Abstract
When searching for an object, we usually avoid items that are visually different from the target and objects or places that have been searched already. Previous studies have shown that neural activity in the lateral intraparietal area (LIP) can be used to guide this behaviour; responses to task irrelevant stimuli or to stimuli that have been fixated previously in the trial are reduced compared with responses to potential targets. Here, we test the hypothesis that these reduced responses have a different genesis. Two animals were trained on a visual foraging task, in which they had to find a target among a number of physically identical potential targets (T) and task irrelevant distractors. We recorded neural activity and local field potentials (LFPs) in LIP while the animals performed the task. We found that LFP power was similar for potential targets and distractors but was greater in the alpha and low beta bands when a previously fixated T was in the response field. We interpret these data to suggest that the reduced single-unit response to distractors is a bottom-up feed-forward result of processing in earlier areas and the reduced response to previously fixated Ts is a result of active top-down suppression.
Keywords: local field potential, attention, saliency map, inhibition of return
1. Introduction
Our capacity for processing visual information for perception and action is limited compared with the amount of information that is received by the eye. This makes it necessary to focus our attention on discrete regions of visual space, which is usually done by moving the eye so that gaze centres on the most important objects in the scene. The underlying mechanism driving attentional selection has been studied for years and is thought to be mainly controlled by a parieto-frontal network, which also includes subcortical oculomotor areas [1–4]. It is thought that these areas may function as priority maps, in which features or locations are represented by levels of neural activity related to the attentional priority at that location [5] and which are used to select the focus of both covert and overt attention [6].
In goal-directed behaviour, such as visual search, these priority maps highlight stimuli similar to the target [7–9] and help keep track of where we have looked [10]. Previous studies have shown that neural activity in the lateral intraparietal area (LIP) of posterior parietal cortex can perform both of these functions: responses are greater for task relevant compared with task irrelevant objects [9,11–13] and responses to stimuli that have been fixated during search are reduced [10]. In both cases, the responses to stimuli that are not the target are lower than the responses to the target, however, it is unclear whether these reductions are driven by the same mechanisms. Here, we hypothesize that the reduced response to task irrelevant distractors is a long-term feed-forward result of processing in earlier areas owing to the animals’ familiarity with the task and stimuli, but that the suppression of items that have been examined previously within the trial is a form of active top-down inhibitory tagging [14,15]. To differentiate between these mechanisms, we examined the local field potential (LFP) activity recorded from LIP while animals performed a visual foraging task. LFP activity is thought to represent both the input and local processing in but not the output of the recorded area [16,17]. Thus, if the two forms of suppression are owing to two separate mechanisms, they should be distinguishable within the LFP.
2. Material and methods
(a). Surgical preparation
Two male rhesus monkeys (9–12 kg) were implanted with head posts, scleral coils and recording cylinders during sterile surgery under general anaesthesia; animals were initially anaesthetized with ketamine and xylazine, and maintained with isofluorane (see [10] for details).
(b). Electrophysiological recording
The experiments were run using the REX system [18] and data were recorded using a Plexon MAP system with an 8-channel Omnetics 0.050 headstage (Plexon Inc., Dallas, TX, USA). Data were analysed using custom code written in Matlab (Mathworks Inc.) and Chronux [19]. Both animals were trained on a standard memory-guided saccade task and the foraging search task (figure 1). We recorded extracellular LFP activity from area LIP using tungsten microelectrodes guided by coordinates from MRI images taken both before and after cylinder implantation. The single-unit activity that was recorded along with LFP activity was reported previously in [20]. Recorded sites were considered to be in LIP if the single-unit activity showed visual, delay and/or peri-saccadic responses during the memory-guided saccade task [21]. After calculating the size and position of the response field for each single neuron (for details see [22]), the foraging task was run and neural data were recorded. Monkeys started each trial of the foraging task by fixating a spot for 450–700 ms. After which, the fixation point was extinguished and an array of potential targets (T) and distractors (+) was presented, with one where the fixation spot had been (figure 1). One of the Ts had a juice reward associated with it, such that if the monkey looked at it for 500 ms within 8 s after start of trial, he would get the reward. As in previous free-viewing visual search studies [23,24], the stimuli were arranged in such a fashion that when the monkey looked at one stimulus, the response field of the isolated LIP neuron usually encompassed another stimulus. The number of targets and distractors varied in each trial. In most sessions, data were recorded in blocks. In one block, the number of potential targets was always three, while the number of objects varied among three, five or seven. In the other block, the total number of objects was always 10, while the number of potential targets varied among three, five or seven. In the remaining sessions all six conditions were pseudorandomly interleaved. The results of the interleaved and blocked sessions were pooled because no clear differences were observed between the two. Because spiking activity in LIP varies greatly as a function of the number of items on the screen [13,20], only trials with less than seven objects were included in the analyses presented here. The same general results were present when all the data were used.
Figure 1.
