Abstract
Regeneration of artificially induced lesions was monitored in nubbins of the branching coral Acropora muricata at two reef-flat sites representing contrasting environments at Réunion Island (21°07′S, 55°32′E). Growth of these injured nubbins was examined in parallel, and compared to controls. Biochemical compositions of the holobiont and the zooxanthellae density were determined at the onset of the experiment, and the photosynthetic efficiency (Fv/Fm) of zooxanthellae was monitored during the experiment. Acropora muricata rapidly regenerated small lesions, but regeneration rates significantly differed between sites. At the sheltered site characterized by high temperatures, temperature variations, and irradiance levels, regeneration took 192 days on average. At the exposed site, characterized by steadier temperatures and lower irradiation, nubbins demonstrated fast lesion repair (81 days), slower growth, lower zooxanthellae density, chlorophyll a concentration and lipid content than at the former site. A trade-off between growth and regeneration rates was evident here. High growth rates seem to impair regeneration capacity. We show that environmental conditions conducive to high zooxanthellae densities in corals are related to fast skeletal growth but also to reduced lesion regeneration rates. We hypothesize that a lowered regenerative capacity may be related to limited availability of energetic and cellular resources, consequences of coral holobionts operating at high levels of photosynthesis and associated growth.
Introduction
Disturbances from multiple biotic and abiotic factors cause recurring losses of living tissues from coral colonies [1] and may result in sporadic severe reductions of living corals at the reef scale [2]. A colony’s integrity is maintained through rapid tissue repair [3], while regrowth from surviving colony parts greatly accelerates the recovery of damaged reefs [4] and may even reverse coral-algal phase shifts [5]. Thus, the growth and regenerative capacities of corals are fundamental to determining the resilience of reefs [5], [6] and are often used as indicators of coral colony condition and generalized to define a reef’s health status [7], [8].
Coral colony growth and regeneration are likely closely related processes, involving calcification and tissue extension [9]. Repair of damaged colony parts (hereafter defined as ‘lesions’) requires energy [10] and interstitial cells [11]–[13] and competes for these limited resources with other essential biological processes, such as growth [14], reproduction [15], disease resistance, and competitive ability [16]. Lesion regeneration is facilitated by the clonal architecture of corals, enabling the transport and reallocation of resources among units [10], [11], to which polyps proximal to the damaged area contribute the most [14], [17]. Lesion regenerative capacity varies with the lesion size and shape [18]–[20], colony morphology and species (see review in [21]), and environmental conditions [8], [22]. Also, coral colony growth is known to vary with these parameters (e.g. in [23]–[25]). While trade-offs between colony growth and lesion repair were documented at the level of individual colonies [17], high skeletal extension rates are generally associated with an efficient lesion-repair capacity (see review in [21]). Hence, fast-growing branching corals have higher lesion regeneration rates with full recovery [26] compared to more-slowly growing massive corals [17].
The present study was designed to investigate the lesion regenerative capacity of the branching coral Acropora muricata at Réunion Island in contrasting natural environments. An earlier study on this species revealed a trade-off between regeneration and growth, and indicated that they were independent of size [27]. We further investigated relationships between skeletal growth and lesion regeneration by monitoring both processes in experimental coral nubbins on two reef flats, Planch’Alizé and Kiosque, selected for their contrasting environmental conditions that induced strong differences in coral growth rates. We not only show that coral growth is inversely related to the rate of lesion repair, but also that high coral growth rates may harm regeneration capacity in A. muricata. We suggest that rapid coral growth, promoted by a high zooxanthellae density and high photosynthetic efficiency, compromised their capacity to invest energy and cellular resources in lesion repair. We hypothesize that maintaining coral-symbiont functioning under conditions that boost zooxanthellae densities and their photosynthetic rate implies their sequestering of an important share of host resources. This may particularly involve the use of the energy-rich photosynthetic compounds produced by the Symbiodinium.
Methods
Ethics Statement
This experiment was conducted and corals were sampled with permission granted by the Réserve Naturelle Marine de la Réunion. All survey procedures carried out were done with proper precautions to minimize impacts to the reefs.
