Abstract
The common view of Alzheimer's disease (AD) is that of an age-related memory disorder, i.e. declarative memory deficits are the first signs of the disease and associated with progressive brain changes in the medial temporal lobes and the default mode network. However, two findings challenge this view. First, new model-based tools of attention research have revealed that impaired selective attention accompanies memory deficits from early pre-dementia AD stages on. Second, very early distributed lesions of lateral parietal networks may cause these attention deficits by disrupting brain mechanisms underlying attentional biased competition. We suggest that memory and attention impairments might indicate disturbances of a common underlying neurocognitive mechanism. We propose a unifying account of impaired neural interactions within and across brain networks involved in attention and memory inspired by the biased competition principle. We specify this account at two levels of analysis: at the computational level, the selective competition of representations during both perception and memory is biased by AD-induced lesions; at the large-scale brain level, integration within and across intrinsic brain networks, which overlap in parietal and temporal lobes, is disrupted. This account integrates a large amount of previously unrelated findings of changed behaviour and brain networks and favours a brain mechanism-centred view on AD.
Keywords: mild cognitive impairment, visual cognition, episodic memory, parietal cortex, functional connectivity, disconnection syndrome
1. Introduction
Imagine a 60-year-old man looking for his car after work. He always parks his car in the same spot on River Street, but is unable to find it there after finishing work. Lately, he has had frequent problems with finding things, so he starts to get nervous and tries to recall the morning's events in more detail. Being agitated, his recollection is a bit jumbled. He searches unsuccessfully for his car along River Street and on neighbouring streets, but repeatedly finds himself back in the same spot, searching in vain and with increasing frustration. After a while, he phones his wife and asks her to pick him up. She calms him down and then reconstructs the morning together with him. ‘You bought some bread in Mountain Street, didn't you?’ she asks. ‘Oh yes, here is the bag in my hand, now I remember. I parked the car in front of the store, and decided to walk to the office because of the sunny weather. I must have overlooked it before.’
This episode is taken from the report of a man who came to the memory clinic of the Technische Universität Munich owing to subjective memory problems. Although not detected by neuropsychological assessment, his wife, friends and colleagues all confirmed his cognitive problems. In fact, during the following 5 years, his memory impairment became more evident. In addition, annual brain scans revealed the first signs of Alzheimer's disease (AD), with increasing parietal hypometabolism and medial temporal lobe (MTL) atrophy.
A typical neuropsychological account of the patient's earliest problems focuses on the memory side, and explains his difficulties with respect to impaired episodic memory due to MTL pathology. Here, we want to extend this ‘conventional view’ for two reasons: first, this analysis does not offer an explanation for several problems of patients with prodromal AD, of which the case described earlier showed some representative features: for instance, why did this patient overlook his car although it was clearly visible to him? And why did his search repeatedly end in that part of River Street with which he was most familiar? Second, the conventional view neglects two types of recent findings concerning attention deficits and extra-MTL brain changes that accompany memory deficits in very early AD. These novel findings suggest that there may be a more general, unifying neurocognitive mechanism that potentially contributes to both memory and attention deficits of patients. Here, we will elaborate on this idea, which is centred on impaired neural interactions within and across brain networks involved in attention and memory and is inspired by the biased competition principle. We will describe this view at two levels of analysis: at the computational level, we suggest that in early AD, subtle and regionally specific cortical lesions induce an imbalance both in the perceptual and the memory domain when representations have to be selected in the presence of competing alternatives. At the large-scale brain level, we propose that the disrupted integration within and across intrinsic brain networks (IBNs), which overlap within the parietal and the temporal lobes, is the cause of the imbalance in selection. We will explain both levels in more detail in §3. In §2, we review recent findings of attention deficits in very early AD and their relation to impaired parietal cortex activity. We start with some basic facts about early AD.
2. Challenges for the conventional memory-focused view on Alzheimer's disease: evidence for lateral parietal lesions, impaired attention and the impact of parietal lesions on attentional selection performance in very early Alzheimer's disease
(a). Alzheimer's disease and being at-risk for Alzheimer's disease
AD is a neurodegenerative disease that accounts for about 60% of age-related dementia cases; worldwide about 80 million cases of dementia are expected by 2040 [1]. AD is characterized by several neuropathological features. First, amyloid plaques are present several years before first symptoms arise; plaque deposition starts in neocortical areas and spreads out to the rest of the brain. Second, neurofibrillary tangles (tau pathology) and cell loss start in the MTLs and spread out in limbic areas followed by the neocortex and the rest of the brain [2]; tau pathology and cell loss start at about that the same time as first cognitive symptoms appear. According to the traditional view of AD, first (pre-dementia) symptoms concern episodic memory, whereas attentional impairments do not become relevant before the stage of mild dementia [3]. The current diagnostic criteria of AD demand the presence of a dementia syndrome [4]; however, the past 10–15 years of research has enabled clinicians to now identify subjects at risk for AD at a stage when dementia is not yet present. These individuals, suffering from mild cognitive impairment (MCI), are characterized by subtle cognitive dysfunction, which lies between normal age-related cognitive decline and dementia [5]. The rate of conversion to AD is approximately 10–15% within 1 year [6] and 19–66% within the following 3–5 years [7]. In vivo biomarkers such as fluorodesoxyglucose positron emission tomography (FDG-PET), Pittsburgh-compound-P (PiB)-PET (sensitive for amyloid plaques) or cerebrospinal fluid-based amyloid-β-42 peptide allow for the identification of those in the MCI population bearing an especially high risk for AD [8]. The term ‘prodromal AD’ describes patients with MCI and at least one positive biomarker for AD, whereas ‘preclinical AD’ is defined by the presence of at least one positive biomarker but an absence of cognitive symptoms [9]. In the following, we use ‘pre-dementia AD’ as an umbrella term for both preclinical and prodromal AD.
(b). Evidence for lateral parietal dysfunction in pre-dementia Alzheimer's disease
(i). Regional lesions
Owing to the simultaneous emergence of both MTL tau pathology and the first cognitive symptoms, tau pathology has been thought of as the critical pathway of AD for a long time [2]. However, molecular research of the past decades revealed that substrates of most genes associated with AD are critically involved in pathways of amyloid pathology, such as amyloid precursor protein (APP) or APP-sensitive enzymes presenelin 1 and 2, which all are linked to familial forms of AD [3], as well as apolipoprotein A allele ε4, which is associated with sporadic AD [10]. According to these findings, it has been suggested that pathways of aberrant amyloid peptide processing might be the initial events in the pathogenesis of AD (the ‘amyloid cascade hypothesis’ [11]). More specifically, aberrant amyloid peptide processing results in amyloid peptide accumulation and plaque formation, both of which are associated with aberrant activity of neighbouring neurons [12,13]. Plaque deposition seems to start 10–30 years before the first obvious symptoms appear, mainly in the areas of the associative neocortex with high levels of both spontaneous (i.e. intrinsic) activity and connectivity (the so-called hubs [14,15]). In vivo PET imaging has demonstrated such a pattern of plaque deposition in both patients with preclinical and prodromal AD [8]. In particular, overlapping hypometabolism and plaque deposition have been consistently observed in patients with MCI, including areas of the lateral posterior parietal cortex (PPC) [16–20]. Importantly, most of these lateral parietal areas are known to be essentially involved in attention functions [21].
(ii). Intrinsic brain network lesions in early Alzheimer's disease
IBNs are characterized by spatially consistent functional connectivity of intrinsic brain activity; in other words, robust large-scale spatial patterns of synchronous ongoing brain activity define an IBN [22]. Most IBNs cover several distinct brain regions, such as parts of the ventromedial prefrontal cortex and the posterior cingulate cortex in the case of the default mode network (DMN). Remote IBN areas synchronize in a frequency range of 0.01–0.1 Hz. Beyond their anatomical extent, IBNs differ with respect to their levels of intrinsic activity (with the highest levels in the DMN, see [23]) and their level of connectivity with other networks, with parts of some networks acting as ‘hubs’ for inter-network synchronization (with the DMN and attention networks having the strongest ‘hubness’, see [23,24]). Because IBNs are consistent across persons, states, development and ageing, and even species [25], they constitute a basic form of the brain's large-scale organization.