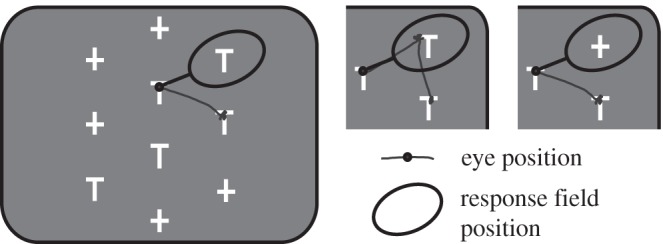
Stimulus array in the foraging task. In this example, there are five potential targets (T) and five distractors (+). One of the Ts has a reward associated with it (the target); the animal obtains the reward if it fixates the target for 500 ms. For the single-unit recordings, the array is aligned such that when looking at one stimulus (small circle), another stimulus is usually in the response field (large oval). In the main panel, a potential target is in the response field, in the middle panel, a previously fixated T is in the response field and in the right panel, a distractor is in the response field.
(c). Data analysis
LFP data were recorded from 25 LIP sites (eight from monkey E and 17 from monkey D) from which 69 single units were recorded simultaneously. Data were qualitatively similar in the two individual animals (see the electronic supplementary material, figure S1) and were pooled for the analyses we present here. We analysed the neural activity during fixations in which there was a single object inside the response field of the single unit being recorded. Each fixation was categorized based on the object inside the response field of the single unit (figure 1): a potential target, which had not been fixated; a T that had been visited before during the trial, which we refer to as a previously fixated T and a distractor, which had not been fixated. Data were aligned by the beginning of fixation, and we analysed the LFP spectrum during fixations. LFP signals were extracted from the extracellular recording on a single tungsten electrode by low-pass filtering at 250 Hz. The baseline period was taken from the 400 ms of fixation before the onset of the array of the objects. We used spectral analyses to characterize the temporal structure in the data. For most of the analyses presented here, the LFP spectrum was estimated in a 100 ms window with 10 Hz resolution using three Slepian data tapers. To confirm the power in the lower bands, we used a 500 ms window with 4 Hz resolution and three data tapers. When averaging across cells, power was normalized per trial by dividing the power trace per frequency by the average power for this frequency in the baseline period. To exclude trials containing possible artefacts in the LFP recordings, maximum and minimum values of the continuous LFP signal and of the time–frequency spectrum were calculated per trial, and trials with minimum signal values below the 5th percentile or maximum values above the 95th percentile were removed. We analysed the LFP power in standard frequency bands: alpha (8–12 Hz); low beta (12–18 Hz); high beta (18–25 Hz) and gamma (25–160 Hz).
3. Results
(a). Single unit data
Consistent with our previous findings [10], the single-unit activity was greatest for potential targets, lower for previously fixated Ts and lowest for distractors. Figure 2 shows the population average response in 20 ms bins for fixations in which a potential target was in the response field (red trace), in which a distractor was in the response field (green trace) and in which a previously fixated T was in the response field (blue trace). Almost immediately following the start of fixation, the response to the distractor was weaker than the response to the Ts, but the response to the previously fixated T did not separate from the response to potential targets until approximately 60 after fixation began. To directly compare with our previous results, we examined the mean spike rate 150–500 ms after fixation onset and found a significant difference among the three conditions (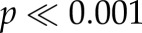 , ANOVA), with all three conditions showing significant post hoc differences in their means.
, ANOVA), with all three conditions showing significant post hoc differences in their means.
Figure 2.
Averaged post-stimulus time histograms (20 ms non-overlapping bins) from all 69 single units collected from both monkeys performing the visual foraging task. Error bars represent the standard error of the mean (s.e.m.). Time is aligned by fixation onset, which represents the completion of the previous saccade. Red denotes target; blue denotes fixated T and green denotes distractor.
(b). Local field potential data
The foraging task in this study allows the animals to make free eye movements to multiple objects on the screen. As a combination of these two conditions has not been studied previously using LFPs, we will first replicate the effects of goal-directed eye movements and task relevance on the LFP separately. Then we will examine the results during ongoing search in the foraging task.
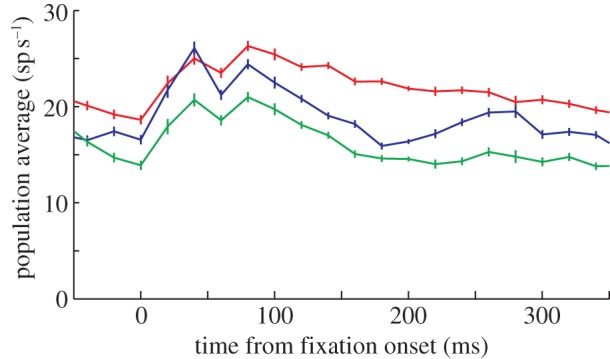
Local field potentials during the memory-guided saccade task were consistent with those seen previously [25,26]. Figure 3 shows the spectrograms averaged across recorded sites during the memory-guided saccade task. The strongest temporal representation of saccades towards the response field was observed in the gamma band. In the population, there was a significant increase in power of all frequency bands (0–200 ms after target presentation) when monkeys made an eye movement towards the centre of the single-unit's response field compared with the other locations (p < 0.001; paired t-tests). A significant difference in the gamma band (p < 0.0125, t-test, Bonferroni-adjusted for the number of bands tested) was seen in 69% of the recording sites. The obvious increase in the alpha and low beta bands when the target was in the single-unit's response field was primarily driven by small but non-significant power in many neurons; significant differences in these bands (p < 0.0125) were only seen in 5% of sites. Each of these results is consistent with previous findings in LIP [25].
