Abstract
One-third of smokers primarily use menthol cigarettes and usage of these cigarettes leads to elevated serum nicotine levels and more difficulty quitting in standard treatment programmes. Previous brain imaging studies demonstrate that smoking (without regard to cigarette type) leads to up-regulation of β2*-containing nicotinic acetylcholine receptors (nAChRs). We sought to determine if menthol cigarette usage results in greater nAChR up-regulation than non-menthol cigarette usage. Altogether, 114 participants (22 menthol cigarette smokers, 41 non-menthol cigarette smokers and 51 non-smokers) underwent positron emission tomography scanning using the α4β2* nAChR radioligand 2-[18F]fluoro-A-85380 (2-FA). In comparing menthol to non-menthol cigarette smokers, an overall test of 2-FA total volume of distribution values revealed a significant between-group difference, resulting from menthol smokers having 9–28% higher α4β2* nAChR densities than non-menthol smokers across regions. In comparing the entire group of smokers to non-smokers, an overall test revealed a significant between-group difference, resulting from smokers having higher α4β2* nAChR levels in all regions studied (36–42%) other than thalamus (3%). Study results demonstrate that menthol smokers have greater up-regulation of nAChRs than non-menthol smokers. This difference is presumably related to higher nicotine exposure in menthol smokers, although other mechanisms for menthol influencing receptor density are possible. These results provide additional information about the severity of menthol cigarette use and may help explain why these smokers have more trouble quitting in standard treatment programmes.
Keywords: Age, nicotine dependence, nicotinic acetylcholine receptor, positron emission tomography, tobacco
Introduction
Tobacco dependence is a leading preventable cause of morbidity and mortality in the United States (CDC, 2009). Despite substantial improvements in tobacco control, the prevalence of cigarette smoking remains high, at 21% in the general population [(Brown, 2009); ∼46 million adults (CDC, 2009)].
Cigarette smoking leads to up-regulation of nicotinic acetylcholine receptors (nAChRs) in the human brain, including the α4β2* nAChR, which is the most common receptor subtype (Whiting & Lindstrom, 1988). Human post-mortem tissue studies show that chronic smokers have increased numbers of α4β2* nAChRs compared to non-smokers (Benwell et al. 1988; Breese et al. 1997) and that former smokers have nAChR densities similar to non-smokers (Breese et al. 1997). Many laboratory animal studies also demonstrate up-regulation of nAChRs in response to chronic nicotine administration (e.g. Marks et al. 2011; Zhang et al. 2002).
Brain imaging studies of human smokers using positron emission tomography (PET) and single photon emission computed tomography (Cosgrove et al. 2009; Mamede et al. 2007; Mukhin et al. 2008; Staley et al. 2006; Wullner et al. 2008; sample sizes of five to 16 smokers) demonstrate up-regulation of available nAChRs across a range of brain regions (e.g. cortex, brainstem and cerebellum) other than thalamus (Mamede et al. 2007; Mukhin et al. 2008; Staley et al. 2006; Wullner et al. 2008). This nAChR up-regulation normalizes to levels of non-smokers following roughly 3 (Mamede et al. 2007; Mukhin et al. 2008) to 12 (Cosgrove et al. 2009) wk abstinence. In one of these studies (Staley et al. 2006), β2-containing nAChR levels did not correlate with the severity of nicotine dependence, severity of withdrawal or the desire to smoke, indicating perhaps that these symptoms are not mediated by up-regulation of these nAChRs.
Menthol flavouring of cigarettes has been shown to affect a smoker's exposure to nicotine (Benowitz et al. 2004; Williams et al. 2007). Approximately 33% of smokers predominantly use menthol cigarettes (Substance Abuse and Mental Health Services Administration, 2009) and a central issue with these cigarettes is that smokers who use them have lower cessation rates in standardized treatment programmes than smokers who use non-menthol cigarettes (Gandhi et al. 2009; Okuyemi et al. 2007; Pletcher et al. 2006). Although many factors have been implicated in the initiation and continued usage of menthol cigarettes (Castro, 2004), studies of biological markers demonstrate that menthol itself inhibits nicotine metabolism (Benowitz et al. 2004) and that menthol cigarette smoking leads to elevated serum nicotine and cotinine levels and greater exhaled carbon monoxide (CO) levels (Williams et al. 2007). These elevated levels would be expected to lead to increased nAChR densities. As an aside, it is noted that two studies found similarities between menthol and non-menthol cigarette usage in cessation rates and bio-markers for smoking (Heck, 2009; Werley et al. 2007).
Recently, our group used PET and the radiotracer 2-[18F]fluoro-3-(2(S)azetidinylmethoxy pyridine (2-FA) to examine brain α4β2* nAChR availability in response to cigarette smoking (Brody et al. 2006, 2009) and secondhand smoke (SHS) exposure (Brody et al. 2011). These studies demonstrate that nAChR occupancy by nicotine is dose-dependent and that the presence of even small amounts of nicotine (from smoking a puff of a cigarette or SHS exposure) leads to substantial α4β2* nAChR occupancy.
In the present examination of a relatively large sample of smokers and non-smokers, we sought to: (1) confirm up-regulation of α4β2* nAChR density in smokers in brain regions other than the thalamus; (2) determine if cigarette type (menthol vs. non-menthol) affects the severity of nAChR up-regulation; (3) explore whether other factors, such as demographic variables, other smoking-related factors, drug/alcohol use or withdrawal/mood symptoms are associated with nAChR density.
Method
Participants and screening methods
Altogether, 114 otherwise healthy adults (22 menthol cigarette smokers, 41 non-menthol cigarette smokers and 51 non-smokers) completed the study and had usable data. Participants were recruited and screened using the same methodology as in our prior report (Brody et al. 2011). For smokers, the central inclusion criteria were current nicotine dependence and smoking levels of 10–40 cigarettes/d, while for non-smokers the central inclusion criterion was no cigarette usage within the past year. Exclusion criteria for all participants were pregnancy, use of a medication or history of a medical condition that might affect the central nervous system at the time of scanning or any history of mental illness or substance abuse/dependence. There was no overlap between the participant group studied here and the groups included in our prior reports (Brody et al. 2006, 2009, 2011).