Early AD appears to be associated with selectively disrupted intrinsic functional connectivity (iFC) of IBNs, particularly in the DMN (figure 1) [26,27]. The DMN covers frontoparietal midline structures, parts of lateral temporal and parietal cortices and the MTL. It is active during self-focused processes such as remembering and deactivates during world-focused processes such as allocating attention to the environment [23]. Particularly, in very early AD, regions of plaque deposition and hypometabolism overlap with the DMN and predict iFC disruptions [16,28]. It has been suggested that elevated intrinsic activity of the DMN predisposes for amyloid pathology in AD, marking AD primarily as a disease of the DMN [15,29]. However, beyond the DMN, very recent imaging studies have demonstrated disrupted iFC also within a lateral frontoparietal IBN, even in prodromal stages of AD [27,30–32]. Such lateral IBNs are called attention or central executive networks, because the areas they cover contribute to attention and cognitive control processes [25,33]. Impaired iFC in such attention networks corresponds with aberrant structural connectivity within these networks [34]. Furthermore, in prodromal AD, there seems to be a close spatial correspondence between plaque deposition and iFC disruption within attention networks [35]. In two recent studies in prodromal AD, impaired iFC of attention networks has been linked to impaired behavioural performance in a selective attention task (conflict processing in a flanker task). It was demonstrated, for the first time, that prodromal AD disrupts effective connectivity within an attention network during conflict processing. Second, it was shown that the relationship between task effective connectivity and resting-state iFC was aberrant in patients [32,36]. In summary, these studies strongly suggest that even in very early AD not only the DMN but also lateral frontoparietal attention networks are disrupted and related to impaired attention
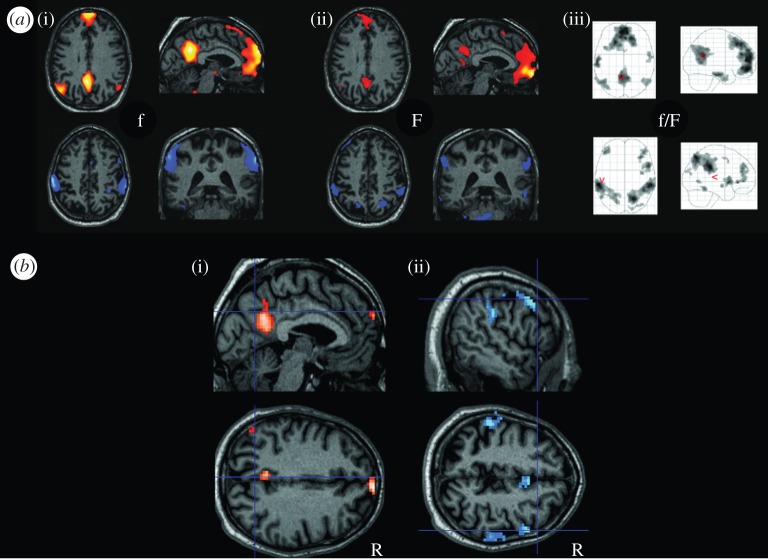
Figure 1.
Selective impact of MCI on intrinsic brain networks. (a) Patterns reflecting areas of significant intrinsic functional connectivity (iFC) of resting-state fMRI activity (blood oxygenation level-dependent signal, one-sample t-test, p < 0.05 FDR corrected). In the first row, the pattern reflects the default mode network (DMN), the pattern in the second row reflects a bilateral attention network (ATN), (i) data derived from 16 healthy elderly, (ii) data from 24 patients with MCI, (iii) corresponding glass brain projection for all subjects). (b) (i), pattern of reduced iFC in the DMN of patients, (ii), reduced iFC in the ATN of patients (two-sample t-test, p < 0.05 FDR-corrected). (Adapted from Buckner et al. [24].) (Online version in colour.)
(c). Evidence for impaired visual attention in pre-dementia Alzheimer's disease
It is well established that tasks involving a high load on visual processing—such as visuoconstruction, complex pattern discrimination or visual search—are already affected in early AD [37–40]. Figure 2 presents an example. During such tasks, visual information has to be sampled and integrated across a series of fixations. Therefore, disorders of visual attention—such as increased interference by distracting visual stimuli or a spatial imbalance during the inspection of the visual array—could decisively contribute to unsuccessful performance (as shown in the example). In fact, several studies have demonstrated impaired visual attention in early stages of AD dementia [41–45], and even in pre-dementia AD [42–47]. Importantly, single cases with AD dementia have been reported to show a pathological spatial preference towards one visual hemi-field [48] that is comparable with the chronic bias of spatial attentional weighting found in patients with visual hemi-neglect following damage to (mostly the right-hemispheric) parietotemporal areas [49–52]. These results are in line with the finding that neurodegeneration is often asymmetric, particularly in temporoparietal regions, in patients with AD [53–58].
Figure 2.
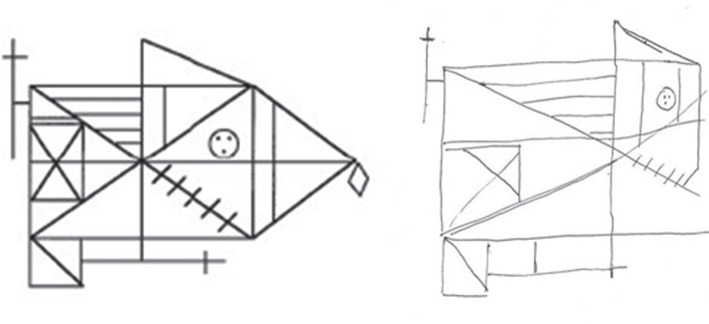
The right-hand side of the figure shows the copy of the Rey complex figure (presented on the left-hand side) by a 58-year-old-male patient with prodromal AD. His performance proves the presence of a visuoconstructive deficit. Besides the displacement of figure details, the patient also clearly neglects parts of the right half of the figure.
To analyse in more detail which visual attentional subprocesses are impaired in patients and how these changes relate to underlying computational principles of biased competition, a series of more comprehensive studies on basic, spatial as well as non-spatial components of visual attention were carried out. The conceptual framework used in this series was the neural interpretation of Bundesen's theory of visual attention (TVA; [59–61]). TVA is a mathematical model with strong relations to the biased competition view of visual attention [49]. On this view, visual objects are processed in parallel and compete for selection, i.e. conscious representation. In TVA, selection of an object is synonymous with its encoding into a visual short-term memory (vSTM) store. This store typically has a limited capacity of around four items in normal participants [62,63]. Competition for selection is decided according to a speed criterion, i.e. those objects with higher processing speed have a higher probability of becoming encoded into vSTM, until the vSTM store is filled. Objects receiving higher attentional weighting (e.g. due to their spatial position or due to their task relevance) gain a speed advantage compared with other competitors and are therefore more likely to be selected. In this way, TVA models the visual selection of objects on the basis of several parameters reflecting aspects of both specific (e.g. spatial or task-related) weighting of attention and general capacity aspects of attention (visual perceptual processing rate and vSTM storage capacity). The neural interpretation of TVA (NTVA [61]) holds that attentional weighting (i.e. the allocation of limited capacity) is reflected by a dynamic remapping of receptive fields of those neurons that are representing the perceived object. Furthermore, NTVA suggests that the processing speed of an object is determined by the number of these neurons (which is related to the attentional weight of the object compared with other objects in the display) and the activation level of those neurons (which is linked to a perceptual bias for important categorizations). In our account, the concept of biased competition will be linked only to the allocation of attentional weights.
