Abstract
Background
Strategies to improve pulmonary endothelial barrier function are needed to reverse the devastating effects of vascular leak in acute respiratory distress syndrome (ARDS). FTY720 is a pharmaceutical analogue of the potent barrier-enhancing phospholipid, sphingosine 1-phosphate (S1P). FTY720 decreases vascular permeability through an incompletely characterized mechanism that differs from S1P. Here we describe its barrier-promoting effects on intracellular signaling and junctional assembly formation in human pulmonary endothelium.
Methods
Permeability of cultured human pulmonary endothelial cells was assessed by transendothelial electrical resistance (TER) and dextran transwell assays. Junctional complex formation was assessed by membrane fractionation and immunofluorescence. Pharmacologic inhibitors and siRNA were utilized to determine the effects of individual components on permeability.
Results
Unlike S1P, FTY720 failed to induce membrane translocation of adherens junction or tight junction proteins. β-catenin, occludin, claudin-5, or ZO-1/ZO-2 siRNAs did not alter FTY720-induced barrier enhancement. FTY720 induced FAK phosphorylation and focal adhesion formation with FAK siRNA partially attenuating the prolonged phase of barrier enhancement. Inhibition of Src, PKA, PKG, PKC, or PP2A failed to alter FTY720-induced barrier enhancement. FTY720 increased c-Abl tyrosine kinase activity, and c-Abl siRNA attenuated peak barrier enhancement after FTY720.
Conclusion
FTY720 enhances endothelial barrier function by a novel pathway involving c-Abl signaling.
Keywords: c-Abl tyrosine kinase, vascular endothelium, FTY720, junctional complexes, vascular permeability
Introduction
In the lung, endothelial cells (EC) serve as a semi-permeable barrier between vascular contents and the pulmonary airspaces and play a critical role in regulating tissue fluid homeostasis and the inflammatory response. Pulmonary endothelial permeability is primarily determined by a paracellular pathway that is regulated by a balance between intracellular contractile forces and adhesive cell-cell and cell-matrix forces [1, 2]. A significant and sustained increase in vascular permeability is a hallmark of acute inflammatory processes such as acute lung injury/acute respiratory distress syndrome (ALI/ARDS) and is associated with significant mortality. Recent data indicate that there are more than 150,000 ALI cases per year in the United States with a mortality rate of ∼35–40% [3]. Effective therapies for preserving or reconstituting the endothelial barrier are lacking despite their theoretic potential for ameliorating the vascular leak that characterizes ALI.
Sphingosine 1-phosphate (S1P), a ubiquitous bioactive sphingolipid, has been identified as a robust barrier-enhancing agent [4]. Treatment of EC monolayers with S1P in vitro causes a rapid and sustained improvement in barrier function in a dose-dependent manner as measured by transendothelial electrical resistance (TER). S1P infusion significantly attenuates lipopolysaccharide (LPS)-induced lung edema and inflammation in murine and canine models of sepsis and ALI [5, 6]. Recent studies have identified transactivation of the S1P1 receptor by other barrier-enhancing agents as a common mechanism for improving endothelial barrier function [7]. Through activation of this Gi-protein coupled S1P1 receptor, S1P induces Rac-dependent peripheral translocation and colocalization of cortactin and non-muscle myosin light chain kinase (nmMLCK), myosin light chain phosphorylation, and cortical actin ring formation to produce improved barrier function [4, 8]. S1P also stimulates tyrosine phosphorylation of FAK at a specific site (Y576) and subsequently causes focal adhesion (FA) formation and redistribution to the cell periphery, which likely contributes to improved barrier function [9, 10]. In addition, S1P may enhance barrier function by facilitating adherens junction (AJ) and tight junction (TJ) assembly [11–13].
FTY720, a structural analogue of sphingosine and S1P [14], is a promising treatment for multiple sclerosis that has been evaluated in recent phase III clinical trials [15, 16]. Like S1P, FTY720 significantly decreases LPS-induced pulmonary leak and inflammation in a mouse model of ALI [5]. We previously reported that FTY720 induced significant but delayed human lung endothelial barrier enhancement in vitro compared to the S1P response [17]. Unlike S1P, FTY720 did not induce MLC phosphorylation and subsequent cortical actin formation. Moreover, reduced expression of cytoskeletal effectors critical for S1P-induced TER elevation, Rac1 and cortactin, did not inhibit FTY720-induced TER elevation. In this prior study, reduction of S1P1 expression attenuated S1P-but not FTY720- induced TER elevations [17]. These results suggest a novel mechanism for FTY720-induced barrier enhancement which remains to be elucidated. There are two important reasons for studying in detail the effects of FTY720 on pulmonary EC barrier function. Unlike S1P, FTY720 has been evaluated in multiple clinical trials as therapy for multiple sclerosis and transplant rejection and soon may be widely available for clinical use. Thus, it has the potential for much more rapid translation into the ICU than S1P as a possible therapy for ALI/ARDS. Secondly, improved understanding of the poorly characterized mechanism responsible for barrier enhancement by FTY720 may identify novel potential targets for the development of ALI therapies. In the current study, we further characterize the barrier promoting effects of FTY720 on intracellular signaling and junctional assembly formation in lung endothelium and provide additional insights into barrier-regulatory pathways.
Materials and methods
Reagents
Unless otherwise specified, reagents were obtained from Sigma (St. Louis, MO). S1P (Biomol, Plymouth Meeting, PA) was dissolved in 4 mg/ml fatty acid free BSA. FTY720 was kindly provided by Novartis. Antibodies: anti-VE-cadherin, anti-β-Catenin, anti-ZO-2, anti-pan FAK, anti-P120, anti-paxillin (Santa Cruz Biotechnology, Santa Cruz, CA), anti-occludin, anti-claudin-5 (Zymed, South San Francisco, CA), anti-vinculin (Sigma), anti-phosphotyrosine 4G10 (Upstate Biotechnology, Lake Placid, NY), anti-phospho-FAK (Y397), anti-ZO-1, anti-c-Abl (8E9) (BD Pharmingen, San Diego, CA), anti-phospho-FAK (Y576) (Cell Signaling Technology, Danvers, MA). Pharmacological inhibitors for Src, PKA, PKC, PKG and PP2A: PP2, Dihydrochloride, 4-cyano-3-methylisoquinoline, Go6983, Ro-32-0432, Go 6850, calphostin c, okadaic acid (EMD Chemicals, Gibbstown, NJ).
Cell culture
Human pulmonary artery endothelial cells (HPAEC) and pulmonary microvascular cells (HLMVEC) (Lonza, Walkersville, MD) were cultured in EBM-2 complete medium (Lonza) with 10% FBS at 37°C in a humidified incubator with 5% CO2 as previously described [4]. Passages 6–9 were used for experimentation.
Immunofluorescence
Confluent EC grown on 35 mm glass-bottom petridishes (MatTek, MA) were treated with various conditions as described for individual experiments. EC were then fixed in 4% paraformaldehyde for 20 min, permeabilized with 0.1% Triton X-100 for 2 min, washed in PBS, blocked with 2% BSA in PBS for 1 h, and incubated with primary antibodies overnight at 4°C. After washing with PBS, EC were incubated with donkey anti–mouse or donkey anti-rabbit IgG antibody conjugated to Alexa Fluor 488 or 568 for 1 h at room temperature. Washed cells were then analyzed using a Nikon Eclipse TE 300 microscope and Sony Digital Photo camera DKC 5000.
Small interference RNA (siRNA) transfection
Negative control siRNA #2 (D-001810-02) and specific siRNAs (except claudin-5) were purchased from Dharmacon. Catalog numbers: si-β-catenin (L-003482-00), si-occludin (L-011345-00), si-ZO-1 (L-007746-00), si-ZO-2 (L-009932-00), si-c-Abl (L-003100-00). siRNA sequence targeting FAK: sense, GCGAUUAUAUGUUAGAGAUAGUU, antisense, CUAUCUCUAACAUAU-AAUCGCUU. si-claudin-5 was purchased from Ambion (Austin, TX): sense, GGCUAAGAAUCUGCUUAGUtt, antisense, ACUAAGCAGAUUCUUAGCCtt. EC (70% confluent) were transfected with 100 nM siRNA using DharmaFECT 1 transfection reagent per manufacturer’s protocol. Silencing efficacy (checked by Western blot) and subsequent experimentation were performed 72 h after siRNA transfection.