During the initial visit, screening data were obtained to verify participant reports and characterize smoking history. Rating scales obtained were the Smoker's Profile Form (containing demographic variables and a detailed smoking history; see Supplementary material), Fagerström Test for Nicotine Dependence (FTND; Fagerstrom, 1978; Heatherton et al. 1991), Hamilton Depression Rating Scale (HAMD; Hamilton, 1967) and Hamilton Anxiety Rating Scale (HAMA; Hamilton, 1969). An exhaled CO level was determined using a MicroSmokerlyzer (Bedfont Scientific Ltd, UK) to verify smoking status [CO ≥8 parts per million (ppm) for active smokers and CO ≤4 ppm for non-smokers]. A breathalyser (AlcoMatePro; AK GlobalTech Corporation, USA) test, urine toxicology screen (Test Country I-Cup Urine Toxicology Kit; TestCountry, USA) and urine pregnancy test (for female participants of childbearing potential; Test Country Cassette Urine Pregnancy Test) were obtained at the screening visit to support the participant's report of no current alcohol or drug dependence and no pregnancy. This study was approved by the local institutional review board and participants provided written informed consent.
Abstinence period and PET protocol
Roughly 1 wk after the initial screening session, participants underwent PET scanning, following the same general procedure as in our prior reports (Brody et al. 2006, 2009, 2011). Participants from the smoker group began smoking/nicotine abstinence two nights prior to each PET session and were monitored as described previously (Brody et al. 2009, 2011), so that nicotine from smoking would not compete with the radiotracer for receptor binding during PET scanning.
At 11:00 hours on the scanning day, participants arrived at the VA Greater Los Angeles Healthcare System and abstinence was verified by participant report and having an exhaled CO ≤4 ppm. Each participant had an i.v. line placed at 11:45 hours in a room adjacent to the PET scanner. At 12:00 hours, bolus plus continuous infusion of 2-FA was initiated, with 147 MBq (3.98±0.06 mCi) 2-FA administered as an i.v. bolus in 5 ml saline over 10 ss. This same amount of 2-FA (147 MBq) was also diluted in 60 ml saline and 51.1 ml was infused over the next 420 min (7.3 ml/h) by a computer-controlled pump (Harvard model 22; Harvard Instruments, USA). Thus, the amount of 2-FA administered as a bolus was equal to the amount that would be infused over 500 min (Kbolus=500 min; Kimes et al. 2008). This Kbolus was effective for reaching an approximate steady state in recent studies by our group and collaborators (Brody et al. 2009, 2011; Kimes et al. 2008). After initiation of the bolus plus continuous infusion, participants remained seated in the room adjacent to the PET scanner for the next 4 h to allow the radio-tracer to reach a relatively steady state in brain. At 16:00 hours, PET scanning commenced and continued for 3 h, with a 10-min break after 90 min scanning. Scans were acquired as series of 10-min frames.
PET scans were obtained using the Philips Gemini TruFlight (Koninklijke Philips Electronics N. V., The Netherlands), a fully 3-dimensional PET-CT scanner, which was operated in non-TOF mode. Reconstruction was done using Fourier rebinning and filtered back projection and scatter and random corrections were applied. The mean spatial resolution (FWHM) for brain scanning is 5.0 mm (transverse) × 4.8 mm (axial). 2-FA was prepared using a published method (Dolle et al. 1998). A magnetic resonance imaging (MRI) scan of the brain was obtained for each participant within 1 wk of PET scanning with the same specifications as in our prior report (Brody et al. 2011).
Blood samples (5 ml) were drawn during PET scanning for determinations of free, unmetabolized 2-FA and nicotine levels in plasma. For 2-FA levels, four samples were drawn as standards prior to 2-FA administration and nine samples were drawn at predetermined intervals during PET scanning. 2-FA levels were determined using previously published methods (Shumway et al. 2007; Sorger et al. 2007). For nicotine levels, blood samples were drawn prior to and following PET scanning. These samples were centrifuged and venous plasma nicotine concentrations were determined in Dr Peyton Jacob's laboratory at UCSF, using a modified version of a published GC-MS method (Jacob et al. 1991). The lower limit of quantification for this method was 0.2 ng/ml. In addition to the participants described in this paper, 19 smokers completed study procedures, but were excluded from the data analysis because their plasma nicotine levels were unacceptably high (>0.4 ng/ml; determined after study participation). This issue of smokers using nicotine/tobacco during the abstinence period of a brain imaging study has also been reported in prior studies (Esterlis et al. 2010a; Staley et al. 2006), presumably related to difficulty in having tobacco-dependent smokers remain abstinent for a prolonged period.
Symptom rating scale administration
In addition to baseline rating scales cited above, symptom rating scales were obtained during the PET scanning procedure. During the 2-FA uptake period, the Profile of Mood States (POMS; McNair et al. 1988) and Shiffman–Jarvik Withdrawal Scale (SJWS; Shiffman & Jarvik, 1976) were administered once. At four time-points during the PET scanning day, the urge to smoke (UTS) craving scale (an analogue scale with 10 craving-related questions) was administered (see Supplementary material).
PET image analysis
After decay and motion correction, each subject's PET scans were co-registered to their MRI using PMOD version 2.9 (http://www.pmod.com/technologies/index.html). Regions of interest (ROIs) were drawn on MRI using PMOD and transferred to the co-registered PET scans. ROIs were the thalamus, prefrontal cortex, brainstem, cerebellum and corpus callosum, which were chosen based on prior reports indicating a range of receptor binding of 2-FA in these regions (Brody et al. 2006; Kimes et al. 2008; Mukhin et al. 2008). The thalamus, brainstem and cerebellum were drawn as whole structures, while representative slices of the prefrontal cortex and genu of the corpus callosum were drawn. ROI placement was visually inspected for each PET frame in order to minimize effects of co-registration errors and movement; this procedure was repeated if there was a noticeable problem.
Total volume of distribution (designated as VT/fP, based on standard nomenclature; Innis et al. 2007) was calculated for each region and used for the central study analyses. VT/fP values were determined from the 17 × 10-min PET frames, as the ratio CT/(CP·fP), where CT is the total concentration of 2-FA in the ROIs, (CP·fP) is the concentration of free 2-FA in plasma and fP is the fraction of free (unbound) 2-FA in plasma. The fraction of free (not protein bound), unmetabolized 2-FA was similar for the smoker and non-smoker groups (47±7 and 47±9%, respectively) and for the menthol and non-menthol smoker subgroups (49±7 and 45±6%, respectively).
