Abstract
Although protracted prefrontal grey matter development is associated with concomitant executive function (EF) development in adolescents, few studies have explored the relationship between white matter and EF. This study examined the relationship between white matter microstructure and two aspects of EF, inhibition and task-switching, in a sample of 84 adolescents using diffusion tensor imaging (DTI). Tract-Based Spatial Statistics (TBSS) were used to examine fractional anisotropy (FA) and mean diffusivity (MD). Adolescents completed the Color-Word Interference task from the Delis-Kaplan Executive Function System, a modification of the Stroop task. Inhibition and task-switching performance were group normalized and measured using both reaction time and errors. Performance and the interaction of age and performance were regressed on FA and MD white matter skeletons, controlling for age and IQ, separately for inhibition and task-switching. Follow up analyses examined the relative contributions of axial and radial diffusivities. Greater FA in the anterior corona radiata (ACR) was associated with better inhibition, independent of age. Greater FA in the SCR and precentral gyrus white matter were associated with better task-switching, regardless of age, whereas an association between FA in the ACR and task-switching was dependent on age. There were no significant associations between MD and performance. Results suggest better inhibition and task-switching are associated with greater integrity of white matter microstructure in regions supporting cross-cortical and cortical-subcortical connections stemming from the prefrontal cortex. These findings are consistent with functional studies of cognitive control and models of EF that propose separate, yet related, latent factors.
Keywords: inhibition, task-switching, adolescents, white matter, executive function, DTI
1. Introduction
Executive function (EF) consists of several related, yet separable, higher-order cognitive processes that exert control over lower-level cognitive functions (Friedman et al., 2008). Together, these abilities allow an individual to exhibit goal-directed behavior and override prepotent responding (Banich, 2009). Research using latent variable analyses suggests two of these processes are inhibition and shifting. Inhibition consists of inhibiting prepotent responding (Friedman et al., 2008; Miyake et al., 2000) and maintaining task-relevant goals and processes (Munakata et al., 2011), whereas shifting involves switching between attentional or task sets (Friedman et al., 2008; Miyake et al., 2000).
Inhibition, particularly in the context of attentional control, has frequently been examined using the Stroop task. The Stroop task consists of a series of color words printed in different colored ink (e.g., “red” in blue ink), and individuals must name the ink color while ignoring the word (Stroop, 1935). This task requires individuals to attend to and process task-relevant color information (ink) and inhibit prepotent processing of conflicting task-irrelevant information (word). Robust behavioral effects on the Stroop task have been found across development (Adleman et al., 2002; for review, see MacLeod, 1991). The dorsolateral prefrontal cortex (DLPFC), middle frontal gyrus (MFG), medial prefrontal cortex (mPFC), and posterior parietal regions are recruited when completing the Stroop task (Banich et al., 2000a, 2000b; Brown et al., 1999; Carter et al., 1995; Milham et al., 2003; Taylor et al., 1997). Similarly, inhibition of irrelevant information during selective attention tasks (Clark et al., 2000; Kirino et al., 2000; McCarthy et al., 1997; Yoshiura et al., 1999), motoric inhibition (Aron et al., 2003, 2004; Garavan et al., 1999; Konishi et al., 1998), and inhibition of information in memory (Anderson & Green, 2001; Depue et al., 2007) are associated with DLPFC, MFG, and mPFC activation, as well as inferior frontal gyrus (IFG).
EF continues to develop over the course of adolescence, with improved EF contributing to better planning, attentional control, and cognitive flexibility (Crone, 2009; Huizinga et al., 2006; Steinberg, 2005). These changes in EF coincide with grey matter maturation of the prefrontal cortex over the course of adolescence, which progresses from more inferior and medial regions to more superior and dorsal regions (Paus, 2005). This is consistent with findings that younger adolescents recruit more medial and inferior frontal regions, as compared to older adolescents and young adults who recruit more dorsolateral frontal regions when performing the Stroop task (Adleman et al., 2002; Andrews-Hanna et al., 2011). Medial regions are more commonly associated with transient control involved in response selection and evaluation, whereas DLPFC is more often associated with sustained control or implementation of an attentional set (Braver et al., 2007; Milham et al., 2003; Silton et al., 2010). Therefore, the development of EF over the course of adolescence likely reflects not only better inhibition, but also more efficient strategy use.
In addition to cortical gray matter changes, white matter continues to develop into the third decade of life. This includes increases in white matter volume and density with age, which is especially robust in the frontal lobes (Giedd et al., 1999a, 1999b; Lebel et al., 2012; Paus et al., 1999). Changes in white matter microstructure have also been detected in adolescents using diffusion tensor imaging (DTI) (Bava et al., 2010; Giorgio et al., 2008; Lebel & Beaulieu, 2011; Lebel et al., 2008, 2012). DTI is a non-invasive imaging technique that allows for the measurement of water diffusion displacement and directionality to make inferences about white matter fiber microstructure (Basser, 1995). A number of outcome variables are obtained to characterize this diffusion, commonly including fractional anisotropy (FA) and mean diffusivity (MD). FA is an index of the degree of restricted, or anisotropic, water diffusion, and MD a measure of overall water diffusion. Higher FA values and lower MD values are believed to represent increased axon caliber, myelination, and/or fiber organization in white matter pathways (Alexander et al., 2007; Beaulieu, 2002), although some studies have found otherwise (for review, see Hasan et al., 2012). FA values reflect both axial (AD) and radial (RD) diffusion parameters (for review, see Alexander et al., 2007). AD quantifies the amount of water diffusion along the primary axis, while RD measures the dispersion perpendicular to the primary axis of diffusion. AD and RD have been implicated in different neurobiological underpinnings (e.g., Song et al., 2002, 2005, but see Wheeler-Kingshott et al., 2009), and therefore are useful in further characterizing water diffusion in white matter.
Although many studies have demonstrated associations between grey matter development and EF, fewer studies have examined the relationship between white matter microstructure and EF in typically developing adolescents. DTI studies of white matter and EF are important, as greater myelination allows for faster conduction of neural information between brain regions and hence may allow for neural circuits to communicate more efficiently or effectively (Casey et al., 2008). Therefore, not only is development of specific brain regions likely important to improved EF, but also improved communication between key brain regions resulting from increased myelination. One DTI study of children and adolescents using a flanker test, which requires inhibition of a prepotent response, found greater intraindividual variability in responding was associated with lower FA values in the cortical spinal tract (CST) and superior longitudinal fasciculus (SLF) (Tamnes et al., 2012). Another study including children through young adults found FA and apparent diffusion coefficient (ADC) in the forceps major was predictive of reaction time (RT) on a flanker test, and FA was also related to variability in responding on conflict trials (Fjell et al., 2012). One other study, which primarily included children and some young adolescents, found better performance on a stop-signal task, a type of motoric inhibition task, was associated with greater FA in right IFG and pre-supplementary motor area (pre-SMA) white matter using region of interest analyses (Madsen et al., 2010). Associations between FA and MD and cognitive processing in children and adolescents have been found using other tasks that involve EF, such as working memory (Nagy et al., 2004; Vestergaard et al., 2011) and delay discounting (Olson et al., 2009).