Transendothelial monolayer electrical resistance (TER)
EC were trypsinized and seeded in polycarbonate wells containing evaporated gold microelectrodes in EBM-2 with 2% FBS. After 24 h, cells were growth to confluence and TER measurements were performed using an electrical cell-substrate impedance sensing system (ECIS) (Applied Biophysics, Troy, NY) as previously described [4]. TER values from each microelectrode were pooled at discrete time points and plotted vs. time as the mean ± S.E.M. Baseline TER values for HPAEC and HLMVEC used in these studies were 1000–1400 ohms prior to agonist stimulation.
Cell fractionation
Cell fractionation was performed as described previously [18]. EC were solubilized in cytoskeleton stabilizing buffer (CSK) (50 mM NaCl, 10 mM PIPES, pH 6.8, 3 mM MgCl2, 0.5% Triton X-100, 300 mM sucrose, protease inhibitor cocktail I) for 20 min at 4 °C. The cells were washed once with CSK buffer and scraped with a rubber policeman. The cell pellet was lysed by SDS buffer (15 mM Tris, pH 7.5, 5 mM EDTA, 2.5 mM EGTA, 1% SDS) and incubated at 100°C for 10 min. After centrifugation at 14,000 g for 10 min, the supernatants were collected as Triton X-100 insoluble fraction and analyzed by Western blot.
In vitro c-Abl kinase assay
After treatment, 700 µg of cell lysates (in RIPA buffer) were immunoprecipitated with anti-c-Abl (8E9) antibody and protein G for 2 h. The beads were washed with lysis buffer once and with washing buffer (20 mM Tris-HCl, 7.2, 25 mM MgCl2, 5mM MnCl2, 0.4 mM EGTA) 3 times. 20 µl kinase reaction buffer (4 µl Src kinase reaction buffer (#20–121, Millipore), 2.5 µl Crk-GST (2 µg/µl, #14–468, Millipore), 1 µl ATP (1 mM), 12.5 µl H2O) were added to each tube. The reaction mixture was kept in 30°C for 20 min while shaking. Tyrosine phosphorylation of Crk-GST was detected by Western blotting with 4G10 antibody (Millipore).
Western blotting
EC were solubilized in RIPA buffer with protease inhibitor cocktail I (for detecting phosphoproteins, phosphatase inhibitor cocktails II and III were added) (Calbiochem, Gibbstown, NJ). Proteins were then separated in 4–15% SDS-PAGE gels and transferred onto nitrocellulose membrane. After blocking for 1 h with 5% non-fat milk at room temperature, the blots were incubated with appropriate primary antibodies overnight at 4°C or 1 h at room temperature followed by incubation with HRP-conjugated second antibodies for 1 h at room temperature. Visualization of immunoreactive protein bands was achieved using enhanced chemiluminescence (Pierce, Rockford, IL).
In vitro dextran permeability assay
We assessed FITC-labeled 40 kDa dextran (Sigma) permeability across EC monolayers plated on transwell inserts per manufacturer’s instructions (Chemicon, Temecula, CA). Transwell inserts were coated with collagen for 1 h at room temperature and EC then seeded at a density of 1×105/well in a final volume of 400 µl EGM-2 with supplements. The inserts were placed into 24 well plates containing 500 µl medium for overnight. To measure permeability, EC were stimulated with agonists for 1 h, and 100 µl FITC-dextran was added into the insert and incubated for 2 h. The insert was then removed and 100 µl medium collected from the bottom chamber. The fluorescent intensity of samples was analyzed on a Titertek Fluoroskan II Microplate Fluorometer (Diversified Equipment, Lorton, VA) at excitation and emission wavelengths of 485 nm and 530 nm, respectively.
Statistics
Student’s t-test was used to compare the means of data from two or more different experimental groups. Results are expressed as means + S.E.M.
Results
Adherens junction and tight junction proteins are not redistributed during barrier enhancement by FTY720
Vascular endothelial cadherin (VE-cadherin) is the major transmembrane protein of AJ in EC and plays a critical role in maintaining and regulating endothelial permeability [19]. β-catenin and p120 catenin bind VE-cadherin and regulate AJ function [20]. FTY720 induces dose-dependent and sustained TER increases with a maximal effect at 1 µM at 1 h [17]. To investigate the effects of FTY720 on endothelial AJ protein distribution, confluent EC monolayers were stimulated with FTY720 and immunostained for VE-cadherin, β-catenin, or P-120 catenin. As shown in Fig. 1A, these AJ proteins are primarily localized at cell-cell boundaries. Unlike S1P [21], FTY720 failed to alter their distribution within 1 h, the timeframe in which maximal barrier enhancement occurs [17].
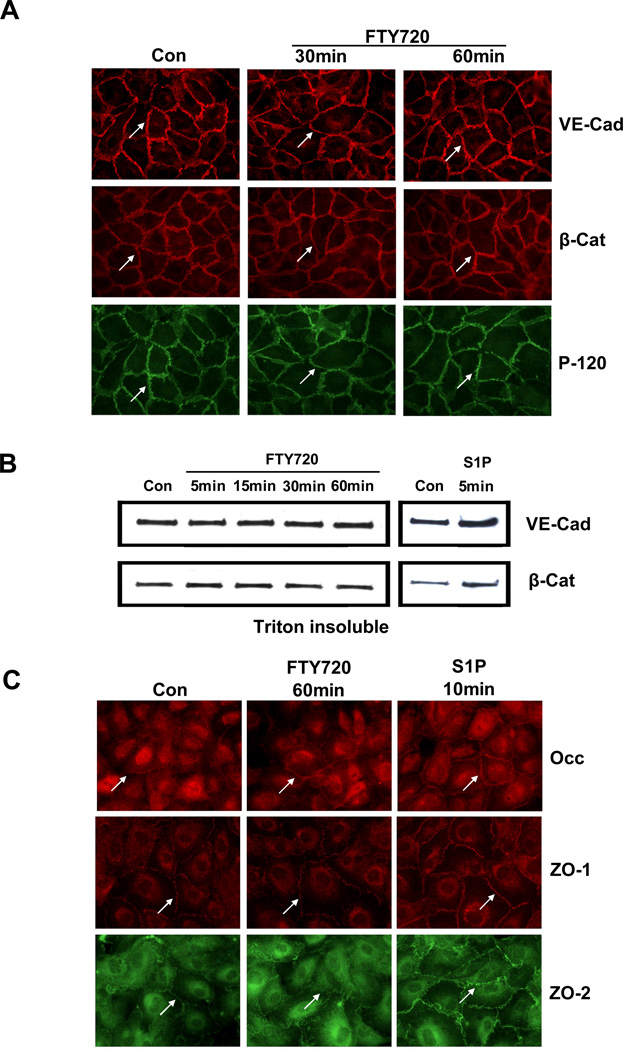
Figure 1. FTY720 does not induce AJ or TJ protein rearrangement.
A) Confluent HPAEC were stimulated with control vehicle (Con) or 1 µM FTY720 for 30 and 60 min and immunostained by anti-VE-Cad (VE-Cad), β-Catenin (β-Cat) and P120 antibodies. Images are representative of several independent experiments. B) Confluent HPAEC were stimulated with 1 µM S1P (5 min) or FTY720 for 5–60 min, and Triton-insoluble proteins were extracted as described. The amount of VE-Cad and β-Cat was detected by Western blot. Blot is representative of 3 independent experiments. C) Confluent HPAEC were stimulated with control vehicle (Con) or 1 µM FTY720 for 60 min or 1 µM S1P for 10 and immunostained by anti-occludin (Occ), ZO-1 and ZO-2 antibodies. Images are representative of several independent experiments.