Study Area and Species
Experiments were conducted on the west coast of Réunion Island (21°07′S, 55°32′E) at two shallow (1∼2 m deep) reef-flat sites 11 km apart from each other: Planch’Alizé and Kiosque. At each site, the seawater surface temperature (SST) was measured at hourly intervals using calibrated underwater temperature loggers (Hobo Water Temp Pro, with an accuracy of 0.2°C, Onset Computer Corp., USA). Solar radiation (J cm−2) data were obtained from the French Meteorological Service on land at station close to each site (stations no. 97415590 and 97413545).
Situated at the la Saline reef, Planch’Alizé is a sheltered site, located downstream of seawater flowing over the 500-m-wide reef flat. Low water flow and high solar radiation contribute to heating the reef water during the day, inducing important daily SST variations (Fig. 1a) and higher average SSTs compared to the second site. In contrast, Kiosque is an exposed site located at the Saint-Leu reef; waves impinging on the narrow reef flat (200 m wide) induce strong water motion and an influx of coastal water that reduces daily SST fluctuations (Fig. 1b). Moreover, solar radiation at this site is often tempered due to cloud formation along the steep slopes above the Saint-Leu reef, especially during the hot season. Further details of environmental conditions at these study sites can be found in [22].
Figure 1. Environmental conditions.
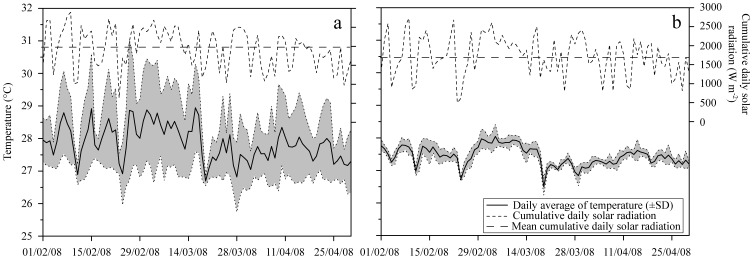
Daily average sea surface temperature (SST; ± SD, gray area) and cumulative daily solar radiation during the experimental period at (a) Planch’Alizé and (b) Kiosque.
The branching coral A. muricata (Linnaeus, 1758), senior synonym of A. formosa [28], is a dominant species of Réunion reef-flat coral communities and a common species in the Indo-Pacific region [28]. At Planch’Alizé, this species often co-occurs with Montipora circumvallata and Porites (Synaraea) rus, forming large thickets. At this site, benthic communities comprise a high proportion of dead corals covered by algal turfs (∼30% in 2008, data from GCRMN monitoring). This site is further characterized by nutrient enrichment originating from N-rich groundwater seepage near the shore [29]. At Kiosque, coral diversity is higher, but the density of A. muricata is lower than at Planch’Alizé [30], [31].
Sample Preparation and Monitoring of Lesion Regeneration and Growth
At each site, 15 nubbins (7 cm long) were sampled from each of 5 haphazardly selected A. muricata colonies separated by >10 m in distance. Nubbins were glued onto numbered acrylic glass tiles, mounted on racks positioned in a representative area at each native reef flat site, and left to recover for 2 months prior to the experiments. On March 8, 2008, 5 replicate nubbins from each colony were randomly selected, and artificial circular lesions of 11.5±0.6 mm in diameter and 3 mm in depth were inflicted using a grinding stone at mid-height on the side of each branch, removing all traces of living tissue. Particular care was taken to induce lesions of a constant size and depth. Nubbins were then left to heal at their respective native sites.
The surface area of lesions and projected vertical surface area of nubbins were monitored until April 30, 2008 and quantified with CPCe software [32] from digital photographs taken with a camera mounted on a support (in order to maintain a constant distance to the sample) equipped with a scale. The monitoring frequency was adjusted to the rate of regeneration.
The relative mass increase (‰ d−1) of nubbins was calculated from the difference between the initial and final projected nubbin surface area multiplied by the mean site-specific skeletal density, divided by the duration of the experiment. The calcification rate, or mass of CaCO3 deposited per unit area per day (g cm−2 d−1), was estimated for each nubbin as the product of its linear extension and skeletal density. The skeletal density (g cm−3) of nubbins was calculated as the dry weight-to-volume ratio, following Bucher et al. [33]. The volume was determined by dipping nubbins in molten paraffin wax to form a water-tight barrier and determining their buoyant mass in distilled water at 20°C.