The aim of our TVA-based studies was to use this framework to investigate in more detail which of the spatial and non-spatial components of visual attention are already affected at the stage of prodromal AD. This was performed by establishing, in each participant, individual estimates of latent attentional parameters that underlie the selective processing of visual information, as conceptualized in TVA. The method applied was partial report of briefly presented letters, where subjects have to report red target letters only, while ignoring green distractor letters. TVA-based modelling of the probability of correct target letter report, both in the left and the right visual hemi-fields, provided estimations of attentional parameters reflecting top-down-related and spatial weighting [50,64].
The results demonstrated specific changes to the parameters relevant for the attentional weighting of incoming information, even at the pre-dementia stage of AD [65]. More precisely, the efficiency of top-down-controlled prioritization of relevant over irrelevant information was already reduced at the MCI stage, and further deteriorated in AD participants. These results suggest that very early AD is associated with changes in competitive attentional selection processes. The impaired integration within and across lateral frontoparietal attention networks might lead to this inability to prioritize relevant over irrelevant visual information. Furthermore, the distribution of spatial attentional weights across the two hemi-fields was already unbalanced in patients with MCI, and was even more lateralized in participants with AD. At the group level, the lateralization in MCI and AD patients primarily favoured the left visual field. However, at the single-case level, evidence was found for a right-sided as well as a left-sided attentional bias. These spatial attentional asymmetries were interpreted as resulting from very early temporoparietal interhemispheric asymmetries. Predominant neurodegeneration within one hemisphere might cause an abnormal spatial bias inducing a constant tendency to favour stimuli from one visual hemi-field over the other.
(d). Relationship between parietal damage and impaired attentional weighting
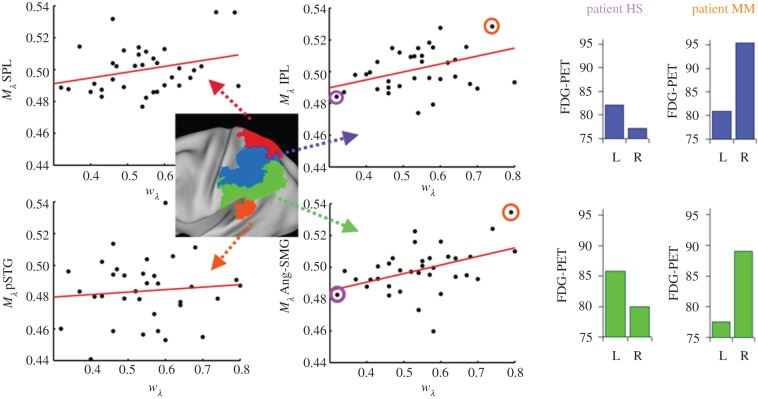
To test directly whether temporoparietal lesions of early AD cause a spatial imbalance of attentional weighting, patients with prodromal AD were assessed by FDG-PET and a TVA-based partial report paradigm. An index for the relative distribution of attentional weights derived by Bundesen's TVA (attentional weight for left hemi-field divided by the sum of attentional weights for both hemi-fields) was taken and correlated to a mathematically identical index which describes the distribution of effective neuronal activity measured by FDG activity across hemispheres (regional activity in the right hemisphere divided by the sum of the activity in homologous regions of both hemispheres). These analyses revealed that the bias of spatial attentional weights was significantly correlated to the bias of activity within two areas of the lateral parietal spatial attention system, i.e. within the intra-parietal lobule and the angular/supramarginal gyri of both hemispheres (figure 3). Importantly, these areas were characterized by significant hypometabolism indicating subtle AD-induced lesions [20]. In terms of NTVA [61], this suggests that in patients with prodromal AD, a left-sided spatial bias is the result of a permanently decreased competitiveness of processing units responsible for the right hemi-field (compared with left hemi-field units). At the neural level, the competition between neuronal populations encoding objects in different hemi-fields seems to be biased in favour of right-hemispheric neurons because of a larger loss of effective neurons in the homologous left-hemispheric populations (see [20] for a more comprehensive discussion).
Figure 3.
Metabolic biases Mλ correlate with spatial bias wλ. In analogy to spatial bias wλ, we defined an index for the lateral bias of metabolism (Mλ) of a given brain region as Mright/(Mright + Mleft), where Mright or Mleft represents the mean metabolism in a right- or left-hemisphere ROI. Metabolic biases Mλ (y-axes) of the angular–supramarginal gyrus (Ang-SMG, lower middle panel; r = 0.44) and the inferior parietal lobe (IPL, upper middle panel; r = 0.41) correlate significantly with wλ (x-axes) (p < 0.05, Bonferroni-corrected for multiple testing); results were based on partial correlation analyses, including both mini-mental-status examination scores and mean metabolism of the posterior cingulate cortex measured by FDG-PET scores as additional covariates, in order to control for effects of general cognitive performance and brain metabolism. The right panel shows left- and right-hemisphere regional metabolism in the IPL (upper row) and Ang-SMG (lower row) in exemplary patients exhibiting either a right- (HS) or left-lateralized (MM) spatial attentional bias. Their location in the scatterplots is circled, HS on the left and MM on the right. (Adapted from Mintun et al. [19].) (Online version in colour.)
(e). Interim summary: parietal damage in prodromal Alzheimer's disease leads to changes in the biased competition of visual attention
We have presented empirical evidence that model-based assessment tools for attentional functions reveal early changes in prodromal AD, especially in visual selection [65,66], which are correlated to activity changes in posterior parietal areas [20,32]. Thus, we suggest that localized neural degeneration within lateral parietal systems and impaired integration within and across frontoparietal brain networks leads to changes in biased competition within the visual system, i.e. both the ability to focus on currently relevant visual target information when presented with additional distracting information, and the ability to optimally balance spatial attention when searching for objects within the visual field. This behavioural consequence might be triggered by an underlying bias of selective weighting processes within functionally connected brain areas resulting from impaired interaction between corresponding neural units.
3. A biased competition account of cognitive symptoms in early Alzheimer's disease
(a). Biased competition changes as a unitary principle of early cognitive symptoms in Alzheimer's disease
In this section, we will consider whether impaired biased competition within and across brain regions may not only be responsible for the behavioural deficits in visual attentional selection. Rather, it might also play a more general role for the cognitive impairments in AD, including early episodic memory deficits. We suggest that it could be worthwhile to consider how the neural lesions in early AD give rise to a variety of cognitive symptoms depending on the affected brain area, even though the nature of the cognitive impairment may be explained by a single neurocognitive mechanism. More specifically, lesion-induced changes in biased competition among interacting brain areas may lead to analogous impairments in different cognitive domains such as memory and attention. First, we will analyse the type of episodic memory deficit in early AD. Then, we will describe new insights into the role of the parietal lobes for episodic memory, allowing us to explain our account at the level of neural implementation (i.e. the level of large-scale brain networks) and of computation (i.e. adapting the biased competition principle for both memory and perception).
(b). What is the precise nature of episodic memory deficits in the prodromal phase of Alzheimer's disease?
In order to theoretically classify the earliest episodic memory deficits in AD, it is important to differentiate between two dissociable phenomena underlying recognition judgements, recollection and familiarity [67–69]. Patients with prodromal AD seem to suffer from a relatively selective impairment in recollection. For example, when presented with a visual item recently encountered (such as a repeatedly displayed picture of an object or a word in a recognition memory test), they might be unable to vividly retrieve, in a conscious and controlled manner, the specific contextual information about this item and about the event in which they encountered it. Owing to this deficit, they tend to rely on familiarity more than healthy participants. Familiarity is a more general non-contextual, automatic feeling of prior exposure which is relatively spared in prodromal AD ([70–75]; but see [76]). Thus, when searching for his/her car in a street, a person with first symptoms of AD (like the patient from our example mentioned in §1) might be guided by the familiarity of the visual environment to a stronger degree than a healthy person. The inability to recollect the particular visual scene encountered in the morning hours might then lead to repeated attempts to search at the most familiar location, i.e. where the car is usually parked.