The anchorage of VE-cadherin through catenins to the actin cytoskeleton stabilizes AJ and results in detergent resistance of AJ proteins. Therefore, EC were stimulated with FTY720 and fractionated by Triton X-100 solution as described [18]. While S1P increased VE-cadherin and β-catenin in the insoluble fraction (Fig. 1B), FTY720 failed to alter the distributions of these AJ proteins (Fig. 1B), suggesting that FTY720 does not increase the association of AJ proteins with the cytoskeleton. Thus, FTY720 induced neither cellular redistribution of VE-cadherin nor the anchorage of VE-cadherin to the cytoskeleton.
Tight junction complexes are another important class of cell-cell junctions that are responsible for regulating paracellular permeability and maintaining cell polarity [22]. TJ are composed of both transmembrane and intracellular molecules including claudins, occludin, junctional adhesion molecules (JAM) and zona occludens proteins (ZO-1–3) [23]. Major transmembrane proteins in TJ include occludin and the claudin family of proteins which consist of more than 20 members [23]. Of these, claudin-5 is expressed in large amounts in lung EC [24]. FTY720 failed to alter the baseline distribution of occludin at 60 min although S1P appeared to modestly increase peripheral localization of occludin by 10 min (Fig. 1C). Similarly, FTY720 treatment failed to alter the baseline distribution of claudin-5 at 60 min (data not shown). Furthermore, FTY720 failed to change the cellular distribution of ZO-1 and ZO-2 within 60 min although S1P increased the membrane localization of both proteins within 10 min (Fig. 1C).
Decreased expression of AJ or TJ proteins fails to inhibit barrier enhancement by FTY720
β-catenin plays a pivotal role in stabilizing and regulating the VE-cadherin complex [20, 25]. To further evaluate the role of AJ in FTY720-induced TER elevation, β-catenin expression was reduced by siRNA (Supp, Fig. 1). β-catenin siRNA decreased basal TER (data not shown) and significantly inhibited S1P-induced barrier enhancement (Fig. 2B) but did not attenuate FTY720-induced TER elevation (Fig. 2A). These data strongly suggest that AJ complex rearrangement is not essential for FTY720-induced TER elevation.
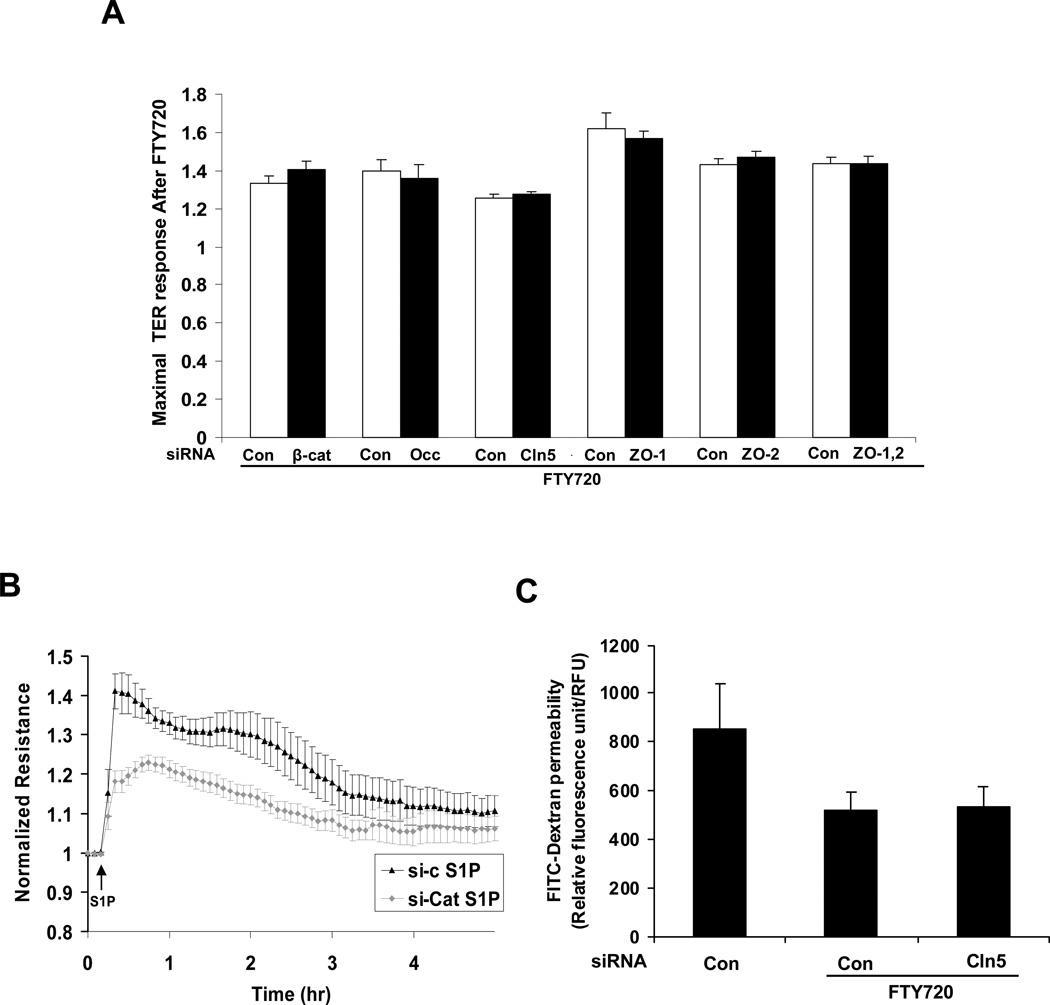
Figure 2. Barrier enhancement by FTY720 does not require β-catenin, occludin, claudin-5, ZO-1 or ZO-2.
A) HPAEC transfected with β-catenin (si-β-cat), occludin (si-occ), claudin-5 (si-cln5), ZO-1, ZO-2, both ZO-1 and ZO-2 siRNA or control siRNA (si-con) were plated on gold microelectrodes and then stimulated with FTY720 (1 µM). Bar graphs represent the maximal TER obtained after FTY720 relative to baseline resistance (mean ± S.E., n = 3∼5 per condition). There is no statistically significant difference between control siRNA and corresponding siRNA (si-β-cat, si-occ, si-cln5, si-ZO-1, si-ZO-2 or si-ZO-1,2) Western blots demonstrate representative downregulation by siRNA (Supp Fig. 1). B) HPAEC transfected with β-catenin (β-cat) siRNA were plated on gold microelectrodes and then stimulated with 1 µM S1P. The TER tracing represents pooled data (± S.E.M) from 3 independent experiments. C) HPAEC were transfected with 100 nM si-cln5 or control siRNA and seeded onto Transwell inserts. After FTY720 stimulation (1 µM), FITC-dextran was added into the top chamber and incubated for 2 h. The fluorescent intensity of the bottom chamber was analyzed by fluorometry as per Methods. n = 3. *p<0.01 vs. control siRNA without FTY720. There is no statistically significant difference between control siRNA and si-cln5 stimulated with FTY720.
To evaluate if TJs participate in FTY720-induced TER elevation, expression of TJ proteins was downregulated by siRNA. Decreased occludin or claudin-5 expression (Supp Fig. 1) did not affect either basal TER (data not shown) or FTY720-induced TER (Fig. 2A). Zona occludens proteins serve to link TJ with the actin cytoskeleton and play an important role in regulating TJ structure. However, ZO-1 or ZO-2 siRNA did not alter basal TER (data not shown) or FTY720-induced TER elevation (Fig. 2A). To exclude functional redundancy between ZO-1 and ZO-2 due to their structural similarity, we also simultaneously reduced the expression of these two proteins. The simultaneous knockdown of ZO-1 and ZO-2 failed to alter FTY720-induced TER elevation (Fig. 2A). Furthermore, Claudin 5 siRNA did not affect the ability of FTY720 to inhibit large molecule (dextran) permeability in a transwell assay (Fig. 2C), which is consistent with the TER data. Thus, we conclude that the TJ proteins occludin, claudin-5, ZO-1 or ZO-2 are not essential for FTY720-induced barrier enhancement.