For quantifying percent group differences in nAChR density, specific binding volume of distribution (VS/fP), (which is proportional to unbound nAChR density) was determined for each participant as the difference between VT/fP and the non-displaceable volume of distribution corrected for the free fraction of plasma 2-FA (VND/fP), such that:
Values for VND/fP were based on data from previously published findings by our group (Brody et al. 2006, 2011), with the assumption that these values did not differ between study groups.
Statistical analysis
For evaluating differences in α4β2* nAChR density between smokers and non-smokers, an overall multivariate analysis of covariance (MANCOVA) was performed using VT/fP values for the five ROIs as dependent measures and group (smoker vs. non-smoker) as a between-subject factor. MANCOVA controls for type 1 error for a multivariate dependent variable, here VT/fP values for the five ROIs. Follow-up analyses of covariance (ANCOVAs) were performed for each of the five brain regions with the same variables as for the MANCOVA. Percent group differences between smokers and non-smokers were then determined using VS/fP values. For comparing menthol vs. non-menthol smokers, the same analytic structure was used, but only data from smokers were included and menthol cigarette status was used as the between-subject factor. In all of these models, years of education and race/ethnicity (Caucasian vs. non-Caucasian) were included as nuisance covariates since the former differed between smokers and non-smokers and the latter differed between menthol and non-menthol smokers as described below. Including both covariates in all models provided the most conservative and comparable results across analyses.
To explore the impact of demographic, smoking-related, drug/alcohol use, and withdrawal/mood symptom measures on α4β2* nAChR density, separate MANCOVAs were performed with each of these variables as a covariate of interest or factor and VT/fP values for the ROIs as the dependent measures. For analyses that included smokers and non-smokers, smoking status (smoker vs. non-smoker) was included as a fixed factor. Significant MANCOVAs were followed up with ANCOVAs for the five ROIs separately. For these exploratory analyses, the following variables were tested: age; gender; race/ethnicity (Caucasian vs. non-Caucasian); marital status (single vs. married); height; weight; educational level (yr); mother's educational level, cigarettes/d; number of years smoking; number of quit attempts; longest lifetime period of abstinence; FTND scores; light vs. regular nicotine cigarette usage; caffeine intake (coffee cup equivalents per day); alcohol drinks/d; marijuana use status (≥1 use per wk); withdrawal/craving ratings (SJWS and UTS scales); anxiety/depression ratings [HAMA, HAMD and Beck Depression Inventory(BDI)]; subscales of the POMS. For these exploratory tests, no statistical corrections were made for multiple comparisons. Statistical tests were performed using PASW/SPSS Statistics version 19.0 (SPSS, Inc., USA).
Results
Demographics and rating scales
The smoker and non-smoker groups were middle-aged, with a slight majority of male participants and roughly equal numbers of Caucasian and non-Caucasian participants (Table 1). The groups did not differ on any demographic or rating scale measures, other than a small but significant difference in highest educational level obtained. Menthol (n=22) and non-menthol (n=41) cigarette smokers did not differ on any demographic or rating scale measure, other than race/ethnicity, where there were more non-Caucasians in the menthol smoker group (77.3 vs. 39.0%, respectively, χ2 test, p<0.05).
Table 1. Demographics and rating scale scores for the smoker and non-smoker groups.
| Variable | Non-smokers (n=51) | Whole smoker group (n=63) | Menthol smoker subgroup (n=22) | Non-menthol smoker subgroup (n=41) |
|---|---|---|---|---|
| Age | 37.0 (±12.0) | 38.8 (±13.7) | 43.1 (±12.9) | 36.4 (±14.2) |
| Gender (% female) | 47.1 | 33.3 | 31.8 | 34.1 |
| Ethnicity (% Caucasian) | 56.9 | 47.6 | 22.7* | 61.0 |
| Height (inches) | 68.3 (±4.0) | 68.7 (±3.5) | 69.1 (±3.2) | 68.5 (±3.7) |
| Weight (lbs) | 168.0 (±35.0) | 174.5 (±37.2) | 180.7 (±29.0) | 171.1 (±40.9) |
| Education (highest completed grade) | 15.5 (±1.9)** | 14.2 (±2.1) | 14.4 (±2.3) | 14.1 (±2.0) |
| Mother's education (highest completed grade) | 14.2 (±2.9) | 14.1 (±2.6) | 14.0 (±2.6) | 14.2 (±2.6) |
| Cigarettes/d | 0 (±0) | 18.9 (±4.6) | 19.9 (±5.8) | 18.4 (±3.7) |
| Fagerstrom Test for Nicotine Dependence | 0 (±0) | 4.0 (±2.3) | 4.1 (±2.1) | 3.9 (±2.4) |
| Hamilton Anxiety Rating Scale | 2.0 (±2.3) | 2.3 (±2.5) | 2.0 (±3.0) | 2.4 (±2.3) |
| Hamilton Depression Rating Scale | 1.6 (±2.0) | 2.0 (±2.3) | 1.7 (±2.7) | 2.2 (±2.0) |
| Beck Depression Inventory | 1.0 (±3.0) | 1.6 (±2.0) | 1.4 (±1.8) | 1.8 (±2.1) |
| Caffeine use (coffee cup equivalents/d) | 1.0 (±1.2) | 1.6 (±1.5) | 1.4 (±1.7) | 1.7 (±1.5) |
| Alcohol drinks/wk | 2.0 (±2.3) | 4.0 (±4.8) | 3.8 (±5.7) | 4.1 (±4.3) |
| Marijuana cigarettes/wk | 0.03 (±0.1) | 0.3 (±1.1) | 0.3 (±0.9) | 0.3 (±1.5) |
p<0.05 between the menthol and non-menthol smoker subgroups, χ2 test.
p<0.005 between the whole non-smoker and smoker groups, Student's t test; other than the difference in ethnicity between menthol and non-menthol smoker subgroups and the difference in education level between non-smokers and smokers, all other statistical tests for between-group differences were non-significant. In this analysis, no statistical corrections were made for multiple comparisons.
All values are presented as means (±S.D.) or percentages.
