47.Hicks N, Bulling MT, Solan M, Raffaelli D, White PCL, Paterson DM. 2011. Impact of biodiversity-climate futures on primary production and metabolism in a model benthic estuarine system. BMC Ecol. 11, 7 (doi:10.1186/1472-6785-11-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lischka S, Riebesell U. 2012. Synergistic effects of ocean acidification and warming on overwintering pteropods in the Arctic. Glob. Change Biol. 18, 3517–3528 (doi:10.1111/gcb.12020) [Google Scholar]
- 49.Pörtner HO. 2001. Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol. A 132, 739–761 (doi:10.1242/jeb.074765) [DOI] [PubMed] [Google Scholar]
- 50.Putnam HM, Edmunds PJ. 2011. The physiological response of reef corals to diel fluctuations in seawater temperature. J. Exp. Mar. Biol. Ecol. 396, 216–223 (doi:10.1016/j.jembe.2010.10.026) [Google Scholar]
- 51.Yvon-Durocher G, et al. 2012. Reconciling the temperature dependence of respiration across timescales and ecosystem types. Nature 487, 472–476 (doi:10.1038/nature11205) [DOI] [PubMed] [Google Scholar]
- 52.Przeslawski R, Zhu Q, Aller R. 2009. Effects of abiotic stressors on infaunal burrowing and associated sediment characteristics. Mar. Ecol. Progr. Ser. 392, 33–42 (doi:10.3354/meps08221) [Google Scholar]
- 53.Sanz-Lazaro C, Valdemarsen T, Marin A, Holmer M. 2011. Effect of temperature on biogeochemistry of marine organic-enriched systems: implications in a global warming scenario. Ecol. Appl. 21, 2664–2677 (doi:10.1890/10-2219.1) [DOI] [PubMed] [Google Scholar]
- 54.Price N, Martz TR, Brainard RE, Smith JE. 2012. Diel variability in seawater pH relates to calcification and benthic community structure on coral reefs. PLoS ONE 7, e43843 (doi:10.1371/journal.pone.0043843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomsen J, Casties I, Pansch C, Kortzinger A, Melzner F. 2013. Food availability outweighs ocean acidification effects in juvenile Mytilus edulis: laboratory and field experiments. Glob. Change Biol. 19, 1017–1027 (doi:10.1111/gcb.12109) [DOI] [PubMed] [Google Scholar]
- 56.Sarker MY, Bartsch I, Olischlager M, Gutow L, Wiencke C. 2013. Combined effects of CO2, temperature, irradiance and time on the physiological performance of Chondrus crispus (Rhodophyta). Bot. Mar. 56, 63–74 (doi:10.1515/bot-2012-0143) [Google Scholar]
- 57.Solan M, Cardinale BJ, Downing AL, Engelhardt KAM, Ruesink JL, Srivastava DS. 2004. Extinction and ecosystem function in the marine benthos. Science 306, 1177–1180 (doi:10.1126/science.1103960) [DOI] [PubMed] [Google Scholar]
- 58.Godbold JA, Bulling MT, Solan M. 2011. Habitat structure mediates biodiversity effects on ecosystem properties. Proc. R. Soc. B 278, 2510–2518 (doi:10.1098/rspb.2010.2414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wootton JT, Pfister CA, Forester JD. 2008. Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset. Proc. Natl Acad. Sci. USA 105, 18 848–18 853 (doi:10.1073/pnas.0810079105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hofmann GE, et al. 2011. High-frequency dynamics of ocean pH: a multi-ecosystem comparison. PLoS ONE 6, e28983 (doi:10.1371/journal.pone.0028983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kitidis V, et al. 2012. Seasonal dynamics of the carbonate system in the Western English Channel. Cont. Shelf Res. 42, 30–40 (doi:10.1016/j.csr.2012.04.012) [Google Scholar]
- 62.Krause E, Wichels A, Gimenez L, Lunau M, Schilhabel MB, Gerdts G. 2012. Small changes in pH have direct effects on marine bacterial community composition: a microcosm approach. PLoS ONE 7, e47035 (doi:10.1371/journal.pone.0047035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gray SEC, DeGrandpre MD, Langdon C, Corredor JE. 2012. Short-term and seasonal pH, pCO2 and saturation state variability in a coral-reef ecosystem. Glob. Biogeochem. Cycles 26, GB3012 (doi:10.1029/2011GB004114) [Google Scholar]
- 64.Alenius B, Munguia P. 2012. Effects of pH variability on the intertidal isopod, Paradella dianae. Mar. Freshwater Behav. Phys. 45, 245–259 (doi:10.1080/10236244.2012.727235) [Google Scholar]