Figure 3.
Averaged normalized LFP spectrograms across all recordings from both monkeys performing a memory-guided saccade task. Time–frequency plots of the spectrogram from trials in which the target appeared in the response field of the single unit being recorded (a) or in which the target appeared out of the response field (b). Power is colour-coded on a log scale.
To study whether the LFP activity varied as a function of stimulus identity and, thus, task relevance, we examined only the first fixation of each trial of the foraging task. During this time, the monkey was looking at the fixation point and the array of potential targets and distractors appeared on the screen (figure 4). For this analysis, only the LFP responses before the eye movements were included, thus only a potential target or a distractor could appear in the response field. Compared with baseline, we found a clear increase in power in the population in all four bands and in both conditions in the first 150 ms after the onset of the array (p < 0.011, paired t-tests, Bonferroni-adjusted for the number of bands tested). This increase in LFP power in response to stimulus presentation lasted for both objects until later times in most frequency bands (p < 0.001, 150–300 ms after array onset, paired t-tests) except for the low beta band in both conditions (target: p = 0.057, distractor: p = 0.071). This robust increase becomes obvious in the spectrograms around 50 ms in the alpha, beta and mid- to upper gamma bands (figure 4). But unlike the single-unit population response, which showed a clear differentiation between potential targets and distractors within 86 ms of array onset [27], we found no significant differences between the conditions in any of the frequency bands in the population during the first 150 ms (p > 0.036; paired t-tests, n.s. with Bonferroni correction). Although we found trends towards significance in the alpha (p = 0.054), low beta (p = 0.0362) and high beta bands (p = 0.066), there was no such trend in the gamma band (p = 0.88). Only after 150 ms, a clear significant difference was seen and that too only in the gamma band (p = 0.003; 150–300 ms, paired t-test); no differences were seen in the remaining three bands (p > 0.73). These data suggest that the single-unit responses to potential targets and distractors are being driven by similar mechanisms.
Figure 4.
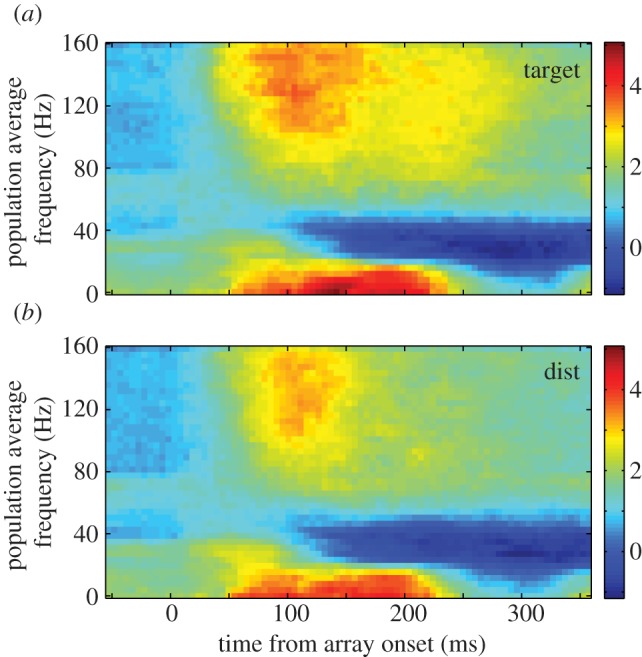
Averaged normalized LFP spectrograms across all recordings from both monkeys during initial fixation and array onset in the foraging task. Time–frequency plots of the spectrogram from trials in which a potential target (a) appeared in the response field of the single unit being recorded or in which a distractor (b) appeared in the response field. Power is colour-coded on a log scale.
During ongoing search, we found the same trend of sustained temporal structure in response to potential targets and distractors at single sites and across the population. We define ongoing search as the time in the trial after the first saccade and before the reward is given, so it represents the time during which the animal continues to forage for the target. In the population (figure 5), there was greater power in all three conditions during ongoing search than baseline in the alpha, low beta and gamma bands (p < 0.001, ANOVA; p < 0.05 HSD post hoc comparisons). Power in the high beta band was not significantly different during the first 200 ms compared with baseline (p = 0.1, ANOVA). These differences were maintained later in the fixation period (200–400 ms) in the gamma band and both beta bands (p < 0.001, ANOVA; p < 0.05 HSD post hoc comparisons) but not in the alpha band (p = 0.14, ANOVA). Consistent with this, a majority of sites showed a significant difference (p < 0.05, paired tests) in power in all four frequency bands compared with baseline in the first 200 ms (table 1). These proportions remained similar in the following 200 ms (table 1) with two key exceptions. First, substantially more sites showed a significant difference in power in the high beta band during the second 200 ms when a distractor or previously fixated T was in the single-unit's response field. Second, the proportion of sites that showed a strong difference between power in the alpha band in response to a previously fixated T and baseline more than halved in the second 200 ms compared with the first 200 ms; a similar, but lesser, decrease was also seen in the low beta band. This drastic reduction shows that the strong power in the alpha and low beta bands seen in the previously fixated T condition in figure 5 was present in many single sites. Note that because we did not use any corrections for multiple comparisons, the data presented in table 1 may be an overestimate of the proportion of the number of significant sites, however this overestimate is present in all conditions, and does not influence the changes in proportions of sites, such as those seen in the alpha and beta bands for the previously fixated T.