To further elaborate on the association between white matter maturation and EF, this study used DTI to examine the relationship between two aspects of EF, inhibition and task-switching, and white matter microstructure in adolescents. Inhibition was measured using a version of the Stroop task, the Color Word Interference subtest from the Delis-Kaplan Executive Function System (D-KEFS) (Delis et al., 2001). Unlike inhibition tasks that have been used in previous DTI studies that include adolescents, the Stroop task involves overriding prepotent processing at a semantic level (color) as opposed to just motoric responding (response). Additionally, the Color Word Interference task from the D-KEFS has different subtests that can help disentangle inhibition and task-switching. Task-switching and inhibition may be confounded in some shifting measures, as previous task sets may need to be inhibited before switching to a new task set. Given that previous studies have demonstrated the anterior corona radiata (ACR) is associated with attentional tasks (Niogi et al., 2010; Tang et al., 2010; Yin et al, 2013) and frontotemporal and frontoparietal fiber tracts are associated with task-switching (Gold et al., 2010; Kucukboyaci et al., 2012), we hypothesized greater FA and lower MD in the ACR would be associated with inhibition and greater FA and lower MD in frontotemporal and frontoparietal fiber tracts would be associated with task-switching in adolescents. Although most white matter tracts demonstrate increased FA and decreased MD from childhood to adulthood (Lebel et al., 2008, 2011), association fibers, such as the SLF, have shown changes most specific to adolescence (Bava et al., 2010; Giorgio et al., 2008; Lebel et al., 2011). Therefore, we predicted there would be an interaction between task performance and age related to white matter integrity of the SLF.
2. Results
2.1. Behavioral Data
Based on scaled score data, participant RT and errors were within the average range for inhibition and task-switching (see Table 1). Greater age was associated with better inhibition (r = −0.29, p = 0.008). This association was driven by the finding that greater age was associated with fewer errors (r = −0.35, p = 0.001), while age was not associated with RT (r = −0.12, p = 0.27). There was no association between age and task-switching (r = −0.021, p = 0.85). However, there was a trend for greater age to be associated with fewer errors (r = −0.21, p = 0.057) and slower RT (r = 0.19, p = 0.087). IQ was not associated with any inhibition or task-switching measures (all p’s > 0.15).
Table 1.
Participant demographics and performance on the Color Word Interference sub-test of the Delis-Kaplan Executive Function System (D-KEFS). Means are presented in each column, with standard deviation presented in parentheses. ScS = scaled score, SD = standard deviation
| Characteristic | Mean (SD) | ||
|---|---|---|---|
| Full Sample | Males | Females | |
| N | 84 | 39 | 45 |
| Age | 13.14 (1.72) | 12.98 (1.69) | 13.28 (1.76) |
| Caucasian (%) | 82.1% | 84.62% | 80% |
| IQa | 116.38 (10.77) | 116.41 (10.11) | 116.36 (11.43) |
| SESb | 27.04 (12.57) | 26.97 (11.20) | 27.09 (13.77) |
| Pubertyc | 3.083 (1.19)* | 2.46 (1.14) | 3.62 (0.96) |
| D-KEFS Color Word Interference Performance (ScS) | |||
| D-KEFS Color Naming | 10.98 (2.19) | 10.97 (2.31) | 10.98 (2.10) |
| D-KEFS Inhibition | 10.98 (2.24) | 11.28 (2.32) | 10.71 (2.17) |
| D-KEFS Task-Switching | 10.89 (2.03) | 10.85 (2.28) | 10.93 (1.81) |
| D-KEFS Inhibition Total Errors | 10.68 (2.15) | 11.00 (2.24) | 10.40 (2.05) |
| D-KEFS Task-Switching Total Errors | 10.61 (1.75) | 10.59 (1.83) | 10.62 (1.70) |
difference between males and females significant at a p < 0.05.
Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999)
Hollingshead Index of Social Position; higher scores indicate lower socioeconomic status; mean scores here are commensurate with upper-middle class status (Hollingshead, 1975)
Pubertal stage measured by the Pubertal Development Scale (Petersen et al., 1988), and shown converted to Tanner Stages (Carskadon & Acebo, 1993)
Males and females did not differ on age, IQ, SES, or ethnicity (all p’s > 0.24). However, females reported more advanced stages of puberty than males (p < 0.001). See Table 1 for details. This is fairly common, as females typically begin puberty earlier than males (McAnarney et al., 1992). Males demonstrated shorter RTs for the inhibition condition (t(82) = 2.031, p = 0.045) than females. There were no other significant differences in any other measure of performance between males and females (all p’s > 0.12). There was a trend for more advanced pubertal development to be associated with fewer inhibition (r = −0.20, p = 0.062) and task-switching (r = −0.18, p = 0.099) errors. However, given similar associations were seen between age and errors and the strong correlation between age and puberty, partial correlations controlling for age were performed. There was no longer an association between puberty and errors for either task (all p’s > 0.1). Puberty was not significantly associated with any other performance measure for inhibition or task-switching (all p’s > 0.2).
2.2. Inhibition
2.2.1. Whole-brain analyses
Two participants were removed from analyses because they demonstrated outlying FA values greater than 2.5 standard deviations from the mean in significant clusters. Results presented exclude these two participants (N = 82). Voxel-wise analyses using performance (inhibition metric) and the interaction of performance and age to predict FA, while controlling for age and IQ, revealed better inhibition was associated with greater FA in the right ACR (Table 2 and Figure 1). Two participants demonstrated potential partial volume effects in the ACR. Excluding these two individuals did not alter the results, and therefore they were retained in the analyses. Follow-up AD and RD analyses found both AD and RD in the ACR predicted performance (Table 2), but only RD was significant after Bonferonni correction. There were no significant clusters for the interaction of age by performance. Similar voxel-wise analyses using performance and the interaction of performance and age to predict MD, while controlling for age and IQ, revealed no significant results.
Table 2.
Inhibition and task-switching relationships with fractional anisotropy (FA), controlling for age and IQ. White matter region, MNI coordinates, and relationship with FA is reported for each significant cluster. Note that for the inhibition and task-switching metric used to quantify performance, better performance corresponds to lower scores. Radial (RD) and axial diffusion (AD) for each significant FA clusters is reported with the β-statistic and associated p-value.
| WM Region | # of Voxels | Relationship with Higher FA | MNI Coordinates | Mean Axial Diffusion (μm2/ms) (λ1) | Mean Radial Diffusion (μm2/ms) ((λ2+λ3)/2) | ||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | β | p-value | β | p-value | |||
| Inhibition | |||||||||
| Right anterior corona radiata | 57 | Better inhibition | 21 | 32 | 25 | −0.23 | 0.038 | 0.30 | 0.005 |
| Task- switching | |||||||||
| Right precentral gyrus WM | 52 | Better inhibition | 17 | −26 | 51 | −0.393 | 0.001 | 0.324 | 0.005 |
| Left splenium of the CC | 48 | Worse inhibition | −18 | −37 | 32 | 0.224 | 0.051 | −0.348 | 0.001 |
| Left superior corona radiata | 38 | Better inhibition | −31 | 16 | 19 | −0.196 | 0.077 | 0.272 | 0.016 |
| Task-switching by age interaction | |||||||||
| Left anterior corona radiata | 53 | -- | −20 | 34 | −8 | 0.332 | 0.004 | −0.247 | 0.031 |
Bold text highlights significant relationships at a p < 0.05 for inhibition and the task-switching by age interaction, and a Bonferroni corrected p < 0.016 for task-switching. WM = white matter.
Figure 1.

Relationship between fractional anisotropy (FA) and inhibition performance in the anterior corona radiata (ACR). A) Higher FA in the ACR was associated with better inhibition, shown in blue (57 voxels). Cluster is overlaid on mean white matter skeleton, shown in green. Cluster is significant at a voxel-wise p < 0.01 and clusterwise α < 0.01, corrected for multiple comparisons. Coordinates are presented in MNI space. B) Graph showing the correlation between higher FA in the ACR and better inhibition. Lower inhibition scores represent better performance. L = left, R = right.
2.2.2. Contribution of RT and error
Examination of the coefficient confidence intervals for RT and errors derived from multiple regression analyses predicting FA revealed neither RT nor errors significantly predicted FA in the ACR cluster (all p’s > 0.10). Therefore, FA was associated with a speed-accuracy trade off, and was not driven primarily by speed of responses or error-responding.