FTY720 induces focal adhesion rearrangement
Recent evidence demonstrates that S1P induces significant FA rearrangement [9, 10]. In the present study, FTY720 appeared to increase the number of FA within 60 min (Fig. 3A) as well as the amount of FA proteins paxillin and vinculin in the Triton X-100-insoluble fraction (Fig. 3B), suggesting increased the formation and stabilization of FA. However, the functional importance of FTY720-induced FA rearrangement remains unclear because neither paxillin nor vinculin siRNA attenuated TER increase by FTY720 (data not shown). The tyrosine kinase FAK plays a critical role in regulating FA structure and function [9]. FAK siRNA slightly reduced the basal TER (data not shown) but did not attenuate the initial rise in TER induced by FTY720 or its maximal elevation (Fig. 3C). However, depletion of FAK significantly attenuated the sustained phase of TER elevation after FTY720 (i.e., after 4 h). It has been reported that phosphorylation of FAK at a specific site (Y576) plays an important role in the FA rearrangement that occurs during S1P-mediated barrier enhancement [9]. Thus, we next determined if FTY720 can induce similar phosphorylation of FAK. As shown in Fig. 4A, FTY720 quickly (within 5 min) induced significant phosphorylation of FAK at Y576 that persisted for at least 60 min (FTY720 did not increase phosphorylation of FAK at the thrombin-associated site, Y397). This phosphorylation is completely inhibited by preincubation with the Src inhibitor PP2 (Fig. 4B). However, PP2 does not inhibit peak barrier enhancement by FTY720 (Fig. 4C), which strongly suggests that Y576 phosphorylation of FAK is not required for the early and most potent effects of FTY720 on EC barrier function. Overall, these data suggest a possible role for FA in sustaining FTY720-induced TER elevation over hours but no critical function during the initial increase in TER after FTY720.
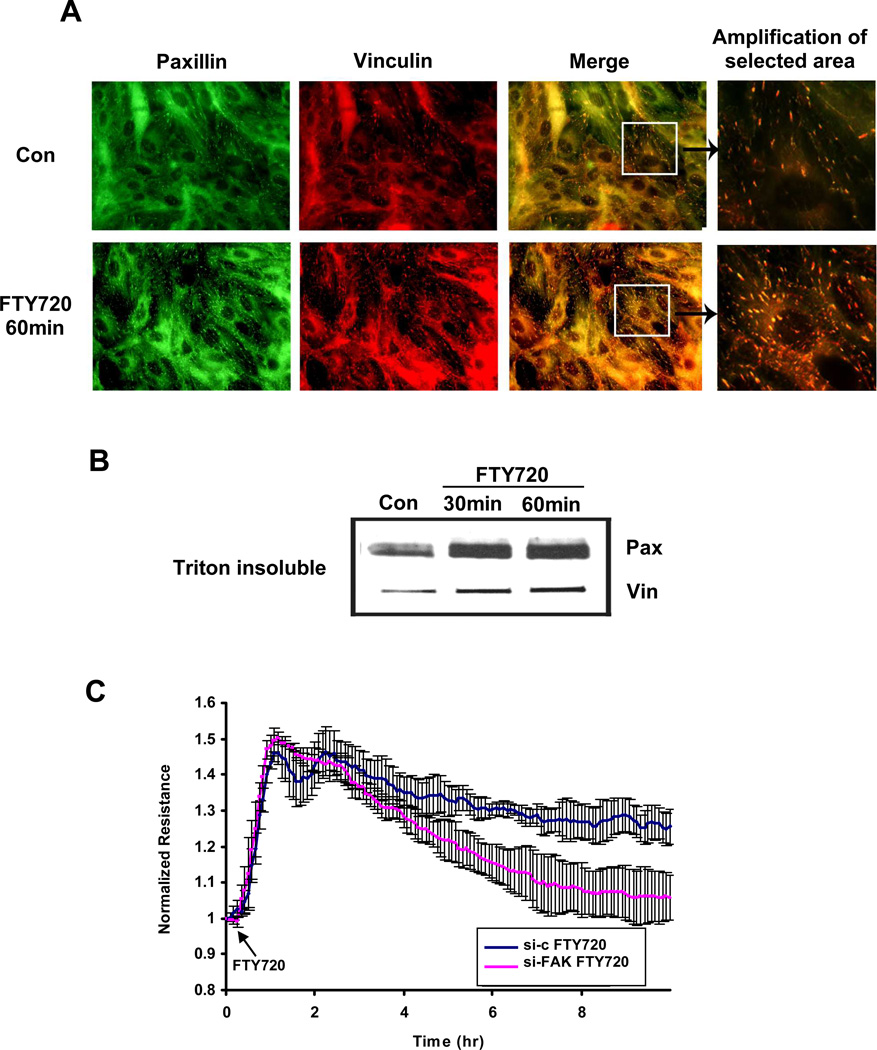
Figure 3. FTY720 induces focal adhesion rearrangement.
A) Confluent HPAEC were stimulated with 1 µM FTY720 for 60 min and then immunostained with anti-paxillin and anti-vinculin antibodies. Amplification of merged images suggests increased focal adhesions after FTY720. Images are representative of several independent experiments. B) Confluent HPAEC were stimulated with 1 µM FTY720 for 30 and 60 min. The Triton-insoluble proteins were extracted as described. The amount of paxillin (Pax) and vinculin (Vin) was detected by Western blot. Blot is representative of 3 independent experiments. C) HPAEC transfected with FAK siRNA or control siRNA (si-c) were plated on gold microelectrodes and then stimulated with FTY720 (1 µM). The TER tracing represents pooled data (± S.E.M) from 3 independent experiments. Western blot demonstrating representative downregulation of FAK by siRNA is shown in Suppl. Fig. 1.
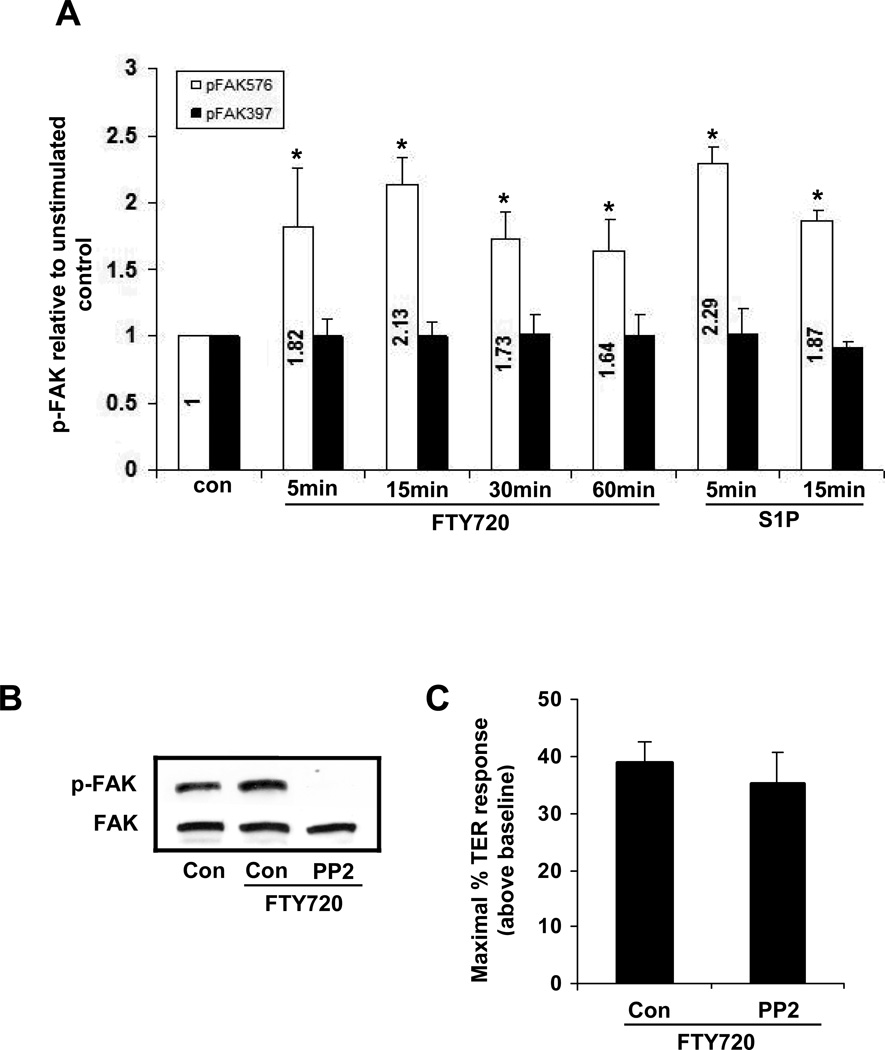
Figure 4. Y576 phosphorylation of FAK induced by FTY720 is not required for TER elevation.