- 65.Langenheder S, Bulling MT, Prosser JI, Solan M. 2012. Role of functionally dominant species in varying environmental regimes: evidence for the performance-enhancing effect of biodiversity. BMC Ecol. 12, 14 (doi:10.1186/1472-6785-12-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sabo JL, Post DM. 2008. Quantifying periodic, stochastic, and catastrophic environmental variation. Ecol. Monogr. 78, 19–40 (doi:10.1890/06-1340.1) [Google Scholar]
- 67.Widdicombe S, Spicer JI. 2008. Predicting the impact of ocean acidification on benthic biodiversity: what can animal physiology tell us? J. Exp. Mar. Biol. Ecol. 366 (SI), 187–197 (doi:10.1016/j.jembe.2008.07.024) [Google Scholar]
- 68.Pörtner HO, Farrell AP. 2008. Physiology and climate change. Science 322, 690–692 (doi:10.1126/science.1163156) [DOI] [PubMed] [Google Scholar]
- 69.Jickells TD. 1998. Nutrient biogeochemistry of the coastal zone. Science 281, 217–222 (doi:10.1126/science.281.5374.217) [DOI] [PubMed] [Google Scholar]
- 70.Johnson KS, Chavez FP, Friederich GE. 1999. Continental-shelf sediment as a primary source of iron for coastal phytoplankton. Nature 398, 697–700 (doi:10.1038/19511) [Google Scholar]
- 71.Gilbertson WW, Solan M, Prosser JI. 2012. Differential effects of microorganism–invertebrate interactions on benthic nitrogen cycling. FEMS Microbiol. Ecol. 82, 11–22 (doi:10.1111/j.1574-6941.2012.01400.x) [DOI] [PubMed] [Google Scholar]
- 72.Laverock B, Kitidis V, Tait K, Gilbert JA, Osborn AM, Widdicombe S. 2013. Bioturbation determines the response of benthic ammonia-oxidizing microorganisms to ocean acidification. Phil. Trans. R. Soc. B 368, 20120441 (doi:10.1098/rstb.2012.0441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ouellette D, Desrosiers G, Gagne JP, Gilbert F, Poggiale JC, Blier PU, Stora G. 2004. Effects of temperature on in vitro sediment reworking processes by a gallery biodiffusor, the polychaete Neanthes virens. Mar. Ecol. Progr. Ser. 266, 185–193 (doi:10.3354/meps266185) [Google Scholar]
- 74.Canuel EA, Cammer SS, McIntosh HA, Pondell CR. 2012. Climate change impacts on the organic carbon cycle at the land–ocean interface. Annu. Rev. Earth Planet. Sci. 40, 685–711 (doi:10.1146/annurev-earth-042711-105511) [Google Scholar]
- 75.Wood HL, Widdicombe S, Spicer JI. 2009. The influence of hypercapnia and the infaunal brittlestar Amphiura filiformis on sediment nutrient flux – will ocean acidification affect nutrient exchange? Biogeosciences 6, 2015–2024 (doi:10.5194/bg-6-2015-2009) [Google Scholar]
- 76.Solan M, Wigham BD, Hudson IR, Kennedy R, Coulon CH, Norling K, Nilsson HC, Rosenberg R. 2004. In situ quantification of bioturbation using time-lapse fluorescent sediment profile imaging (f-SPI), luminophore tracers and model simulation. Mar. Ecol. Progr. Ser. 271, 1–12 (doi:10.3354/meps271001) [Google Scholar]
- 77.Schiffers K, Teal LR, Travis JMJ, Solan M. 2011. An open source simulation model for soil and sediment bioturbation. PLoS ONE 6, e28028 (doi:10.1371/journal.pone.0028028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Forster S, Glud RN, Gundersen JK, Huettel M. 1999. In situ study of bromide tracer and oxygen flux in coastal sediment. Estuar. Coast Shelf Sci. 49, 813–827 (doi:10.1006/ecss.1999.0557) [Google Scholar]
- 79.Findlay HS, Kendall MA, Spicer JI, Turley C, Widdicombe S. 2008. Novel microcosm system for investigating the effects of elevated carbon dioxide and temperature on intertidal organisms. Aquat. Biol. 3, 51–62 (doi:10.3354/ab00061) [Google Scholar]
- 80.Dickson AG, Sabine CL, Christian JR. (eds) 2007. Guide to best practices for ocean CO2 measurements, vol. 3, 191 pp. PICES Special Publication [Google Scholar]
- 81.Dickson AG. 2010. The carbon dioxide system in seawater: equilibrium chemistry and measurements. In Guide to best practices for ocean acidification research and data reporting (eds Riebesell U, Fabry VJ, Hansson L, Gattuso J-P.), 260 pp. Luxembourg: Publications Office of the European Union [Google Scholar]
- 82.Robbins LL, Hansen ME, Kleypas JA, Meylan SC.2010. CO2calc—a user-friendly seawater carbon calculator for Windows, Max OS X, and iOS (iPhone). US Geological Survey Open-File Report 2010–1280, 17 pp.