2.2.3. Sex and puberty
Exploratory hierarchical regression analyses, with sex and puberty entered in the first step followed by a full model including sex, puberty, age, IQ, and performance, found that sex and puberty entered alone did not account for a significant portion of variance in FA in the ACR (R2 = 0.011, F(2,81) = 0.43, p = 0.65). Although the full model was not significant (R2 = 0.10, F(5,81) = 1.70, p = 0.14), performance was the only significant predictor of FA (β = −0.30, t(76) = −2.55, p = 0.013). Males and females did not have significantly different FA values in the ACR (p = 0.35).
2.3. Task-switching
2.3. 1 Whole-brain analyses
Voxelwise analyses demonstrated better task-switching was associated with greater FA in right precentral gyrus white matter (PrG-WM) and left superior corona radiata (SCR). Worse task-switching was associated with greater FA in the left splenium of the corpus callosum (SCC; Table 2 and Figure 2). AD and RD analyses showed both diffusion parameters in PrG-WM predicted task-switching, whereas only RD in the SCC predicted task-switching. Although RD in the SCR predicted task-switching, it was not significant after Bonferonni correction (Table 2). Similar voxelwise analyses using performance and the interaction of performance and age to predict MD, while controlling for age and IQ, revealed no significant results.
Figure 2.
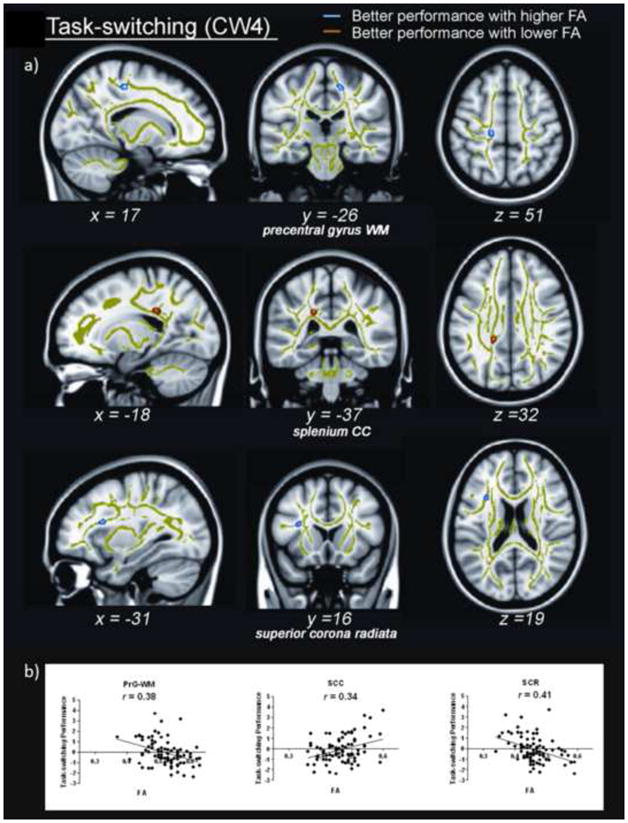
White matter regions demonstrating an association between fractional anisotropy (FA) and task-switching performance. A) Top: Higher FA in precentral gyrus white matter (PrG-WM) was associated with better task-switching, shown in blue (52 voxels). Middle: Higher FA in the splenium of the corpus callosum (SCC) was associated with worse task-switching, shown in red (48 voxels). Bottom: Higher FA in superior corona radiata (SCR) was associated with better task-switching, shown in blue (38 voxels). Clusters are overlaid on mean white matter skeleton, shown in green. Clusters are significant at a voxel-wise p < 0.01 and clusterwise α < 0.01, corrected for multiple comparisons. Coordinates are presented in MNI space. B) Graphs showing the correlation between FA and task-switching. Left: Correlation between higher FA in PrG-WM and better task-switching. Middle: Correlation between lower FA in the SCC and better task-switching. Right: Correlation between higher FA in the SCR and better task-switching. Lower task-switching scores represent better performance. L = left, R = right.
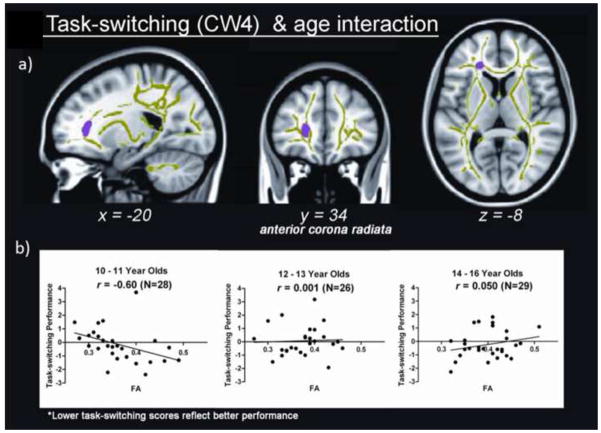
A cluster in the left ACR demonstrated an age by performance interaction effect (Table 2 and Figure 3). Two participants demonstrated potential partial volume effects. Excluding these two individuals did not alter the results, therefore the original results are reported. Visual inspection of the data showed a potential bivariate outlier, which was confirmed using Mahalanobis Distances with a significance value of p < 0.001. One participant (male, age 14) met this criterion and was removed from follow-up analyses only, as running the original analyses without this individual did not affect the results. In younger adolescents, age 10–11 (N = 28, 15 male), greater FA in the ACR was associated with better task-switching (r = 0.60, p <0.001), whereas adolescents age 12–13 (N = 26, 11 male) and 14–16 (N = 29, male = 13) did not demonstrate a relationship between FA in the ACR and task-switching (12–13: r = 0.001, p = 0.88; 14–16: r = 0.050, p = 0.24) (Figure 3). Mean AD and RD analyses showed that both AD and RD predicted task-switching performance, although only AD was significant after Bonferroni correction (Table 2).
Figure 3.
White matter regions demonstrating an interaction between age and task-switching performance in the anterior corona radiata (ACR). A) Cluster in the ACR demonstrating an interaction between age and task-switching performance, shown in purple (53 voxels). Cluster is overlaid on mean white matter skeleton, shown in green. All clusters are significant at a voxel-wise p < 0.01 and clusterwise α < 0.01, corrected for multiple comparisons. Coordinates are presented in MNI space. B) Graphs showing the correlation between FA in the ACR cluster and task-switching performance. The sample is divided into three age groups, from left to right, 10–11 year olds, 12–13 year olds, and 14–16 year olds. Task-switching metric is on the x-axis, and was calculated by adding normalized reaction time and normalized errors. Higher scores represent worse performance. L = left, R = right.
2.3.2. Contribution of RT and error
Examination of the coefficient confidence intervals for RT and errors derived from multiple regression analyses revealed RT (β = 0.025, CI = [0.018 – 0.032], p = 0.001) was a better predictor of FA in the SCC than errors (β; = 0.010, CI = [0.003 to 0.017], p = 0.14). Neither errors nor RT differentially predicted FA in PrG-WM (RT:β = −0.019, CI = [−0.026 to −0.012], p = 0.009; errors:β = −0.012, CI = [−0.019 to −0.005], p = 0.067) or SCR (RT:β = −0.018, CI = [−0.025 to −0.011], p = 0.008; errors:β = −0.015, CI = [−0.022 to −0.008], p = 0.026). For the age by performance interaction cluster in the ACR, neither RT nor errors predicted FA (all p’s > 0.28).
