Abstract
Several decades of patient, functional imaging and neurophysiological studies have supported a model in which the lateral prefrontal cortex (PFC) acts to suppress unwanted saccades by inhibiting activity in the oculomotor system. However, recent results from combined PFC deactivation and neural recordings of the superior colliculus in monkeys demonstrate that the primary influence of the PFC on the oculomotor system is excitatory, and stands in direct contradiction to the inhibitory model of PFC function. Although erroneous saccades towards a visual stimulus are commonly labelled reflexive in patients with PFC damage or dysfunction, the latencies of most of these saccades are outside of the range of express saccades, which are triggered directly by the visual stimulus. Deactivation and pharmacological manipulation studies in monkeys suggest that response errors following PFC damage or dysfunction are not the result of a failure in response suppression but can best be understood in the context of a failure to maintain and implement the proper task set.
Keywords: prefrontal cortex, inhibition, eye movements, primates
1. Introduction
But in order that a highly evolved function can adequately serve its purpose it is essential that it can control subordinate mechanisms, so the frontal centre became not merely an organ from which ocular movements could be evoked, it acquired also the power of inhibiting inappropriate and undesirable activities of the occipital lobes [1, p. 12].
The view expressed here by the eminent British neurologist Sir Gordon Holmes, that the frontal cortex controls saccadic eye movements by inhibiting activity in other brain areas, is a widely accepted concept in the oculomotor field and a crucial component of models of saccade control. An inhibitory model of frontal function, particularly of the lateral prefrontal cortex (PFC), has traditionally been supported by a diverse body of evidence, including patients with PFC lesions, functional imaging studies and neurophysiology in the non-human primate model. Despite the compelling support for this model, some of which has come from our own experiments over the past 15 years, we have come to a view radically different from this classical idea—that the PFC does not inhibit unwanted saccades, but instead facilitates goal-directed ones. Espousing a radical view requires reckoning with existing facts, and so we begin with a brief critical discussion of the patient, functional imaging and single neuron recording studies commonly interpreted as supporting the inhibition model. We then discuss potential neural mechanisms by which the PFC could inhibit saccade-related activity in other brain areas, and present data from our own recent studies in which we simultaneously deactivated the principal sulcus region of the PFC in rhesus macaques and recorded the activity of single saccade-related neurons in the superior colliculus (SC), a midbrain oculomotor structure critical for saccade initiation. These new findings are irreconcilable with the view that the PFC inhibits saccade-related activity in the SC, and suggest instead that the PFC contributes an excitatory signal that facilitates generation of goal-directed saccades. We conclude by discussing an alternative, more general model in which the PFC maintains and implements task rules that guide the flow of neural activity, and in which erroneous saccades are a consequence not of attenuated inhibition, but a failure to facilitate the appropriate behavioural goal.
2. Prefrontal cortex lesions and inhibitory saccade control
The seminal evidence for inhibitory saccade control by the PFC comes from investigations in patients with PFC lesions. Studies using the antisaccade task have been particularly influential in this regard [2–4]. In this task, participants are instructed to generate a saccade to the location opposite that of a suddenly appearing peripheral visual stimulus. Performance, in this task, is generally contrasted to that in a prosaccade task, in which a saccade towards the peripheral stimulus is the correct response. Two constituent voluntary processes are believed to be essential for correct antisaccade performance; inhibitory control, to override the natural tendency to saccade towards the visual stimulus, and goal-directed saccade generation, to direct a saccade to an empty location in the visual field. Psychophysical evidence from both human participants and non-human primates supports the notion of an inhibitory component in this task. Error rates and reaction times in the antisaccade task exceed those in the prosaccade task, suggesting involvement of an additional fallible and time-consuming inhibitory process. Further, the reaction times of antisaccade errors—responses in which a saccade is directed towards rather than away from the visual stimulus—are similar to that observed on prosaccade trials, suggesting that these errant responses are triggered by the visual stimulus and represent failures of the inhibitory process. Especially in the so-called gap condition, in which the initial fixation point disappears 200 ms before stimulus onset, humans [5] and non-human primates [6,7] often generate response errors with express saccade latencies [8], i.e. saccades with very short reaction times that approach the minimal conduction times in the oculomotor system.
The foundational evidence for the link between PFC damage and inhibitory saccade control comes from work carried out by Guitton et al. [9], who used a variant of the antisaccade task to investigate saccade suppression and generation in patients who had undergone unilateral excisions of frontal cortex for treatment of intractable epilepsy. Although this study is one of the most highly cited papers in the oculomotor field, often ignored is the fact that this study used a sort of dual-task design by combining the antisaccade task with a visual discrimination requirement, and that this manipulation impacted substantially the frequency of erroneous reflexive saccades. In this task, subjects were required to report the location of a gap in a briefly flashed square discrimination stimulus presented at various durations subsequent to a flashed cue stimulus. The cue and discrimination stimuli were presented at either the same—on prosaccade trials, or opposite locations—on antisaccade trials. For most patients, the frequency of errors on antisaccade trials diminished or returned to normal as the duration between cue and square presentation increased, or if the discrimination requirement was omitted entirely. This suggests that errors might result not from a failure to inhibit a reflexive saccade per se, but rather an increase in task demands. Surprisingly, most patients exhibited strong bilateral deficits, despite confinement of lesions to a single hemisphere. Many patients also showed substantial increases in the reaction times of correct antisaccades that could be attributed to a difficulty in generating the required voluntary saccade away from the cue stimulus. In many cases, reaction times were consistent with a visual triggering of the correct antisaccade by the appearance of the square discrimination stimulus, not a voluntary internal command. On the whole, the data suggest a complex deficit characterized by a task-demand-mediated deficit in suppression of inappropriate saccades and initiation of voluntary saccades. Noteworthy, in this regard, is the fact that the PFC lesions included the frontal eye fields (FEFs) in most of the patients. In fact, Guitton and co-workers hypothesized that lesions of the FEF or possibly supplementary eye fields lead to difficulties in antisaccade initiation, whereas the ability to suppress inappropriate saccades would require the integrity of a large portion of the frontal lobe. They referred to erroneous cue-directed saccades as ‘reflexive saccades’ and hypothesized that the lesions resulted in a delay in the generation of a cancellation signal which would act to suppress the so-called visual grasp reflex, a term coined initially by Hess and co-workers based on their experiments in the SC of the cat.
Subsequently, Pierrot-Deseilligny et al. [10] sought to determine directly whether response suppression deficits seen in frontal lobe patients were related to lesions of the FEF or PFC. To this end, they compared error rates and reaction times of antisaccades in a gap task between groups of patients with recent, relatively small, unilateral cortical infarcts. They reported that PFC, but not FEF, supplementary eye field (SEF), or posterior parietal lesion groups showed an increased frequency of antisaccade errors. Although the PFC lesions were exclusively unilateral, the laterality of deficits exhibited by the patients varied substantially. Some failed to suppress saccades towards ipsiversive stimuli, others towards contraversive stimuli, and many demonstrated bilateral suppression deficits.
Despite the seeming consistency of these results with a specific role of the PFC in the suppression of erroneous responses in the antisaccade task, it is now clear that the FEF was mislocalized in this study, with the result that the lesions of some patients in the PFC group also extended into the FEF. Working prior to the advent of high-field functional magnetic resonance imaging (fMRI), the authors placed their region of interest slightly too far ventral in the precentral sulcus. We now know from a large number of fMRI studies that, in humans, the FEF is located at the junction of the superior frontal sulcus and precentral sulcus [11–13], further medial than Pierrot-Deseilligny and co-workers had surmised. Two subsequent studies from the same group that examined patients with more restricted prefrontal lesions avoided this problem and localized a putative saccade suppression region in the middle portions of the middle frontal gyrus [14,15], which corresponds to Brodmann's area 46 or area 9/46 in the more recent cytoarchitectonic maps of Petrides & Pandya [16]. Patients with unilateral lesions in this area exhibited bilateral increases in error rates, with a notable bias in errors towards the contralesional hemifield. Patients with cortical lesions dorsal to the superior frontal gyrus or ventral to the inferior frontal gyrus, but sparing the middle frontal gyrus exhibited normal error rates in the antisaccade task. This result has also been observed in other studies [17,18]. To account for the findings of lesion studies, Pierrot-Deseilligny et al. [10] proposed a model in which the PFC, in particular, the region of the middle frontal gyrus, suppresses inappropriate reflexive saccades via projections that inhibit the SC, a midbrain structure critical for saccade initiation. We henceforth refer to this idea as the ‘inhibition model’ (figure 1).