The remaining undamaged nubbins (9 or 10 per colony) were used as controls, and their growth (relative increases in the buoyant mass and surface area) was monitored simultaneously.
Photosynthetic Efficiency, Symbiodinium Identification, Density of Zooxanthellae, and Biochemical Composition of Holobionts
The photosynthetic efficiency of nubbins was quantified using the maximum dark-adapted quantum yield, Fv/Fm (Fv is the difference between F0, the initial fluorescence, and Fm, the maximum fluorescence [34]). Measurements were made with a diving-PAM (Walz, Germany) at night, 1 h after sunset in order to maximize the frequency of open photosystem II reaction centres, using a custom-built nubbin holder to ensure a constant probe distance and measurement location. Following diving-PAM settings were used along the experiment: saturating intensity = 8, saturating width = 0.6, gain = 4, damping = 2. On injured nubbins, Fv/Fm was assessed at each monitoring time, about 2 cm away from the lesion borders. Fv/Fm of control nubbins was determined at the beginning, half-way, and end of the experiment.
For 3 of the 5 colonies per site, 1 undamaged nubbin was sampled before the beginning of the experiment (February 28, 2008), snap frozen in liquid nitrogen and stored at −80°C. Genomic DNA was then extracted from a subsample using a Qiagen® Blood and Tissue Kit (Santa Clarita, CA, USA). The internal transcribed spacer (ITS)-2 region of Symbiodinium ribosomal (r) DNA was amplified using the primers “ITSintfor2” and “ITS2revclamp” [35] under the following conditions: an initial denaturing step of 94°C for 3 min followed by 35 cycles of 1 min at 94°C, 1 min at 58°C, and 1 min at 74°C, followed by a single cycle of 7 min at 74°C. Amplified DNA was then analyzed by denaturing gradient gel electrophoresis (DGGE; CBS Scientific, Del Mar, CA, USA) using a denaturant gradient of 35% to 75%. Prominent bands characterizing different profiles were excised, re-amplified, and sequenced as described by LaJeunesse [36]. Sequences were identified using BLAST searches of GenBank, and exact matches were reported using the nomenclature established by LaJeunesse [35].
Tissues were removed from the skeleton using a dental jet (Waterpik Technologies, Fort Collins, CO, US) with recycled freshly filtered seawater [37], and the obtained coral blastate was homogenized with a potter homogenizer (5 min, 2000 rpm). Zooxanthellae densities were determined from an aliquot of the homogenate by first separating zooxanthellae from host tissue by centrifugation, suspension, and homogenization of the pellet in 2 mL of a formalin solution (5%). Zooxanthellae were then counted in 5 subsamples (0.2 mm3 each) using a hemocytometer at 400×magnification. The chlorophyll (Chl) a concentration was determined by spectrometric absorbance at 664 nm [38]. The protein concentration was determined from 0.1 mL of homogenate following a modified protocol [39] of the Lowry method [40], using the Folin reagent with phenol and bovine serum albumin as standards. Finally, total lipids were extracted using a monophasic solvent mixture (CH2Cl2: CH3OH: H2O; 1∶2: 0.8 v/v/v) [41] with 100 µL of an internal standard (hexadecanone, GC grade, Sigma Chemical, St. Louis, MO, USA) during 12 h at 4°C. CH2Cl2 and H2O (1∶1 v/v) were added to the supernatant to create a biphasic mixture. The organic (dichloromethane) phase was collected in glass bottles with Teflon caps, evaporated under nitrogen, and stored under a nitrogen atmosphere at −20°C until being analyzed. The total lipid content was then determined using thin-layer chromatography coupled with flame-ionization detection (TLC-FID) on an Iatroscan apparatus model MK6-s (with hydrogen flow of 160 mL min−1 and air flow of 2000 mL min−1) and an i-Chromstar 6.1 integration system (SCPA, Bremen, Germany).
Statistical Analysis
Regeneration of each nubbin was quantified by fitting the remaining lesion size (%) over time using a least-squared regression and an exponential decay model, allowing full recovery [20]. We used the following formula:
where size is the remaining lesion size (%), sizereg is the maximum area that can be fully regenerated, slope is the regression slope, and t is the time in days. Since there is no natural logarithm for 0 (when a lesion is completely healed), +1 was added to the remaining lesion size for the calculations.