2.3.3. Sex and puberty
Exploratory hierarchical regression analyses, with sex and puberty entered in the first step followed by a full model including sex, puberty, age, IQ, and performance, found that sex and puberty entered alone did not account for a significant portion of the variance in FA in the Main Effect clusters in PrG-WM (R2 = 0.003, F(2,83) = 0.12, p = 0.89) or the SCR (R2 = 0.072, F(2,83) = 0.21, p = 0.81). For both PrG-WM and the SCR, the full model was significant (PrG-WM: R2 = 0.23, F(5,83) = 4.60, p = 0.001; SCR: R2 = 0.20, F(5,83) = 3.90, p = 0.003). Task performance remained a significant predictor in both these models (PrG-WM: β = −0.34, t(78) = −3.37, p = 0.001; SCR: β = −0.39, t(81) = −3.76, p < 0.001). There was a trend for the model of just sex and puberty to predict FA in the Main Effect cluster in the SCC (R2 = 0.26, F(2,81) = 2.92, p = 0.059). The full model was significant (R2 = 0.24, F(5,83) = 5.03, p < 0.001). Task performance (β = 0.36, t(81) = 3.58, p = 0.001) was significant, and there was a trend for sex (β = 0.23, t(81) = 1.94, p = 0.056). For the age by performance interaction cluster in the ACR, sex and puberty accounted for a significant portion of the variance in FA (R2 = 0.079, F(2,83) = 3.48, p = 0.035). Both sex(β = 0.26, t(81) = 2.20, p = 0.030) and puberty (β = 0.25, t(81) = 2.20, p = 0.031) significantly predicted FA. The full model was also significant (R2 = 0.30, F(5,83) = 6.72, p < 0.001). However, neither sex (p = 0.11) nor puberty (p = 0.64) remained significant. The interaction term remained a significant predictor of FA in the full model ( β = 0.37, t(78) = 3.77, p < 0.001). Males (M = 0.48, SD = 0.50) demonstrated higher FA values in the SCC than females (M = 0.45, SD = 0.57; p = 0.018). No other regions demonstrated significant sex differences in FA values (all p’s > 0.15).
2.4. Exploratory Overlap Analyses
To explore the degree to which white matter tracts identified in the FA voxelwise analyses were specific to inhibition or task-switching, exploratory multiple regression analyses were run. For the ACR inhibition Main Effect cluster, task-switching performance was entered to predict FA, controlling for age and IQ. The full model was not significant (R2 = 0.008, F(3,81) = 0.21, p = 0.89). A similar multiple regression analysis was run for each of the three Main Effect clusters for task-switching, using inhibition performance to predict FA. The full model was not significant for the SCC (R2 = 0.050, F(3,83) = 1.41, p = 0.025) and SCR (R2 = 0.064, F(3,83) = 1.83, p = 0.15). The full model was significant for the PrG-WM (R2 = 0.11, F(3,83) = 3.36, p = 0.023), but inhibition was not a significant predictor (p = 0.95). Therefore, this suggests FA findings are not reflective of a task-general network.
3. Discussion
This study examined the relationship between white matter microstructure and inhibition and task-switching in adolescents. As predicted, greater white matter integrity in the right ACR was associated with better inhibitory skills independent of age. In contrast to our predictions, greater white matter integrity of the right PrG-WM and left SCR were associated with better task-switching, whereas greater white matter integrity of the SCC was associated with worse task-switching. Unlike inhibition, age moderated the association between task-switching and white matter integrity in the left ACR, such that performance and white matter integrity were associated in younger but not older adolescents.
3.1. Inhibition
The ACR was the only region that demonstrated an association between white matter integrity and inhibition, namely greater white matter integrity was associated with better inhibition, regardless of age. The ACR is a subsection of the corona radiata, which is comprised of both projection and commissural fibers in the cerebral cortex (Oishi et al., 2011; Wanaka et al., 2004). Projection fibers fan out across the cortex and converge into the internal capsule (Oishi et al., 2011) and are included in multiple tracts, including thalamocortical, corticothalamic, corticopontine, and corticospinal tracts. Commissural fibers connect many bilateral prefrontal regions, including DLPFC, MFG, and mPFC (Oishi et al., 2011; Wanaka et al., 2004). Based on available tractography and grey matter anatomy, the ACR likely contains projections in the prefrontal cortical regions, including the DLPFC, IFG, and dorsal anterior cingulate cortex (ACC). Grey matter in these regions has been implicated in both motoric and cognitive inhibition, as well as inhibition of conflicting or irrelevant information (Anderson & Green, 2001; Aron et al., 2003, 2004; Banich, 2009; Barch et al., 2001; Botvonick et al., 2004; Depue et al., 2007; Garavan et al., 1999; Konishi et al., 1998).
Greater FA in the ACR is associated with better attention to relevant stimuli and inhibition of irrelevant stimuli in both adults (Niogi et al., 2008, 2010) and adolescents (Yin et al., 2013). Additionally, meditation training in adults increases FA in the ACR (Tang et al., 2010). Other DTI studies using ROI and tract-based approaches that did not specifically include the corona radiata have found increased FA in frontal white matter and tracts including projection fibers found in the corona radiata, such as the thalamocortical and corticospinal tracts, are associated with better attention and inhibition (Madsen et al., 2010; Tamnes et al., 2012). Furthermore, decreased FA in the ACR has been found in individuals with disorders characterized by inattention and impulsivity, such as ADHD (for review, see van Ewijk et al., 2012) and bipolar disorder (Pavuluri et al., 2009).
Given that inhibition likely involves allocation of attention towards task-relevant representations and responding (Munakata et al., 2011), our results are consistent with the ACRs associations with attention (Niogi et al., 2008, 2010; Tang et al., 2010; Yin et al., 2013) and the functional role of grey matter regions bordering the ACR, namely the DLPFC and ACC, in the cognitive control of attention (Banich et al., 2000a, 2000b; Clark et al., 2000; Kirino et al., 2000; Milham et al., 2003). Additionally, FA in the ACR was not uniquely predicted by RT or errors on the inhibition task, but rather a speed-accuracy trade off. This is consistent with the ACCs role in error detection and recruitment of prefrontal regions to enhance top-down control when needed (Banich, 2009; Barch et al., 2001; Botvinick et al., 2004; Kerns et al., 2004; MacDonald et al., 2000; Milham et al., 2003; Silton et al., 2010).
3.2. Task-switching
Greater FA in the SCR and PrG-WM, white matter tracts that border frontal and parietal grey matter regions that have been implicated in task-switching, were associated with better task-switching, independent of age. Similar to the ACR, the SCR contains projections from the ACC and frontal regions. However, the SCR likely includes projections from posterior dorsal ACC and posterior frontal regions (Jellison et al., 2004; Oishi et al., 2011; Wanaka et al., 2004). Similar to the SCR, projections from PrG-WM also connect to the CST and internal capsule (Oishi et al., 2011; Wanaka et al., 2004). Additionally, PrG-WM fibers extend to other regions, including the corpus callosum, superior frontal gyrus, and parietal regions (Holodny et al., 2001). Posterior frontal regions bordering the SCR, such as the pre-SMA, and parietal regions bordering the PrG-WM are associated with task-switching (for review, see Monsell, 2003). Consistent with functional studies, DTI studies examining task-switching using ROI-based approaches have found greater FA in pericallosal frontal white matter is associated with task-switching (Gold et al., 2010). Taken together, these results suggest that white matter tracts facilitating communication within prefrontal regions or between prefrontal and parietal regions are associated with task-switching.
Similar to inhibition, FA in the ACR was associated with task-switching. However, this relationship was moderated by age. Greater white matter integrity of the ACR was associated with better task-switching only in young adolescents (10–11 years), but was not associated with task-switching for the rest of the sample (12–16 years). Recent studies have found that commissural and projection fibers, which run through the ACR, show age-related changes during childhood and early adolescence, but not during later adolescence (Lebel & Beaulieu, 2011; Lebel et al., 2012). It is possible age only moderated the relationship between FA in the ACR and task performance for task-switching, and not inhibition, because of differences in the developmental trajectory for these two cognitive processes. Inhibition develops earlier than task-switching, which has been attributed to inferior frontal regions developing earlier than dorsal and middle frontal regions (for review, see Durston & Casey, 2006).