Comparison of α4β2* nAChR density between smokers and non-smokers
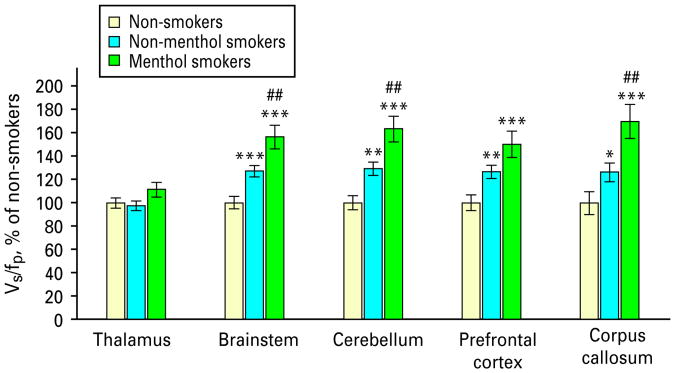
The overall test of VT/fP values revealed a significant difference between smokers and non-smokers (MANCOVA; F=22.8; d.f.=5,106; p<0.0005; Figs. 1 and 2). Follow-up tests for individual brain regions revealed significant between-group differences for the prefrontal cortex, brainstem, cerebellum and corpus callosum (ANCOVAs, F values = 16.1, 25.9, 24.1 and 11.7; all d.f.=1, 113; all p values <0.0005, except corpus callosum, where p=0.001; Table 2). VT/fP values for the thalamus were not significantly different between groups (ANCOVA, p=0.6). For regions found significant in this analysis (prefrontal cortex, brainstem, cerebellum and corpus callosum), α4β2* nAChR levels were higher in smokers than non-smokers by 36, 37, 42 and 40%, respectively. For the thalamus, α4β2* nAChR levels were 3% higher in smokers than non-smokers.
Fig. 1.
Both non-menthol and menthol cigarette smokers have higher α4β2* nicotinic acetylcholine receptor (nAChR) densities than non-smokers in the brainstem, cerebellum, prefrontal cortex and corpus callosum. Levels of nAChRs for the non-menthol and menthol smoker groups are compared to the non-smoker group (100%) and to one another. For comparisons with the non-smoker group, * p<0.05, ** p<0.005 and *** p<0.0005 (uncorrected). For comparisons between the non-menthol and menthol smoker groups, ## p<0.01 (uncorrected). Statistics are generally the same for specific binding volume of distribution (Vs/fp) values in this figure as for the Vt/fp values in Table 2, because the same non-displaceable volume of distribution (VND) values are subtracted from each participant's Vt/fp values to obtain Vs/fp values.
Fig. 2.
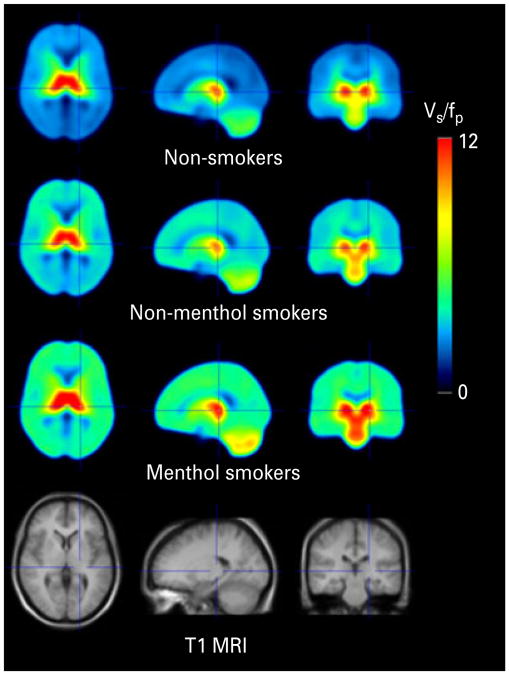
Menthol cigarette smokers have greater α4β2* nicotinic acetylcholine receptor (nAChR) up-regulation than non-menthol smokers. Averages of spatially normalized parametric images [specific binding volume of distribution (VS/fP)] obtained in the study from 51 non-smokers, 41 non-menthol cigarette smokers and 22 menthol cigarette smokers are shown. From left to right are transaxial, sagittal and coronal brain slices. The bottom row shows the mean T1-weighted magnetic resonance image (MRI) of study participants.
Table 2. Total binding volumes of distribution (VT/fP) in the brain regions of interest for non-smokers and smokers (and the non-menthol smoker and menthol smoker subgroups).
| Brain region | VT/fP values – non-smokers (n=51) | VT/fP values – smokers (n=63) | VT/fP values – non-menthol smoker subgroup (n=41) | VT/fP values – menthol smoker subgroup (n=22) |
|---|---|---|---|---|
| Thalamus | 16.0 (±3.6) | 16.3 (±3.1) | 15.7 (±2.8) | 17.3 (±3.4) |
| Prefrontal cortex | 7.1 (±1.4) | 8.1 (±1.4)*** | 7.9 (±1.2)** | 8.6 (±1.6)*** |
| Brainstem | 9.6 (±2.1) | 11.6 (±2.2)*** | 11.1 (±1.7)*** | 12.7 (±2.6)*** |
| Cerebellum | 8.2 (±1.8) | 9.9 (±1.8)*** | 9.4 (±1.5)** | 10.8 (±2.1)*** |
| Corpus callosum | 6.0 (±1.6) | 7.0 (±1.4)*** | 6.6 (±1.2)* | 7.6 (±1.6)*** |
p≤0.05,
p≤0.005,
p≤0.0005, compared to non-smokers (uncorrected).
All values are mean±s.d.
To confirm that prior smoking history did not affect study results, the non-smoker group was divided into never (<20 cigarettes lifetime; n = 30) and former (>1 yr abstinent; n = 21) smokers. Regional VT/fP values were compared between the subgroups. No significant differences were found for any regions (range of p values −0.4 to 0.7).