Figure 5.
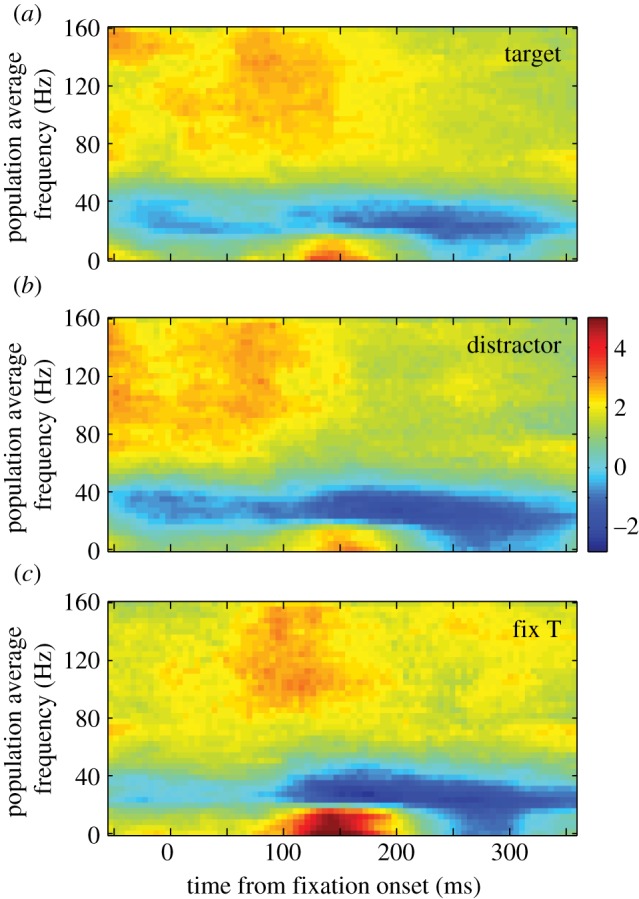
Averaged normalized LFP spectrograms across all recordings from both monkeys during ongoing search in the foraging task. Time–frequency plots of the spectrogram from fixations in which a potential target (a) was brought into the response field of the single unit being recorded, in which a distractor (b) was brought into the response field or in which a previously fixated T (fix T (c)) was brought into the response field. Power is colour coded on a log scale. Time is aligned by fixation onset, which represents the completion of the previous saccade.
Table 1.
Proportion of sites exhibiting a significant difference (p < 0.05, paired t-tests) between conditions during two different epochs: 0–200 ms and 200–400 ms after fixation onset. T, target; D, distractor; Fix T, fixated T.
| 0–200 ms |
200–400 ms |
|||||||
|---|---|---|---|---|---|---|---|---|
| alpha (%) | low beta (%) | high beta (%) | gamma (%) | alpha (%) | low beta (%) | high beta (%) | gamma (%) | |
| T versus baseline | 76 | 68 | 72 | 100 | 72 | 68 | 72 | 84 |
| D versus baseline | 56 | 36 | 52 | 92 | 52 | 48 | 88 | 84 |
| Fix T versus baseline | 79 | 67 | 37 | 88 | 33 | 46 | 71 | 70 |
An examination of the spectrograms in figure 5 shows that there were very few differences in power among the conditions. At the population level, there were no significant differences in the alpha, low beta and gamma bands between potential targets and distractors in the first 200 ms (p > 0.05, HSD post hoc comparisons) and only a small, but subtle, difference in the high beta band (p < 0.05). There were also no significant differences in the high beta and gamma bands between the previously fixated Ts and the other two stimulus classes in the first 200 ms (p > 0.05). However, there was a clear and strong increase in the alpha and low beta bands when a previously fixated T was in the response field during this period (p < 0.05). This is seen as the red patch between 100 and 200 ms in figure 5c. When we examine power traces for each band independently (see figure 6 and electronic supplementary material, figure S1), it is clear that the difference in power between a previously fixated T (blue traces) and the other two conditions started around the time that fixation began in both the alpha and low beta bands and peaked around 150 ms later. As such, we found the differences in power between the previously fixated T and potential targets or distractors to be significant in these two bands during the first 200 ms of fixation (p < 0.05, HSD post hoc comparisons). The small, early difference seen in the high beta band was not enough to produce a significant difference (p > 0.05). To confirm that the increase in power in the alpha and low beta bands were real and not an artefact of using a short (100 ms) window, we re-analysed the data in all three conditions using a 500 ms window. We found that the difference remained highly significant in both the alpha and low beta bands (p = 0.001, ANOVA). These data suggest that a unique neural mechanism may be involved in response to a T that has been fixated previously within the trial.
Figure 6.