Conformity with parametric assumptions was visually assessed from residual plots. A potential site difference in the initial lesion size was tested using a 1-way analysis of variance (ANOVA). Possible effects of the initial lesion size on the regression slopes were analyzed using an analysis of covariance (ANCOVA) with the initial lesion size as the co-variable. A site difference in model slopes was investigated using an ANOVA, with “colony” as the random factor. For each site, mean slopes were used to simulate regeneration during 300 days. The difference in relative growth between sites and treatments was compared using a 2-way ANOVA, and Tukey’s honest significant difference (HSD) test was used for post-hoc comparisons.
Welch’s t-test was used to compare biochemical properties of nubbins between sites at the onset of the lesion regeneration experiment. As a parametric assumption could not be met, non-parametric Mann-Whitney U-test was used to compare Fv/Fm of injured and control nubbins at the beginning, middle, and end of the experiment, and was also used to assess site differences in Fv/Fm of injured nubbins. Friedman’s ANOVA was used to test temporal variations in Fv/Fm, where values obtained from the same nubbin over time were considered dependent. Results are presented as the mean ± standard deviation (SD).
Results
Acropora muricata nubbins were consistently associated with C3 Symbiodinium. Corals from the sheltered site Planch’Alizé were characterized by a higher zooxanthellae density, Chl a concentration, and total lipid content, but also not significant lower tissue biomass and protein content compared to those from the exposed site Kiosque (Table 1).
Table 1. Biochemical properties of nubbins of Acropora muricata at the onset of the lesion regeneration experiment.
| Parameter | Planch’Alizé | Kiosque |
| Tissue biomass (mg dry weight cm−2) | 3.27 (±0.49) | 4.13 (±0.83) |
| Zooxanthellae density (106 cells cm−2)* | 2.11 (±0.08) | 1.85 (±0.60) |