A) Confluent HPAEC were stimulated with 1 µM FTY720 for 5, 15, 30 or 60 min or 1 µM S1P for 5 or 15 min. Phosphorylation of FAK (Y576 or Y397) was detected by phosphorylation antibody. Bar graphs represent the phosphorylation fold relative to control phosphorylation (mean ± S.E., n = 3). *, p<0.01 compared to control phosphorylation (Y576) There is no statistically significant difference for FAK phosphorylation at Y397. B) Confluent HPAEC were pretreated with 10 µM PP2 for 60 min and stimulated with 1 µM FTY720 for 15 min. Phosphorylation of FAK (Y576) was detected by phosphorylation antibody. Blot is representative of 3 independent experiments. C) Confluent HPAEC plated on gold microelectrodes were pretreated with 10 µM PP2 for 60min and then stimulated with FTY720 (1 µM). Bar graphs represent the maximal TER obtained after FTY720 relative to baseline resistance (mean ± S.E., n = 3). There is no statistically significant difference between control and PP2 pretreated cells.
Kinase/phosphatase signaling in FTY720-induced barrier enhancement: involvement of c-Abl tyrosine kinase
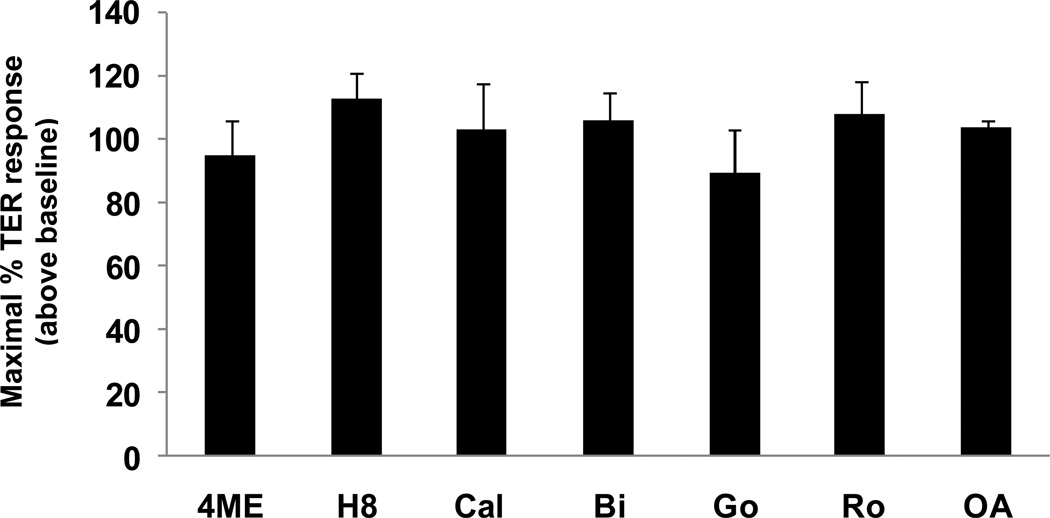
Previous investigations have demonstrated that intracellular signaling pathways involving PKA, PKC, PKG and PP2A regulate endothelial barrier function under various conditions [26–29]. Thus, we utilized multiple pharmacological inhibitors to inhibit these well known intracellular signaling pathways: 4-cyano-3-methylisoquinoline (PKA inhibitor), Dihydrochloride (PKA and PKG inhibitor), Go6983, Ro-32-0432, Go 6850 and Calphostin C, (which combine to inhibit most PKC isoforms) and okadaic acid (PP2A inhibitor). None of these inhibitors significantly attenuated maximal FTY720-induced barrier enhancement (Fig. 5).
Figure 5. Barrier enhancement by FTY720 does not require PKA, PKC, PKG, or PP2A intracellular signaling.
HPAEC plated on gold microelectrodes were pretreated with the following inhibitors or vehicle for 1 h: 4-cyano-3-methylisoquinoline (4ME) (25 µM, PKA inhibitor), Dihydrochloride (H8) (60 µM, PKA and PKG inhibitor), Go6983 (Go) (1 µM), Ro-32-0432 (Ro) (1 µM), Go 6850 (Bi) (1 µM) and Calphostin C (Cal) (1 µM), (which combine to inhibit most PKC isoforms) and okadaic acid (OA) (2.5 nM, PP2A inhibitor). The range of inhibitor concentrations used is 10∼100 times of IC50 provided by the manufacture. Cells were then stimulated with FTY720 (1 µM). The maximal TER increase of vehicle control plus FTY720 was set at 100, n=3 per condition. There are no statistically significant differences between any of the conditions shown.
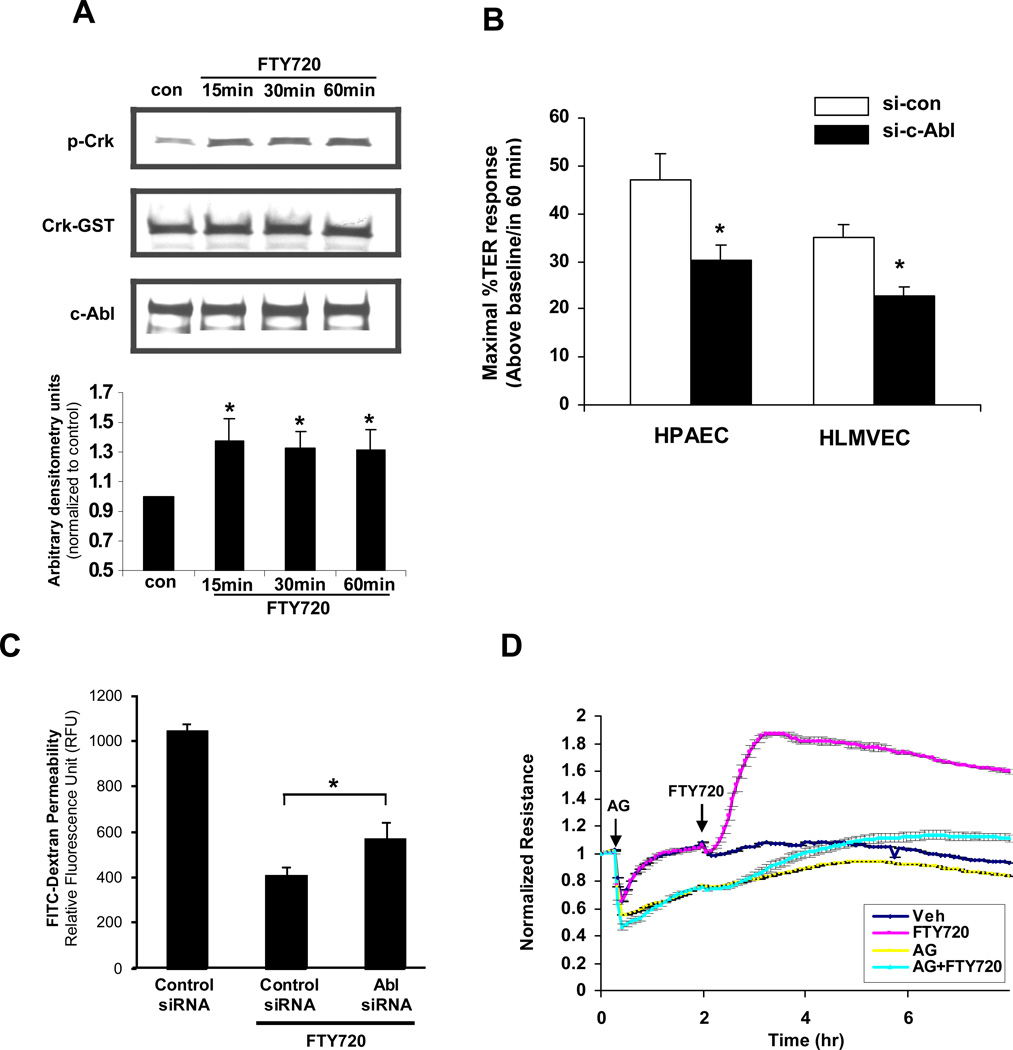
Our previously published data implicate tyrosine kinase signaling in FTY720-induced TER elevation as the nonspecific tyrosine kinase inhibitor genistein significantly attenuated this response [17]. c-Abl is a ubiquitously expressed non-receptor tyrosine kinase that is involved in the regulation of cell growth, survival and morphology [30]. FTY720 stimulation (1 µM for 15–60 min) of HPAEC significantly increased the capacity of immunoprecipitated c-Abl to phosphorylate recombinant Crk-GST (a known c-Abl target [31]), demonstrating that FTY720 increases c-Abl kinase activity during the timeframe within which it increases TER (Fig. 6A). Importantly, c-Abl siRNA significantly attenuated FTY720-induced peak TER elevation by ∼40% both in human pulmonary arterial endothelial cells (HPAEC) and human lung microvascular endothelial cells (HLMVEC) (Fig. 6B) without changing the basal TER (data not shown). Well-described phenotypic differences have been described in the barrier regulatory responses of pulmonary macrovascular and microvascular endothelium to other barrier-altering agonists such as thapsigargin [32] and thrombin [33], but our data demonstrate the similarity of the FTY720 response across these vascular beds within the lung. Furthermore, c-Abl siRNA significantly attenuated the ability of FTY720 to inhibit large molecule (dextran) permeability in a transwell assay (Fig. 6C), confirming its importance in barrier regulation in a complementary approach to TER measurements. Finally, the pharmacologic c-Abl inhibitor, AG957 (20 µM), attenuated the FTY720 TER response (Fig. 6D).