Figure 1.

Schematic of the inhibitory control model. According to this model, the lateral prefrontal cortex (PFC) inhibits saccade-related activity in the ipsilateral superior colliculus (SC). Because cortical output neurons are excitatory, they have to terminate either on fixation neurons (FN) in the rostral SC or on interneurons (IN) to inhibit the activity of saccade-related neurons (SN) that activate long-lead burst neurons (LLBN) in reticular formation in the opposite hemisphere.
3. Evidence from functional imaging
A role of the PFC in saccade suppression has been supported by positron emission tomography and fMRI studies, which have reported increased activation for the performance of antisaccades compared with prosaccades in the PFC (for review, see [19]). In most of these studies, the locus of activation was in the middle frontal gyrus, although some also found activation in the superior frontal gyrus. Other studies, however, have failed to find any activation in the PFC. Although the reason for these conflicting results is presently unclear, differences in task design may be a potential explanatory factor [20,21]. Dyckman et al. [21] found higher blood-oxygenation level dependent (BOLD) activation for anti- than prosaccades when the tasks were performed in separate blocks, but not when they were randomly interleaved. They hypothesized that sustained activation of the lateral PFC may be present throughout the mixed task owing to the increased difficulty and more complex response selection requirements engendered by continually switching between the two responses. Consistent with this, subjects occasionally made erroneous responses on prosaccade trials that followed antisaccade trials. While most functional imaging studies could not address whether differences in activity for anti- and prosaccades occur prior to or post-saccade, some event-related studies using long instruction periods [22,23], or rapid designs with catch trials [24], reported that the PFC was more active for anti- than prosaccades during the instruction but not the response period. A direct comparison of the BOLD signal during the late preparatory period also found higher activation in the PFC (as well as in the anterior cingulate cortex and pre-SEF) for correct trials compared with error trials [23]. Greater pre-stimulus activation in the frontal cortex on correct when compared with error trials has also been reported by an event-related potential study [25]. Recently, an electroencephalogram study reported a decrease in ongoing beta-power (20–30 Hz) in frontal cortex on error trials during the preparatory period on antisaccade trials [26]. In general, data from human imaging studies indicate that increased PFC activation, particularly of the region of the middle frontal gyrus, is associated with correct performance on the antisaccade task. This evidence is has been interpreted as supporting the inhibition model.
4. Neurophysiology in the superior colliculus: fingerprints of inhibitory control
The SC is a midbrain structure with laminar organization that is critical for the initiation of saccades. The intermediate and deep layers receive convergent projections from all cortical saccade-related areas, including the PFC, and project to the saccade generating circuit in the brainstem (for reviews, see [27,28]). These layers contain a retinotopic map of saccade vectors for the contralateral hemifield. The influence of cortical areas on the saccade generation process is mediated by direct and indirect projections to the SC. Although some cortical projections target the brainstem saccade generator directly, this pathway alone is insufficient for initiating saccades [29]. The SC is thus the site of convergence and integration of cortical signals and directs, in turn, oculomotor behaviour. This, coupled with the proposal that the PFC inhibits this structure, makes it an ideal point of departure for studies investigating the role of inhibitory cortical signals for saccade suppression in the antisaccade task.
The SC contains neurons exhibiting a variety of response properties, including fixation-related activity, low-frequency ‘build-up’ activity preceding the onset of saccades, responses to visual stimuli and robust saccade-related bursts of activity. The link between SC activity and saccade generation has often been conceptualized as a complementary relationship between neurons with fixation-related activity, located in the rostrolateral pole of the SC, and those with saccade-related activity, in more caudal SC regions [30,31]. In the late 1990s, Munoz and Everling investigated the activity of single SC neurons in macaque monkeys performing pro- and antisaccade trials [7,32]. Animals were instructed which of the two responses to perform based on the colour of a central fixation stimulus. Fixation and saccade-related neurons exhibited complementary activity that differed between pro- and antisaccade trials. During the instruction period prior to onset of the peripheral visual stimulus, in which animals prepared to perform the correct response, but had insufficient information to programme the direction of the forthcoming saccade, fixation neurons exhibited tonic activity that was greater on anti- than prosaccade trials. By contrast, build-up neurons, a subtype of SC saccade-related neuron characterized by a steady increase of low-frequency activity prior to the onset of saccades, exhibited the inverse pattern; activity was lower on antisaccade than prosaccade trials. Many trials included a gap manipulation, in which the fixation stimulus was extinguished just before the onset of the peripheral visual stimulus. During the gap, the tonic activity of fixation neurons declined, and the difference in activity between pro- and antisaccade trials was reduced. Conversely, the steady increase in activity exhibited by build-up neurons was enhanced, and activity differences between pro- and antisaccade trials were maintained. The level of this pre-stimulus build-up activity was negatively correlated to the reaction times of saccades; greater activity was associated with reduced reaction times. No such relationship was observed for fixation neurons.
When a visual stimulus is presented within the circumscribed region of space in the contralateral hemifield that defines the response field of an SC neuron, a subset of intermediate layer SC saccade-related neurons discharge a transient burst of action potentials. This visual discharge is accompanied by a suppression of activity of saccade-related neurons in the SC on the opposite side of the brain. This push–pull relationship between activity in the left and right SCs is mediated by intercollicular inhibition [33,34], and prevents simultaneous programming of incompatible saccades. On prosaccade trials, this visual response was followed closely by a burst of activity beginning just prior to generation of the saccade into the neuron's response field. By contrast, on antisaccade trials, requiring a saccade away from the visual stimulus in the response field, the visual response was attenuated, and followed by a similarly attenuated motor burst in the opposite SC, accompanying generation of the correct saccade away from the peripheral visual stimulus. During this time, the activity of fixation neurons declined to minimal levels for both types of trials. To evaluate directly the link between SC activity and antisaccade performance, activity on erroneous antisaccade trials, in which animals made saccades towards the visual stimulus, was compared with that on correct antisaccade trials [7]. Response errors were characterized by an increase in the pre-stimulus activity of build-up neurons and a burst of action potentials that occurred at roughly the same time as the visual response on correct trials but was of significantly greater magnitude. Very similar discharge patterns have been observed in FEF neurons [35,36], including those electrophysiologically identified as projecting to the SC [35].
These findings indicate that two critical conditions must be met for correct antisaccade performance. First, the activity of saccade-related neurons preceding stimulus onset must be reduced. Second, the visual responses of saccade-related neurons stimuli must be attenuated. Munoz & Everling [3] formalized these conditions in a thresholded accumulator model. According to this model, activity of saccade-related neurons grows towards a fixed threshold, and a saccade is triggered when this level of activity is exceeded. For correct antisaccade execution, pre-stimulus and visual activity must be reduced so that they do not exceed threshold and trigger an erroneous prosaccade. The link between attenuated activity and correct antisaccade performance suggested an inhibitory mechanism that suppresses the activity of saccade-related neurons. Such a mechanism could be either local, in the form of fixation neuron activity, or distant, in the form of a top-down signal (figure 1). A local fixation neuron-based explanation seems unlikely, as single neuron recordings of fixation neuron activity have failed to find differences between correct and erroneous antisaccade trials, as would be expected if these neurons were responsible for local suppression of saccade-related neurons (S. Everling & D. P. Munoz 1998, unpublished data). This contrasts with observations in the saccade countermanding task, in which saccades cancelled successfully in response to an imperative stop signal are associated with an increase in fixation neuron activity [37]. It is possible, however, that this activity is simply a response to the reappearance of the foveal fixation stimulus, which serves as the stop signal in this task. Altogether, the results of SC recordings in the anti saccade task are suggestive of an extra-collicular inhibitory influence on saccade-related activity that enables correct task performance.