SCC was the only white matter region to demonstrate a relationship between greater FA and worse performance. Although the SCC has not been associated with task-switching, greater FA in the cingulum has been associated with better sustained attention (Takahashi et al., 2010) and found to be decreased in individuals with poorer sustained attention, such as those with mild traumatic brain injury (Bonnelle et al., 2011). Given we are unable to determine the differential contribution of set maintenance (e.g., “name the ink” trial followed by a “name the ink” trial) and switch cost (e.g., “name the ink” trial followed by a “name the word” trial), it’s possible that sustained attention to one task-set may actually increase the time cost for switching to a new task-set, and therefore increase overall RT. For the SCC, only RT, and not errors, predicted FA. Additionally, cognitive and motor training studies have demonstrated increased FA in white matter is associated with improved performance, including faster RT, after even short periods of training (Mackey et al., 2012; Scholz et al., 2009; Takeuchi et al., 2010; Tang et al., 2010). Therefore, the association between FA in the SCC and task-switching may reflect better initial task-set maintenance and hence greater difficulty in switching.
3.3. Implications for EF
Decreased RD was associated with performance in all white matter regions demonstrating a relationship with EF. Additionally, some regions also demonstrated increased AD. Longitudinal studies have found increases in FA over adolescence and into young adulthood are accompanied by decreased RD, and to a lesser extent increased AD, which has been posited to relate to increased myelination (Lebel et al., 2011). During adolescence, increased myelination of white matter occurs and is thought to reflect enhanced conduction and communication between brain regions (Casey et al., 2008). In addition to maturation of frontal regions, enhanced communication between brain regions is also likely to be important for the development of EF during this time period. Alternatively, it is also possible that increased application and strategic use of EF enhances communication between brain regions. Cognitive training studies have found increased FA, often driven by decreased RD, after training (Mackey et al., 2012). Furthermore, greater white matter integrity was associated with both inhibition and task-switching regardless of age, with only one region demonstrating a relationship between FA and performance that was dependent on age. If age was the main factor underlying the relationship between white matter microstructure and EF, we would have expected more regions to demonstrate an age by performance interaction. Our results suggest changes in microstructure in white matter connecting prefrontal regions, associated with cognitive control, across hemispheres and with the thalamus and basal ganglia may be related to individual development of EF across adolescence.
Our results are consistent with the proposal that EF consists of disparate, yet related, constructs (Friedman et al., 2008; Miyake et al., 2000). Although greater FA of the ACR was associated with both better inhibition and task-switching, this relationship was age-dependent for task-switching. Additionally, regression analyses revealed white matter regions associated with inhibition were not predicted by task-switching performance and vice versa. Previous studies of the three factor model of EF have demonstrated that although inhibition, shifting, and updating are separate latent factors, they are also correlated. This is especially true of inhibition, which is a general aspect of EF that is likely involved in most EF tasks (Friedman et al., 2008; Miyake et al., 2000). Given the presence of both projection and commissural fibers in the corona radiata, as well as the fact that projection fibers are part of multiple fiber tracts, it is possible that different tracts of the corona radiata are more or less integral to different factors. Further tractography studies are necessary to better discern which types of fibers and fiber tracts are associated with each factor.
3.4. Limitations
There are some limitations to the current study. First, although we were able to control for age and IQ, there are other aspects of development that might influence or interact with age to influence EF and the development of associated brain processes. Results of exploratory analyses suggested neither sex nor puberty, two potential confounding factors, were driving the observed associations. However, the results did indicate that sex may be an important factor to consider when examining brain and behavior relationships. Future studies should be conducted that directly explore the impact of these other relevant factors on the relationship between white matter diffusivity and EF. Secondly, there was only one measure of inhibition and task-switching. Multiple measures of both constructs are needed to further generalize the results to the latent constructs of inhibition and shifting. Additionally, the current measure only examined sustained performance, as RT and errors were computed for a series of trials. Future measures should be administered in such a way that trial-by-trial and sustained performance can be examined and compared. Another factor that may have affected our ability to detect interaction effects was the age range of our sample. Given the continued development of projection fibers, including the SLF, through late adolescence and young adulthood (Lebel & Beaulieu, 2011; Lebel et al., 2012), it is possible interactions of age and performance were not evident because we only captured a portion of a larger trajectory of white matter development.
3.5. Conclusion
In conclusion, the current study used DTI to examine the relationship between white matter integrity and two EF factors, inhibition and shifting, in adolescents. Results suggest increased white matter integrity of the ACR, frequently associated with attention and cognitive control, are also associated with inhibition and task-switching. However, the relationship between the ACR and EF was differentially influenced by age. Although greater FA was associated with better inhibition across all adolescents, only younger adolescents demonstrated an association with better task-switching. Greater integrity of PrG-WM and the SCR, white matter regions in the frontal cortex that border frontal and parietal grey matter regions that have been implicated in task-switching, were associated with better task-switching as well. The results suggest white matter microstructure in areas connecting bilateral cognitive control regions, as well as interconnections with subcortical regions responsible for motor control and sensory modulation, is associated with both inhibition and task-switching performance in adolescents. Although some relationships between FA and inhibition and task-switching were similar, they were not overlapping. In conceptualizing the impact of brain development on adolescent behaviors related to inhibition and task-switching, such as impulsivity or decision-making, we need to consider how connectivity between brain regions, not just regional development, may impact EF.
4. Experimental Procedure
4.1. Participants
Participants consisted of 89 youth between the ages of 10 and 16. Five participants were not included in analyses because they demonstrated outlying behavior that was greater than 2.5 standard deviations above the mean for their age on one of the two cognitive tasks. Therefore, the final sample consisted of 84 youth (age: M = 13.14, SD = 1.72). After obtaining written consent and assent from all youth and their parents in accordance with the Oregon Health & Science University (OHSU) Institutional Review Board, separate structured telephone interviews were conducted with both the youth and one of their parents. Exclusionary criteria for youth included lifetime personal history of a diagnosed DSM-IV psychiatric disorder (Diagnostic Interview Schedule for Children Predictive Scales) (Lucas et al., 2001), significant alcohol/substance use ( >10 lifetime alcoholic drinks or >2 drinks/occasion, >5 uses of marijuana, any other drug use, or >4 cigarettes per day) (Customary Drinking and Drug Use Record) (Brown et al., 1998), neurological illness, significant head trauma (loss of consciousness >2 minutes), serious medical problems, mental retardation or learning disability, prenatal exposure to drugs or alcohol (Structured Clinical Interview) (Brown et al., 1994), left-handedness (Edinburgh Handedness Inventory) (Oldfield, 1971), irremovable metal, and pregnancy. See Table 1 for participant demographics.
4.2. Participant Characteristics
Youth were administered the 2-subtest version of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) to provide an estimate of overall IQ, as previous studies have demonstrated a relationship between IQ and white matter integrity (Schmithorst et al., 2005). Additionally, youth completed the self-report Pubertal Development Scale (PDS) (Petersen et al., 1988). Previous studies have found relationships between FA and puberty (Herting et al., 2012). Information was gathered on socioeconomic status by administering the Hollingshead Index of Social Position to parents, which determines socioeconomic status based on occupation and educational attainment of each parent (Hollingshead, 1975). See Table 1 for participant characteristics.
4.3. Executive Function
Youth completed the Color Word Interference sub-test of the D-KEFS (Delis et al., 2001). This sub-test consists of four conditions, color naming (Condition 1), word reading (Condition 2), inhibition (Condition 3), and inhibition/task-switching (Condition 4). For all four conditions, participants are presented with one sheet containing rows of color patches (Condition 1) or color words (Conditions 2–4). Conditions 1 and 2 serve as control conditions, requiring the participant to name the patches of color or read color words printed in black ink, respectively. Condition 3 consists of color words printed in different colored ink (e.g., the word “red” printed in blue ink), and participants are instructed to name the ink color the word is printed in and not to read the word. This condition requires the participant to inhibit automatic processing of the irrelevant color information (word) and instead process the relevant color information (ink). In Condition 4, color words printed in different colored ink are again presented. 50% of the trials contain a box around the word. Participants are instructed to do exactly the same thing as Condition 3 for trials with no box, and to read the word and ignore the ink color on trials with a box. This condition requires participants to engage in task-switching as well as inhibition. Each condition is timed and the number of errors is recorded. For all conditions, participants are instructed to perform the task as quickly as they can without making mistakes.
