Abstract
Fat infiltration within the fascial envelope of the thigh or intermuscular adispose tissue (IMAT), has been shown to be associated with both adverse metabolic and mobility impairments in older individuals. More recent findings suggest these fat deposits may be associated with increasing age and inactivity; and perhaps exercise may be able to counter or mitigate this increase in IMAT. This brief report summarizes the literature with respect to IMAT and its relationship to increasing age, physical activity levels, muscle strength, mobility and metabolism in the elderly. Further, we present preliminary data suggesting that IMAT is associated with increasing age in individuals across disease states (r=0.47, p<0.05), and that resistance exercise can decrease IMAT in older individuals with a variety of co-morbid conditions.
Keywords: Intermuscular adipose tissue, resistance exercise, muscle, fat
Introduction and Review of Literature
Age-related changes in the prevalence and magnitude of fat within the fascial envelope of locomotor muscle, termed intermuscular adipose tissue (IMAT), likely contributes to impaired muscle force production, mobility and metabolic status in the elderly. The independent univariate effect of aging on skeletal muscle fat infiltration however, is not fully understood. There are reports of a positive association between age and skeletal muscle fat (1–4), though others have not found this to be the case (5, 6). Reasons for the contradictory findings include the different types of skeletal muscle fat assessed, the age range of the subjects, and their varying physical activity levels. Of interest, the two studies (5, 6) that have not found increased skeletal muscle fat with increased age did not include individuals older than 70 years, a fact that may mask any increase in skeletal muscle fat with increasing age.
Moreover, it is difficult to determine the extent to which the accumulation of fat in muscle is directly attributable to aging and which to physical inactivity since muscle fatty infiltration has been unambiguously linked to physical inactivity in healthy younger individuals and in those with muscle impairments. Accumulation of IMAT occurs in the muscles of healthy young individuals after only four weeks of immobilization (7). Greater fat deposition has been reported in the paretic limb of chronic stroke survivors (8) and muscle fat content is 126% greater in patients six-weeks post spinal cord injury compared to able-bodied controls (9). We also have observed that unilateral muscle impairments and resultant inactivity are associated with increased IMAT (unpublished data).
It is well established that even in the healthy elderly, strength declines outweigh the loss of lean muscle tissue mass by up to three times (10–17). Further, maintenance or even gains in lean mass do not necessarily prevent this loss of strength (12); and the force produced by skeletal muscle per unit of muscle mass decreases with advancing age (12, 18, 19). While these facts do not diminish the importance of maintaining muscle mass as one ages, they do underscore that there is more to this loss of age-related muscle strength than simply a loss of lean tissue. The lipotoxic effect of fatty infiltration of muscle has recently gained attention and now joins the ranks of age-related neurological changes, hormonal and metabolic alterations, and the presence of pro-inflammatory cytokines as potential contributors to the loss of muscle force producing capabilities (20).
Recent evidence suggests that skeletal muscle lipid content influences muscle strength and mobility function (3, 21) as well as increased risk of future mobility loss in older men and women (22). The additional burden of age-associated co-morbid disease conditions appear to strengthen the association between skeletal muscle fat and mobility as obese individuals with diabetes and peripheral neuropathy demonstrate a strong inverse association between distal lower extremity IMAT and physical performance test scores (23). Lending support to this assertion is data from our laboratory (unpublished data) indicating that relative to mobility function (six minute walk and habitual physical activity), thigh IMAT is a more important muscle structure variable than thigh lean tissue in older cancer survivors.
Central body fat distribution has long been associated with metabolic abnormalities like type 2 diabetes (24–26), though adipose tissue in muscle is becoming increasingly important as it relates to metabolism, specifically its negative and independent impact on insulin resistance (27–31). Obese individuals with type 2 diabetes have higher amounts of IMAT but less subcutaneous adipose than obese individuals with normal glucose tolerance (32), suggesting that increased IMAT may intensify the risk of insulin resistance.