(c). Level of implementation: large-scale brain network interaction
(i). Role of posterior parietal cortex for episodic memory retrieval
Lateral parietal cortex lesions do not lead to retrograde or anterograde amnesia as is known from patients with MTL lesions [77], and also considered as the hallmark of AD-related memory symptoms. However, they seem to induce more subtle impairments during free recall of episodic memory events (see [78] for a comprehensive review). For example, a single-case report study on a patient with bilateral posterior parietal damage following closed head injury [79] who was not able to spontaneously recall autobiographical incidents from his life prior to the accident, suggested that the integrity of lateral parietal cortex is critical for the conscious retrieval of episodic memory. Using more sophisticated episodic memory paradigms, it was shown that patients with bilateral ventral parietal lesions have impoverished autobiographical memory reports with a lack of detail (e.g. specific perceptual features). However, when recall is guided by explicit probe questions about memory details, no impairment is found [80]. Similar results were obtained in a source memory test [81]. Here, patients with unilateral left or right-sided lateral parietal lesions reported not to have the subjective impression of remembering previously encountered items but performed normally when required to judge the familiarity with the stimulus material. To summarize, evidence is accumulating to suggest that certain, multimodal, episodic memory retrieval deficits are found after parietal damage. They occur especially in conditions with low-retrieval support which require top-down-controlled memory search for previous events. Interestingly, these deficits bear striking similarities to the earliest episodic memory retrieval deficits seen in AD.
The PPC has direct anatomical connections to the dorsolateral prefrontal cortex, temporal cortex and medial parietal cortex, as well as to the entorhinal, parahippocampal and hippocampal structures of the MTL [82]. Coherent spontaneous activity implies a hippocampal–parietal functional network [83]. Parietal activations are seen frequently in fMRI studies of episodic memory, for example, in recollection versus familiarity conditions and in source memory versus item memory tasks [84,85], i.e. especially in the conditions of explicit memory that require the intention to remember and to voluntarily direct attention to memory contents (see [86]).
On the basis of these findings and a comprehensive review of the neuropsychological and functional imaging literature, Cabeza et al. [86] proposed that the lateral PPC is critical for attentional processing for remembered episodic information during memory retrieval in a similar vein as it is for incoming sensory information during visual perception (‘attention-to-memory (AtoM) model’, but see [84,87] for related ideas). For the visual domain, it is well established that the PPC is critically involved when spatial attention has to be intentionally redirected, because a target stimulus cannot be accessed automatically, such as after presentation of an invalid cue [88]. Comparably, for the memory domain, the PPC is thought to play a decisive role in indirect episodic retrieval, when a target memory is not automatically elicited by a retrieval cue, but has to be recovered by effortful memory search processes. Moreover, distinct functions are attributed to the dorsal and ventral PPC, respectively, complementary to their differential roles in visual attention [89]. In the AtoM model, it is assumed that top-down scheduling of attentional resources during effortful memory searches is a function of the dorsal PPC, whereas the ventral PPC is captured, in a bottom-up manner, by automatically elicited and thus directly retrieved memories mediated by MTL that signal the necessity of attention shifts. Hence, according to this view, the dorsal PPC exerts a top-down influence on MTL-activity, while the ventral PPC is driven by the MTL (and dorsal PPC) in a bottom-up manner. Although some studies have questioned the exact anatomical overlap of regions involved in the control of attention and memory [90,91], recent neuroimaging and lesion-mapping evidence supports the AtoM model view of the dorsal PPC as a domain-general top-down controller for biased memory retrieval and attentional allocation [92–97].
(ii). A network perspective on attention and memory deficits in early Alzheimer's disease
Relating the neuroanatomical considerations of the AtoM model to AD, we predict that the lateral parietal damage in prodromal AD reviewed above plays a decisive role not only in visual perceptual processing but also in memory retrieval. We have already concluded that the dorsal PPC is impaired in prodromal AD, and that this impairment should lead to difficulties of top-down control. This could also explain the memory deficits of prodromal AD: if the dorsal PPC cannot control the MTL to retrieve currently relevant episodes from memory, then a patient's recollection will be driven by the bottom-up familiarity signals which primarily require the MTL (and possibly the ventral parietal cortex) but not the dorsal PPC.
Importantly, the involvement of the domain-general dorsal PPC control network in AD can be best understood via the concept of IBNs. Neuroimaging in healthy humans suggests that the dorsal PPC is part of neuroanatomically and functionally distinct networks centred on the superior parietal lobule (and possibly parts of the intra-parietal sulcus [25,98,99]), specifically the dorsal attention network and the central executive network [89,91]. These two networks are distinct from the ventral attention network, which covers the ventral PPC, including the temporoparietal junction and is associated with bottom-up attention [89,100,101]. In spite of their independence, these networks can connect to different domain-specific areas depending on current task demands, such as the MTL and ventral DMN during memory retrieval [102], and visual cortex during externally oriented tasks [103,104]. The iFC among these networks is disrupted in early AD [27,30,31,105], and parts of these networks are affected by amyloid plaque deposition and hypometabolism in early AD [16,20]. The prevalence of these lesions in early AD supports the view that impaired interactions among these attention networks are relevant for patients' impaired memory and visual attention.
Next, we will propose for the computational level that the same underlying imbalance of attentional weighting processes that have been demonstrated for selective visual information processing might also play a central role in the earliest episodic memory retrieval deficits in AD.
(d). Level of computation: biased competition principle in memory and perception
A computational basis for specific predictions about the relationship between changes in visual attention in AD that were found by TVA-based assessment [20,61] and changes in episodic memory is provided by Logan's instance theory of attention and memory (ITAM; [106]). ITAM suggests that attention as well as memory depends on one and the same choice process which can be modelled as a parallel race between competing alternatives. In accordance with the TVA model [59], ITAM assumes that attentional selection into vSTM and categorization are simultaneous choice processes that follow the assumptions of a biased competition model [49]. The former is an outward choice between perceptual objects presented in the environment and the latter an inward choice among categories available in memory. ITAM assumes that objects are represented in a multi-dimensional similarity space and that similarity between objects is an (exponential) function of distance within this space. In attentional selection, the race is driven by similarity between displayed objects and multi-dimensional target representations. In memory selection, the race is driven by similarity between a displayed object and category exemplars of alternative memory categories. Attentional weighting processes determine the outcome of the object as well as the category selection race. In the healthy brain, biased competition is assumed to optimize memory selection by allocating attentional weights preferentially towards relevant dimensions in a multi-dimensional similarity space. Attended dimensions are ‘stretched’, so that exemplars of these dimensions are more distinguishable from each other. At the same time, owing to the limited amount of available attentional capacity, unattended dimensions are necessarily ‘shrunk’.1
Although specific tasks (such as identification, classification or recall) might differ with respect to optimal weight allocation, the central prediction that follows for prodromal AD, i.e. for a brain state characterized by reduced efficiency of top-down control [65], is straightforward: these patients should suffer from several deficits in parallel. That is, they should be similarly less efficient in favouring relevant over irrelevant dimensions during memory retrieval as they are during visual selection. As in the visual domain, the choice process is triggered to a larger degree by bottom-up saliency also in the memory domain, in this case, within a multi-dimensional memory space. Thus, salient memory events, such as, for example, highly familiar visual scenes that have been encountered many times before, get more likely to interfere with the selection of a particular episodic visual memory instance that has been encountered only once. Therefore, owing to the inability to allocate, via top-down control, attentional weights preferentially on memory dimensions relevant for events encountered during that same day, the patient with prodromal AD in our example instead retrieved images of his car parked in a visual scenery he has seen many times before. An external (attentional) cue (given by his wife during the phone call) which activates the correct memory dimension leads to a competitive advantage of this dimension and to correct memory retrieval.
In this way, the computational assumptions of TVA and ITAM provide a basis to consider AD-related deficits as significant changes in attentional bias parameters that affect both perceptual and memory processing.
4. Conclusion: unified biased competition account of visual attention and memory deficits in Alzheimer's disease
Combining the AtoM model [86] with ITAM [106] offers the opportunity for a unifying neurocognitive account of both memory and attention deficits in AD that is based on the biased competition principle. More precisely, this account explains the memory deficits emerging in prodromal AD within an attention framework and its neurocognitive mechanisms. In this way, when considering prodromal AD, the focus shifts from a strict ‘memory deficit’ view to an ‘attentional weighting deficit’ view of early AD.