- 83.Mehrbach C, Culberson CH, Hawley JE, Pytkowicz RM. 1973. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, 897–907 (doi:10.4319/lo.1973.18.6.0897) [Google Scholar]
- 84.Dickson AG, Millero FJ. 1987. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res. A 34, 1733–1743 (doi:10.1016/0198-0149(87)90021-5) [Google Scholar]
- 85.Dickson AG. 1990. Standard potential of the reaction AgCl(s)+0.5H2 (g)=Ag(s)+HCl(aq) and the standard acidity constant of the ion HSO4—in synthetic sea water from 273.15 to 318.15 K. J. Chem. Thermodyn. 22, 113–127 (doi:10.1016/0021-9614(90)90074-Z) [Google Scholar]
- 86.Pinheiro JC, Bates DM. 2000. Mixed-effects models in S and S-plus. New York, NY: Springer [Google Scholar]
- 87.West BT, Welch KB, Gatecki AT. 2007. Linear mixed models. A practical guide using statistical software. London, UK: Chapman and Hall [Google Scholar]
- 88.Diggle PJ, Heagerty P, Liang KY, Zeger SL. 2002. Analysis of longitudinal data. Oxford, UK: Oxford University Press [Google Scholar]
- 89.R Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/ [Google Scholar]
- 90.Pinheiro J, Bates D, DebRoy S, Sarkar D, the R Development Core Team 2012. nlme: linear and nonlinear mixed effects models. R package version 3.1-105 See http://cran.r-project.org/package=nlme.
- 91.Morales M, with code developed by the R Development Core Team, with general advice from the R-help listserv community and especially Duncan Murdoch 2012. sciplot: scientific graphing functions for factorial designs. R package version 1.1-0 See http://CRAN.R-project.org/package=sciplot.
- 92.Wickham H. 2009. ggplot2: elegant graphics for data analysis. New York, NY: Springer [Google Scholar]
- 93.Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, Turner SM, Rowley SJ, Tedesco D, Buia MC. 2008. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454, 96–99 (doi:10.1038/nature07051) [DOI] [PubMed] [Google Scholar]
- 94.Manzello DP, Kleypas JA, Budd DA, Eakin CM, Glynn PW, Langdon C. 2008. Poorly cemented coral reefs of the eastern tropical Pacific: possible insights into reef development in a high-CO2 world. Proc. Natl Acad. Sci. USA 105, 10 450–10 455 (doi:10.1073/pnas.0712167105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hendriks IE, Duarte CM, Alvarez M. 2010. Vulnerability of marine biodiversity to ocean acidification: a meta-analysis. Estuar. Coast. Shelf Sci. 86, 157–164 (doi:10.1016/j.ecss.2009.11.022) [Google Scholar]
- 96.Liu JW, Weinbauer MG, Maier C, Dai MH, Gattuso JP. 2010. Effect of ocean acidification on microbial diversity and on microbe-driven biogeochemistry and ecosystem functioning. Aquat. Microb. Ecol. 61, 291–305 (doi:10.3354/ame01446) [Google Scholar]
- 97.Hahn S, Rodolfo-Metalpa R, Griesshaber E, Schmahl WW, Buhl D, Hall-Spencer JM, Baggini C, Fehr KT, Immenhauser A. 2012. Marine bivalve shell geochemistry and ultrastructure from modern low pH environments: environmental effect versus experimental bias. Biogeosciences 9, 1897–1914 (doi:10.5194/bg-9-1897-2012) [Google Scholar]
- 98.Deschênes J, Desrosiers G, Ferron J, Cloutier R, Stora G. 2005. Environmental influence on activity levels and behavioural allocation in the polychaete Nereis virens (Sars). J. Exp. Mar. Biol. Ecol. 317, 203–212 (doi:10.1016/j.jembe.2004.11.017) [Google Scholar]