The purpose of this brief report is to present a suite of findings that confirm IMAT is positively associated with age across a broad spectrum of impaired individuals, and to present for the first time that resistance exercise training can decrease thigh IMAT in older individuals.
Methods
Participants
Aim 1
In order to examine the association of age with IMAT, we sought to include participants with a wide range of ages, mobility levels, and co-morbid diseases. The accessible population from which the sample was drawn was the participants who were enrolled in studies of lower extremity exercise training and their pre-training test results. The common inclusion criterion for these studies was a disease state or disability that resulted in muscle weakness or loss of muscle mass in the lower extremities. All subjects also had to be ambulatory. Subjects were excluded only if they had unstable medical conditions such as severe cardiac disease (New York Heart Association class III or uncontrolled hypertension (SBP > 165/95 mmHg); orthopedic problems that limited their ability to use exercise equipment without pain; diabetic retinopathy; myopathy; an inability to concentrate, follow directions or work independently; impaired knee flexion < 90°, or extreme claustrophobia.
Aim 2
In order to explore the impact of resistance exercise on thigh IMAT in older individuals, the sample was drawn from all participants in the age and IMAT analysis. To be included in the cohort for aim 2, participants had to be over 55 years old and have participated in a 12-week resistance-training program.
Measurements
Age, sex, and body mass index were gathered from all participants. Bilateral MRI scans of the thighs were obtained. Both IMAT and lean tissue cross-sectional areas were calculated from the MRI scans. Subjects were placed supine in a 1.5 Tesla whole body MR imager (Signa Lightening LX 8.4; General Electric Medical Systems, Milwaukee, WI). To establish the region of interest (ROI), a coronal fast spoiled gradient echo scout scan was used to identify the superior and inferior boundaries of the scans (the femoral head and the tibiofemoral joint line). Once the ROI was established, axial T1 weighted images were acquired in the standard body coil using a fast-spin echo sequence with repetition time/time to echo = 550/9.2, 8-mm slice thickness, 15-mm interslice distance, and a 320 X 320 matrix. Four images from the middle 1/3 of each thigh were used to determine average cross-sectional area (cm2) of IMAT and lean tissue using custom written image analysis software (MatLab; Mathworks, Natick, MA). Manual tracing eliminated subcutaneous fat and bone and isolated the fascial border of the thigh to create a subfascial ROI. The total number of pixels within the ROI, a frequency distribution and a histogram of all pixels and signal intensities produced two different peaks that differentiated lean tissue from IMAT. The dependent variable of percentage IMAT was calculated by dividing the cross sectional area of IMAT by the total thigh cross sectional area. The same investigator, blinded to time point of the scan and slice location, performed measurements of individual participants before and after training. This technique has demonstrated high levels of intrarater reliability (36), test-retest reliability (9, 37), and concurrent validity when compared to imaging of a cadaveric phantom limb (36).
Intervention
The resistance training consisted of high-force eccentric lower extremity extensor muscle contractions for 3 nonconsecutive days per week for 12 weeks. A physical therapist or exercise physiologist supervised all exercise sessions. Ratings of perceived exertion (RPE), using the Borg scale (38), were obtained from the subject at the end of each training session. This eccentric resistance program was titrated to progressively increase in intensity while avoiding muscle damage. The progression proceeded from a perceived exertion level of “very light” to “somewhat hard,” and from a duration of 5 minutes to 30 minutes per day and was performed on a high-force eccentric ergometer, described previously (39). As the ergometer pedals moved, subjects attempted to slow the pedals by applying force, resulting in high-force eccentric muscle contractions of the lower extremity extensor muscles. A visual analog scale (VAS) was used to monitor muscle pain prior to each session and heart rate and RPE were collected at the half-way point of each session.
Statistical Analyses
Descriptive statistics were calculated for demographic variables (Table 1). The distributions of all dependent variables were examined to insure that the assumptions of parametric statistical tests were met. To examine the relationship of age and locomotor muscle adiposity, bivariate Pearson product moment correlations were performed between age and the thigh IMAT. In order to examine the effect of resistance training on thigh tissue morphology, separate paired t-tests were utilized to compare pre and post training IMAT and lean tissue. All statistical tests were interpreted with an a priori alpha level of p<.05.