In support of the latter view, we suggest that the same cognitive mechanism underlies deficits in different functions. While so far we have presented evidence for an inefficiency in task-specific allocation of attentional weights in visual selection in prodromal AD [20,65], we assume that similar weight allocation changes also impede the task-based selection in memory retrieval. As a result, patients in the prodromal stage of AD are less efficient in prioritizing the most relevant memory categories during retrieval processes. Thereby, there is an increased probability that non-relevant information from multiple dimensions in memory space is pre-activated so that multiple competitors enter the mnemonic race and a clear-cut access to the most relevant category is rendered impossible. In case there is high similarity between exemplars of different memory categories, competition may in fact even be so strong as to prevent access to the relevant target category. Then, the affected individual will fail to remember the identity of a presented object unless further retrieval cues are provided.
The ‘attention-centred’ perspective on early AD is also a ‘brain mechanism-centred’ one: it suggests that distributed and progressive neurodegeneration affects interactions of distant brain regions, resulting in changes of biased competition in neural activity between these regions. This perspective is complemented by an IBN view on AD which fits the current assumption that AD is a disconnection syndrome [109]. It also links network-level explanations of neurodegeneration to varieties of cognitive dysfunction, allowing not only for new explanations for different variants of AD but also for a novel dementia taxonomy similar to the one introduced by Seeley et al. [110].
The change in perspective can foster a genuine neurocognitive description and understanding of the neurodegeneration related to AD. This may not only significantly enhance the predictive value of assessment early in the disease but also represent a critical source of evidence for theories of normal information processing. Testing patients with documented changes in the biased competition process of attentional selection offers a critical methodology in order to test theoretical assumptions on visual attention and mnemonic processing that are made by cognitive models. For instance, the critical assumption of the ITAM model [106] that attentional selection and memory categorization rely on the same competitive race, could be tested by the use of paradigms that allow us to quantify parameters for attentional selection [50] as well as memory categorization [111]. Furthermore, the neuroanatomical AtoM model [86] could be tested by the assessment of patients with relatively homogeneous neurodegeneration within the parietal lobe system when neuroscientific methods (e.g. of brain connectivity measures in AD [20]) are combined with the assessment of attentional as well as mnemonic processing.
5. Caveats
In presenting our ideas, it should be emphasized that we by no means want to neglect the severe memory storage deficits in AD patients, i.e. forgetfulness [112]. For example, Ivanoiu et al. [113] have shown that prodromal AD is particularly linked to delayed recall deficits which persist in spite of reminder cues. In addition, in predicting conversion to AD, it is specifically amnestic MCI which is a good predictor [5].
We do not reject the amnestic nature of early AD/MCI. We merely suggest that a substantial portion of patients subsumed under the global label of ‘amnestic MCI’ have memory problems that are more attentional in nature, in the way we have specified in our paper. In our opinion, this possibility may have been underestimated hitherto, and therefore not specifically investigated in amnestic MCI subjects. As a result, whether the attentional biased competition view on memory has a potential for predicting conversion of MCI patients that is comparable with the traditional ‘amnestic’ view, is currently an unresolved issue and an empirical question. If it is true that parietal dysfunction is present at least as early as MTL pathology, and the parietal lobe is involved in memory processes, then memory problems of parietal origin should be detectable from early on, and may independently or additionally contribute to the memory problems of AD patients. Thus, specific memory problems may already prevail before a strict storage deficit has fully developed.
Funding statement
K.F. and C.S. receive support from the Alzheimer Foundation Initiative (AFI). N.M. is supported by the Wellcome Trust.
Endnote
Proper selection between competing representations, which is the central assumption of the biased competition approach, requires that these representations are activated in a stable and reliable manner. Put differently, if representations are less reliable and stable, then they will be less distinguishable, and competition between them will be amplified. As a result, selection will be rendered more difficult. Modulatory neurotransmitter effects, resulting from the corticopetal projections of, for example, acetylcholine and noradrenaline, will certainly play a decisive role in this regard [107,108]. However, a neurochemical discussion of attentional capacity is beyond the scope of this paper and also appears dispensable in advancing the basic ideas of our view.
References
- 1.Ferri CP, et al. 2006. Global prevalence of dementia: a Delphi consensus study. Lancet 366, 2112–2117 (doi:10.1016/S0140-6736(05)67889-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braak H, Braak E. 1991. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1, 213–216 (doi:10.1111/j.1750-3639.1991.tb00661.x) [DOI] [PubMed] [Google Scholar]
- 3.Blennow K, de Leon MJ, Zetterberg H. 2006. Alzheimer's disease. Lancet 368, 387–403 (doi:10.1016/S0140-6736(06)69113-7) [DOI] [PubMed] [Google Scholar]
- 4.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. 1984. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34, 939–944 (doi:10.1212/WNL.34.7.939) [DOI] [PubMed] [Google Scholar]
- 5.Gauthier S, et al. 2006. Mild cognitive impairment. Lancet 367, 1262–1270 (doi:10.1016/S0140-6736(06)68542-5) [DOI] [PubMed] [Google Scholar]
- 6.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. 1999. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 56, 303–308 (doi:10.1001/archneur.56.3.303) [DOI] [PubMed] [Google Scholar]
- 7.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. 2001. Mild cognitive impairment represents early-stage Alzheimer disease. Arch. Neurol. 58, 397–405 (doi:10.1001/archneur.58.3.397) [DOI] [PubMed] [Google Scholar]
- 8.Jack CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen C, Trojanowski JQ. 2010. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 9, 119–128 (doi:10.1016/S1474-4422(09)70299-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubois B, et al. 2010. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 9, 1118–1127 (doi:10.1016/S1474-4422(10)70223-4) [DOI] [PubMed] [Google Scholar]
- 10.Genin E, et al. 2011. APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Mol. Psychiatry 16, 903–907 (doi:10.1038/mp.2011.52) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardy J, Selkoe DJ. 2002. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 (doi:10.1126/science.1072994) [DOI] [PubMed] [Google Scholar]
- 12.Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O. 2008. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science 321, 1686–1689 (doi:10.1126/science.1162844) [DOI] [PubMed] [Google Scholar]
- 13.Cirrito JR, et al. 2005. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron 48, 913–922 (doi:10.1016/j.neuron.2005.10.028) [DOI] [PubMed] [Google Scholar]