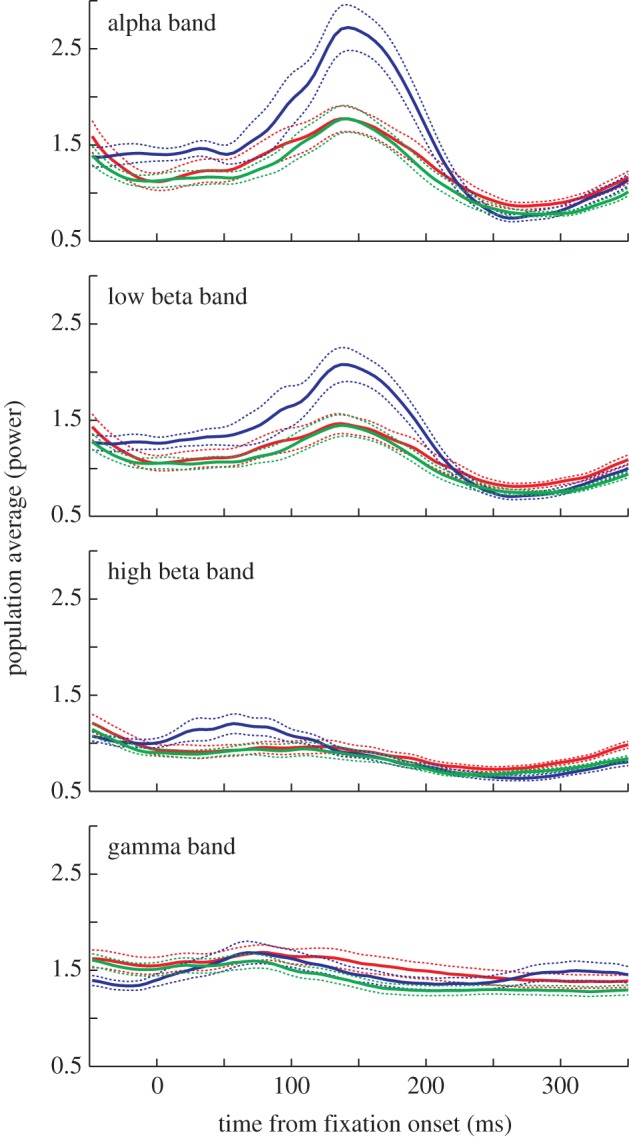
Averaged normalized LFP power traces across all recordings from both monkeys during ongoing search in the foraging task. Power traces in response to a potential target (red), a distractor (green) or a previously fixated T (Fix T; blue) being brought into the response field by a saccade are plotted across four frequency bands: (a) alpha (8–12 Hz); (b) low beta (12–18 Hz); (c) high beta (18–25 Hz) and (d) gamma (25–160 Hz). Dotted lines show the s.e.m., representing the variance across recording sites.
4. Discussion
In this study, we examined single-unit activity and LFPs recorded from LIP while monkeys were performing a visual foraging task. Consistent with previous studies, we found that single-unit responses to distractors and previously fixated potential targets were reduced compared with the responses to potential targets that had not been examined [9,10,12,13]. However, the LFPs recorded during search showed a different pattern: while power in the gamma band was similar across all three conditions, activity when the previously fixated T was in the single-unit's response field was unique in the alpha and low beta bands, showing significantly stronger power. We suggest that this enhanced power represents top-down inhibitory feedback, which we will refer to as inhibitory tagging [14,28].
Our interpretation is consistent with the broader view of lower band functions in attention. Here, we view our low beta band as being part of these lower bands; only part of our high beta overlaps with the ‘middle’ frequencies, which usually range from approximately 20–35 Hz, thought to be involved in top-down enhancement [29]. In visual cortex, focusing attention leads to a concurrent suppression of low-frequency synchrony [30], and increased alpha power has been related to the behavioural inhibition of unattended stimuli [31–33] or the disengagement of spatial attention [34]. If LIP acts as a priority map, which guides the allocation of attention, then a reduction in activity in LIP will result in a reduced probability of attending that location [35]. Also consistent is the finding that low current microstimulation of the frontal eye field (FEF) increases alpha power in LIP but only under behavioural conditions in which a distractor is in the LIP response field [33]. It is important to note that FEF has neurons that actively respond when animals suppress saccades to objects within their response fields [36]. Thus, these ‘don't look’ neurons could be the source of the signal driving the suppression in LIP. Indeed, we have found a distinct subpopulation of FEF neurons that respond more to previously fixated Ts than to targets or distractors in monkey E (K. Mirpour and J. W. Bisley 2013, unpublished data).