5. Linking inhibitory control and neural mechanisms
Based on the above-described data from patients with PFC lesions, functional imaging results, and single neuron recordings in the SC of macaques during performance of the antisaccade task, Munoz & Everling [3] hypothesized that lesions of the PFC would result in an elevation of pre-stimulus activity in the SC, which would sum with the incoming visual response to trigger direction errors. This conceptualization was consistent with the inhibition model as proposed by Pierrot-Deseilligny et al. [10,38], and extended it by making explicit the potential neurophysiological mechanisms instantiating the inhibitory process. This extended model proposed a direct mapping between the cognitive process of inhibitory control and physiological states in the PFC and SC, and can thus be considered a linking proposition [39,40]. This could be formally stated as follows: the cognitive process of inhibitory control corresponds to some physiological state of activity in the PFC that engages a mechanism causing inhibition of saccade-related activity.
At least three potential inhibition mechanisms have been proposed: (i) the PFC inhibits the FEF through corticocortical projections, (ii) the PFC inhibits the SC directly through corticotectal projections, and (iii) the PFC inhibits the SC by activating an indirect saccade suppression pathway through the basal ganglia. Although all three pathways could, in principle, transmit an inhibition signal to saccade-related neurons in the SC, the plausibility of any neurophysiological explanation is constrained by the structural architecture of the system in question. Put another way, if one structure is to inhibit another, then it must be connected to it in a manner that that allows it to do so. Long-range corticocortical projections are made by pyramidal neurons, which are exclusively excitatory [41]. Inhibition of FEF by the PFC, as proposed above, would require that PFC neurons predominately target inhibitory FEF interneurons. This possibility seems unlikely given the results of a recent study that examined the reverse projection, from FEF to area 46, and found that 95% of retrogradely labelled synapses were with dendritic spines of pyramidal neurons in area 46 [42]. Only the remaining 5% of synapses targeted smooth (putative γ-aminobutyric acid (GABA)ergic) and spiny neurons in about equal proportions. Similarly, the great majority of corticotectal fibres—projecting from the cortex to the SC—target the ipsilateral SC [43–45]. These projections originate from pyramidal neurons and are thus also excitatory [41,46]. This architecture dictates that PFC-mediated inhibition would have to occur in the ipsilateral SC, and that suppression of SC saccade neurons must be carried out via excitation of either fixation neurons in the rostral SC or inhibitory interneurons in the ipsilateral SC (figure 1). Although proposed by several investigators [47,48], an activation of fixation neurons seems an unlikely mechanism, for reasons discussed above. This suggests that, if the inhibition model is correct, excitatory cortical inputs to SC inhibitory interneurons is the mechanism of suppression of SC saccade-related activity.
Relatively little is still known about the pathway through the basal ganglia [49]. The majority of corticostriatal PFC fibres terminate in the ipsilateral caudate nucleus [50]. There are, however, a smaller number of axon collaterals that cross the midline and terminate in the contralateral caudate nucleus [50]. Caudate projection neurons are inhibitory and usually silent, but respond phasically to specific task events, including stimulus and saccade onset [51,52]. Roughly half of these medium spiny neurons in the caudate suppress directly the tonic inhibitory activity of the substantia nigra pars reticulata (SNr) which, in turn, facilitates saccade generation (direct pathway). The other half of the medium spiny neurons project to the globus pallidus external segment (GPe; indirect pathway) [53]. Because GPe neurons are inhibitory [54], their inhibition leads to an increase in activity in the subthalamic nucleus (STN). Activation of the STN excites SNr neurons via an excitatory projection from STN to SNr [55,56]; thus, the SNr increases tonic inhibition on neurons in the SC, thereby suppressing saccade initiation.
Ford & Everling [57] proposed that the PFC inhibits contralateral saccades by projections to the ipsilateral indirect pathway. By contrast, Watanabe & Munoz [49] have suggested that voluntary cortical saccade commands are sent to both the ipsilateral direct pathway and to the contralateral indirect pathway. More work is required to determine the potential contribution of basal ganglia pathways to the inhibition of saccades.
6. Putative suppression signals and single neuron activity in the lateral prefrontal cortex
To evaluate the link between PFC activity and inhibitory control in the antisaccade task, it is obviously necessary to investigate directly the responses of PFC neurons during antisaccade performance. Several single neuron recording studies have shown that about a quarter of randomly sampled neurons in the lateral PFC have different levels of activity between pro- and antisaccade trials during the preparatory period prior to stimulus presentation [58,59]. To rule out the possibility that these differences might be related to the visual properties of the fixation stimulus, the colour of which instructed the animals which task to perform in previous studies, Everling & Desouza [58] designed a task paradigm in which monkeys performed alternate blocks of pro- and antisaccade trials in the absence of an explicit instruction cue. In this paradigm, the task rule was acquired by trial and error based on the delivery or omission of reward after each trial. PFC neurons exhibited task-selective preparatory activity that persisted throughout the task blocks and discriminated between correct and erroneous responses. No clear preference for either the pro- or antisaccade task was found in the population of PFC neurons; roughly half had greater activity on prosaccade than antisaccade trials, with the other half exhibiting the inverse pattern of selectivity. A subsequent analysis sorted the population of PFC neurons into subgroups of pyramidal and putative interneurons using the waveform durations of the extracellularly recorded action potentials [60]. This analysis revealed evidence for opponent coding of task-selective responses of PFC neurons. Putative pyramidal neurons exhibited greater activity on anti- than prosaccade trials, whereas putative interneurons showed the inverse pattern of activity. Because pyramidal neurons are the output neurons of the cortex, enhanced responses on antisaccade trials could represent a suppression signal consistent with the inhibition model, if sent to the SC. This is, indeed, the case. Enhanced activity on antisaccade trials is observed in PFC neurons electrophysiologically identified as sending a projection to the SC [48].
Studies investigating the responses of PFC neurons in the antisaccade task demonstrate selective responses consistent with a reduction of activity in oculomotor circuits prior to visual stimulus onset, and a suppression signal that follows peripheral stimulus presentation. Activity differences are observed during preparatory periods, in which animals have acquired knowledge of the type of trial to be performed, but not the direction of the upcoming response. Following stimulus onset, this selective PFC activity is maintained until roughly 130 ms following stimulus onset, at which time activity rises selectively on antisaccade trials [48,60]. Prima facie, when considered together with data from single neuron recordings in the SC, such responses certainly ‘look like’ what would be expected on the basis of the inhibition model—a sustained increase in activity of PFC output neurons prior to stimulus presentation, and a more phasic response following stimulus presentation, seemingly consistent with a suppression or cancellation signal, coupled with reduced preparatory and stimulus-related activity in the SC. Indeed, we hypothesized initially that this was the case [48,60]. This putative link between neural activity and the cognitive process of inhibitory control begins to fray under closer scrutiny however, particularly when the temporal relationship between the phasic post-stimulus PFC response and SC activity is considered. Figure 2 shows a direct comparison between the timing of responses of task-related putative pyramidal PFC neurons [60] and SC neurons on pro- and antisaccade trials. These data were recorded from adult rhesus monkeys in the same behavioural setups with the same amplifiers in our laboratory. For the SC recordings, monkeys were cued by the colour of the central fixation point which task to perform, whereas in the PFC recordings they had to alternate between uncued blocks of pro- and antisaccade trials. Note that although the animals performed different versions of the pro- antisaccade paradigm, saccadic reaction times were not longer for the PFC recordings. This direct comparison of the time courses of neural activity on pro- and antisaccade trials in the PFC and SC shows that differences between the two tasks emerged later in the PFC than in the SC. When measured with a sliding receiver operating characteristic (ROC) analysis on baseline-corrected neural activity in 10 ms intervals [60], significant differences in the stimulus-related response of SC neurons emerged already at the onset of the phasic response (54 ms after stimulus onset), whereas the putative PFC suppression signal began much later at about 155 ms after stimulus onset. The transmission time between PFC and SC averages approximately 6 ms [48]. Therefore, the PFC signal arrives at the SC too late to influence the initial visual response. Thus, PFC neurons are logically incapable of providing a post-stimulus cancellation signal.