- 14.Bateman RJ, et al. 2012. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N. Engl. J. Med. 367, 795–804 (doi:10.1056/NEJMoa1202753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckner RL, et al. 2005. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J. Neurosci. 25, 7709–7717 (doi:10.1523/JNEUROSCI.2177-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drzezga A, et al. 2011. Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden. Brain 134, 1635–1646 (doi:10.1093/brain/awr066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engler H, et al. 2006. Two-year follow-up of amyloid deposition in patients with Alzheimer's disease. Brain 129, 2856–2866 (doi:10.1093/brain/awl178) [DOI] [PubMed] [Google Scholar]
- 18.Kemppainen NM, et al. 2007. PET amyloid ligand [11C]PIB uptake is increased in mild cognitive impairment. Neurology 68, 1603–1606 (doi:10.1212/01.wnl.0000260969.94695.56) [DOI] [PubMed] [Google Scholar]
- 19.Mintun MA, et al. 2006. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 67, 446–452 (doi:10.1212/01.wnl.0000228230.26044.a4) [DOI] [PubMed] [Google Scholar]
- 20.Sorg C, et al. 2012. Asymmetric loss of parietal activity causes spatial bias in prodromal and mild Alzheimer's disease. Biol. Psychiatry 71, 798–804 (doi:10.1016/j.biopsych.2011.09.027) [DOI] [PubMed] [Google Scholar]
- 21.Wojciulik E, Kanwisher N. 1999. The generality of parietal involvement in visual attention. Neuron 23, 747–764 (doi:10.1016/S0896-6273(01)80033-7) [DOI] [PubMed] [Google Scholar]
- 22.Fox MD, Raichle ME. 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 9, 700–711 (doi:10.1038/nrn2201) [DOI] [PubMed] [Google Scholar]
- 23.Buckner RL, Andrews-Hanna JR, Schacter DL. 2008. The brain's default network: anatomy, function, and relevance to disease. Ann. NY Acad. Sci. 1124, 1–38 (doi:10.1196/annals.1440.011) [DOI] [PubMed] [Google Scholar]
- 24.Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. 2009. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J. Neurosci. 29, 1860–1873 (doi:10.1523/JNEUROSCI.5062-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith SM, et al. 2009. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl Acad. Sci. USA 106, 13 040–13 045 (doi:10.1073/pnas.0905267106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greicius MD, Srivastava G, Reiss AL, Menon V. 2004. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc. Natl Acad. Sci. USA 101, 4637–4642 (doi:10.1073/pnas.0308627101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorg C, et al. 2007. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc. Natl Acad. Sci. USA 104, 18 760–18 765 (doi:10.1073/pnas.0708803104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sperling RA, et al. 2009. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 63, 178–188 (doi:10.1016/j.neuron.2009.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheline YI, Raichle ME. In press Resting state functional connectivity in preclinical Alzheimer's disease. Biol. Psychiatry. (doi:10.1016/j.biopsych.2012.11.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agosta F, Pievani M, Geroldi C, Copetti M, Frisoni GB, Filippi M. 2012. Resting state fMRI in Alzheimer's disease: beyond the default mode network. Neurobiol. Aging 33, 1564–1578 (doi:10.1016/j.neurobiolaging.2011.06.007) [DOI] [PubMed] [Google Scholar]
- 31.Li R, Wu X, Fleisher AS, Reiman EM, Chen K, Yao L. 2012. Attention-related networks in Alzheimer's disease: a resting functional MRI study. Hum. Brain Mapp. 33, 1076–1088 (doi:10.1002/hbm.21269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neufang S, Akhrif A, Riedl V, Förstl H, Kurz A, Zimmer C, Sorg C, Wohlschläger AM. In press Predicting effective connectivity from resting-state networks in healthy elderly and patients with prodromal Alzheimer's disease. Hum. Brain Mapp. (doi:10.1002/hbm.22226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laird AR, et al. 2011. Behavioral interpretations of intrinsic connectivity networks. J. Cogn. Neurosci. 23, 4022–4037 (doi:10.1162/jocn_a_00077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hahn K, Myers N, Prigarin S, Rodenacker K, Kurz A, Förstl H, Zimmer C, Wohlschläger AM, Sorg C. 2013. Selectively and progressively disrupted structural connectivity of functional brain networks in Alzheimer's disease: revealed by a novel framework to analyze edge distributions of networks detecting disruptions with strong statistical evidence. NeuroImage 81, 96–109 (doi:10.1016/j.neuroimage.2013.05.011) [DOI] [PubMed] [Google Scholar]
- 35.Sorg C, et al. In press Graded spatial correlation between amyloid-β plaque deposition and functional connectivity within and across intrinsic networks in Alzheimer's disease. Abstract presented at Neuroscience 2013, Society for Neuroscience Annual Meeting. [Google Scholar]
- 36.Neufang S, Akhrif A, Riedl V, Förstl H, Kurz A, Zimmer C, Sorg C, Wohlschläger AM. 2011. Disconnection of frontal and parietal areas contributes to impaired attention in very early Alzheimer's disease. J. Alzheimers Dis. 25, 309–321 (doi:10.3233/JAD-2011-102154) [DOI] [PubMed] [Google Scholar]
- 37.Binetti G, Cappa SF, Magni E, Padovani A, Bianchetti A, Trabucchi M. 1998. Visual and spatial perception in the early phase of Alzheimer's disease. Neuropsychology 12, 29–33 (doi:10.1037/0894-4105.12.1.29) [DOI] [PubMed] [Google Scholar]
- 38.Jefferson AL, Barakat LP, Giovannetti T, Paul RH, Glosser G. 2006. Object perception impairments predict instrumental activities of daily living dependence in Alzheimer's disease. J. Clin. Exp. Neuropsychol. 28, 884–897 (doi:10.1080/13803390591001034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendez MF, Mendez MA, Smyth MR, Whitehouse PJ. 1990. Complex visual disturbances in Alzheimer's disease. Neurology 40, 439–443 (doi:10.1212/WNL.40.3_Part_1.439) [DOI] [PubMed] [Google Scholar]
- 40.Rizzo M, Anderson SW, Dawson J, Myers R, Ball K. 2000. Visual attention impairments in Alzheimer's disease. Neurology 54, 1954–1959 (doi:10.1212/WNL.54.10.1954) [DOI] [PubMed] [Google Scholar]
- 41.Deary IJ, Hunter R, Langan SJ, Goodwin GM. 1991. Inspection time, psychometric intelligence and clinical estimates of cognitive ability in presenile Alzheimer's disease and Korsakoff's psychosis. Brain 114, 2543–2554 (doi:10.1093/brain/114.6.2543) [DOI] [PubMed] [Google Scholar]
- 42.Foldi NS, Lobosco JJ, Schaefer LA. 2002. The effect of attentional dysfunction in Alzheimer's disease: theoretical and practical implications. Semin. Speech Lang. 23, 139–150 (doi:10.1055/s-2002-24990) [DOI] [PubMed] [Google Scholar]
- 43.Parasuraman R, Haxby JV. 1993. Attention and brain functions in Alzheimer's disease. A review. Neuropsychology 7, 243–273 (doi:10.1037/0894-4105.7.3.242) [Google Scholar]
- 44.Perry RJ, Hodges JR. 1999. Attention and executive deficits in Alzheimer's disease: a critical review. Brain 122, 383–404 (doi:10.1093/brain/122.3.383) [DOI] [PubMed] [Google Scholar]
- 45.Vasquez BP, Buck BH, Black SE, Leibowitch FS, Lobaugh NJ, Caldwell CB, Behrmann M. 2011. Visual attention deficits in Alzheimer's disease: relationship to HMPAO SPECT cortical hypoperfusion. Neuropsychologia 49, 1741–1750 (doi:10.1016/j.neuropsychologia.2011.02.052) [DOI] [PubMed] [Google Scholar]
- 46.Alescio-Lautier B, Michel BF, Herrera C, Elahmadi A, Chambon C, Touzet C, Paban V. 2007. Visual and visuospatial short-term memory in mild cognitive impairment and Alzheimer disease: role of attention. Neuropsychologia 45, 1948–1960 (doi:10.1016/j.neuropsychologia.2006.04.033) [DOI] [PubMed] [Google Scholar]