Comparison of α4β2* nAChR density between menthol and non-menthol cigarette smokers
The overall test of VT/fP values revealed a significant difference between menthol and non-menthol smokers (MANCOVA; F=2.7; d.f.=5, 55; p<0.05). Follow-up tests revealed between-group differences for the brainstem, cerebellum and corpus callosum (ANCOVAs, F values=8.1, 8.3 and 8.9; all d.f.=1, 62; p values=0.006, 0.006 and 0.004, respectively; Table 2). VT/fP values for the thalamus and prefrontal cortex did not reach significance (ANCOVAs; F values=3.5 and 3.8; d.f.=1, 62; p values=0.07 and 0.06, respectively). For regions found significant in this analysis (brainstem, cerebellum and corpus callosum), α4β2* nAChR levels were higher in menthol than non-menthol cigarette smokers by 21, 25 and 28%, respectively. For regions not found significant in this analysis (thalamus and prefrontal cortex), α4β2* nAChR levels were 9 and 19% higher, respectively, in menthol cigarette smokers.
For completeness, we performed MANCOVAs comparing menthol smokers to non-smokers and non-menthol smokers to non-smokers. We found that both smoker subgroups had significant up-regulation of nAChRs compared to non-smokers (MANCOVAs, F=24.8; d.f.=5, 65; p<0.0005 for the menthol smoker subgroup and F=18.6; d.f.=5, 84; p<0.0005 for the non-menthol smoker subgroup).
Exploratory analysis: α4β2* nAChR density and demographic measures
For the exploration of relationships between α4β2* nAChR density and demographic variables, the MANCOVA for age was significant (F=7.4, p<0.0005) with greater age being associated with lower nAChR density, independent of smoking status. For the non-smoker group, there were significant negative correlations between age and nAChR density for the brainstem (Spearman's ρ=−0.39, p=0.005) and corpus callosum (Spearman's ρ=−0.32, p=0.02), with trend level negative correlations for the cerebellum (Spearman's ρ=−0.25, p=0.07) and prefrontal cortex (Spearman's ρ=−0.27, p=0.06) but not thalamus (Spearman's ρ=−0.19, p=0.18). Across regions found significant in the preceding analysis, the change in nAChR density averaged −3.4% per decade. These findings are consistent with prior research indicating that α4β2* nAChR density decreases with age (Mitsis et al. 2009). MANCOVAs for gender, race/ethnicity, marital status, height, weight, education completed and mother's education completed were non-significant (p values = 0.2–0.9).
Exploratory analysis: α4β2* nAChR density and smoking-related measures
The overall MANCOVA for cigarettes/d did not reach significance (F=2.0; d.f.=5, 57; p=0.10), but individual ANCOVAs for the brainstem (F=4.2; d.f.=1, 62; p<0.05) and corpus callosum (F=6.1; d.f.=1, 62; p<0.05) were suggestive that a greater number of cigarettes/d was associated with greater up-regulation of nAChRs. There were no significant findings for number of years smoking, number of quit attempts, longest lifetime period of abstinence, FTND scores or light (vs. regular) nicotine cigarettes (MANCOVAs, F values = 0.3−1.7; d.f.=5, 57; p values = 0.1−0.9). The last finding with light nicotine cigarettes is consistent with prior research demonstrating similar biomarker exposure for light and regular nicotine cigarette smokers (Bernert et al. 2005).
Exploratory analysis: α4β2* nAChR density and drug/alcohol use
Caffeine use (coffee cup equivalents per day) had a significant relationship with α4β2* nAChR density (MANCOVA; F=2.8; d.f.=5, 107; p<0.05), with values for the thalamus and brainstem reaching significance (ANCOVAs; F values = 8.8 and 5.3;p values = 0.004 and 0.02, respectively), indicating that greater caffeine intake was associated with lower α4β2* nAChR density. Values for other regions did not reach significance (p values = 0.06−0.13). For marijuana, participants who reported current use (≥1 use per wk; n = 14) had significantly higher α4β2* nAChR densities than participants who did not use marijuana, after controlling for smoking status (MANCOVA; F=2.6; d.f.=5, 107; p<0.05). α4β2* nAChR density was numerically higher for the marijuana users in all regions studied and reached significance in the corpus callosum (ANCOVA; F=4.7; d.f.=1, 113; p<0.05). For alcohol use, the MANCOVA was not significant (p=0.6).
Exploratory analysis: α4β2* nAChR density and withdrawal/mood symptom factors
In the smoker group, no significant associations were found between withdrawal symptoms and α4β2* nAChR density for the overall SJWS score (or its subscales) (MANCOVAs; F's = 0.6–2.0; d.f. =5, 57; p's = 0.1–0.7) or UTS score (MANCOVA; F=1.2; d.f.=5, 57; p=03). For mood/anxiety symptoms in the whole study sample, no associations were found between α4β2* nAChR density and HAMA, HAMD or BDI scores (MANCOVAs; F's = 0.5–1.3; d.f.=5, 107; p's = 0.3–0.8). For normal mood states in the whole study sample, there were no significant associations between α4β2* nAChR density and POMS subscale scores (MANCOVAs; F's=0.2–1.2; d.f.=5, 107; p's=0.3–1.0).
Discussion
The study results demonstrate that: (1) α4β2* nAChRs are up-regulated in cigarette smokers (compared to non-smokers) in all regions studied other than thalamus; (2) α4β2* nAChR up-regulation is greater in menthol than non-menthol cigarette smokers. Other significant findings included an association between increasing age and decreasing α4β2* nAChR density, a suggestion of an association between number of cigarettes smoked per day and greater nAChR up-regulation in two brain regions and associations between caffeine use and marijuana use with decreased and increased α4β2* nAChR density, respectively. Based on the highly significant p values for the smoker vs. non-smoker comparisons and reports that menthol cigarette smokers have higher levels of nicotine exposure than non-menthol cigarette smokers, the study findings indicate that nicotine exposure is a potent determinant of α4β2* nAChR density.
For differences between smokers and non-smokers, this study supports much prior basic and clinical research demonstrating up-regulation of α4β2* nAChRs in brain regions other than the thalamus following nicotine (Marks et al. 2011; Yates et al. 1995; Zhang et al. 2002) or cigarette smoke (Mamede et al. 2007; Mukhin et al. 2008; Staley et al. 2006; Wullner et al. 2008) exposure. The mechanism of nAChR up-regulation has been examined (for comprehensive reviews of this topic, see Govind et al. 2009; Lester et al. 2009; Quick & Lester, 2002), with this research demonstrating that nAChR up-regulation is post transcriptional (Bencherif et al. 1995; Marks et al. 1992) and that nicotine acts as a stabilizing pharmacological chaperone for nascent α4β2* nAChRs in the endoplasmic reticulum (Srinivasan et al. 2011). The pattern of α4β2* nAChR up-regulation in smokers found in this and prior studies (robust in all regions other than thalamus) may be due to a ceiling effect, given the normally high density of nAChRs in this region.