Figure 6. Barrier enhancement by FTY720 involves c-Abl tyrosine kinase.
A) HPAEC were stimulated with FTY720 (1 µM) or vehicle control for 15, 30, or 60 min. After treatment, c-Abl was immunoprecipitated with anti-c-Abl (8E9) antibody and incubated with Crk-GST in kinase buffer as described in Methods. The tyrosine phosphorylation of Crk-GST was detected by Western blot as a measure of c-Abl activity. The bar graph represents pooled densitometric results relative to vehicle control from 3 independent experiments. *p<0.01 vs. vehicle control. B) HPAEC or HLMVEC transfected with c-Abl siRNA or control siRNA (si-con) were plated on gold microelectrodes and then stimulated with FTY720 (1 µM). Bar graphs represent the maximal TER obtained after FTY720 relative to baseline resistance (mean ± S.E., n = 3 per condition). *, P<0.05 compared to control siRNA. Western blot demonstrating representative downregulation of c-Abl by siRNA is shown in Suppl. Fig. 1. C) HPAEC were transfected with 100 nM c-Abl or control siRNA and seeded onto Transwell inserts. After FTY720 stimulation (1 µM), FITC-dextran was added into the top chamber and incubated for 2 h. The fluorescent intensity of the bottom chamber was analyzed by fluorometry as per Methods. n = 4. *p<0.031 vs. control siRNA. D) HPAEC were preincubated for 1 h with the c-Abl inhibitor, AG957 (20 µM) or vehicle control and then stimulated with FTY720 (1 µM) or vehicle. Data are a representative of 3 independent experiments.
These combined data strongly suggest that c-Abl expression and activity are necessary for maximal FTY720-induced barrier enhancement in human pulmonary EC. As an initial exploration of the possible c-Abl downstream targets responsible for mediating barrier enhancement by FTY720, we downregulated expression of the well described c-Abl target, Crk [31]. Although Crk siRNA greatly decreased basal TER, it did not inhibit FTY720-induced TER elevation (data not shown). Thus, the downstream targets of c-Abl responsible for mediating this FTY720 effect remain unclear. However, the well-known ability of c-Abl to regulate F-actin structures may be involved because depolymerization of F-actin with cytochalasin D partially inhibits TER elevation by FTY720 (Supplemental Fig. 2).
Discussion
FTY720 is currently in clinical trials as an immunomodulator for multiple sclerosis treatment and soon may be available as a therapeutic agent [15, 16]. Recently, we reported that FTY720 improved endothelial barrier function by a novel pathway different from S1P [17]. We now further characterize the novel barrier-promoting effects of FTY720 on intracellular signaling and junctional assembly formation in human pulmonary EC.
Our current studies clearly demonstrate that the VE-cadherin complex does not participate in FTY720-induced barrier enhancement despite its well known role in EC barrier function. Although recent work by Sarai et al [34] reported increased peripheral localization of both VE-cadherin and β-catenin in HMVEC after prolonged exposure to FTY720 (3 h), FTY720 in our study did not cause redistribution of AJ proteins within the timeframe of maximal TER elevation (Fig. 1A), nor did it induce tyrosine phosphorylation of VE-cadherin (data not shown) or increase the linkage between VE-cadherin complex and cytoskeleton (Fig. 1B). Importantly, β-catenin siRNA did not attenuate FTY720-induced barrier enhancement even after 3 h of stimulation (Fig. 2A). Interestingly, the direct functional role of VE-cadherin in mediating S1P-induced endothelial barrier enhancement remains incompletely understood as well. In HUVEC, S1P significantly increases the abundance of VE-cadherin and β-catenin at cell-cell contact regions [11]. However, recent data suggests that VE-cadherin is not necessary for the immediate TER increase (within minutes) after S1P but may participate in the sustained phase that lasts for several hours [12].
Our data also suggest that TJ proteins are not essential for FTY720-induced barrier enhancement. TJ participate in endothelial barrier function to variable degrees along different segments of the vasculature [35]. For example, decreased expression of some claudins, the major transmembrane constituents of TJ [36], has been associated with decreased blood–brain barrier (BBB) function [37]. In particular, claudin-5 is specifically and highly expressed in EC [24], and claudin-5-deficient mice are selectively leaky for molecules smaller than 800 Da in BBB [37]. However, in our study claudin-5 siRNA did not change the basal TER of HPAEC or attenuate FTY720-induced barrier enhancement (Fig. 2A), nor did it alter permeability to larger molecules (dextan) after FTY720 in a transwell assay (Fig. 2C). Downregulation of occludin, another transmembrane component of TJ [38], has been observed in BBB disruption and in VEGF-treated retinal endothelial cells [39]. Our data indicate that HPAEC express occludin, but FTY720 did not induce its redistribution within these cells (Fig. 1C). Furthermore, occludin siRNA neither changed the basal TER nor attenuated FTY720-induced barrier enhancement (Fig. 2A).
Claudins and occludin are linked to numerous intracellular partners, including ZO-1, −2 and −3, AF-6, PAR-3, cingulin and 7H6 [40], which provide important connections to the underlying actin cytoskeleton. In HUVEC, S1P treatment caused ZO-1 redistribution to the lamellipodia and cell-cell junctions via the S1P1/Gi/Akt/Rac pathway, while the enhanced barrier function induced by S1P was attenuated by ZO-1 siRNA [13]. In the current study in HPAEC, we found that FTY720 failed to induce ZO-1 and ZO-2 redistribution (Fig. 1C), and siRNA for ZO-1 and ZO-2 failed to attenuate FTY720-induced barrier enhancement (Fig. 2A).