- 47.Bonney KR, Almeida OP, Flicker L, Davies S, Clarnette R, Anderson M, Lautenschlager NT. 2006. Inspection time in non-demented older adults with mild cognitive impairment. Neuropsychologia 44, 1452–1456 (doi:10.1016/j.neuropsychologia.2005.12.002) [DOI] [PubMed] [Google Scholar]
- 48.Bartolomeo P, Dalla Barba G, Boissé MF, Bachoud-Lévi AC, Degos JD, Boller F. 1998. Right-side neglect in Alzheimer's disease. Neurology 51, 1207–1209 (doi:10.1212/WNL.51.4.1207) [DOI] [PubMed] [Google Scholar]
- 49.Desimone R, Duncan J. 1995. Neural mechanisms of selective visual attention. Annu. Rev. Psychol. 18, 193–222 (doi:10.1146/annurev.ne.18.030195.001205) [DOI] [PubMed] [Google Scholar]
- 50.Duncan J, Bundesen C, Olson A, Humphreys G, Chavda S, Shibuya H. 1999. Systematic analysis of deficits in visual attention. J. Exp. Psychol. Gen. 128, 450–478 (doi:10.1037/0096-3445.128.4.450) [DOI] [PubMed] [Google Scholar]
- 51.Finke K, Matthias E, Keller I, Müller HJ, Schneider WX, Bublak P. 2012. How does phasic alerting improve performance in patients with unilateral neglect? A systematic analysis of attentional processing capacity and spatial weighting mechanisms. Neuropsychologia 50, 1178–1189 (doi:10.1016/j.neuropsychologia.2012.02.008) [DOI] [PubMed] [Google Scholar]
- 52.Kerkhoff G. 2001. Spatial hemineglect in humans. Prog. Neurobiol. 63, 1–27 (doi:10.1016/S0301-0082(00)00028-9) [DOI] [PubMed] [Google Scholar]
- 53.Derflinger S, Sorg C, Gaser C, Myers N, Arsic M, Kurz A, Zimmer C, Wohlschläger A, Mühlau M. 2011. Grey-matter atrophy in Alzheimer's disease is asymmetric but not lateralized. J. Alzheimers Dis. 25, 347–357 (doi:10.3233/JAD-2011-110041) [DOI] [PubMed] [Google Scholar]
- 54.Haxby JV, Duara R, Grady CL, Cutler NR, Rapoport SI. 1985. Relations between neuropsychological and cerebral metabolic asymmetries in early Alzheimer's disease. J. Cereb. Blood Flow. Metab. 5, 193–200 (doi:10.1038/jcbfm.1985.25) [DOI] [PubMed] [Google Scholar]
- 55.Li F, Iseki E, Kato M, Adachi Y, Akagi M, Kosaka K. 2000. An autopsy case of Alzheimer's disease presenting with primary progressive aphasia: a clinicopathological and immunohistochemical study. Neuropathology 20, 239–245 [DOI] [PubMed] [Google Scholar]
- 56.Thompson PM, et al. 2003. Dynamics of gray matter loss in Alzheimer's disease. J. Neurosci. 23, 994–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ueyama K, Fukuzako H, Fukuzako T, Hokazono K, Takeuchi K, Hashiguchi T, Takigawa M, Yamanaka T, Matsumoto M. 1994. CT study in senile dementia of Alzheimer type. Int. J. Geriatr. Psychiatry 9, 919–924 (doi:10.1002/gps.930091109) [Google Scholar]
- 58.Volkow ND, Zhu W, Felder CA, Mueller K, Welsh TF, Wang GJ, de Leon MJ. 2002. Changes in brain functional homogeneity in subjects with Alzheimer's disease. Psychiatry Res. 114, 39–50 (doi:10.1016/S0925-4927(01)00130-5) [DOI] [PubMed] [Google Scholar]
- 59.Bundesen C. 1990. A theory of visual attention. Psych. Rev. 97, 523–547 (doi:10.1037/0033-295X.97.4.523) [DOI] [PubMed] [Google Scholar]
- 60.Bundesen C. 1998. A computational theory of visual attention. Phil. Trans. R. Soc. Lond. B 353, 1271–1281 (doi:10.1098/rstb.1998.0282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bundesen C, Habekost T, Kyllingsbæk S. 2005. A neural theory of visual attention: bridging cognition and neurophysiology. Psychol. Rev. 112, 291–328 (doi:10./0033-295X.112.2.291) [DOI] [PubMed] [Google Scholar]
- 62.Luck SJ, Vogel EK. 1997. The capacity of visual working memory for features and conjunctions. Nature 390, 279–281 (doi:10.1038/36846) [DOI] [PubMed] [Google Scholar]
- 63.Todd JJ, Marois R. 2004. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature 428, 751–754 (doi:10.1038/nature02466) [DOI] [PubMed] [Google Scholar]
- 64.Bublak P, Redel P, Finke K. 2006. Spatial and non-spatial attention deficits in neurodegenerative diseases: assessment based on Bundesen's theory of visual attention (TVA). Restor. Neurol. Neurosci. 24, 287–301 [PubMed] [Google Scholar]
- 65.Redel P, Bublak P, Sorg C, Kurz A, Förstl H, Müller HJ, Schneider WX, Perneczky R, Finke K. 2012. Deficits of spatial and task-related attentional selection in mild cognitive impairment and Alzheimer's disease. Neurobiol. Aging 33, 27–42 (doi:10.1016/j.neurobiolaging.2010.05.014) [DOI] [PubMed] [Google Scholar]
- 66.Bublak P, Redel P, Sorg C, Kurz A, Förstl H, Müller HJ, Schneider WX, Finke K. 2011. Staged decline of visual processing capacity in mild cognitive impairment and Alzheimer's disease. Neurobiol. Aging 32, 1219–1230 (doi:10.1016/j.neurobiolaging.2009.07.012) [DOI] [PubMed] [Google Scholar]
- 67.Jacoby LL. 1991. A process-dissociation framework: separating automatic from intentional uses of memory. J. Mem. Lang. 30, 513–541 (doi:10.1016/0749-596X(91)90025-F) [Google Scholar]
- 68.Mandler G. 1980. Recognizing: the judgement of previous occurrence. Psych. Rev. 87, 252–271 (doi:10.1037/0033-295X.87.3.252) [Google Scholar]
- 69.Yonelinas AP, Jacoby LL. 1995. The relation between remembering and knowing as bases for recognition: effects of size congruency. J. Mem. Lang. 34, 622–643 (doi:10.1006/jmla.1995.1028) [Google Scholar]
- 70.Ally BA, Gold CA, Budson AE. 2009. An evaluation of recollection and familiarity in Alzheimer's disease and mild cognitive impairment using receiver operating characteristics. Brain Cogn. 69, 504–513 (doi:10.1016/j.bandc.2008.11.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anderson ND, Ebert PL, Jennings JM, Grady CL, Cabeza R, Graham SJ. 2008. Recollection- and familiarity-based memory in healthy aging and amnestic mild cognitive impairment. Neuropsychology 22, 177–187 (doi:10.1037/0894-4105.22.2.177) [DOI] [PubMed] [Google Scholar]
- 72.Embree LM, Budson AE, Ally BA. 2012. Memorial familiarity remains intact for pictures but not for words in patients with amnestic mild cognitive impairment. Neuropsychologia 50, 2333–2340 (doi:10.1016/j.neuropsychologia.2012.06.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hudon C, Belleville S, Gauthier S. 2009. The assessment of recognition memory using the remember/know procedure in amnestic mild cognitive impairment and probable Alzheimer's disease. Brain Cogn. 70, 171–179 (doi:10.1016/j.bandc.2009.01.009) [DOI] [PubMed] [Google Scholar]
- 74.Serra L, Bozzali M, Cercignani M, Perri R, Fadda L, Caltagirone C, Carlesimo GA. 2010. Recollection and familiarity in amnesic mild cognitive impairment. Neuropsychology 24, 316–326 (doi:10.1037/a0017654) [DOI] [PubMed] [Google Scholar]
- 75.Westerberg CE, Paller KA, Weintraub S, Mesulam MM, Holdstock JS, Mayes AR, Reber PJ. 2006. When memory does not fail: familiarity based recognition in mild cognitive impairment and Alzheimer's disease. Neuropsychology 20, 193–205 (doi:10.1037/0894-4105.20.2.193) [DOI] [PubMed] [Google Scholar]
- 76.Wolk DA, Signoff ED, Dekosky ST. 2008. Recollection and familiarity in amnestic mild cognitive impairment: a global decline in recognition memory. Neuropsychologia 46, 1965–78 (doi:10.1016/j.neuropsychologia.2008.01.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Squire LR, Zola-Morgan S. 1991. The medial temporal lobe memory system. Science 253, 1380–1386 (doi:10.1126/science.1896849) [DOI] [PubMed] [Google Scholar]
- 78.Berryhill ME. 2012. Insights from neuropsychology: pinpointing the role of the posterior parietal cortex in episodic and working memory. Front. Integr. Neurosci. 6, 31 (doi:10.3389/fnint.2012.00031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hunkin NM, Parkin AJ, Bradley VA, Burrows EH, Aldrich FK, Jansari A, Burdon-Cooper C. 1995. Focal retrograde amnesia following closed head injury: a case study and theoretical account. Neuropsychologia 33, 509–523 (doi:10.1016/0028-3932(94)00136-D) [DOI] [PubMed] [Google Scholar]