Figure 2.

(a) Temporal activity profile of a population of task-related neurons putative pyramidal neurons in the lateral prefrontal cortex (PFC) contralateral to the stimulus [60] and (b) saccade-related neurons in the superior colliculus (SC) on antisaccade (red/light) and prosaccade (blue/dark) trials when the stimulus is presented into the neuron's response field [61]. Activation waveforms were obtained by convolving each spike with an asymmetric function that resembled a postsynaptic potential [62,63]. Shaded envelopes depict within-subjects standard error of the mean discharge rate. Arrows indicate the mean reaction times of pro- and antisaccades in the two datasets. The onset of significant differences between pro- and antisaccade trials in the populations of PFC and SC neurons is indicated by vertical dashed lines. Onset was determined by bootstrap analyses on data from 10 ms sliding ROC analyses (for details, see [60]). (Online version in colour.)
7. Challenging the inhibitory model: prefrontal cortex deactivation and stimulation
While the studies that we have reviewed so far are consistent with a role of the PFC in the suppression of preparatory activity in the SC, two studies that manipulated PFC activity in non-human primates have yielded results that are difficult to reconcile with the inhibition model. These and the following studies have focused on the caudal principal sulcus, the homologue of the middle frontal gyrus in humans [16,64]. To understand the mismatch between these results and the inhibition model, it is helpful to consider in detail the neural circuit, putative inhibitory mechanism and predictions of the model (figure 1). Consistent with the known corticotectal projections of the PFC, the inhibition model proposes that a suppression signal, instantiated by enhanced PFC activity, is sent to the ipsilateral SC and leads to a reduction in activity of SC saccade-related neurons [10,15,38,48,60]. The sign inversion necessary to transform an excitatory PFC input to an inhibitory effect is proposed to be mediated by excitation of either SC fixation neurons or inhibitory interneurons. Recalling that the SC contains a vector map of saccades to the contralateral hemifield, the model predicts a PFC-mediated inhibition of the ipsilateral SC, which would suppress contraversive saccades. A secondary effect would be a reduction of intercollicular inhibition resulting in increased activity of the contralateral SC, and a facilitation of ipsiversive saccades. According to the inhibition model then, unilateral deactivations of the PFC should result in increased activity in the ipsilateral SC—facilitating contraversive saccades, and decreased activity in the contralateral SC—suppressing ipsiversive saccades. Unilateral deactivations of the PFC by injections of the GABA agonist muscimol into the ventral or dorsal bank of the caudal principal sulcus resulted in effects opposite this prediction; a failure to suppress task-inappropriate ipsiversive saccades in the antisaccade task, whereas the ability to suppress inappropriate contraversive saccades improved [65]. In addition, electrical microstimulation of sites in the dorsal bank or fundus of the caudal principal sulcus, which would be expected to enhance the inhibitory effects of the PFC, facilitated contraversive pro- and antisaccades, in the form of decreased reaction times, and increased the frequency of antisaccade errors when the stimulus was presented on the contraversive side [66]. These results are not consistent with the inhibitory model, but suggest an alternative conceptualization, in which the PFC either excites the ipsilateral SC or inhibits the contralateral SC.
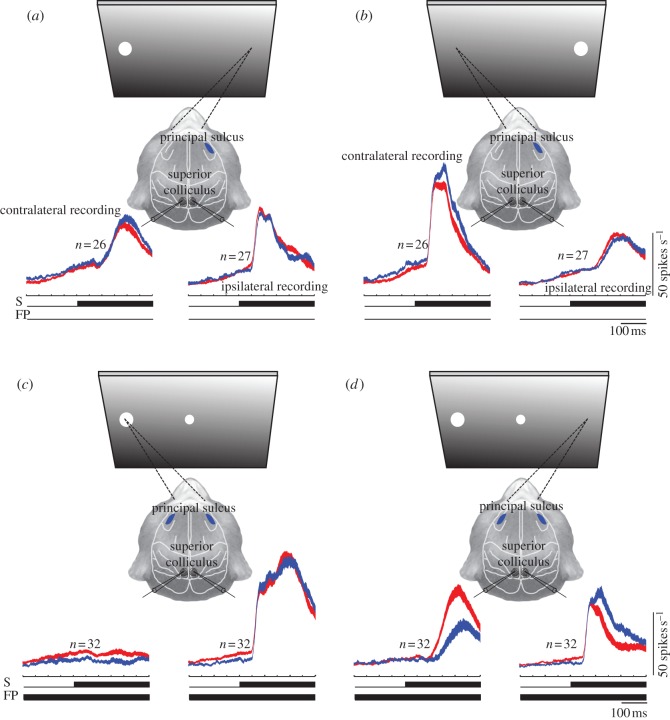
The most direct test of the contribution of the individual components of a neural circuit to the output of the circuit as a whole is to deactivate one component while simultaneously monitoring circuit output. To assess directly the inhibition model, we carried out a series of studies in which we cryogenically deactivated the PFC while simultaneously recording the activity of SC saccade-related neurons in monkeys performing pro- and antisaccades [61]. We found that unilateral deactivation of the PFC resulted in changes in SC activity, which were not consistent with the inhibition model [67]. Contrary to this model, which would predict removal of a net inhibitory input to result in increased activity in the ipsilateral SC, and a decrease in the contralateral SC, we found that the activity of many build-up neurons in the ipsilateral SC decreased, whereas those in the contralateral SC showed an increase in activity during the pre-stimulus period on pro- and antisaccade trials (figure 3a,b). We found also that the onset of saccade-related activity in the ipsilateral SC was delayed significantly (figure 3b). Finally, we found that the stimulus-related response increased in the contralateral SC on antisaccade trials, and that it decayed more slowly during cooling (figure 3b). These changes in neural activity were mirrored by an increase in saccadic reaction times for contraversive saccades and a decrease for ipsiversive saccades. Error rates were increased on trials in which stimuli were presented in the hemifield ipsilateral to the cooled hemisphere and decreased for trials on which the stimulus was presented to the contralateral hemisphere. These results demonstrate that, contrary to the proposal of the inhibition model, unilateral PFC deactivation leads to activity decreases in the ipsilateral, and increases in the contralateral SC. We hypothesized that the mechanism responsible for this change was a neural imbalance between the two SCs effected by removal of an excitatory input to the ipsilateral SC.
Figure 3.
Effects of caudal principal sulcus cooling on the activity of saccade-related neurons in the superior colliculus on control (red/light) and cooling (blue/dark) trials. Blue patches indicate the deactivation sites. Activity was combined for correct and error trials (same stimulus location but opposite saccade direction). (a) Activity on antisaccade trials on which the stimulus was presented contralateral to the cooled principal sulcus. Data were obtained in a gap saccade task [68]. (b) Same as (a) but on trials on which the stimulus was presented ipsilateral to the cooled principal sulcus. (c) Activity on prosaccade trials in the bilateral deactivation condition in a task that required monkeys to retain the task instruction for 500–700 ms [67]. (d) Same as (c) but on antisaccade trials. S, stimulus; FP, fixation point. (Online version in colour.)