| Chl a concentration (µg cm−2)** | 9.68 (±1.88) | 8.74 (±2.09) |
| Protein content (mg cm−2) | 1.99 (±0.19) | 2.30 (±0.34) |
| Total lipids in holobiont (mg C cm−2)* | 0.81 (±0.26) | 0.51 (±0.10) |
p<0.05;
p<0.01;
p<0.001.
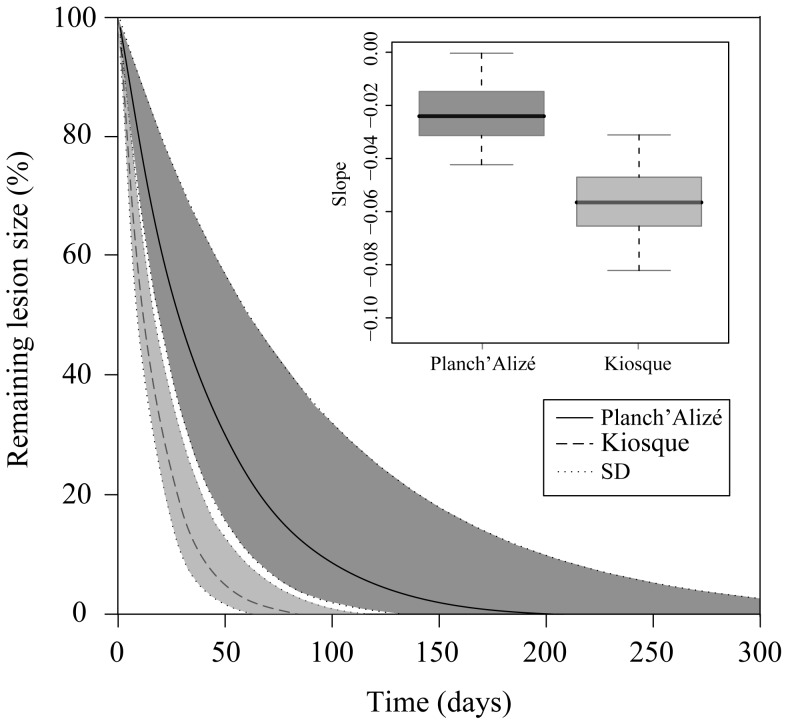
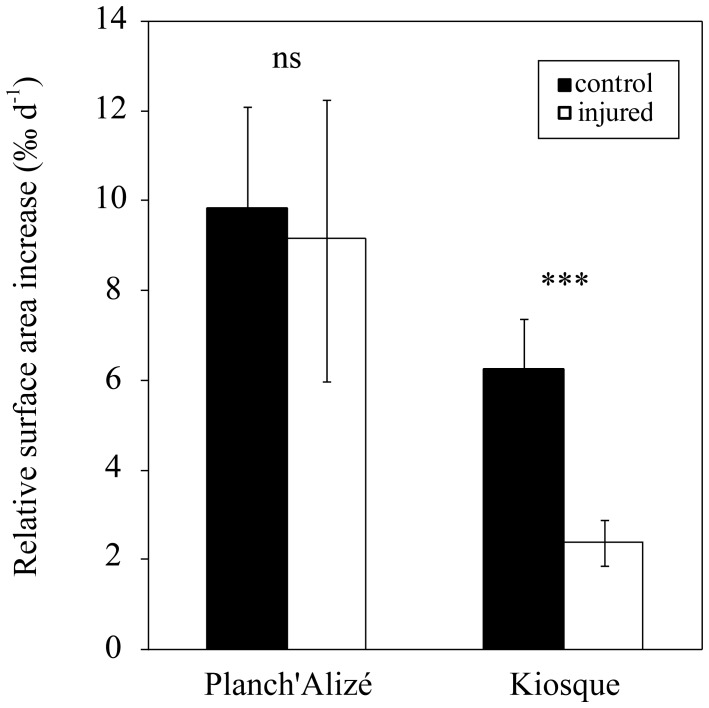
The initial lesion size was 104±11 mm2 (n = 48) and did not differ between sites (F 1,47 = 2.21, p>0.05) or colonies (F 9,39 = 1.98, p>0.05). Two nubbins at Kiosque died before the onset of regeneration monitoring; these were not included in the analysis. There was no mortality of nubbins during the experimental period. The lesion size decreased rapidly over time (Fig. 2). After 53 days, lesions in nubbins at Kiosque were at 3% of their initial size, while those in nubbins at Planch’Alizé were still at 24% of their initial size. Based on the outcomes of the exponential decay model, lesions healed completely after on average 81 days at Kiosque and 192 days at Planch’Alizé (Fig. 3). Regression slopes significantly differed between Planch’Alizé (−0.024±0.012, n = 25) and Kiosque (−0.057±0.017, n = 23; F 1,8 = 32.89, p<0.001, inset in Fig. 3), and the difference was not related to colony (F 8,38 = 2.17, p>0.05) or initial lesion size (F 1,43 = 1.79, p>0.05). Increases in the relative surface area of nubbins (Fig. 4) varied with site (F 1,142 = 121.04, p<0.001) and treatment (F 1,142 = 25.10, p<0.001), with a significant interaction term observed between the two factors (F 1,142 = 7.15, p<0.01).
Figure 2. Acropora muricata.
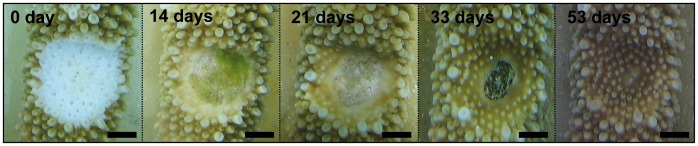
Lesion regeneration pattern at Kiosque, almost completely healed after 53 days. Scale bar = 5 mm.
Figure 3. Lesion regeneration of Acropora muricata.
Predicted size of artificial lesions over time in nubbins at Planch’Alizé and Kiosque according to slopes estimated from the regeneration model (inset).
Figure 4. Growth of Acropora muricata.
The mean (± SD) relative increase in the projected surface area (‰ d−1) of control and injured nubbins by site.