Our data are unable to directly address the question of whether the increased synchrony in the lower bands caused the reduction in spiking activity in response to a previously fixated T, however, there are several facts that strongly support the hypothesis. First, transcranial magnetic stimulation over human parietal cortex at 10 Hz, which is within our alpha band, impairs visual detection within the contralateral visual field but enhances it in the ipsilateral visual field [37]. This suggests that there is a causal link between synchrony in the alpha band and a suppression of attention in the affected visual field. Second, within our data, we found no evidence that the greater activity to potential targets was actively driven, suggesting that the difference in response between a potential target and a previously fixated T must be owing to suppression of the previously fixated T response. When we examined the LFP for the potential target, we found no unique LFP signature, even when comparing the potential target with the distractor, which had a much lower spike rate. This suggests that the only way to create a lower spiking response to a previously fixated T, which is physically identical to the potential target and, thus, should have the same feed-forward connections, would be by a suppressive mechanism. Because the tagging of fixated objects must be active and ongoing, a hard-wired feed-forward process would be inappropriate. Third, the timing of the burst in power slightly leads the time during which we saw reductions in spiking responses to a previously fixated T. An examination of the data shows that the increased synchrony began in the alpha and beta bands around the onset of fixation and peaked around 150 ms later (figure 6), whereas the single-unit activity differentiated between a potential target and previously fixated T within 60 ms after the onset of fixation and peaked around 150 ms later (figure 2). Thus, the timing is consistent with the idea that the increase in synchronization in the lower bands represents a local mechanism that could inhibit the response to a previously fixated T.
Although the strongest difference in spike rate was between the potential target and the distractor, we found few differences in LFP power between these conditions. These differences were primarily seen in the higher gamma bands shortly after array onset and are not too surprising given that these higher bands tend to most closely resemble the spiking output in LIP [25,26]. We found no obvious differences between these conditions during ongoing visual search, when a clear significant difference in spiking activity was seen. During this period, there was a clear increase in power compared with baseline in both conditions. Thus, the lack of difference between the conditions suggests that the mechanism generating the spikes may be similar. It has previously been shown that with extensive training, animals can learn to ignore task irrelevant distractors, even if they are highly salient [38], yet this training does not lead to an excessive over representation of neurons tuned for these stimuli in earlier visual areas [39]. In our task, the animals had performed many tens of thousands of trials with the same target and distractor stimuli and had never been rewarded for fixating a distractor. We suggest that with over-training, the weight given to the feed-forward signal representing distractors is reduced leading to a feed-forward global reduction in priority, represented in the spike rate in LIP [5].
Our results and interpretations fit well into most models of visual attention. We have suggested that the increase in low band synchrony represents a top-down suppression, which we refer to as inhibitory tagging, but which is often referred to as inhibition of return [28,40]. This is a mechanism explicitly used in the saliency map models of Koch and co-workers [41–43], thus our results fit perfectly with their model. Because LIP is thought to be involved in allocating visual attention, modulation of LIP responses biases the way that attention or attentional weights will be distributed. Thus, our findings also fit into many other models of attention. For example, within the normalization model of attention [44], the responses in LIP would be part of the network creating the attentional field, so reducing the responses to distractors and previously fixated Ts would reduce their gain, thus reducing the effect that attention would have on the responses in earlier visual areas. And within the neural theory of visual attention (NTVA) model [45,46], the responses in LIP would represent the priority map, which is used to compute the attentional weights. Thus, top-down suppression of responses to previously fixated Ts would reduce the attentional weights associated with those stimuli.
Our data may be an underestimate of the synchronization power in this task. Previous studies of LFPs in LIP have used the presentation of no more than four stimuli positioned around a central fixation point [25,26,33,47,48]. In this study, we analysed data from trials in which we varied the number of stimuli on the screen from three to seven and had varying array arrangements with substantially more crowding. Because LFPs are thought to represent the input and local processing across a broader area of cortex than the single-unit activity [49,50], it is likely that the variance and crowding in our stimulus set reduces our ability to identify changes in power. Indeed, while the LFPs cover an area with similar response fields to those we mapped for the single-unit recording, it is likely that they are far broader and will be impacted by the identity of stimuli close to single-unit's response field. Given this, it is possible that under more constrained conditions, more differences in power could be illuminated. So it is possible that the difference in the feed-forward weights for potential targets and distractors may be present, but is too weak to be clearly identified under these conditions.
Acknowledgements
We thank the members of the UCLA DLAM for their superb animal care.
All experiments and protocols were approved by the Chancellor's Animal Research Committee at UCLA as complying with the guidelines established in the Public Health Service Guide for the Care and Use of Laboratory Animals.
Funding statement
This work was supported by a McKnight Scholar Award and the National Eye Institute (R01 EY019273-01).