To avoid the neural imbalance problems associated with unilateral deactivations, we also carried out bilateral PFC deactivations while recording SC activity [61]. Bilateral PFC deactivation significantly reduced the levels of pre-stimulus activity in the SC on both prosaccade (figure 3c) and antisaccade trials (figure 3d) and effectively abolished the normally robust differences in SC preparatory activity between the two tasks. The initial magnitude of the stimulus-related response did not vary between cooling and non-cooling trials in the antisaccade task (figure 3d); however, the stimulus-related activity decayed more slowly on cooling trials. Moreover, the motor burst for the antisaccade was delayed on cooling trials (figure 3d). Reaction times of pro- and antisaccades were increased, and error rates increased on antisaccade trials. Error rates increased particularly on those trials which required the animals to retain temporarily the task instruction in working memory prior to the onset of the peripheral stimulus (figure 4a). An important finding was that although the monkeys generated a large proportion of errors on these memory antisaccade trials (figure 4a), those errors had latencies outside of the range of express saccades (figure 4b), suggesting that errors were not the result of a visual triggering of incorrect saccades towards the visual stimulus. Even when we tested the effects of bilateral principal sulcus cooling on the performance of a gap saccade task in one monkey, we found that although cooling increased the number of errors (figure 4c), it did not increase the percentage of errors with express saccade latencies (figure 4d). When we compared directly the activity on correct and error trials, we found that pre-stimulus activity was reduced on error trials, and that the magnitude of the initial stimulus-related response was not increased but rather prolonged in such a manner that it did not slowly decline as on correct trials, but increased until it culminated in a saccadic motor burst [61]. We found no indication in the activity of SC saccade-related neurons that the antisaccade was prepared when monkeys made performance errors. In addition to increased reaction times and error rates on antisaccade trials, we found also that monkeys were generally less vigilant during task performance, failing to initiate or aborting trials more often during cooling.
Figure 4.
Behavioural effects of PFC deactivation. (a) Mean per cent errors for 54 experimental sessions from two monkeys on prosaccade (thick lines) and antisaccade trials (thin lines) on overlap trials (solid lines) in which the coloured fixation stimulus is visible throughout the trial and on memory trials (dashed lines) in which the coloured fixation stimulus changed to a neutral stimulus 500–700 ms prior to stimulus onset. (b) Cumulative distribution of all direction errors in the memory condition on antisaccade trials. (c) Mean per cent errors for 17 experimental sessions from one monkey on gap prosaccade trials (solid line) and gap antisaccade trials (dashed line). (d) Cumulative distribution of all direction errors in the gap condition on antisaccade trials. (e) Mean per cent errors for 12 sessions on antisaccade trials with a short delay of 500–700 ms (solid line) and mean per cent errors for 10 sessions with a long delay of 1000–1200 ms (dashed line) obtained in same the monkey. PRE, period before cooling; COOL, during bilateral cooling of the prefrontal cortex; POST, after cooling.
The inhibition hypothesis predicts that PFC deactivation should lead to increased activity in the ipsilateral and decreased activity in the contralateral SC. We found that unilateral PFC deactivation resulted in the opposite pattern of results [67], and that bilateral deactivations resulted in decreases in preparatory activity in the SC [61]. These findings demonstrate that the caudal principal sulcus region provides a direct or indirect excitatory drive to saccade-related SC neurons, rather than activating ipsilaterally projecting intratectal inhibitory neurons or commissural intratectal inhibitory neurons. We hypothesize that motor activity for antisaccades leads, via commissural inhibition, to the suppression of the stimulus-related response. On cooling trials, the antisaccade motor activity is delayed, thereby allowing the stimulus-related activity in the other SC to increase towards saccade threshold. Based on these experiments, we suggest that errors in schizophrenia and frontal lobe patients occur, because the antisaccade programme often starts too late, which allows the system to default to a prosaccade mode.
8. If not inhibition, then what? prefrontal cortex encodes the task rule
Evidence from studies using a variety of methods to investigate the neural basis of inhibitory control using the antisaccade task had supported the inhibition model. Our recent findings, however, are incompatible with this model. Single neuron recordings of PFC neurons have revealed that the increases in PFC activity we have observed on antisaccade trials begin too late to arrive at the SC in time to provide a saccade suppression or cancellation signal. Studies in which we have recorded SC neurons while deactivating the PFC have revealed that neural activity in the SC is altered in a manner opposite to that predicted by the inhibition model. We therefore conclude that the lateral PFC does not inhibit automatic saccades by suppressing SC activity. We support instead a heterodox view in which the PFC provides an excitatory input to the SC that acts to facilitate behavioural goals. We argue that these facilitatory signals provide a mechanism for implementing behavioural rules, and as such are consistent with another current view of PFC function; that it is responsible for the encoding and implementation of task sets and task rules [68,69]. If the lateral PFC does not provide a saccade suppression signal, why do patients with PFC lesions generate more response errors in the antisaccade task? Why have functional imaging studies found higher activation for anti- than prosaccade trials in the lateral PFC and more activation for correct responses than errors, and why do monkey PFC neurons exhibit task-selectivity for pro- and antisaccades?
The increased errors of patients with lateral PFC lesions have been interpreted as failures to suppress reflexive or automatic saccades [9,10,14,15,38]. Our deactivation experiments have shown that the increased errors have reaction times outside of the range of express saccades and that the initial stimulus-related response is not increased on error trials [61,67] (figure 4b,d). Unfortunately, almost none of the human lesion studies have reported the reaction times of response errors. However, inspection of some of the eye traces in Guitton et al.'s [9] study shows that very few errors were in the range of express saccades and Walker et al. [18] reported that their patient, who was completely unable to perform antisaccades, had mean response error latencies of 162 ms, i.e. clearly longer than express saccades [70]. Similarly, although often described as automatic or pre-potent responses, response errors of schizophrenic patients in the antisaccade task are also typically not in the express saccade range (R. Lencer 1998, personal communication).
It has been known for a long time that patients with frontal lobe damage may understand and remember task instructions, but are unable to use the instructions to control behaviour [71,72]. This deficit has been termed ‘goal neglect’ [73,74]. Our data suggest that response errors of patients with frontal lobe damage or schizophrenia are not the result of an inhibition deficit, but of a failure to maintain and implement the proper task set. Neural correlates of task set failure have been observed in the FEF in a modified visual search task that required monkeys to make either a prosaccade towards a colour singleton, an antisaccade to the location opposite the singleton, or, on no-saccade trials, to withhold a saccade. In this study, the task rule was instructed by the shape of the colour singleton. Interestingly, the majority of errors on no-saccade trials were antisaccades, and this behaviour was consistent with the unnecessary selection of movement endpoints by FEF neurons which was often observed on no-saccade trials [75]. It seems plausible to suggest that erroneous antisaccades in this case resulted from a failure to implement the ‘no saccade’ rule, perhaps because extensive training biased activity in favour of antisaccade performance. Our own data also support the notion of task set failure. We observed that errors are increased during bilateral prefrontal cooling when monkeys have to briefly memorize the task instruction (figure 4a). In fact, we hypothesize that the increase in error rate in the gap task during cooling is also owing to the brief delay (200 ms) between the disappearance of the coloured central fixation stimulus, which serves as the task cue, and the appearance of the peripheral stimulus (figure 4c).
According to our hypothesis, performance should deteriorate further if the delay interval during which the animal has to maintain the task rule is increased. Preliminary data obtained from one animal seem to support this hypothesis (figure 4e). This monkey generated more response errors on antisaccade trials in the long (1000–1200 ms) than short delay (500–700 ms) condition.