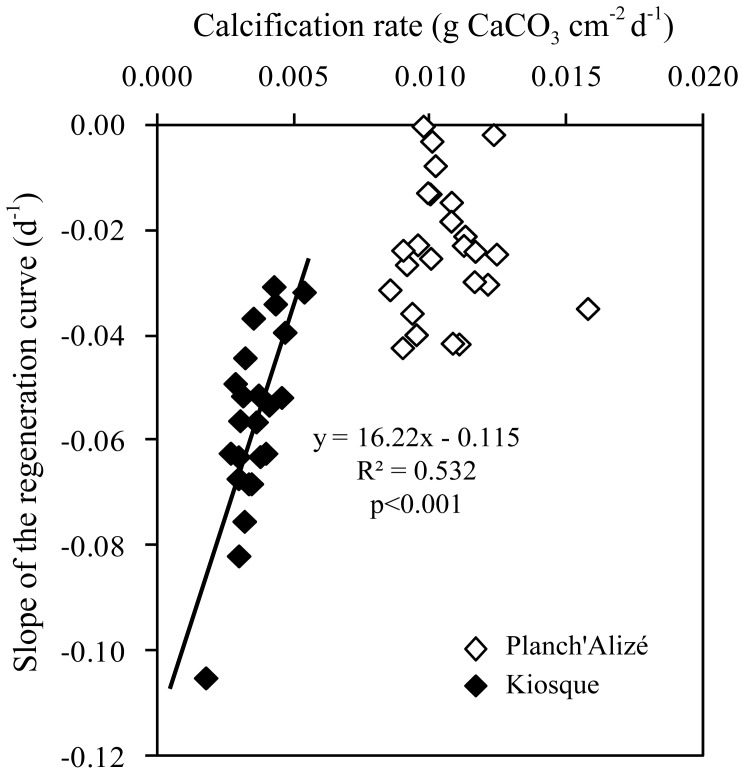
At Kiosque, the calcification rates of the injured nubbins were lower than that of the controls (p<0.001), and rates of calcification and lesion regeneration were negatively correlated (R2 = 0.532, p<0.001; Fig. 5). At Planch’Alizé, no difference in growth was observed between injured and control nubbins (p = 0.3). At this latter site, lesion regeneration rates were slow and not related to growth (Fig. 5).
Figure 5. Calcification and lesion healing of Acropora muricata.
Relationship between calcification rates and lesion healing for injured nubbins from Planch’Alizé and Kiosque.
Fv/Fm values of injured and control nubbins were respectively 0.677±0.016 (n = 25) and 0.684±0.021 (n = 50) at Planch’Alizé, and 0.682±0.020 (n = 23) and 0.690±0.016 (n = 50) at Kiosque. Fv/Fm did not significantly differ between sites or treatments (Mann-Whitney U-tests, p>0.05), and no significant temporal variation was observed for injured nubbins (Planch’Alizé: X2 = 9.64, p>0.05, df = 6; Kiosque: X2 = 6.82, p>0.05, df = 6).
Discussion
While the existence of a trade-off between growth and regeneration was clearly identified for some coral species [17], it is not known whether this relationship varies with environmental conditions. Here, we identified that a specific environment conducive to high zooxanthellae densities in coral tissues may favour growth regardless of damage to the colony.
Environmental conditions at Planch’Alizé, including low water flow, high mean SST and high SST variation, combined with nutrient enrichment from N-rich groundwater seepage [29], likely boost zooxanthellae densities and chlorophyll concentrations [42]. Preponderance of autotrophic energy sources in A. muricata at this site is further reflected in the high lipid and low protein content of the nubbins. Indeed, lipids are mainly derived from excess carbon fixed by zooxanthellae [43]. Due to its sheltered location and high irradiance, stressful conditions for corals may also be expected to occur frequently here (Fig. 1a). Lipids represent important energy at reserves during stressful periods [44], and integrated symbionts-host lipogenesis is considered as a photoprotective mechanism during periods of excess irradiance [45].
At Kiosque, the tissue biomass of nubbins tended to be higher than at Planch’Alizé, although the difference was not significant. Higher tissue biomass may reflect the opportunities corals have for heterotrophic feeding (see review in [46]). The exposed site Kiosque, which receives a regular influx of coastal water, provides this advantage and allows corals to maintain vital processes during periods when photosynthetic function is compromised. In an earlier study on the lesion regeneration in massive corals at same sites, the potential importance of heterotrophic feeding at Kiosque reef flat during the summer season [22] was already highlighted.