For the finding of more pronounced up-regulation of nAChRs in menthol (compared to non-menthol) cigarette smokers, this result is consistent with prior research demonstrating that menthol smokers have relatively high levels of nicotine exposure (Benowitz et al. 2004; Williams et al. 2007), although other potential mechanisms are also possible. For example, menthol cigarette smoke exposure has been shown to increase the ratio of cotinine:nicotine (Abobo et al. 2012) and cotinine has been shown to interact with α4β2*nAChRs to result in dopamine release (O'Leary et al. 2008). Therefore, it is possible that menthol cigarette smoking leads to increased exposure to cotinine, which could lead to greater up-regulation of nAChRs. Additionally, recent work suggests that menthol may alter nAChR functioning directly (Hans et al. 2012).
The demonstration of greater nAChR up-regulation in menthol cigarette smokers may help explain why these smokers have more difficulty than non-menthol cigarette smokers in quitting smoking (Gandhi et al. 2009; Okuyemi et al. 2007; Pletcher et al. 2006). Marketing of menthol cigarettes is at least partly aimed at younger smokers (Kaufman et al. 2004) because the menthol flavouring may make these cigarettes more palatable than non-menthol cigarettes (McClernon et al. 2007) and tobacco smoke may be milder for inexperienced smokers (Kreslake et al. 2008). Tobacco industry documents also demonstrate that menthol cigarettes have been specifically marketed to urban smokers in minority racial/ethnic groups (Gardiner, 2004; Sutton & Robinson, 2004), which is consistent with our study sample composition. Studies of African-American smokers (Allen & Unger, 2007; Castro, 2004) indicate that a combination of physiological, psychological and societal factors results in elevated levels of menthol cigarette usage. Because of these issues, several research groups have called for a better understanding of the physiological mechanism of menthol cigarette usage (e.g. Hyland et al. 2002). The present study presents (to our knowledge) the first evidence of greater α4β2* nAChR up-regulation in menthol cigarette smokers, which has implications for the underlying neurobiology of menthol cigarette usage.
The suggestion of an association between number of cigarettes/d and α4β2* nAChR density in brainstem and corpus callosum (and non-significant trends in the other regions) is consistent with higher levels of nicotine exposure being associated with greater α4β2* nAChR up-regulation. For the present study, all regions may not have reached significance due to the narrow range of smoking levels in the sample, which was selected to obtain a relatively homogeneous population of smokers.
Findings in the exploratory analyses that caffeine and marijuana use were associated with decreased and increased α4β2* nAChR density, respectively, were unexpected. However, because smoking status was controlled for, these analyses suggest that caffeine and marijuana have effects on α4β2* nAChR density that are independent of nicotine exposure. While these results could be due to type I error, it is also possible that these other substances affect nAChR density through their pharmacological effects, although more work is needed to confirm and explain these effects. The absences of findings with alcohol use or smoking-related symptoms are consistent with prior research (Esterlis et al. 2010b; Staley et al. 2006), which also did not have significant findings with these variables.
A central limitation of the study was that nicotine/cotinine levels were not available from smokers during their habitual smoking, so it was not possible to confirm that menthol cigarette smokers indeed had greater nicotine exposure than non-menthol smokers, as was demonstrated in past research (Benowitz et al. 2004; Williams et al. 2007). Another limitation was the difficulty in having smokers maintain abstinence for a prolonged period (over two nights) prior to PET scanning, despite compensation for such abstinence. While participants met a relatively stringent criterion (CO ≤4 ppm) for abstinence on the day of scanning, some participants still had measurable levels of plasma nicotine on the day of scanning. It is possible that even more stringent CO levels (e.g. ≤2 or 3 ppm) at the time of scanning or in-patient hospitalization prior to scanning would have assisted in reaching nicotine levels low enough not to interfere with 2-FA binding.
In summary, cigarette smoking leads to up-regulation of α4β2* nAChRs and menthol cigarette smoking leads to greater up-regulation of these receptors than non-menthol cigarette smoking. These findings may help explain the relative severity of dependence on menthol cigarettes.
Supplementary Material
Acknowledgments
This study was supported by the Tobacco-Related Disease Research Program [A.L.B. (19XT-0135)], the National Institute on Drug Abuse [A.L.B. (R01 DA20872)] and a Veterans Affairs Type I Merit Review Award (A.L.B.), as well as by an endowment from the Richard Metzner Chair in Clinical Neuropharmacology (A.L.B.). The sponsors had no role in the design and conduct of the study, collection, management, analysis and interpretation of the data or preparation, review or approval of the manuscript.
Footnotes
Statement of Interest: Drs Brody and Mandelkern were previously co-investigators on a grant funded by Pfizer, Inc., which was unrelated to this study. Dr Mukhin is a co-investigator on a grant from Phillip Morris, Inc., for research unrelated to this study.