References
- 1.Corbetta M, Patel G, Shulman GL. 2008. The reorienting system of the human brain: from environment to theory of mind. Neuron 58, 306–324 (doi:10.1016/j.neuron.2008.04.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bisley JW, Goldberg ME. 2010. Attention, intention, and priority in the parietal lobe. Annu. Rev. Neurosci. 33, 1–21 (doi:10.1146/annurev-neuro-060909-152823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noudoost B, Chang MH, Steinmetz NA, Moore T. 2010. Top-down control of visual attention. Curr. Opin. Neurobiol. 20, 183–190 (doi:10.1016/j.conb.2010.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krauzlis R, Lovejoy LP, Zenon A. 2013. Superior colliculus and visual spatial attention. Annu. Rev. Neurosci. 36, 165–182 (doi:10.1146/annurev-neuro-062012-170249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ipata AE, Gee AL, Bisley JW, Goldberg ME. 2009. Neurons in the lateral intraparietal area create a priority map by the combination of disparate signals. Exp. Brain Res. 192, 479–488 (doi:10.1007/s00221-008-1557-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bisley JW. 2011. The neural basis of visual attention. J. Physiol. 589, 49–57 (doi:10.1113/jphysiol.2010.192666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McPeek RM, Keller EL. 2002. Saccade target selection in the superior colliculus during a visual search task. J. Neurophysiol. 88, 2019–2034 [DOI] [PubMed] [Google Scholar]
- 8.Sato TR, Watanabe K, Thompson KG, Schall JD. 2003. Effect of target–distractor similarity on FEF visual selection in the absence of the target. Exp. Brain Res. 151, 356–363 (doi:10.1007/s00221-003-1461-1) [DOI] [PubMed] [Google Scholar]
- 9.Thomas NW, Pare M. 2007. Temporal processing of saccade targets in parietal cortex area LIP during visual search. J. Neurophysiol. 97, 942–947 (doi:10.1152/jn.00413.2006) [DOI] [PubMed] [Google Scholar]
- 10.Mirpour K, Arcizet F, Ong WS, Bisley JW. 2009. Been there, seen that: a neural mechanism for performing efficient visual search. J. Neurophysiol. 102, 3481–3491 (doi:10.1152/jn.00688.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ipata AE, Gee AL, Goldberg ME, Bisley JW. 2006. Activity in the lateral intraparietal area predicts the goal and latency of saccades in a free-viewing visual search task. J. Neurosci. 26, 3656–3661 (doi:10.1523/JNEUROSCI.5074-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oristaglio J, Schneider DM, Balan PF, Gottlieb J. 2006. Integration of visuospatial and effector information during symbolically cued limb movements in monkey lateral intraparietal area. J. Neurosci. 26, 8310–8319 (doi:10.1523/JNEUROSCI.1779-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balan PF, Oristaglio J, Schneider DM, Gottlieb J. 2008. Neuronal correlates of the set-size effect in monkey lateral intraparietal area. PLoS Biol. 6, e158 (doi:10.1371/journal.pbio.0060158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein R. 1988. Inhibitory tagging system facilitates visual search. Nature 334, 430–431 (doi:10.1038/334430a0) [DOI] [PubMed] [Google Scholar]
- 15.Shariat Torbaghan S, Yazdi D, Mirpour K, Bisley JW. 2012. Inhibition of return in a visual foraging task in non-human subjects. Vis. Res. 74, 2–9 (doi:10.1016/j.visres.2012.03.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Logothetis NK. 2003. The underpinnings of the BOLD functional magnetic resonance imaging signal. J. Neurosci. 23, 3963–3971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buzsaki G, Anastassiou CA, Koch C. 2012. The origin of extracellular fields and currents: EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 13, 407–420 (doi:10.1038/nrn3241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hays AV, Richmond BJ, Optican LM. 1982. A UNIX-based multiple process system for real-time data acquisition and control. WESCON Conf. Proc. 2, 1–10 [Google Scholar]
- 19.Bokil H, Andrews P, Kulkarni JE, Mehta S, Mitra PP. 2010. Chronux: a platform for analyzing neural signals. J. Neurosci. Methods 192, 146–151 (doi:10.1016/j.jneumeth.2010.06.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirpour K, Bisley JW. 2012. Dissociating activity in the lateral intraparietal area from value using a visual foraging task. Proc. Natl Acad. Sci. USA 109, 10 083–10 088 (doi:10.1073/pnas.1120763109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA. 1991. Saccade-related activity in the lateral intraparietal area. I. Temporal properties; comparison with area 7a. J. Neurophysiol. 66, 1095–1108 [DOI] [PubMed] [Google Scholar]
- 22.Mirpour K, Ong WS, Bisley JW. 2010. Microstimulation of posterior parietal cortex biases the selection of eye movement goals during search. J. Neurophysiol. 104, 3021–3028 (doi:10.1152/jn.00397.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazer JA, Gallant JL. 2003. Goal-related activity in V4 during free viewing visual search. Evidence for a ventral stream visual salience map. Neuron 40, 1241–1250 (doi:10.1016/S0896-6273(03)00764-5) [DOI] [PubMed] [Google Scholar]
- 24.Bichot NP, Rossi AF, Desimone R. 2005. Parallel and serial neural mechanisms for visual search in macaque area V4. Science 308, 529–534 (doi:10.1126/science.1109676) [DOI] [PubMed] [Google Scholar]