Table 1.
Demographic characteristics of all 88 participants. (IGT = impaired glucose tolerance)
| Disability (n) | Mean Age (years) | Sex (F/M) | Mean BMI (kg/m2) |
|---|---|---|---|
| Chronic Stroke Survivors (10) | 63.7 | 5/5 | 29.2 |
| Post-Menopausal with IGT (5) | 57.2 | 5/0 | 29.2 |
| Cancer Survivors (38) | 73.8 | 26/12 | 28.8 |
| Anterior Cruciate Deficient (13) | 30.2 | 5/8 | 24.9 |
| Multiple Sclerosis (5) | 59.0 | 2/3 | 30.3 |
| Total Knee Arthroplasty (17) | 67.4 | 12/5 | 32.1 |
Results
Aim 1
The participants included for examination of the relationship of age to IMAT included 88 subjects, aged 18–87 years, and included chronic 10 stroke survivors, 5 post-menopausal women with impaired glucose tolerance, 38 cancer survivors, 13 anterior cruciate ligament deficient individuals, 5 individuals with multiple sclerosis, and 17 individuals two years after a total knee replacement (Table 1).
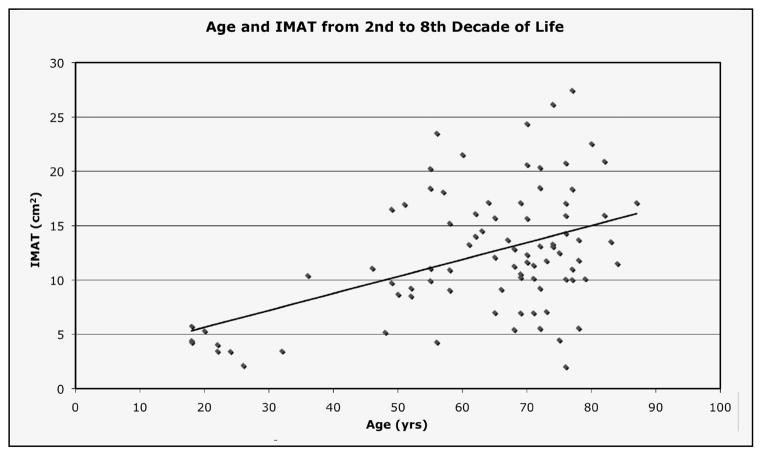
A significant (p<0.01) positive relationship exists between age and percentage of thigh IMAT (r=0.47), suggesting that aging is associated with an increase in thigh IMAT (Figure 1).
Figure 1.
Relationship between age and percent thigh intermuscular fat (IMAT) in 88 individuals with a variety of co-morbid conditions, r=0.47, p<0.05
Aim 2
The subset of the individuals utilized for Aim 1 comprised 32 individuals (mean age 69 years, 22 women, 10 men) who were either cancer survivors (n=15), chronic stroke survivors (n=4), had impaired glucose tolerance (n=2), multiple sclerosis (n=2), or total knee arthroplasty (n=9).
A significant (p<0.05) decrease in thigh IMAT (11.0%), and increase in thigh lean tissue (7.0%) was found in individuals 55 years and older who participated in a 12-week resistance training program.
Discussion
This investigation has demonstrated that aging is accompanied by an increase in muscle fatty infiltration, and that this fatty infiltration is amenable to change after 12 weeks of thrice-weekly resistance training in a group of older individuals with a variety of co-morbid conditions. The association of muscle fatty infiltration with increasing age in our sample validates and adds to the body of literature that describes this phenomenon in sedentary, healthy individuals (1, 2, 4, 40). Our finding of a reduction in thigh IMAT following 12 weeks of resistance training is novel and demonstrates a newly discovered adaptation to resistance training in this diverse group. Further, our results suggest an important role for resistance exercise in mitigating muscle fatty infiltration in the elderly across disease states.