Supplementary material: For supplementary material accompanying this paper, visit http://dx.doi.org/10.17/S1461145712001022
References
- Abobo CV, Ma J, Liang D. Effect of menthol on nicotine pharmacokinetics in rats after cigarette smoke inhalation. Nicotine and Tobacco Research. 2012;14:801–808. doi: 10.1093/ntr/ntr287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen B, Jr, Unger JB. Sociocultural correlates of menthol cigarette smoking among adult African Americans in Los Angeles. Nicotine and Tobacco Research. 2007;9:447–451. doi: 10.1080/14622200701239647. [DOI] [PubMed] [Google Scholar]
- Bencherif M, Fowler K, Lukas RJ, Lippiello PM. Mechanisms of up-regulation of neuronal nicotinic acetylcholine receptors in clonal cell lines and primary cultures of fetal rat brain. Journal of Pharmacology Experimental Therapeutics. 1995;275:987–994. [PubMed] [Google Scholar]
- Benowitz NL, Herrera B, Jacob P., 3rd Mentholated cigarette smoking inhibits nicotine metabolism. Journal of Pharmacology and Experimental Therapeutics. 2004;310:1208–1215. doi: 10.1124/jpet.104.066902. [DOI] [PubMed] [Google Scholar]
- Benwell ME, Balfour DJK, Anderson JM. Evidence that tobacco smoking increases the density of (-)-[ 3 H]nicotine binding sites in human brain. Journal of Neurochemistry. 1988;50:1243–1247. doi: 10.1111/j.1471-4159.1988.tb10600.x. [DOI] [PubMed] [Google Scholar]
- Bernert JT, Jain RB, Pirkle JL, Wang L, et al. Urinary tobacco-specific nitrosamines and 4-aminobiphenyl hemoglobin adducts measured in smokers of either regular or light cigarettes. Nicotine and Tobacco Research. 2005;7:729–738. doi: 10.1080/14622200500259762. [DOI] [PubMed] [Google Scholar]
- Breese CR, Marks MJ, Logel J, Adams CE, et al. Effect of smoking history on [3H]nicotine binding in human postmortem brain. Journal of Pharmacology and Experimental Therapeutics. 1997;282:7–13. [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Costello MR, Abrams AL, et al. Brain nicotinic acetylcholine receptor occupancy: effect of smoking a denicotinized cigarette. International Journal of Neuropsychopharmacology. 2009;12:305–316. doi: 10.1017/S146114570800922X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Khan A, et al. Effect of secondhand smoke on occupancy of nicotinic acetylcholine receptors in brain. Archives of General Psychiatry. 2011;68:953–960. doi: 10.1001/archgenpsychiatry.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Olmstead RE, et al. Cigarette smoking saturates brain alpha4beta2 nicotinic acetylcholine receptors. Archives of General Psychiatry. 2006;63:907–915. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DW. Smoking prevalence among US veterans. Journal of General Internal Medicine. 2009;25:147–149. doi: 10.1007/s11606-009-1160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro FG. Physiological, psychological, social, and cultural influences on the use of menthol cigarettes among Blacks and Hispanics. Nicotine and Tobacco Research. 2004;6(Suppl. 1):S29–S41. doi: 10.1080/14622200310001649487. [DOI] [PubMed] [Google Scholar]
- CDC. Cigarette smoking among adults and trends in smoking cessation – United States 2008. MMWR Morbidity and Mortality Weekly Report. 2009;58:1227–1232. [PubMed] [Google Scholar]
- Cosgrove KP, Batis J, Bois F, Maciejewski PK, et al. beta2-nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Archives of General Psychiatry. 2009;66:666–676. doi: 10.1001/archgenpsychiatry.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolle F, Valette H, Bottlaender M, Hinnen F, et al. Synthesis of 2-[F-18]fluoro-3-[2(S)-2-azetidinylmethoxy] pyridine, a highly potent radioligand for in vivo imaging central nicotinic acetylcholine receptors. Journal of Labelled Compounds & Radiopharmaceuticals. 1998;41:451–463. [Google Scholar]
- Esterlis I, Cosgrove KP, Batis JC, Bois F, et al. Quantification of smoking-induced occupancy of beta2-nicotinic acetylcholine receptors: estimation of nondisplaceable binding. Journal of Nuclear Medicine. 2010a;51:1226–1233. doi: 10.2967/jnumed.109.072447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterlis I, Cosgrove KP, Petrakis IL, McKee SA, et al. SPECT imaging of nicotinic acetylcholine receptors in nonsmoking heavy alcohol drinking individuals. Drug and Alcohol Dependence. 2010b;108:146–150. doi: 10.1016/j.drugalcdep.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom KO. Measuring the degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive Behaviors. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Gandhi KK, Foulds J, Steinberg MB, Lu SE, et al. Lower quit rates among African American and Latino menthol cigarette smokers at a tobacco treatment clinic. International Journal of Clinical Practice. 2009;63:360–367. doi: 10.1111/j.1742-1241.2008.01969.x. [DOI] [PubMed] [Google Scholar]
- Gardiner PS. The African Americanization of menthol cigarette use in the United States. Nicotine and Tobacco Research. 2004;6(Suppl. 1):S55–S65. doi: 10.1080/14622200310001649478. [DOI] [PubMed] [Google Scholar]
- Govind AP, Vezina P, Green WN. Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochemical Pharmacology. 2009;78:756–765. doi: 10.1016/j.bcp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Diagnosis and rating of anxiety. British Journal of Psychiatry. 1969;3:76–79. [Google Scholar]
- Hans M, Wilhelm M, Swandulla D. Menthol suppresses nicotinic acetylcholine receptor functioning in sensory neurons via allosteric modulation. Chemical Senses. 2012;37:463–469. doi: 10.1093/chemse/bjr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heck JD. Smokers of menthol and nonmenthol cigarettes exhibit similar levels of biomarkers of smoke exposure. Cancer Epidemiology Biomarkers and Prevention. 2009;18:622–629. doi: 10.1158/1055-9965.EPI-08-0550. [DOI] [PubMed] [Google Scholar]
- Hyland A, Garten S, Giovino GA, Cummings KM. Mentholated cigarettes and smoking cessation: findings from COMMIT. Community Intervention Trial for Smoking Cessation. Tobacco Control. 2002;11:135–139. doi: 10.1136/tc.11.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of Cerebral Blood Flow and Metabolism. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Jacob P, 3rd, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3k,3k-d2 in humans. Biology Mass Spectrometry. 1991;20:247–252. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- Kaufman NJ, Castrucci BC, Mowery P, Gerlach KK, et al. Changes in adolescent cigarette-brand preference, 1989 to 1996. American Journal of Health Behavior. 2004;28:54–62. doi: 10.5993/ajhb.28.1.6. [DOI] [PubMed] [Google Scholar]