- 99.Godbold JA, Solan M. 2009. Relative importance of biodiversity and the abiotic environment in mediating an ecosystem process. Mar. Ecol. Progr. Ser. 396, 273–282 (doi:10.3354/meps08401) [Google Scholar]
- 100.Widdicombe S, Dashfield SL, McNeill CL, Needham HR, Beesley A, McEvoy A, Oxnevad S, Clarke KR, Berge JA. 2009. Effects of CO2 induced seawater acidification on infaunal diversity and sediment nutrient fluxes. Mar. Ecol. Prog. Ser. 379, 59–75 (doi:10.3354/meps07894) [Google Scholar]
- 101.Hale R, Calosi P, McNeill L, Mieszkowska N, Widdicombe S. 2011. Predicted levels of future ocean acidification and temperature rise could alter community structure and biodiversity in marine benthic communities. Oikos 120, 661–674 (doi:10.1111/j.1600-0706.2010.19469.x) [Google Scholar]
- 102.Suggett DJ, Hall-Spencer JM, Rodolfo-Metalpa R, Boatman TG, Payton R, Pettay DT, Johnson VR, Warner ME, Lawson T. 2012. Sea anemones may thrive in a high CO2 world. Glob. Change Biol. 18, 3015–3025 (doi:10.1111/j.1365-2486.2012.02767.x) [DOI] [PubMed] [Google Scholar]
- 103.Koch M, Bowes G, Ross C, Zhang XH. 2013. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob. Change Biol. 19, 103–132 (doi:10.1111/j.1365-2486.2012.02791.x) [DOI] [PubMed] [Google Scholar]
- 104.Dupont S, Dorey N, Thorndyke M. 2010. What meta-analysis can tell us about vulnerability of marine biodiversity to ocean acidification? Estuar. Coast Shelf Sci. 89, 182–185 (doi:10.1016/j.ecss.2010.06.013) [Google Scholar]
- 105.Koo BJ, Kwon KK, Hyun J-H. 2007. Effect of environmental conditions on variation in the sediment-water interface created by complex macrofaunal burrows on a tidal flat. J. Sea Res. 58, 302–312 (doi:10.1016/j.seares.2007.07.002) [Google Scholar]
- 106.Riisgard HU, Vedel A, Boye H, Larsen PS. 1992. Filter-net structure and pumping activity in the polychaete Nereis diversicolor—effects of temperature and pump-modeling. Mar. Ecol. Progr. Ser. 83, 79–89 (doi:10.3354/meps083079) [Google Scholar]
- 107.Nguyen HD, Doo SS, Soars NA, Byrne A. 2012. Noncalcifying larvae in a changing ocean: warming, not acidification/hypercapnia, is the dominant stressor on development of the sea star Meridiastra calcar. Glob. Change Biol. 18, 2466–2476 (doi:10.1111/j.1365-2486.2012.02714.x) [Google Scholar]
- 108.Chua CM, Leggat W, Moya A, Baird AH. 2013. Temperature affects the early life history stages of corals more than near future ocean acidification. Mar. Ecol. Prog. Ser. 475, 85–92 (doi:10.3354/meps10077) [Google Scholar]
- 109.Corti S, Masciandaro G, Ceccanti B, Lardicci C. 2006. Interactions between bacteria and polychaetes in the sediment and the role of temperature: a preliminary experiment. Vie Milieu 56, 43–47 [Google Scholar]
- 110.Dijkstra JA, Westerman EL, Harris LG. 2011. The effects of climate change on species composition, succession and phenology: a case study. Glob. Change Biol. 17, 2360–2369 (doi:10.1111/j.1365-2486.2010.02371.x) [Google Scholar]
- 111.Ibanez I, et al. 2013. Moving forward in global-change ecology: capitalizing on natural variability. Ecol. Evol. 3, 170–181 (doi:10.1002/ece3.433) [DOI] [PMC free article] [PubMed] [Google Scholar]