Functional imaging studies have shown that the lateral PFC is not just active when subjects have to inhibit responses as in the antisaccade task or no-go task, but that the area in and around the inferior frontal sulcus is activated together with the anterior insula/frontal operculum, the dorsomedial frontal cortex, and cortex in and around the intraparietal sulcus across tasks with a multitude of different cognitive demands, including response selection, executive control, working memory, episodic memory and problem solving [76]. Because of its activation across a wide variety of cognitive demands, this network of cortical areas has been termed the multi-demand (MD) system. Duncan [77] suggested that the MD system organizes relevant facts, rules and requirements into a ‘task model’ that then drives activity in large parts of the brain to perform the required task. This idea is consistent with experiments on single neurons in the primate lateral PFC in non-human primates. These studies have found that a large proportion of PFC neurons code various aspects of whatever task a monkey has been trained to perform (stimuli, responses, rules, rewards) and that the coding changes when the animal must perform a different task [78]. We propose that the task-selective activity that we observed in the monkey lateral PFC for pro- and antisaccades is the neural instantiation of the behavioural rules for these two oculomotor tasks. A recent neural modelling study has shown that neurons which are connected with random synaptic strengths to neurons representing the task rule, and neurons representing sensory inputs neurons resemble the activity profiles of recorded lateral PFC neurons [79]. Although our results stand in opposition to the inhibitory model of antisaccade performance, it is unclear whether this result extends to other oculomotor tasks, or inhibitory control in general [80]. The saccade countermanding task requires withholding a saccade in response to an imperative stop signal [81]. In contrast to the antisaccade task, successful countermanding requires rapid triggering of a stop process after a peripheral visual stimulus has been presented, and a saccade to its location prepared. Our PFC recordings suggest that phasic responses in this area are too sluggish to effectively implement a stop process; the stop signal reaction time in the countermanding task is typically less than 100 ms in macaque monkeys [81], whereas the latencies of the signals we observed were well more than 100 ms. The relevant experiments investigating the effects of PFC deactivation on countermanding performance have not been carried out however, so it remains an open question as to whether the PFC is involved in engagement of the stop process, or encodes more contextual aspects of this task. Combined neurophysiological and deactivation based investigations of the role of the PFC in other aspects of cognitive control that may depend on an inhibitory substrate such as interference control [82] similarly await completion. Such studies, particularly if extended to include other PFC subregions, promise to clarify the long-held view that inhibitory control is a cardinal function of the PFC.
Funding statement
This work was supported by a grant from the Canadian Institutes of Health Research (FRN 44067) to S.E.
References
- 1.Holmes G. 1938. The cerebral integration of the ocular movements. Br. Med. J. 108, 107–112 (doi:10.1136/bmj.2.4045.107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Everling S, Fischer B. 1998. The antisaccade: a review of basic research and clinical studies. Neuropsychologia 136, 885–899 (doi:10.1016/S0028-3932(98)00020-7) [DOI] [PubMed] [Google Scholar]
- 3.Munoz DP, Everling S. 2004. Look away: the anti-saccade task and the voluntary control of eye movement. Nat. Rev. Neurosci. 5, 218–228 (doi:10.1038/nrn1345) [DOI] [PubMed] [Google Scholar]
- 4.Hutton SB, Ettinger U. 2006. The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology 43, 302–313 (doi:10.1111/j.1469-8986.2006.00403.x) [DOI] [PubMed] [Google Scholar]
- 5.Fischer B, Weber H. 1992. Characteristics of ‘anti’ saccades in man. Exp. Brain Res. 89, 415–424 (doi:10.1007/BF00228257) [DOI] [PubMed] [Google Scholar]
- 6.Bell AH, Everling S, Munoz DP. 2000. Influence of stimulus eccentricity and direction on characteristics of pro- and antisaccades in non-human primates. J. Neurophysiol. 84, 2595–2604 [DOI] [PubMed] [Google Scholar]
- 7.Everling S, Dorris MC, Munoz DP. 1998. Reflex suppression in the anti-saccade task is dependent on prestimulus neural processes. J. Neurophysiol. 80, 1584–1589 [DOI] [PubMed] [Google Scholar]
- 8.Fischer B, Boch R. 1983. Saccadic eye movements after extremely short reaction times in the monkey. Brain Res. 260, 21–26 (doi:10.1016/0006-8993(83)90760-6) [DOI] [PubMed] [Google Scholar]
- 9.Guitton D, Buchtel HA, Douglas RM. 1985. Frontal lobe lesions in man cause difficulties in suppressing reflexive glances and in generating goal-directed saccades. Exp. Brain Res. 58, 455–472 (doi:10.1007/BF00235863) [DOI] [PubMed] [Google Scholar]
- 10.Pierrot-Deseilligny C, Rivaud S, Gaymard B, Agid Y. 1991. Cortical control of reflexive visually-guided saccades. Brain 114, 1473–1485 (doi:10.1093/brain/114.3.1473) [DOI] [PubMed] [Google Scholar]
- 11.Amiez C, Kostopoulos P, Champod AS, Petrides M. 2006. Local morphology predicts functional organization of the dorsal premotor region in the human brain. J. Neurosci. 26, 2724–2731 (doi:10.1523/JNEUROSCI.4739-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luna B, Thulborn KR, Strojwas MH, McCurtain BJ, Berman RA, Genovese CR, Sweeney JA. 1998. Dorsal cortical regions subserving visually guided saccades in humans: an fMRI study. Cereb. Cortex 8, 40–47 (doi:10.1093/cercor/8.1.40) [DOI] [PubMed] [Google Scholar]
- 13.Paus T. 1996. Location and function of the human frontal eye-field: a selective review. Neuropsychologia 34, 475–483 (doi:10.1016/0028-3932(95)00134-4) [DOI] [PubMed] [Google Scholar]
- 14.Ploner CJ, Gaymard BM, Rivaud-Pechoux S, Pierrot-Deseilligny C. 2005. The prefrontal substrate of reflexive saccade inhibition in humans. Biol. Psychiatry 57, 1159–1165 (doi:10.1016/j.biopsych.2005.02.017) [DOI] [PubMed] [Google Scholar]
- 15.Gaymard B, Francois C, Ploner CJ, Condy C, Rivaud-Pechoux S. 2003. A direct prefrontotectal tract against distractibility in the human brain. Ann. Neurol. 53, 542–545 (doi:10.1002/ana.10560) [DOI] [PubMed] [Google Scholar]
- 16.Petrides M, Pandya DN. 1999. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur. J. Neurosci. 11, 1011–1036 (doi:10.1046/j.1460-9568.1999.00519.x) [DOI] [PubMed] [Google Scholar]
- 17.Fukushima J, Fukushima K, Miyasaka K, Yamashita I. 1994. Voluntary control of saccadic eye movement in patients with frontal cortical lesions and parkinsonian patients in comparison with that in schizophrenics. Biol. Psychiatry 36, 21–30 (doi:10.1016/0006-3223(94)90058-2) [DOI] [PubMed] [Google Scholar]
- 18.Walker R, Husain M, Hodgson TL, Harrison J, Kennard C. 1998. Saccadic eye movement and working memory deficits following damage to human prefrontal cortex. Neuropsychologia 36, 1141–1159 (doi:10.1016/S0028-3932(98)00004-9) [DOI] [PubMed] [Google Scholar]