Decreasing IMAT with a resistance training-only stimulus is a unique outcome, though recent evidence from our laboratory suggests resistance training combined with aerobic exercise may alter IMAT in middle-aged individuals with diabetes (33), and others (34, 35) have begun to report loss of other lower extremity fat depots with resistance exercise. In previously trained elderly individuals, withdrawal of resistance exercise substantially decreased the radiological density of skeletal muscle, indicating an increase in skeletal muscle lipid content. Further, when this group of 13 individuals participated in a 12-week re-training period it resulted in a significant decrease in muscle lipid content (35). In a separate study, a decrease of nearly 7% thigh fat was a significant but unexpected finding of a recent eccentric ergometer resistance-training program in 80 year-old healthy elders (34). Because the decreased fat was measured by densitometry it is unknown whether this fat was lost from the subcutaneous depot or within the fascial border of the thigh muscles (34).
Other evidence suggesting that changing the way a muscle is used, rather than aging itself, may play an important role in the level of fatty infiltration seen with age is found in studies of inactivity. A recent randomized controlled trial with a one-year follow-up determined that moderate increases in physical activity, made up of primarily brisk walking, prevented age-associated muscle fat infiltration in elderly individuals (41). This novel finding was especially interesting since the prevention of fat gain was specific to the sub-fascial fat depot and subcutaneous gluteal-femoral fat was not significantly different between the physical activity group and the controls.
Muscle fatty infiltration has been shown to have a negative impact on muscle strength and mobility (21–23, 40) in the elderly. A recent review paper suggests that muscle fat infiltration may in fact be more important than muscle lean when referring to mobility function (43). Though muscle fat infiltration was not specifically implicated, older individuals with type 2 diabetes are reported to have lower muscle quality, or a decreased ability to produce muscle force per unit of muscle mass, than age matched control subjects, despite having higher leg and arm muscle mass (44). Because those with diabetes have higher amounts of both total adipose tissue and IMAT, muscle fatty infiltration has been suggested as a potential contributory factor to the lower muscle quality seen in this population (12). Resistance training that results in improved muscle composition may increase not only muscle size, but muscle’s ability to produce force, or muscle quality and hence the ability to perform important mobility functions. Our data, in addition to the available literature suggests that fatty infiltration into muscle is a dynamic process that is responsive to exercise, a countermeasure that can prevent or reverse its occurrence.
Improving muscle composition by decreasing fatty infiltration may also positively impact metabolism. Several linkages have been found between muscle fat infiltration and insulin sensitivity in those with and without type 2 diabetes (27–31). Resistance training may be an especially attractive alternative or adjunct to the more traditional aerobic training that has come to be associated with diabetes care since it typically requires lower energetic output and perceived exertion. In fact, resistance training has recently been found to have a positive impact on insulin sensitivity in those with diabetes (45), and though the mechanisms of these improvements remains elusive, participation in regular resistance training is now recommended for those with type 2 diabetes (46, 47).
Conclusions
Fat infiltration in skeletal muscle is associated with both metabolic and mobility impairments in older individuals. IMAT increases with age and inactivity. While our data is consistent with previous reports of increased IMAT in older individuals, our results are novel in that we report IMAT responds to resistance training in older individuals with a variety of co-morbid conditions. This finding underscores the importance of further exploring the impact of exercise training on changes in IMAT, muscle strength, and mobility in the elderly and other mobility impaired individuals. Exercise that mitigates the accumulation of IMAT in older individuals has the potential to positively impact both metabolic and mobility impairments. Resistance training, in addition to increasing lean tissue, may improve muscle composition in older adults. Determining the functional and metabolic impact of these changes is an alluring proposition that deserves future research consideration.
Footnotes
Financial disclosure: None of the authors had any financial interest or support for this paper.