Focal adhesions (FA) are complex protein structures consisting of integrins, actin-binding proteins, and focal adhesion kinase (FAK) [41] that contribute to EC barrier function by linking EC to the underlying matrix [42]. Both depletion of FAK expression and expression of a kinase-deficient FAK mutant can impair EC barrier function [43]. Our data now suggest that FTY720 increased the formation and stabilization of FA (Fig. 3). Furthermore, FAK siRNA inhibited the prolonged elevation in TER induced by FTY720 (>4 h) but did not alter peak TER occurring within the first hour. To further explore the possible role of FAK in FYT720-induced barrier enhancement, we determined if FTY720 induced phosphorylation of FAK at the specific site (Y576) known to mediate FA rearrangement that occurs during EC barrier enhancement by S1P [9]. Although FTY720 significantly induced phosphorylation of FAK at Y576 within the 1 h timeframe of maximal TER enhancement (Fig. 4A), this phosphorylation can be completely inhibited by the Src inhibitor PP2 (Fig. 4B) without affecting peak barrier enhancement by FTY720 (Fig. 4C). These data strongly suggest that Y576 phosphorylation of FAK is not required for the early and most potent effects of FTY on EC barrier function. Overall, our data suggest that FA rearrangements and FAK may participate in the sustained phase of FTY720-induced barrier enhancement, but the mechanisms involved remain unclear and require further investigation.
PKC isoforms are important regulators of junctional permeability [26]. For example, PKCδ has been reported to have a barrier-protective function by increasing FA contacts [44]. The activation of PKA promotes endothelial barrier integrity partially through its ability to stabilize EC cytoskeletal and adhesive structures [27], while PKG also has barrier protective effects [45]. In this study, we used multiple pharmacologic inhibitors of PKA, PKG, and PKC to screen for potential roles in regulation of EC barrier function by FTY720. However, inhibitors for all of these pathways failed to attenuate FTY720-induced barrier enhancement (Fig. 5). FTY720 is known to activate protein phosphatase 2A (PP2A) activity [46], another potential barrier regulating protein [47]. However, inhibition of PP2A (via okadaic acid) had no effect on FTY720-induced TER elevation (Fig. 5).
The nonreceptor tyrosine kinase c-Abl is ubiquitously expressed in mammalian cells and plays a critical role in the regulation of cell growth, survival and morphogenesis [30]. c-Abl can directly bind F-actin and interacts with a number of target effectors that regulate barrier-regulatory cytoskeletal structures such as FA, lamellipodia, filopodia and membrane ruffles [30]. Abl/arg knockout mouse embryos exhibit a significant defect in the actin latticework [48]. During cell adhesion, activation of c-Abl causes down-regulation of Rap1-GTP and cell rounding and detachment when Rho-ROCK1 is simultaneously activated [31]. The PDGF-induced dorsal-wave response requires c-Abl to phosphorylate the actin binding protein cortactin on three critical tyrosine residues [49]. All of these observations strongly indicate that c-Abl is a critical mediator in regulating cytoskeletal organization.
Current knowledge about the role of c-Abl in regulating EC barrier function is limited. One of the mechanisms by FTY720 may have utility in treating leukemia is through the dephosphorylation and inactivation of BCR/Abl fusion protein [50]. However, it is not clear how FTY720 regulates c-Abl in normal cells. Here we describe a novel role for c-Abl in mediating FTY720-induced pulmonary EC barrier enhancement. FTY720 significantly increased c-Abl kinase activity in cultured HPAEC (Fig. 6A). Downregulation of c-Abl expression significantly inhibited FTY720-induced TER elevation in both human pulmonary arterial EC and human lung microvascular EC (Fig. 6B) as well as the ability of FTY720 to inhibit large molecule permeability in a transwell assay (Fig. 6C). These data demonstrate an important role for c-Abl in achieving maximal barrier enhancement after FTY720, but it is not essential for all aspects of the FTY720 response because significant barrier enhancement still occurs when c-Abl expression is suppressed.
The mechanism by which c-Abl mediates this effect on EC barrier function is unclear. multiple published studies have demonstrated the ability of c-Abl to regulate F-actin and cytoskeletal structures, including FA, AJ, lamellipodia, membrane ruffles, and filopodia (reviewed in [30, 51]). Because our study implicates c-Abl in EC barrier enhancement by FTY720, and because EC barrier function is determined in large part by cytoskeletal structure, it is reasonable to hypothesize that c-Abl mediates the FTY720 effect via cytoskeletal rearrangement. For example, c-Abl has long been known to localize to FA and to phosphorylate various FA-associated proteins. However, our data indicate that FA proteins (FAK, vinculin, paxillin) are not essential for the early peak TER effect in which c-Abl participates (Fig. 6). Similarly, inhibition of c-Abl results in disruption of cadherin-based AJ [52], but our data indicate that EC cadherin complexes are not involved in barrier enhancement by FTY720 in pulmonary EC (Figs. 1–2). Therefore, it is unlikely that c-Abl contributes to EC barrier enhancement by FTY720 through regulation of FA or AJ junctional structures.
Another possible mechanism involves regulation of peripheral F-actin structures by c-Abl that could theoretically mediate improved barrier function by FTY720. Multiple studies have demonstrated a role for c-Abl in modulating such structures. For example, cells deficient in Abl exhibit dramatically reduced membrane ruffling in response to PDGF, possibly because of decreased phosphorylation of the actin-binding protein, cortactin [53]. Rapid peripheral cortical actin rearrangement and lamellipodial formation mediated via Rac and cortactin are critical aspects for EC barrier enhancement by S1P [8]. Moreover, c-Abl activity is known to regulate Rac and cortactin function [51], and our group has recently demonstrated a role for c-Abl in the S1P barrier enhancing response in part via cortactin phosphorylation and cortical actin rearrangement [54]. In contrast, our previous work has demonstrated no role for Rac and cortactin in the FTY720 response, and immunofluorescent studies do not reveal peripheral actin rearrangement during EC barrier enhancement by FTY720 [17]. These observations suggest that dramatic peripheral actin rearrangement is not required for the FTY720 response, but the possibility remains that FTY720 may induce a modest amount of peripheral ruffling mediated via c-Abl that participates in barrier enhancement but is too subtle to be detected in confluent EC by standard immunofluorescence imaging. The partial inhibition of FTY720-induced TER elevation by the actin disrupting agent, cytochalasin D, is consistent with this hypothesis (Supplemental Fig. 2).
To summarize, FTY720 enhances pulmonary EC barrier function by a novel pathway that does not require AJ or TJ protein complexes but does involve c-Abl signaling. Although the downstream targets of c-Abl responsible for this effect remain unclear and are the subject of ongoing investigation, these results suggest that modulation of c-Abl activity may represent a new therapeutic approach for regulating vascular permeability.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Heart Lung Blood Institute NIH grant P01 HL 58064 (JGNG) and R01 HL 88144 (SMD). The authors thank Lakshmi Natarajan and Sara M. Camp for superb technical support.