- 19.McDowell JE, Dyckman KA, Austin BP, Clementz BA. 2008. Neurophysiology and neuroanatomy of reflexive and volitional saccades: evidence from studies of humans. Brain Cogn. 68, 255–270 (doi:10.1016/j.bandc.2008.08.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ethridge LE, Brahmbhatt S, Gao Y, McDowell JE, Clementz BA. 2009. Consider the context: blocked versus interleaved presentation of antisaccade trials. Psychophysiology 46, 1100–1107 (doi:10.1111/j.1469-8986.2009.00834.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dyckman KA, Camchong J, Clementz BA, McDowell JE. 2007. An effect of context on saccade-related behavior and brain activity. Neuroimage 36, 774–784 (doi:10.1016/j.neuroimage.2007.03.023) [DOI] [PubMed] [Google Scholar]
- 22.Desouza JF, Menon RS, Everling S. 2003. Preparatory set associated with pro-saccades and anti-saccades in humans investigated with event-related fMRI. J. Neurophysiol. 89, 1016–1023 (doi:10.1152/jn.00562.2002) [DOI] [PubMed] [Google Scholar]
- 23.Ford KA, Goltz HC, Brown MR, Everling S. 2005. Neural processes associated with antisaccade task performance investigated with event-related fMRI. J. Neurophysiol. 94, 429–440 (doi:10.1152/jn.00471.2004) [DOI] [PubMed] [Google Scholar]
- 24.Brown MR, Vilis T, Everling S. 2007. Frontoparietal activation with preparation for antisaccades. J. Neurophysiol. 98, 1751–1762 (doi:10.1152/jn.00460.2007) [DOI] [PubMed] [Google Scholar]
- 25.Everling S, Spantekow A, Krappmann P, Flohr H. 1998. Event-related potentials associated with correct and incorrect responses in a cued antisaccade task. Exp. Brain Res. 118, 27–34 (doi:10.1007/s002210050252) [DOI] [PubMed] [Google Scholar]
- 26.Hamm JP, Dyckman KA, McDowell JE, Clementz BA. 2012. Pre-cue fronto-occipital alpha phase and distributed cortical oscillations predict failures of cognitive control. J. Neurosci. 32, 7034–7041 (doi:10.1523/JNEUROSCI.5198-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wurtz RH, Goldberg ME. 1989. The neurobiology of saccadic eye movements. Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 28.Johnston K, Everling S. 2008. Neurophysiology and neuroanatomy of reflexive and voluntary saccades in non-human primates. Brain Cogn. 68, 271–283 (doi:10.1016/j.bandc.2008.08.017) [DOI] [PubMed] [Google Scholar]
- 29.Hanes DP, Wurtz RH. 2001. Interaction of the frontal eye field and superior colliculus for saccade generation. J. Neurophysiol. 85, 804–815 [DOI] [PubMed] [Google Scholar]
- 30.Munoz DP, Dorris MC, Paré M, Everling S. 2000. On your mark, get set: brainstem circuitry underlying saccadic initiation. Can. J. Physiol. Pharmacol. 78, 934–944 (doi:10.1139/y00-062) [PubMed] [Google Scholar]
- 31.Dorris MC, Paré M, Munoz DP. 1997. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J. Neurosci. 17, 8566–8579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Everling S, Dorris MC, Klein RM, Munoz DP. 1999. Role of primate superior colliculus in preparation and execution of anti-saccades and pro-saccades. J. Neurosci. 19, 2740–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munoz DP, Istvan PJ. 1998. Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. J. Neurophysiol. 79, 1193–1209 [DOI] [PubMed] [Google Scholar]
- 34.Takahashi M, Sugiuchi Y, Izawa Y, Shinoda Y. 2005. Commissural excitation and inhibition by the superior colliculus in tectoreticular neurons projecting to omnipause neuron and inhibitory burst neuron regions. J. Neurophysiol. 94, 1707–1726 (doi:10.1152/jn.00347.2005) [DOI] [PubMed] [Google Scholar]
- 35.Everling S, Munoz DP. 2000. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J. Neurosci. 20, 387–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato TR, Schall JD. 2003. Effects of stimulus-response compatibility on neural selection in frontal eye field. Neuron 38, 637–648 (doi:10.1016/S0896-6273(03)00237-X) [DOI] [PubMed] [Google Scholar]
- 37.Paré M, Hanes DP. 2003. Controlled movement processing: superior colliculus activity associated with countermanded saccades. J. Neurosci. 23, 6480–6489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pierrot-Deseilligny C, Muri RM, Ploner CJ, Gaymard B, Demeret S, Rivaud-Pechoux S. 2003. Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain 126, 1460–1473 (doi:10.1093/brain/awg148) [DOI] [PubMed] [Google Scholar]
- 39.Schall JD. 2004. On building a bridge between brain and behavior. Ann. Rev. Psychol. 55, 23–50 (doi:10.1146/annurev.psych.55.090902.141907) [DOI] [PubMed] [Google Scholar]
- 40.Teller DY. 1984. Linking propositions. Vision Res. 24, 1233–1246 (doi:10.1016/0042-6989(84)90178-0) [DOI] [PubMed] [Google Scholar]
- 41.Tsumoto T. 1990. Excitatory amino acid transmitters and their receptors in neural circuits of the cerebral neocortex. Neurosci. Res. 9, 79–102 (doi:10.1016/0168-0102(90)90025-A) [DOI] [PubMed] [Google Scholar]
- 42.Anderson JC, Kennedy H, Martin KA. 2011. Pathways of attention: synaptic relationships of frontal eye field to V4, lateral intraparietal cortex, and area 46 in macaque monkey. J. Neurosci. 31, 10 872–10 881 (doi:10.1523/JNEUROSCI.0622-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldman PS, Nauta WJ. 1976. Autoradiographic demonstration of a projection from prefrontal association cortex to the superior colliculus in the rhesus monkey. Brain Res. 116, 145–149 (doi:10.1016/0006-8993(76)90256-0) [DOI] [PubMed] [Google Scholar]
- 44.Fries W. 1984. Cortical projections to the superior colliculus in the macaque monkey: a retrograde study using horseradish peroxidase. J. Comp. Neurol. 230, 55–76 (doi:10.1002/cne.902300106) [DOI] [PubMed] [Google Scholar]
- 45.Leichnetz GR, Spencer RF, Hardy SG, Astruc J. 1981. The prefrontal corticotectal projection in the monkey; an anterograde and retrograde horseradish peroxidase study. Neuroscience 6, 1023–1041 (doi:10.1016/0306-4522(81)90068-3) [DOI] [PubMed] [Google Scholar]
- 46.Dori I, Dinopoulos A, Cavanagh ME, Parnavelas JG. 1992. Proportion of glutamate- and aspartate-immunoreactive neurons in the efferent pathways of the rat visual cortex varies according to the target. J. Comp. Neurol. 319, 191–204 (doi:10.1002/cne.903190202) [DOI] [PubMed] [Google Scholar]
- 47.Noorani I, Carpenter RH. 2012. Antisaccades as decisions: LATER model predicts latency distributions and error responses. Eur. J. Neurosci. 37, 330–338 (doi:10.1111/ejn.12025) [DOI] [PubMed] [Google Scholar]
- 48.Johnston K, Everling S. 2006. Monkey dorsolateral prefrontal cortex sends task-selective signals directly to the superior colliculus. J. Neurosci. 26, 12 471–12 478 (doi:10.1523/JNEUROSCI.4101-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watanabe M, Munoz DP. 2009. Neural correlates of conflict resolution between automatic and volitional actions by basal ganglia. Eur. J. Neurosci. 30, 2165–2176 (doi:10.1111/j.1460-9568.2009.06998.x) [DOI] [PubMed] [Google Scholar]
- 50.Leichnetz GR. 1981. The median subcallosal fasciculus in the monkey: a unique prefrontal corticostriate and corticocortical pathway revealed by anterogradely transported horseradish peroxidase. Neurosci. Lett. 21, 137–142 (doi:10.1016/0304-3940(81)90371-2) [DOI] [PubMed] [Google Scholar]