References
- 1.Cree MG, Newcomer BR, Katsanos CS, et al. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab. 2004;89(8):3864–71. doi: 10.1210/jc.2003-031986. [DOI] [PubMed] [Google Scholar]
- 2.Gallagher D, Kuznia P, Heshka S, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81(4):903–10. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90(6):2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa Y, Hattori M, Harada K, Shirase R, Bando M, Okano G. Age-related changes in intramyocellular lipid in humans by in vivo H-MR spectroscopy. Gerontology. 2007;53(4):218–23. doi: 10.1159/000100869. [DOI] [PubMed] [Google Scholar]
- 5.Machann J, Thamer C, Schnoedt B, et al. Age and gender related effects on adipose tissue compartments of subjects with increased risk for type 2 diabetes: a whole body MRI/MRS study. MAGMA. 2005;18(3):128–37. doi: 10.1007/s10334-005-0104-x. [DOI] [PubMed] [Google Scholar]
- 6.Martin JC, Farrar RP, Wagner BM, Spirduso WW. Maximal power across the lifespan. J Gerontol A Biol Sci Med Sci. 2000;55(6):M311–6. doi: 10.1093/gerona/55.6.m311. [DOI] [PubMed] [Google Scholar]
- 7.Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz-Snyder LL, Harris TB. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr. 2007;85(2):377–84. doi: 10.1093/ajcn/85.2.377. [DOI] [PubMed] [Google Scholar]
- 8.Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil. 2002;83(12):1703–7. doi: 10.1053/apmr.2002.36399. [DOI] [PubMed] [Google Scholar]
- 9.Gorgey AS, Dudley GA. Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord. 2007;45(4):304–9. doi: 10.1038/sj.sc.3101968. [DOI] [PubMed] [Google Scholar]
- 10.Evans WJ, Cyr-Campbell D. Nutrition, exercise, and healthy aging. J Am Diet Assoc. 1997;97(6):632–8. doi: 10.1016/S0002-8223(97)00160-0. [DOI] [PubMed] [Google Scholar]
- 11.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88(4):1321–6. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 12.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–64. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 13.Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA. Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr. 2002;76(2):473–81. doi: 10.1093/ajcn/76.2.473. [DOI] [PubMed] [Google Scholar]
- 14.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 2000;89(1):81–8. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 15.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137(4):231–43. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 16.Roubenoff R. Sarcopenic obesity: does muscle loss cause fat gain? Lessons from rheumatoid arthritis and osteoarthritis. Ann N Y Acad Sci. 2000;904:553–7. doi: 10.1111/j.1749-6632.2000.tb06515.x. [DOI] [PubMed] [Google Scholar]
- 17.Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25(1):17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- 18.Brooks SV, Faulkner JA. Skeletal muscle weakness in old age: underlying mechanisms. Med Sci Sports Exerc. 1994;26(4):432–9. [PubMed] [Google Scholar]
- 19.Metter EJ, Lynch N, Conwit R, Lindle R, Tobin J, Hurley B. Muscle quality and age: cross-sectional and longitudinal comparisons. J Gerontol A Biol Sci Med Sci. 1999;54(5):B207–18. doi: 10.1093/gerona/54.5.b207. [DOI] [PubMed] [Google Scholar]
- 20.Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63(8):829–34. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- 21.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50(5):897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 22.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60(3):324–33. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 23.Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther. 2008;88(11):1336–44. doi: 10.2522/ptj.20080079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjostrom L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed) 1984;289(6454):1257–61. doi: 10.1136/bmj.289.6454.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsson B, Svardsudd K, Welin L, Wilhelmsen L, Bjorntorp P, Tibblin G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed) 1984;288(6428):1401–4. doi: 10.1136/bmj.288.6428.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr. 1956;4(1):20–34. doi: 10.1093/ajcn/4.1.20. [DOI] [PubMed] [Google Scholar]