- 25.Pesaran B, Pezaris JS, Sahani M, Mitra PP, Andersen RA. 2002. Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nat. Neurosci. 5, 805–811 (doi:10.1038/nn890) [DOI] [PubMed] [Google Scholar]
- 26.Premereur E, Vanduffel W, Janssen P. 2012. Local field potential activity associated with temporal expectations in the macaque lateral intraparietal area. J. Cogn. Neurosci. 24, 1314–1330 (doi:10.1162/jocn_a_00221) [DOI] [PubMed] [Google Scholar]
- 27.Mirpour K, Bisley JW. 2012. Anticipatory remapping of attentional priority across the entire visual field. J. Neurosci. 32, 16 449–16 457 (doi:10.1523/JNEUROSCI.2008-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein RM. 2000. Inhibition of return. Trends Cogn. Sci. 4, 138–147 (doi:10.1016/S1364-6613(00)01452-2) [DOI] [PubMed] [Google Scholar]
- 29.Buschman TJ, Miller EK. 2007. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 315, 1860–1862 (doi:10.1126/science.1138071) [DOI] [PubMed] [Google Scholar]
- 30.Fries P, Reynolds JH, Rorie AE, Desimone R. 2001. Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291, 1560–1563 (doi:10.1126/science.1055465) [DOI] [PubMed] [Google Scholar]
- 31.Klimesch W, Sauseng P, Hanslmayr S. 2007. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res. Rev. 53, 63–88 (doi:10.1016/j.brainresrev.2006.06.003) [DOI] [PubMed] [Google Scholar]
- 32.Haegens S, Handel BF, Jensen O. 2011. Top-down controlled alpha band activity in somatosensory areas determines behavioral performance in a discrimination task. J. Neurosci. 31, 5197–5204 (doi:10.1523/JNEUROSCI.5199-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Premereur E, Vanduffel W, Roelfsema PR, Janssen P. 2012. Frontal eye field microstimulation induces task-dependent gamma oscillations in the lateral intraparietal area. J. Neurophysiol. 108, 1392–1402 (doi:10.1152/jn.00323.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thut G, Nietzel A, Brandt SA, Pascual-Leone A. 2006. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J. Neurosci. 26, 9494–9502 (doi:10.1523/JNEUROSCI.0875-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bisley JW, Goldberg ME. 2003. Neuronal activity in the lateral intraparietal area and spatial attention. Science 299, 81–86 (doi:10.1126/science.1077395) [DOI] [PubMed] [Google Scholar]
- 36.Hasegawa RP, Peterson BW, Goldberg ME. 2004. Prefrontal neurons coding suppression of specific saccades. Neuron 43, 415–425 (doi:10.1016/j.neuron.2004.07.013) [DOI] [PubMed] [Google Scholar]
- 37.Romei V, Gross J, Thut G. 2010. On the role of prestimulus alpha rhythms over occipito-parietal areas in visual input regulation: correlation or causation? J. Neurosci. 30, 8692–8697 (doi:10.1523/JNEUROSCI.0160-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ipata AE, Gee AL, Gottlieb J, Bisley JW, Goldberg ME. 2006. LIP responses to a popout stimulus are reduced if it is overtly ignored. Nat. Neurosci. 9, 1071–1076 (doi:10.1038/nn1734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ipata AE, Gee AL, Goldberg ME. 2012. Feature attention evokes task-specific pattern selectivity in V4 neurons. Proc. Natl Acad. Sci. USA 109, 16 778–16 785 (doi:10.1073/pnas.1215402109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Klein RM. 2010. Searching for inhibition of return in visual search: a review. Vis. Res. 50, 220–228 (doi:10.1016/j.visres.2009.11.013) [DOI] [PubMed] [Google Scholar]
- 41.Itti L, Koch C, Niebur E. 1998. A model of saliency-based visual attention for rapid scene analysis. IEEE Trans. Pattern Anal. Mach. Intell. 20, 1254–1259 (doi:10.1109/34.730558) [Google Scholar]
- 42.Koch C, Ullman S. 1985. Shifts in selective visual attention: towards the underlying neural circuitry. Hum. Neurobiol. 4, 219–227 [PubMed] [Google Scholar]
- 43.Walther D, Koch C. 2006. Modeling attention to salient proto-objects. Neural Netw. 19, 1395–1407 (doi:10.1016/j.neunet.2006.10.001) [DOI] [PubMed] [Google Scholar]
- 44.Reynolds JH, Heeger DJ. 2009. The normalization model of attention. Neuron 61, 168–185 (doi:10.1016/j.neuron.2009.01.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bundesen C, Habekost T, Kyllingsbaek S. 2005. A neural theory of visual attention: bridging cognition and neurophysiology. Psychol. Rev. 112, 291–328 (doi:10.1037/0033-295X.112.2.291) [DOI] [PubMed] [Google Scholar]
- 46.Bundesen C, Habekost T, Kyllingsbaek S. 2011. A neural theory of visual attention and short-term memory (NTVA). Neuropsychologia 49, 1446–1457 (doi:10.1016/j.neuropsychologia.2010.12.006) [DOI] [PubMed] [Google Scholar]
- 47.Buneo CA, Jarvis MR, Batista AP, Andersen RA. 2003. Properties of spike train spectra in two parietal reach areas. Exp. Brain Res. 153, 134–139 (doi:10.1007/s00221-003-1586-2) [DOI] [PubMed] [Google Scholar]
- 48.Hagan MA, Dean HL, Pesaran B. 2012. Spike-field activity in parietal area LIP during coordinated reach and saccade movements. J. Neurophysiol. 107, 1275–1290 (doi:10.1152/jn.00867.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitzdorf U. 1985. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol. Rev. 65, 37–100 [DOI] [PubMed] [Google Scholar]
- 50.Kajikawa Y, Schroeder CE. 2011. How local is the local field potential? Neuron. 72, 847–858 (doi:10.1016/j.neuron.2011.09.029) [DOI] [PMC free article] [PubMed] [Google Scholar]