Inhibition was calculated by first normalizing the RT for Conditions 1 and 3 separately. Condition 1 was subtracted from Condition 3 in order to assess inhibition while controlling for overall response rate. This difference was then normalized and added to the normalized number of errors on Condition 3. Task-switching was calculated by first normalizing RT for Conditions 3 and 4. Condition 3 was subtracted from Condition 4 in order to assess task-switching while controlling for inhibitory processes. This difference was normalized and added to the normalized number of errors on Condition 4. A metric including RT and errors was used for both performance measures as it represents a speed-accuracy trade-off. Although better performance on these EF tasks is often conceptualized based on RT, this is tempered by the accuracy of responding. Normalized raw RT, as opposed to scaled scores for conditions (presented in Table 1), was used to better capture variability and allow for the creation of a composite that reflected a speed-accuracy trade off. For both metrics, higher scores indicate worse performance.
4.4. Imaging Procedures
Images were acquired on a 3.0 Tesla Siemens Magnetom Tim Trio system (Siemens Medical Solutions, Erlangen, Germany) with a 12 channel head coil at OHSU’s Advanced Imaging Research Center. Whole-brain, high-resolution structural anatomical images were acquired in the sagittal plane using a T1-weighted magnetization prepared rapid gradient echo (MPRAGE) scanning sequence (T1 = 900 ms, flip angle = 10°, time echo (TE) = 3.58 ms, time repetition (TR) = 2300 ms, acquisition matrix = 256 × 240, slice thickness = 1.1 mm, scan time = 9:14). T2-weighted diffusion weighted images (DWIs) were acquired oblique to the anterior commissure-posterior commissure, using a high-angular resolution echo planar imaging (EPI) sequence (TR = 9500 ms, TE = 95 ms, field of view = 240 mm2, 72 slices, slice thickness = 2 mm, scan time = 15:04). Gradient encoding pulses were applied in 20 directions with a b-value of 1000 s/mm2, 4 diffusion-weighted runs were collected with 3 b0 (nondiffusion weighted) images per run. Past research has demonstrated 20 diffusion gradient directions allow for calculating robust and reliable FA measurements (Li et al., 2005; Ni et al., 2006).
4.5. Imaging Processing
Image processing is similar to previously published protocols (Herting et al., 2010, 2012). Eddy current effects, magnetic field inhomogeneities, and head motion were corrected using FMRIB’s Diffusion Toolbox and Utility for Geometrically Unwarping EPIs (Jenkinson, 2003). The 4 diffusion weighted images runs were aligned using linear (affine) registration to one another using FMRIB’s Linear Image Registration Tool (Jenkinson et al., 2002), averaged, and brain-extracted using BET (Smith, 2002). Based on the 6 motion parameters established by the affine registration of the b0s from each of the 4 runs, an average root mean square was determined. All participants had less than 2 mm RMS of movement for at least three co-registrations. AFNI (Cox, 1996) was then used to calculate the diffusion tensor and identify the eigenvalues of the tensor (λ1,λ2,λ3) for each voxel. FA and MD were determined for each voxel using AFNIs nonlinear computational algorithm (3dDWItoDT) (Cox, 1996).
Voxel-wise statistical analyses were performed on FA maps using TBSS (see Smith et al., 2006, 2007). Using individual FA maps, a common registration target image was identified and affine aligned to standard MNI space and each participant’s FA map was nonlinearly registered to this common target using FMRIB’s Non-linear Image Registration Tool (Andersson et al., 2007). Aligned FA images were averaged to create a group-wise mean FA map and a white matter skeleton, representing only the major tracts common across all participants (Smith et al., 2006, 2007). A mean FA threshold of 0.3 was applied to the white matter skeleton to reduce partial volume effects (Smith et al., 2006). Group level statistics were comprised of each participant’s aligned FA image projected onto the white matter skeleton. To examine MD, MD images were also af3ne aligned to standardized space, the nonlinear registration parameters determined by FMRIB’s Non-linear Image Registration Tool were applied to the MD maps, and MD images were merged and projected onto the FA-derived white matter skeleton to perform statistical comparisons.
4.6. Statistical Analysis
4.6. 1 Behavior
Statistical analyses were performed in PASW Statistics 18 (PASW, Chicago, Illinois) and a p <0.05 was used, unless otherwise indicated. Descriptive statistics for the scaled scores, as provided in the D-KEFS manual (Delis et al., 2001), were computed. Correlations were performed for the three inhibition performance measures, sample normalized contrast of RT for Condition 3 compared to Condition 1 (inhibition RT), sample normalized errors on Condition 3 (inhibition errors), and sample normalized inhibition metric, and age. Similarly, correlations were performed for the three task-switching measures, sample normalized contrast of RT for Condition 4 compared to Condition 3 (task-switching RT), sample normalized errors on Condition 4 (task-switching errors), and sample normalized task-switching metric, and age. IQ was correlated with RT, errors, and task metric for both inhibition and task-switching. Additional correlations were also performed between puberty, as measured by the PDS, and all performance measures for inhibition and task-switching. Independent samples t-tests comparing performance on all three performance measures for inhibition and task-switching between males and females was performed.
4.6.2. Imaging
Separate models examining the relationship between FA and task performance and the interaction of age and task performance, while controlling for age and IQ, were performed separately for inhibition and task-switching. The same models were also performed using MD data. Previous reports have described associations between age and FA and MD (Bava et al., 2010; Giorgio et al., 2008; Lebel & Beaulieu, 2011; Lebel et al., 2008, 2012) and IQ and FA (Schmithorst et al., 2005), and therefore these variables were controlled for in these analyses. Separate models examining age and IQ were performed for both FA and MD, and these results are reported in the Supplemental Information (SI Table 1 and SI Table 2). Using AlphaSim (Cox, 1996), Monte Carlo simulations were performed using both a voxel and cluster threshold (Forman et al., 1995) to correct for multiple comparisons. For all analyses, 35 contiguous voxels with a voxel-wise threshold of p < 0.01 (cluster volume ≥ 35 μl, α < 0.01) were necessary to consider a cluster significant using a 2.5-mm full width at half maximum intrinsic smoothing inherent to the DWI data. White matter tracts were identified using the MRI Atlas of Human White Matter, 2nd Edition (Oishi et al., 2011). Mean FA and MD values for significant effects of performance and interaction clusters were extracted for each participant in order to directly examine the relationship between FA and respective inhibition and task-switching performance. Of note, a negative correlation indicates better performance was associated with higher FA or MD values (see 4.3). Due to the sensitivity of TBSS to outliers, FA and MD values for each significant cluster were examined for potential outliers greater than 2.5 standard deviations from the mean for their age. Analyses were performed again excluding participants with FA values greater than 2.5 standard deviations and those results are reported. Furthermore, to ensure the results were not driven by partial volume effects, we overlaid the group effects on each individual participant’s skeleton and visually inspected that the cluster was in white matter. If any participants demonstrated partial volume effects, the analyses were re-run excluding them.
In order to better examine significant interactions, participants were divided into three approximately equal age groups (10–11, 12–13, and 14–16) and the relationship between FA or MD and age examined. Similar analyses were done grouping participants by single year, which produced similar results. For ease of presentation and interpretation, results are presented using the three age groups.
Additional analyses were conducted to explore the specificity of the contribution of inhibition or task-switching to the identified significant performance and interaction clusters. Multiple regression analyses were performed entering inhibition or task-switching performance, controlling for age and IQ, to predict FA or MD in the significant clusters predicted by the other performance metric.