- 80.Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. 2007. Parietal lobe and episodic memory: bilateral damage causes impaired free recall of autobiographical memory. J. Neurosci. 27, 14 415–14 423 (doi:10.1523/JNEUROSCI.4163-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davidson PS, Anaki D, Ciaramelli E, Cohn M, Kim AS, Murphy KJ, Troyer AK, Moscovitch M, Levine B. 2008. Does lateral parietal cortex support episodic memory: evidence from focal lesion patients. Neuropsychologia 46, 1743–1755 (doi:10.1016/j.neuropsychologia.2008.01.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, Wedeen VJ. 2007. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain 130, 630–653 (doi:10.1093/brain/awl359) [DOI] [PubMed] [Google Scholar]
- 83.Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. 2006. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J. Neurophysiol. 96, 3517–3531 (doi:10.1152/jn.00048.2006) [DOI] [PubMed] [Google Scholar]
- 84.Wagner AD, Shannon BJ, Kahn I, Buckner RL. 2005. Parietal lobe contributions to episodic memory retrieval. Trends Cogn. Sci. 9, 445–453 (doi:10.1016/j.tics.2005.07.001) [DOI] [PubMed] [Google Scholar]
- 85.Uncapher MR, Wagner AD. 2009. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol. Learn. Mem. 91, 139–154 (doi:10.1016/j.nlm.2008.10.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cabeza R, Ciaramell E, Olson IR, St. Moscovitch M. 2008. The parietal cortex and episodic memory: an attentional account. Nat. Rev. Neurosci. 9, 613–625 (doi:10.1038/nrn2459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Naghavi HR, Nyberg L. 2005. Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration? Conscious Cogn. 14, 390–425 (doi:10.1016/j.concog.2004.10.003) [DOI] [PubMed] [Google Scholar]
- 88.Fan J, McCandliss BD, Fossela J, Flombaum JE, Posner ME. 2005. The activation of attentional networks. Neuroimage 26, 471–479 (doi:10.1016/j.neuroimage2005.02.004) [DOI] [PubMed] [Google Scholar]
- 89.Corbetta M, Shulman GL. 2002. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 3, 201–215 (doi:10.1038/nrn755) [DOI] [PubMed] [Google Scholar]
- 90.Hutchinson JB, Uncapher MR, Wagener AD. 2009. Posterior parietal cortex and episodic retrieval: convergent and divergent effects of attention and memory. J. Learn Mem. 16, 343–356 (doi:10.1101/lm.919109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sestieri C, Shulman GL, Corbetta M. 2010. Attention to memory and the environment: functional specialization and dynamic competition in human posterior parietal cortex. J. Neurosci. 30, 8445–8456 (doi:10.1523/JNEUROSCI.4719-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chun MM, Golomb JD, Turk-Browne NB. 2011. A taxonomy of external and internal attention. Annu. Rev. Psychol. 62, 73–101 (doi:10.1146/annurev.psych.093008.100427) [DOI] [PubMed] [Google Scholar]
- 93.Dobbins IG, Jaeger A, Studer B, Simons JS. 2012. Use of explicit memory cues following parietal lobe lesions. Neuropsychologia 50, 2992–3003 (doi:10.1016/j.neuropsychologia.2012.07.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Elman JA, Cohn-Sheehy BI, Shimamura AP. 2012. Dissociable parietal regions facilitate successful retrieval of recently learned and personally familiar information. Neuropsychologia 51, 573–583 (doi:10.1016/j.neuropsychologia.2012.12.013) [DOI] [PubMed] [Google Scholar]
- 95.Guerin SA, Robbins CA, Gilmore AW, Schacter DL. 2012. Interactions between visual attention and episodic retrieval: dissociable contributions of parietal regions during gist-based false recognition. Neuron 75, 1122–1134 (doi:10.1016/j.neuron.2012.08.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hutchinson JB, Uncapher MR, Weiner KS, Bressler DW, Silver MA, Preston AR, Wagner AD. In press Functional heterogeneity in posterior parietal cortex across attention and episodic memory retrieval. Cereb. Cortex (doi:10.1093/cercor/bhs278) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kuhl BA, Chun MM. 2012. Attending to the present when remembering the past. Neuron 75, 944–947 (doi:10.1016/j.neuron.2012.09.002) [DOI] [PubMed] [Google Scholar]
- 98.Margulies DS, et al. 2009. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc. Natl Acad. Sci. USA 106, 20 069–20 074 (doi:10.1073/pnas.0905314106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nelson SM, et al. 2010. A parcellation scheme for human left lateral parietal cortex. Neuron 67, 156–170 (doi:10.1016/j.neuron.2010.05.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Corbetta M, Patel G, Shulman GL. 2008. The reorienting system of the human brain: from environment to theory of mind. Neuron 58, 306–324 (doi:10.1016/j.neuron.2008.04.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. 2006. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl Acad. Sci. USA 103, 10 046–10 051 (doi:10.1073/pnas.0604187103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Burianová H, Ciaramelli E, Grady CL, Moscovitch M. 2012. Top-down and bottom-up attention-to-memory: mapping functional connectivity in two distinct networks that underlie cued and uncued recognition memory. Neuroimage 63, 1343–1352 (doi:10.1016/j.neuroimage.2012.07.057) [DOI] [PubMed] [Google Scholar]
- 103.Chadick JZ, Gazzaley A. 2011. Differential coupling of visual cortex with default or frontal-parietal network based on goals. Nat. Neurosci. 14, 830–832 (doi:10.1038/nn.2823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lauritzen TZ, D'Esposito M, Heeger DJ, Silver MA. 2009. Top-down flow of visual spatial attention signals from parietal to occipital cortex. J. Vis. 9, 18 (doi:10.1167/9.13.18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brier M, Thomas J, Snyder A, Fagan A, Grant E, Benzinger T, Morris JC, Ances B. 2011. The effects of Alzheimer's disease (AD) on blood oxygen level dependent resting state functional connectivity (BOLD rsFC) networks. Neurology 76, A190 [Google Scholar]
- 106.Logan GD. 2002. An instance theory of attention and memory. Psychol. Rev. 109, 376–400 (doi:10.1037/0033-295X.109.2.376) [DOI] [PubMed] [Google Scholar]
- 107.Aston-Jones G, Cohen JD. 2005. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450 (doi:10.1146/annurev.neuro.28.061604.135709) [DOI] [PubMed] [Google Scholar]
- 108.Furey ML. 2011. The prominent role of stimulus processing: cholinergic function and dysfunction in cognition. Curr. Opin. Neurol. 24, 364–370 (doi:10.1097/WCO.0b013e328348bda5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li R, Wu X, Chen K, Fleisher AS, Reiman EM, Yao L. 2013. Alterations of directional connectivity among resting-state networks in Alzheimer disease. Am. J. Neuroradiol. 34, 340–345 (doi:10.3174/ajnr.A3197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. 2009. Neurodegenerative diseases target large-scale human brain networks. Neuron 62, 42–52 (doi:10.1016/j.neuron.2009.03.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nosofsky RM, Little DR, James TW. 2012. Activation in the neural network responsible for categorization and recognition reflects parameter changes. Proc. Natl Acad. Sci. USA 109, 333–338 (doi:10.1073/pnas.1111304109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dubois B, et al. 2007. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS–ADRDA criteria. Lancet Neurol. 6, 734–746 (doi:10.1016/S1474-4422(07)70178-3) [DOI] [PubMed] [Google Scholar]
- 113.Ivanoiu A, Adam S, Van der Linden M, Salmon E, Juillerat A-C, Mulligan R, Seron X. 2005. Memory evaluation with a new cued recall test in patients with mild cognitive impairment and Alzheimer's disease. J. Neurol. 252, 47–55 (doi:10.1007/s00415-005-0597-2) [DOI] [PubMed] [Google Scholar]