References
- 1.Wang L, Dudek SM. Regulation of vascular permeability by sphingosine 1-phosphate. Microvasc Res. 2009;77(1):39–45. doi: 10.1016/j.mvr.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91(4):1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 4.Garcia JG, et al. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108(5):689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng X, et al. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169(11):1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 6.McVerry BJ, et al. Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am J Respir Crit Care Med. 2004;170(9):987–993. doi: 10.1164/rccm.200405-684OC. [DOI] [PubMed] [Google Scholar]
- 7.Singleton PA, et al. Transactivation of sphingosine 1-phosphate receptors is essential for vascular barrier regulation. Novel role for hyaluronan and CD44 receptor family. J Biol Chem. 2006;281(45):34381–34393. doi: 10.1074/jbc.M603680200. [DOI] [PubMed] [Google Scholar]
- 8.Dudek SM, et al. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem. 2004;279(23):24692–24700. doi: 10.1074/jbc.M313969200. [DOI] [PubMed] [Google Scholar]
- 9.Shikata Y, et al. Involvement of site-specific FAK phosphorylation in sphingosine-1 phosphate- and thrombin-induced focal adhesion remodeling: role of Src and GIT. FASEB J. 2003;17(15):2240–2249. doi: 10.1096/fj.03-0198com. [DOI] [PubMed] [Google Scholar]
- 10.Romer LH, Birukov KG, Garcia JG. Focal adhesions: paradigm for a signaling nexus. Circ Res. 2006;98(5):606–616. doi: 10.1161/01.RES.0000207408.31270.db. [DOI] [PubMed] [Google Scholar]
- 11.Lee MJ, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99(3):301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 12.Xu M, et al. Sphingosine 1-phosphate rapidly increases endothelial barrier function independently of VE-cadherin but requires cell spreading and Rho kinase. Am J Physiol Cell Physiol. 2007;293(4):C1309–C1318. doi: 10.1152/ajpcell.00014.2007. [DOI] [PubMed] [Google Scholar]
- 13.Lee JF, et al. Dual roles of tight junction-associated protein, zonula occludens-1, in sphingosine 1-phosphate-mediated endothelial chemotaxis and barrier integrity. J Biol Chem. 2006;281(39):29190–29200. doi: 10.1074/jbc.M604310200. [DOI] [PubMed] [Google Scholar]
- 14.Mandala S, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296(5566):346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 15.Cohen JA, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 362(5):402–415. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- 16.Kappos L, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 362(5):387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 17.Dudek SM, et al. Pulmonary endothelial cell barrier enhancement by FTY720 does not require the S1P1 receptor. Cell Signal. 2007;19(8):1754–1764. doi: 10.1016/j.cellsig.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinck L, et al. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biol. 1994;125(6):1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86(1):279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 20.Pokutta S, Weis WI. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu Rev Cell Dev Biol. 2007;23:237–261. doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- 21.Sun X, et al. Enhanced interaction between focal adhesion and adherens junction proteins: involvement in sphingosine 1-phosphate-induced endothelial barrier enhancement. Microvasc Res. 2009;77(3):304–313. doi: 10.1016/j.mvr.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84(3):869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 23.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2(4):285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 24.Morita K, et al. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147(1):185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmeliet P, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98(2):147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 26.Farhadi A, et al. The role of protein kinase C isoforms in modulating injury and repair of the intestinal barrier. J Pharmacol Exp Ther. 2006;316(1):1–7. doi: 10.1124/jpet.105.085449. [DOI] [PubMed] [Google Scholar]
- 27.Birukova AA, et al. Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J Cell Physiol. 2008;215(3):715–724. doi: 10.1002/jcp.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia JG, et al. Diperoxovanadate alters endothelial cell focal contacts and barrier function: role of tyrosine phosphorylation. J Appl Physiol. 2000;89(6):2333–2343. doi: 10.1152/jappl.2000.89.6.2333. [DOI] [PubMed] [Google Scholar]
- 29.Rentsendorj O, et al. Role of vasodilator-stimulated phosphoprotein in cGMP-mediated protection of human pulmonary artery endothelial barrier function. Am J Physiol Lung Cell Mol Physiol. 2008;294(4):L686–L697. doi: 10.1152/ajplung.00417.2007. [DOI] [PubMed] [Google Scholar]
- 30.Woodring PJ, Hunter T, Wang JY. Regulation of F-actin-dependent processes by the Abl family of tyrosine kinases. J Cell Sci. 2003;116(Pt 13):2613–2626. doi: 10.1242/jcs.00622. [DOI] [PubMed] [Google Scholar]
- 31.Huang X, et al. Induction of cell retraction by the combined actions of Abl-CrkII and Rho-ROCK1 signaling. J Cell Biol. 2008;183(4):711–723. doi: 10.1083/jcb.200801192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu S, et al. Essential role of a Ca2+-selective, store-operated current (ISOC) in endothelial cell permeability: determinants of the vascular leak site. Circ Res. 2005;96(8):856–863. doi: 10.1161/01.RES.0000163632.67282.1f. [DOI] [PubMed] [Google Scholar]
- 33.Troyanovsky B, et al. Thrombin enhances the barrier function of rat microvascular endothelium in a PAR-1-dependent manner. Am J Physiol Lung Cell Mol Physiol. 2008;294(2):L266–L275. doi: 10.1152/ajplung.00107.2007. [DOI] [PubMed] [Google Scholar]
- 34.Sarai K, et al. Endothelial barrier protection by FTY720 under hyperglycemic condition: involvement of focal adhesion kinase, small GTPases, and adherens junction proteins. Am J Physiol Cell Physiol. 2009;297(4):C945–C954. doi: 10.1152/ajpcell.00606.2008. [DOI] [PubMed] [Google Scholar]
- 35.Vandenbroucke E, et al. Regulation of endothelial junctional permeability. Ann N Y Acad Sci. 2008;1123:134–145. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 36.Van Itallie CM, Anderson JM. The role of claudins in determining paracellular charge selectivity. Proc Am Thorac Soc. 2004;1(1):38–41. doi: 10.1513/pats.2306013. [DOI] [PubMed] [Google Scholar]
- 37.Nitta T, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161(3):653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furuse M, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123(62 Pt 2):1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang W, Dentler WL, Borchardt RT. VEGF increases BMEC monolayer permeability by affecting occludin expression and tight junction assembly. Am J Physiol Heart Circ Physiol. 2001;280(1):H434–H440. doi: 10.1152/ajpheart.2001.280.1.H434. [DOI] [PubMed] [Google Scholar]
- 40.Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. 2008;1778(3):794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6(1):56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 42.Wu MH. Endothelial focal adhesions and barrier function. J Physiol. 2005;569(Pt 2):359–366. doi: 10.1113/jphysiol.2005.096537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braren R, et al. Endothelial FAK is essential for vascular network stability, cell survival, and lamellipodial formation. J Cell Biol. 2006;172(1):151–162. doi: 10.1083/jcb.200506184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrington EO, et al. PKCdelta regulates endothelial basal barrier function through modulation of RhoA GTPase activity. Exp Cell Res. 2005;308(2):407–421. doi: 10.1016/j.yexcr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Moldobaeva A, et al. Role of protein kinase G in barrier-protective effects of cGMP in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290(5):L919–L930. doi: 10.1152/ajplung.00434.2005. [DOI] [PubMed] [Google Scholar]
- 46.Liu Q, et al. FTY720 demonstrates promising preclinical activity for chronic lymphocytic leukemia and lymphoblastic leukemia/lymphoma. Blood. 2008;111(1):275–284. doi: 10.1182/blood-2006-10-053884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tar K, et al. Role of protein phosphatase 2A in the regulation of endothelial cell cytoskeleton structure. J Cell Biochem. 2006;98(4):931–953. doi: 10.1002/jcb.20829. [DOI] [PubMed] [Google Scholar]
- 48.Koleske AJ, et al. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21(6):1259–1272. doi: 10.1016/s0896-6273(00)80646-7. [DOI] [PubMed] [Google Scholar]
- 49.Matsubara T, et al. Critical role of cortactin in actin ring formation and osteoclastic bone resorption. J Bone Miner Metab. 2006;24(5):368–372. doi: 10.1007/s00774-006-0701-4. [DOI] [PubMed] [Google Scholar]
- 50.Neviani P, et al. FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. J Clin Invest. 2007;117(9):2408–2421. doi: 10.1172/JCI31095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bradley WD, Koleske AJ. Regulation of cell migration and morphogenesis by Abl-family kinases: emerging mechanisms and physiological contexts. J Cell Sci. 2009;122(Pt 19):3441–3454. doi: 10.1242/jcs.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zandy NL, Playford M, Pendergast AM. Abl tyrosine kinases regulate cell-cell adhesion through Rho GTPases. Proc Natl Acad Sci U S A. 2007;104(45):17686–17691. doi: 10.1073/pnas.0703077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyle SN, et al. A critical role for cortactin phosphorylation by Abl-family kinases in PDGF-induced dorsal-wave formation. Curr Biol. 2007;17(5):445–451. doi: 10.1016/j.cub.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 54.Dudek SM, et al. Abl tyrosine kinase phosphorylates non-muscle myosin light chain kinase to regulate endothelial barrier function. Mol Biol Cell. 2010 doi: 10.1091/mbc.E09-10-0876. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.