- 51.Hikosaka O, Sakamoto M, Usui S. 1989. Functional properties of monkey caudate neurons. I. Activities related to saccadic eye movements. J. Neurophysiol. 61, 780–798 [DOI] [PubMed] [Google Scholar]
- 52.Hikosaka O, Sakamoto M, Usui S. 1989. Functional properties of monkey caudate neurons. II. Visual and auditory responses. J. Neurophysiol. 61, 799–813 [DOI] [PubMed] [Google Scholar]
- 53.Feger J, Crossman AR. 1984. Identification of different subpopulations of neostriatal neurones projecting to globus pallidus or substantia nigra in the monkey: a retrograde fluorescence double-labelling study. Neurosci. Lett. 49, 7–12 (doi:10.1016/0304-3940(84)90127-7) [DOI] [PubMed] [Google Scholar]
- 54.Smith Y, Bolam JP. 1990. The output neurones and the dopaminergic neurones of the substantia nigra receive a GABA-containing input from the globus pallidus in the rat. J. Comp. Neurol. 296, 47–64 (doi:10.1002/cne.902960105) [DOI] [PubMed] [Google Scholar]
- 55.Hammond C, Deniau JM, Rizk A, Feger J. 1978. Electrophysiological demonstration of an excitatory subthalamonigral pathway in the rat. Brain Res. 151, 235–244 (doi:10.1016/0006-8993(78)90881-8) [DOI] [PubMed] [Google Scholar]
- 56.Nakanishi H, Kita H, Kitai ST. 1987. Intracellular study of rat substantia nigra pars reticulata neurons in an in vitro slice preparation: electrical membrane properties and response characteristics to subthalamic stimulation. Brain Res. 437, 45–55 (doi:10.1016/0006-8993(87)91525-3) [DOI] [PubMed] [Google Scholar]
- 57.Ford KA, Everling S. 2009. Neural activity in primate caudate nucleus associated with pro- and antisaccades. J. Neurophysiol. 102, 2334–2341 (doi:10.1152/jn.00125.2009) [DOI] [PubMed] [Google Scholar]
- 58.Everling S, Desouza JF. 2005. Rule-dependent activity for prosaccades and antisaccades in the primate prefrontal cortex. J. Cogn. Neurosci. 17, 1483–1496 (doi:10.1162/0898929054985455) [DOI] [PubMed] [Google Scholar]
- 59.Johnston K, Everling S. 2006. Neural activity in monkey prefrontal cortex is modulated by task context and behavioral instruction during delayed-match-to-sample and conditional prosaccade–antisaccade tasks. J. Cogn. Neurosci. 18, 749–765 (doi:10.1162/jocn.2006.18.5.749) [DOI] [PubMed] [Google Scholar]
- 60.Johnston K, De Souza JFX, Everling S. 2009. Monkey prefrontal cortical pyramidal and putative interneurons exhibit differential patterns of activity between pro- and antisaccade tasks. J. Neurosci. 29, 5516–5524 (doi:10.1523/JNEUROSCI.5953-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koval MJ, Lomber SG, Everling S. 2011. Prefrontal cortex deactivation in macaques alters activity in the superior colliculus and impairs voluntary control of saccades. J. Neurosci. 31, 8659–8668 (doi:10.1523/JNEUROSCI.1258-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanes DP, Schall JD. 1996. Neural control of voluntary movement initiation. Science 274, 427–430 (doi:10.1126/science.274.5286.427) [DOI] [PubMed] [Google Scholar]
- 63.Thompson KG, Hanes DP, Bichot NP, Schall JD. 1996. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J. Neurophysiol. 76, 4040–4055 [DOI] [PubMed] [Google Scholar]
- 64.Hutchison RM, Gallivan JP, Culham JC, Gati JS, Menon RS, Everling S. 2012. Functional connectivity of the frontal eye fields in humans and macaque monkeys investigated with resting-state fMRI. J. Neurophysiol. 107, 2463–2474 (doi:10.1152/jn.00891.2011) [DOI] [PubMed] [Google Scholar]
- 65.Condy C, Wattiez N, Rivaud-Pechoux S, Tremblay L, Gaymard B. 2007. Antisaccade deficit after inactivation of the principal sulcus in monkeys. Cereb. Cortex 17, 221–229 (doi:10.1093/cercor/bhj140) [DOI] [PubMed] [Google Scholar]
- 66.Wegener SP, Johnston K, Everling S. 2008. Microstimulation of monkey dorsolateral prefrontal cortex impairs antisaccade performance. Exp. Brain Res. 190, 463–473 (doi:10.1007/s00221-008-1488-4) [DOI] [PubMed] [Google Scholar]
- 67.Johnston K, Koval MJ, Lomber SG, Everling S. In press. Macaque dorsolateral prefrontal cortex does not suppress saccade-related activity in the superior colliculus. Cereb. Cortex. (doi:10.1093/cercor/bhs424) [DOI] [PubMed] [Google Scholar]
- 68.Sakai K. 2008. Task set and prefrontal cortex. Annu. Rev. Neurosci. 31, 219–245 (doi:10.1146/annurev.neuro.31.060407.125642) [DOI] [PubMed] [Google Scholar]
- 69.Bunge SA, Wallis JD, Parker A, Brass M, Crone EA, Hoshi E, Sakai A. 2005. Neural circuitry underlying rule use in humans and nonhuman primates. J. Neurosci. 25, 10 347–10 350 (doi:10.1523/JNEUROSCI.2937-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fischer B, Ramsperger E. 1984. Human express saccades: extremely short reaction times of goal directed eye movements. Exp. Brain Res. 57, 191–195 (doi:10.1007/BF00231145) [DOI] [PubMed] [Google Scholar]
- 71.Milner B. 1963. Effects of different brain lesions on card sorting: the role of the frontal lobes. Arch. Neurol. 9, 90–100 (doi:10.1001/archneur.1963.00460070100010) [Google Scholar]
- 72.Luria AR. 1966. Higher cortical functions in man. New York, NY: Basic Books [Google Scholar]
- 73.Duncan J, Emslie H, Williams P, Johnson R, Freer C. 1996. Intelligence and the frontal lobe: the organization of goal-directed behavior. Cognit. Psychol. 30, 257–303 (doi:10.1006/cogp.1996.0008) [DOI] [PubMed] [Google Scholar]
- 74.Duncan J, Parr A, Woolgar A, Thompson R, Bright P, Cox S, Bishop S, Nimmo-Smith I. 2008. Goal neglect and Spearman's g: competing parts of a complex task. J. Exp. Psychol. 137, 131–148 (doi:10.1037/0096-3445.137.1.131) [DOI] [PubMed] [Google Scholar]
- 75.Schall JD. 2004. On the role of frontal eye field in guiding attention and saccades. Vision Res. 44, 1453–1467 (doi:10.1016/j.visres.2003.10.025) [DOI] [PubMed] [Google Scholar]
- 76.Duncan J, Owen AM. 2000. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 23, 475–483 (doi:10.1016/S0166-2236(00)01633-7) [DOI] [PubMed] [Google Scholar]
- 77.Duncan J. 2010. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn. Sci. 14, 172–179 (doi:10.1016/j.tics.2010.01.004) [DOI] [PubMed] [Google Scholar]
- 78.Duncan J. 2001. An adaptive coding model of neural function in prefrontal cortex. Nat. Rev. Neurosci. 2, 820–829 (doi:10.1038/35097575) [DOI] [PubMed] [Google Scholar]
- 79.Rigotti M, Ben Dayan Rubin D, Wang XJ, Fusi S. 2010. Internal representation of task rules by recurrent dynamics: the importance of the diversity of neural responses. Front. Comput. Neurosci. 4, 1–29 (doi:10.3389/fncom.2010.00024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O'Reilly RC. 2011. A unified framework for inhibitory control. Trends Cogn. Sci. 15, 453–459 (doi:10.1016/j.tics.2011.07.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hanes DP, Schall JD. 1995. Countermanding saccades in macaque. Vis. Neurosci. 12, 929–937 (doi:10.1017/S0952523800009482) [DOI] [PubMed] [Google Scholar]
- 82.Nigg JT. 2000. On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychol. Bull. 126, 220–246 (doi:10.1037/0033-2909.126.2.220) [DOI] [PubMed] [Google Scholar]