AD and RD values were extracted from each significant cluster for the effects of performance and interactions for each participant. In order to better determine the degree to which AD and RD were related to performance, multiple regression analyses were performed. AD and RD values were extracted from each significant cluster and entered into a regression predicting respective task performance, while controlling for age and IQ. In order to correct for multiple comparisons for the three Main Effect of task-switching clusters, a Bonferroni-adjusted p < 0.016 was used for these analyses.
Given the metrics for inhibition and task-switching included both RT and errors, exploratory analyses were run to examine whether results related to performance were driven by RT, errors, or their unique combination. Multiple regression analyses were performed separately for each significant performance and interaction cluster entering the respective normalized RT difference and the normalized errors, controlling for age and IQ, to predict FA or MD in the region. As RT and errors were both included in the performance metric that was used to generate the FA results, we compared confidence intervals (95%) for the coefficients for RT and errors to determine if RT or errors differentially predicted FA, as opposed to examining whether each factor was a significant predictor.
Additional exploratory analyses were performed to examine whether sex and puberty (measured by the PDS) were contributing to the significant findings. Hierarchical multiple regression analyses were performed for each significant cluster, with the following variables used to predict FA or MD in the identified cluster. In the first step, sex and puberty were entered, and in the second step age, IQ, and relevant performance metric (inhibition, task-switching, or interaction) were entered. Additionally, t-tests contrasting FA or MD values for males and females in significant clusters were performed.
Supplementary Material
Examined white matter integrity and inhibition and task switching in adolescents
FA measure obtained from DTI using TBSS and correlated with performance
FA in anterior and superior corona radiata associated with inhibition
FA in superior corona radiata and precentral gyrus associated with task switching
FA in anterior corona radiata demonstrated an age by task switching interaction
Acknowledgments
The authors would like to thank Madison Stroup, Nate Spofford, Emily Maxwell, Richard Bruno, and Jill Waldman for their contributions to subject recruitment and data collection. This work and the authors were supported by a grant from the National Institute of Neurological Disorders and Stroke, K08 NS52147 (BJN), the Dana Foundation (BJN), and grants from the National Institute on Alcohol Abuse and Alcoholism, T32 AA007468-24 (KMS) and F31-AA019866 (MMH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kristen L. Mackiewicz Seghete, Email: mackiewi@ohsu.edu.
Megan M. Herting, Email: mherting@chla.usc.edu.
Bonnie J. Nagel, Email: nagelb@ohsu.edu.
References
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL. A developmental fMRI study of the Stroop color-word task. NeuroImage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Alexander AL, Eun Lee J, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapuetics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MC, Green C. Suppressing unwanted memories by executive control. Nature. 2001;410:366–369. doi: 10.1038/35066572. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka spatial normalization. FMIRB technical report TR07JA2. 2007 Available from: URL http://www.fmirb.ox.ac.uk/analysis/techrep.
- Andrews-Hanna JR, Mackiewicz Seghete KL, Claus ED, Burgess GC, Ruzic L, Banich MT. Cognitive control in adolescence: neural underpinnings and relation to self-report behaviors. PLoS ONE. 2011;6:e21598. doi: 10.1371/journal.pone.0021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Banich MT. Executive function: The search for an integrated account. Curr Dir Psychol Sci. 2009;18:89–94. [Google Scholar]
- Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang ZP, Wright A, Shenker J, Magin R. fMRI studies of stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. J Cog Neurosci. 2000a;12:988–1000. doi: 10.1162/08989290051137521. [DOI] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang ZP, Barad V, Gullett D, Shah C, Brown C. Prefrontal regions play a predominant role in imposing and attentional ‘set’: evidence from fMRI. Cog Brain Res. 2000b;10:1–9. doi: 10.1016/s0926-6410(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A. Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex. 2001;11:837–848. doi: 10.1093/cercor/11.9.837. [DOI] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Res. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bonnelle V, Leech R, Kinnunen KM, Ham TE, Beckman CF, De Boissezon X, Greenwood RJ, Sharp DJ. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J Neurosci. 2011;31:13442–13451. doi: 10.1523/JNEUROSCI.1163-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: dual mechanisms of cognitive control. In: Conway A, Jarold C, Kane M, Miyake A, Towse J, editors. Variation in Working Memory. Oxford University Press; Oxford: 2007. pp. 76–106. [Google Scholar]
- Brown GG, Kindermann SS, Siegle GJ, Granholm E, Wong EC, Buxton RB. Brain activation and pupil response during covert performance of the Stroop color word task. J Int Neuropsych Soc. 1999;54:308–319. doi: 10.1017/s1355617799544020. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Mott MA, Vik PW. Correlates of success following treatment for adolescent substance abuse. Appl Prev Psychol. 1994;3:61–73. [Google Scholar]
- Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J Adolesc Health. 1993;14:190–195. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Cohen JD. Interference and facilitation effects during selective attention: an H215O PET study of Stroop task performance. NeuroImage. 1995;24:264–272. doi: 10.1006/nimg.1995.1034. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann NY Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark VP, Fannon S, Lai S, Benson R, Bauer L. Responses to rare visual target and distractor stimuli using event-related fMRI. J Neurophysiol. 2000;83:3133–3139. doi: 10.1152/jn.2000.83.5.3133. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crone E. Executive function in adolescence: inferences from brain and behavior. Developmental Sci. 2009;12:825–830. doi: 10.1111/j.1467-7687.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) Psych Corp; San Antonio, TX: 2001. [Google Scholar]
- Depue BE, Curran T, Banich MT. Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science. 2007;317:215–219. doi: 10.1126/science.1139560. [DOI] [PubMed] [Google Scholar]
- Durston S, Casey BJ. What have we learned about cognitive development from neuroimaging? Neuropsychologia. 2006;44:2149–2157. doi: 10.1016/j.neuropsychologia.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Beate Walhovd K, Brown TT, Kuperman JM, Chung Y, Hagler DJ, Jr, Venkatraman V, Roddey JC, Erhart M, McCabe C, Akshoomoff N, Amaral DG, Bloss CS, Libiger O, Darst BF, Schork NJ, Casey BJ, Chang L, Ernst TM, Gruen JR, Kaufmann WE, Kenet T, Frazier J, Murray SS, Sowell ER, van Zigl P, Mostofsky S, Jernigan TL, Dale AM. Multimodal imaging of the self-regulating developing brain. Proc Natl Acad Sci. 2012;109:19620–19625. doi: 10.1073/pnas.1208243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. J Exp Psychol Gen. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999a;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Berry YC, Tobin M, Nelson J, Castellanos FX. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Prog Neuro-Psychoph. 1999b;23:571–588. doi: 10.1016/s0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, Matthews PM, Smith SM, Johansen-Berg H. Changes in white matter microstructure during adolescence. NeuroImage. 2008;39:52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jicha GA, Smith CD. Age-related slowing of task switching is associated with decreased integrity of frontoparietal white matter. Neurobiol Aging. 2010;3:512–522. doi: 10.1016/j.neurobiolaging.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Molfese DL, Walimuni IS, Stuening KK, Papanicolaou AC, Narayana PA, Fletcher JM. Diffusion tensor quantification and cognitive correlates of the macrostructure and microstructure of the corpus callosum in typically developing and dyselxic children. NMR Biomed. 2012;25:1263–1270. doi: 10.1002/nbm.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Maxwell EC, Irvine C, Nagel BJ. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb Cortex. 2012;22:1979–1992. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Schwartz D, Mitchell SH, Nagel BJ. Delay discounting behavior and white matter microsctructure abnormalities in youth with a family history of alcoholism. Alcohol Clin Exp Res. 2010;34:1590–1602. doi: 10.1111/j.1530-0277.2010.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. Yale University; New Haven, CT: 1975. [Google Scholar]