- Kimes AS, Chefer SI, Matochik JA, Contoreggi CS, et al. Quantification of nicotinic acetylcholine receptors in the human brain with PET: bolus plus infusion administration of 2-[18F]F-A85380. Neuroimage. 2008;39:717–727. doi: 10.1016/j.neuroimage.2007.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreslake JM, Wayne GF, Alpert HR, Koh HK, et al. Tobacco industry control of menthol in cigarettes and targeting of adolescents and young adults. American Journal of Public Health. 2008;98:1685–1692. doi: 10.2105/AJPH.2007.125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester HA, Xiao C, Srinivasan R, Son CD, et al. Nicotine is a selective pharmacological chaperone of acetylcholine receptor number and stoichiometry. Implications for drug discovery. American Association of Pharmaceutical Scientists Journal. 2009;11:167–177. doi: 10.1208/s12248-009-9090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Westman EC, Rose JE, Lutz AM. The effects of foods, beverages, and other factors on cigarette palatability. Nicotine and Tobacco Research. 2007;9:505–510. doi: 10.1080/14622200701243177. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr D, Droppelman LF. Manual for the Profile of Mood States. San Diego, CA: Educational Testing Services; 1988. [Google Scholar]
- Mamede M, Ishizu K, Ueda M, Mukai T, et al. Temporal change in human nicotinic acetylcholine receptor after smoking cessation: 5IA SPECT study. Journal of Nuclear Medicine. 2007;48:1829–1835. doi: 10.2967/jnumed.107.043471. [DOI] [PubMed] [Google Scholar]
- Marks MJ, McClure-Begley TD, Whiteaker P, Salminen O, et al. Increased nicotinic acetylcholine receptor protein underlies chronic nicotine-induced up-regulation of nicotinic agonist binding sites in mouse brain. Journal of Pharmacology and Experimental Therapeutics. 2011;337:187–200. doi: 10.1124/jpet.110.178236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, et al. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. Journal of Neuroscience. 1992;12:2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsis EM, Cosgrove KP, Staley JK, Bois F, et al. Age-related decline in nicotinic receptor availability with [(123)I]5-IA-85380 SPECT. Neurobiology of Aging. 2009;30:1490–1497. doi: 10.1016/j.neurobiolaging.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhin AG, Kimes AS, Chefer SI, Matochik JA, et al. Greater nicotinic acetylcholine receptor density in smokers than in nonsmokers: a PET study with 2-18F-FA-85380. Journal of Nuclear Medicine. 2008;49:1628–1635. doi: 10.2967/jnumed.108.050716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary K, Parameswaran N, McIntosh JM, Quik M. Cotinine selectively activates a subpopulation of alpha3/alpha6beta2 nicotinic receptors in monkey striatum. Journal of Pharmacology and Experimental Therapeutics. 2008;325:646–654. doi: 10.1124/jpet.108.136838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyemi KS, Faseru B, Sanderson Cox L, Bronars CA, et al. Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction. 2007;102:1979–1986. doi: 10.1111/j.1360-0443.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Pletcher MJ, Hulley BJ, Houston T, Kiefe CI, et al. Menthol cigarettes, smoking cessation, atherosclerosis, and pulmonary function: the coronary artery risk development in young adults (CARDIA) study. Archives of Internal Medicine. 2006;166:1915–1922. doi: 10.1001/archinte.166.17.1915. [DOI] [PubMed] [Google Scholar]
- Quick MW, Lester RA. Desensitization of neuronal nicotinic receptors. Journal of Neurobiology. 2002;53:457–478. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- Shiffman SM, Jarvik ME. Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology (Berlin) 1976;50:35–39. doi: 10.1007/BF00634151. [DOI] [PubMed] [Google Scholar]
- Shumway DA, Pavlova OA, Mukhin AG. A simplified method for the measurement of nonmetabolized 2-[18F]F-A-85380 in blood plasma using solid-phase extraction. Nuclear Medicine Biology. 2007;34:221–228. doi: 10.1016/j.nucmedbio.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Sorger D, Becker GA, Patt M, Schildan A, et al. Measurement of the alpha4beta2* nicotinic acetylcholine receptor ligand 2-[(18)F]Fluoro-A-85380 and its metabolites in human blood during PET investigation: a methodological study. Nuclear Medicine Biology. 2007;34:331–342. doi: 10.1016/j.nucmedbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Pantoja R, Moss FJ, Mackey ED, et al. Nicotine up-regulates alpha4beta2 nicotinic receptors and ER exit sites via stoichiometry-dependent chaperoning. Journal of General Physiology. 2011;137:59–79. doi: 10.1085/jgp.201010532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, et al. Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. Journal of Neuroscience. 2006;26:8707–8714. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. The NSDUH Report: Use of Menthol Cigarettes. Rockville, MD: Office of Applied Studies; 2009. [Google Scholar]
- Sutton CD, Robinson RG. The marketing of menthol cigarettes in the United States: populations, messages, and channels. Nicotine and Tobacco Research. 2004;6(Suppl. 1):S83–S91. doi: 10.1080/14622203310001649504. [DOI] [PubMed] [Google Scholar]
- Werley MS, Coggins CR, Lee PN. Possible effects on smokers of cigarette mentholation: a review of the evidence relating to key research questions. Regulatory Toxicology and Pharmacology. 2007;47:189–203. doi: 10.1016/j.yrtph.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Whiting PJ, Lindstrom JM. Characterization of bovine and human neuronal nicotinic acetylcholine receptors using monoclonal antibodies. Journal of Neuroscience. 1988;8:3395–3404. doi: 10.1523/JNEUROSCI.08-09-03395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Gandhi KK, Steinberg ML, Foulds J, et al. Higher nicotine and carbon monoxide levels in menthol cigarette smokers with and without schizophrenia. Nicotine and Tobacco Research. 2007;9:873–881. doi: 10.1080/14622200701484995. [DOI] [PubMed] [Google Scholar]
- Wullner U, Gundisch D, Herzog H, Minnerop M, et al. Smoking upregulates alpha4beta2* nicotinic acetylcholine receptors in the human brain. Neuroscience Letters. 2008;430:34–37. doi: 10.1016/j.neulet.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Yates SL, Bencherif M, Fluhler EN, Lippiello PM. Up-regulation of nicotinic acetylcholine receptors following chronic exposure of rats to mainstream cigarette smoke or alpha 4 beta 2 receptors to nicotine. Biochemical Pharmacology. 1995;50:2001–2008. doi: 10.1016/0006-2952(95)02100-0. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tian JY, Svensson AL, Gong ZH, et al. Chronic treatments with tacrine and (-)-nicotine induce different changes of nicotinic and muscarinic acetylcholine receptors in the brain of aged rat. Journal of Neural Transmission. 2002;109:377–392. doi: 10.1007/s007020200030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.