- 27.Albu JB, Kovera AJ, Allen L, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr. 2005;82(6):1210–7. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26(2):372–9. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 29.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71(4):885–92. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 30.Perseghin G, Lattuada G, Danna M, et al. Insulin resistance, intramyocellular lipid content, and plasma adiponectin in patients with type 1 diabetes. Am J Physiol Endocrinol Metab. 2003;285(6):E1174–81. doi: 10.1152/ajpendo.00279.2003. [DOI] [PubMed] [Google Scholar]
- 31.Yim JE, Heshka S, Albu J, et al. Intermuscular adipose tissue rivals visceral adipose tissue in independent associations with cardiovascular risk. Int J Obes (Lond) 2007;31(9):1400–5. doi: 10.1038/sj.ijo.0803621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallagher D, Kelley DE, Yim JE, et al. Adipose tissue distribution is different in type 2 diabetes. Am J Clin Nutr. 2009;89(3):807–14. doi: 10.3945/ajcn.2008.26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcus RL, Smith S, Morrell G, et al. Comparison of combined aerobic and high-force eccentric resistance exercise with aerobic exercise only for people with type 2 diabetes mellitus. Phys Ther. 2008;88(11):1345–54. doi: 10.2522/ptj.20080124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mueller M, Breil FA, Vogt M, et al. Different response to eccentric and concentric training in older men and women. Eur J Appl Physiol. 2009 doi: 10.1007/s00421-009-1108-4. [DOI] [PubMed] [Google Scholar]
- 35.Taaffe DR, Henwood TR, Nalls MA, Walker DG, Lang TF, Harris TB. Alterations in muscle attenuation following detraining and retraining in resistance-trained older adults. Gerontology. 2009;55(2):217–23. doi: 10.1159/000182084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dibble LE, Hale TF, Marcus RL, Droge J, Gerber JP, LaStayo PC. High-intensity resistance training amplifies muscle hypertrophy and functional gains in persons with Parkinson’s disease. Mov Disord. 2006;21(9):1444–52. doi: 10.1002/mds.20997. [DOI] [PubMed] [Google Scholar]
- 37.Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA. Intramuscular fat and glucose tolerance after spinal cord injury--a cross-sectional study. Spinal Cord. 2004;42(12):711–6. doi: 10.1038/sj.sc.3101652. [DOI] [PubMed] [Google Scholar]
- 38.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2):92–8. [PubMed] [Google Scholar]
- 39.Marcus RL, Lastayo PC, Dibble LE, Hill L, McClain DA. Increased strength and physical performance with eccentric training in women with impaired glucose tolerance: a pilot study. J Womens Health (Larchmt) 2009;18(2):253–60. doi: 10.1089/jwh.2007.0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86(12):5755–61. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- 41.Goodpaster BH, Chomentowski P, Ward BK, et al. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol. 2008;105(5):1498–503. doi: 10.1152/japplphysiol.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorgey AS, Dudley GA. Spasticity may defend skeletal muscle size and composition after incomplete spinal cord injury. Spinal Cord. 2008;46(2):96–102. doi: 10.1038/sj.sc.3102087. [DOI] [PubMed] [Google Scholar]
- 43.Kidde JM, RL, Dibble L, Smith S, LaStayo P. Regional muscle and whole body composition factors related to an older individual’s level of mobility: A review. Physiotherapy Canada. doi: 10.3138/physio.61.4.197. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes. 2006;55(6):1813–8. doi: 10.2337/db05-1183. [DOI] [PubMed] [Google Scholar]
- 45.Gordon BA, Benson AC, Bird SR, Fraser SF. Resistance training improves metabolic health in type 2 diabetes: a systematic review. Diabetes Res Clin Pract. 2009;83(2):157–75. doi: 10.1016/j.diabres.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 46.Albright A, Franz M, Hornsby G, et al. American College of Sports Medicine position stand. Exercise and type 2 diabetes. Med Sci Sports Exerc. 2000;32(7):1345–60. doi: 10.1097/00005768-200007000-00024. [DOI] [PubMed] [Google Scholar]
- 47.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(6):1433–8. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]