- Holodny AI, Ollenschleger MD, Liu WC, Schulder M, Kalnin NJ. Identification of the corticospinal tracts achieved using blood-oxygen-level dependent and diffusion functional MR imaging in patients with brain tumors. Am J Neuroradiol. 2001;22:83–88. [PMC free article] [PubMed] [Google Scholar]
- Huizinga M, Dolan CV, Van der Molen MW. Age-related change in executive function: developmental trends and a latent variable analyses. Neuropsychologia. 2006;44:2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Jellison BJ, Field AS, Medow J, Lazar M, Salamat SM, Alexander AL. Diffusion tensor imaging of cerebral white matter: a pictoral review of physics, fiber tract anatomy, and tumor imaging patterns. Am J Neuroradiol. 2004;25:356–369. [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2003;49:193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kirino E, Belger A, Goldman-Rakic P, McCarthy G. Prefrontal activation evoked by infrequent target and novel stimuli in a visual target detection task: an event-related functional magnetic resonance imaging study. J Neurosci. 2000;20:6612–6618. doi: 10.1523/JNEUROSCI.20-17-06612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Sekihara K, Miyashita Y. No-go dominant brain activity in human inferior prefrontal cortex revealed by functional magnetic resonance imaging. Eur J Neurosci. 1998;10:1209–1213. doi: 10.1046/j.1460-9568.1998.00167.x. [DOI] [PubMed] [Google Scholar]
- Kucukboyaci NE, Girad HM, Hagler DJ, Jr, Kuperman J, Tecoma ES, Iragui VJ, Halgren E, McDonald CR. Role of frontotemporal fiber tract integrity in task-switching performance of healthy controls and patients with temporal lobe epilepsy. J Int Neuropsych Soc. 2012;18:57–67. doi: 10.1017/S1355617711001391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker T, Leemans A, Phillips L, Beaulieu C. Microstuctural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Li TQ, Mathews VP, Wang Y, Dunn D, Kronenberger W. Adolescents with disruptive behavior disorder investigated using an optimized MR diffusion tensor imaging protocol. Ann N Y Acad Sci. 2005;1064:184–192. doi: 10.1196/annals.1340.034. [DOI] [PubMed] [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, Dulcan MK, Canino G, Rubio-Stipec M, Lahey BB, Friman P. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mackey AP, Whitaker KJ, Bunge SA. Experience-dependent plasticity in white matter microstructure: reasoning training alters structural connectivity. Front Neuroanat. 2012;6:Article 32. doi: 10.3389/fnana.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Madsen KS, Baaré WFC, Vestergaard M, Skimminge A, Ejersbo LR, Ramsøy TZ, Gerlach C, Åkeson P, Paulson OB, Jernigan TL. Response inhibition is associated with white matter microstructure in children. Neuropsychologia. 2010;48:854–862. doi: 10.1016/j.neuropsychologia.2009.11.001. [DOI] [PubMed] [Google Scholar]
- McAnarney ER, Kreipe RE, Orr DP, Comerci GD. Textbook of adolescent medicine. Philadelphia: W.B. Saunders Company; 1992. [Google Scholar]
- McCarthy G, Luby M, Gore J, Goldman-Rakic P. Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. J Neurophysiol. 1997;77:1630–1634. doi: 10.1152/jn.1997.77.3.1630. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Barad V. Competition for priority in processing increases prefrontal cortex’s involvement in top-down control: an event-related fMRI study of the stroop task. Cog Brain Res. 2003;17:212–222. doi: 10.1016/s0926-6410(03)00108-3. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cog Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends Cogn Sci. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O’Reilly RC. A unified framework for inhibitory control. Trends Cog Sci. 2011;15:453–459. doi: 10.1016/j.tics.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Ni H, Kavcic V, Zhu T, Ekholm S, Zhong J. Effects of number of diffusion gradient directions on derived diffusion tensor imaging indices in human brain. AJNR Am J Neuroradiol. 2006;27:1776–1781. [PMC free article] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P, Ghajar J, Johnson CE, Kolster R, Lee H, Suh M, Zimmerman RD, Manley GT, McCandliss BD. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 2008;131:3209–3221. doi: 10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- Niogi S, Mukherjee P, Ghajar J, McCandliss BD. Individual differences in distinct components of attention are linked to anatomical variations in distinct white matter tracts. Front Neuroanat. 2010;4:2. doi: 10.3389/neuro.05.002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Faria A, van Zijl PC, Mori S. MRI atlas of human white matter. 2. Oxford: Elsevier; 2011. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in 9- to 23-year olds: a diffusion tensor imaging study. J Cogn Neurosci. 2009;21:1406–1421. doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Yang S, Kamineri K, Passarotti AM, Srinivasan G, Harral EM, Sweeney JA, Zhou XJ. Diffusion tensor imaging study of white matter fiber tracts in pediatric bipolar disorder and attention deficit/hyperactivity disorder. Biol Psychiat. 2009;65:586–593. doi: 10.1016/j.biopsych.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Hum Brain Mapp. 2005;26:139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neuroscie. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silton RL, Heller W, Towers DN, Engels AS, Spielberg, Edgar C, Sass SM, Stewart JL, Sutton BP, Banich MT, Miller GA. The time course of activity in dorsolateral prefrontal cortex and anterior cingulate cortex during top-down attentional control. NeuroImage. 2010;50:1292–1302. doi: 10.1016/j.neuroimage.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith M, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, Robson MD, Jones DK, Klein JC, Bartsch AJ, Behrens TEJ. Acquisition and voxelwise analysis multi-subject diffusion data with tract-based spatial statistics. Nat Protoc. 2007;2:499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Demyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- Takahashi M, Iwamoto K, Fukatsu H, Naganawa S, Iidaka T, Ozaki N. White matter microstructure of the cingulum and cerebellar peduncle is related to sustained attention and working memory: a diffusion tensor imaging study. Neurosci Lett. 2010;477:72–76. doi: 10.1016/j.neulet.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Sekiguchi A, Taki Y, Yokoyama S, Yomogida Y, Komuro N, Yamanouchi T, Suzuki S, Kawashima R. Training of working memory impacts structural connectivity. J Neurosci. 2010;30:3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Fjell AM, Westlye LT, Østby Y, Walhovd KB. Becoming consistent: developmental reductions in intraindividual variability in reaction time are related to white matter integrity. J Neurosci. 2012;32:972–982. doi: 10.1523/JNEUROSCI.4779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Lu Q, Geng X, Stein EA, Yang Y, Posner MI. Short-term meditation induced white matter changes in the anterior cingulate cortex. Proc Natl Acad Sci. 2010;107:15649–15652. doi: 10.1073/pnas.1011043107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Kornblum S, Lauber EJ, Minoshima S, Koeppe RA. Isolation of specific interference processing in the Stroop task: PET activation studies. NeuroImage. 1997;6:81–92. doi: 10.1006/nimg.1997.0285. [DOI] [PubMed] [Google Scholar]
- van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlan J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. 2012 doi: 10.1016/j.neubiorev.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Vestergaard M, Madsen KS, Baaré WFC, Skimminge A, Ejersbo LR, Ramsøy TZ, Gerlach C, Åkerson P, Paulson OB, Jernigan TL. White matter microstructure in superior longitudinal fasiculus associated with spatial working memory performance in children. J Cogn Neurosci. 2011;23:2135–2146. doi: 10.1162/jocn.2010.21592. [DOI] [PubMed] [Google Scholar]
- Wanaka S, Jiang H, Nagae-Poetscher LM, van Zijl PCM, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corp; San Antonio, TX: 1999. [Google Scholar]
- Wheeler-Kingshott CAM, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- Yin X, Han Y, Ge H, Xu W, Huang R, Zhang D, Xu J, Fan L, Pang Z, Liu S. Inferior frontal white matter asymmetry correlates with executive control of attention. Hum Brain Mapp. 2013;34:796–813. doi: 10.1002/hbm.21477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiura T, Zhong J, Shibata DK, Kwok WE, Shrier DA, Numaguchi Y. Functional MRI study of auditory and visual oddball tasks. NeuroReport. 1999;10:1683–1688. doi: 10.1097/00001756-199906030-00011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.