While the contrasted light and temperature regimes between Planch’Alizé and Kiosque were expected to affect the photosynthetic efficiency in corals, Fv/Fm values measured in A. muricata nubbins did not differ between these sites. Furthermore, lesion infliction did not affect Fv/Fm values in nubbins, in spite of the stress this likely represents. High Fv/Fm values were maintained throughout the experiment. Measured photosynthetic efficiencies were higher than those of A. muricata nubbins from the Great Barrier Reef kept at a non-stressful temperature [47]. This further suggests that specific environmental conditions at Planch’Alizé did not impair the photosynthesis of A. muricata at this site and may not have been stressful for nubbins there. Differences in the photosynthetic efficiency may also be related to differences in zooxanthellae genotypes [48]. However, while in Chagos Archipelago, A. muricata is commonly associated with C1-Symbiodinium [49], on the Great Barrier Reef, C3-Symbiodinium is the predominant subclade present in Acropora spp. [50], [51] as identified here.
Homogeneity of lesion sizes avoids an important confounding factor that complicates drawing viable inferences about the regeneration capacity of corals under different environmental conditions [19]. Lesion size in A. muricata decreased exponentially over time, which corresponds to the common pattern observed for corals [14], [17], [20]. Despite significantly lower skeletal growth at Kiosque, the mean time required for complete lesion healing was shorter here (81 days) than at Planch’Alizé (192 days). This result is remarkable because it contradicts the general positive relationship found between coral growth and lesion repair ability (see review in [21]). Most studies on lesion regeneration in corals have used massive species (see review in [21]), and only a few studies on Acropora spp. are available to compare the slopes of the exponential decay model obtained here. Slopes observed at Planch’Alizé were higher than those of the Caribbean A. palmata regenerating similar-sized lesions (−0.040±0.007 [52]), indicating a lower regenerative capacity than that for this flattened branching coral. In contrast, the very steep slopes obtained for A. muricata nubbins at Kiosque attest to favourable environmental conditions for coral regeneration at this exposed site.
Vigorous skeletal growth, both in terms of linear extension and calcification, of nubbins from Planch’Alizé was not affected by lesion infliction and may be driven by a high supply of photosynthetic products from zooxanthellae. Indeed, a previous study suggested the stimulating role of photosynthesis on calcification induced by nutrient enrichment at this site [41]. Whereas the lesion regenerations rates at Planch’Alizé were slower and not related to growth, at Kiosque both the reduced skeletal growth of injured lesions compared to controls and the negative relationship between calcification and lesion regeneration rates suggest competition for limited resources between these vital processes. While a high lesion regeneration rate is generally assumed in fast-growing corals [21], our observations suggest that environmental conditions that promote fast skeletal growth may compromise rather than boost the lesion regeneration capacity. This observation may help explain the paradox that while fast growing corals such as Acropora spp. are capable of fast tissue regeneration [5], [14], [53], they may nevertheless be prone to substantial partial mortality in their natural environment [54], [55].
Energetic reserves such as lipids are considered to uphold vital life processes [43]. Despite A. muricata nubbins from Planch’Alizé having high lipid content, they regenerated lesions slowly. Tissue biomass of nubbins may be a better predictor of the regenerative capacity, as nubbins from Kiosque showed both high tissue biomass and fast regeneration. Essential resources required for lesion regeneration not only involve energy but also interstitial cells, which are shared among different life functions, including reproduction, growth, repair [11], [56] and mucus production. The trade-off between growth and lesion regeneration observed in nubbins from Kiosque complement this view. Very high skeletal extension rates, as observed in nubbins from Planch’Alizé may exert an important drain on interstitial cell availability for tissue repair and may compromise a coral’s regenerative capacity. As proposed by Rinkevich [12], the availability (or depletion) of stem cells will ultimately control observed trade-offs among life-history traits, which are independent of energy availability.
The fact that corals living with high zooxanthellae densities under high irradiation levels require more photosynthetically derived energy to maintain the stability of this symbiosis may provide an alternative explanation for the reduced regeneration capacity of nubbins at Planch’Alizé. While CO2-concentrating mechanisms are highly energy consuming, they may play an essential role in preventing CO2 limitation of zooxanthellae and its deleterious consequences for both photosystem II and the coral host [57]. Such energetic demands could interfere with lesion regeneration. At Planch’Alizé, where environmental conditions boost zooxanthellae densities alongside with high photosynthetic and calcification rates, our results suggest that growth demands have priority over other life processes. At Kiosque, where zooxanthellae densities, light levels and photosynthetic demands are lower, corals show the expected trade-off between growth and regeneration rates.
Eventually, competition with organisms (such as algae or microbes) that settle inside lesions may hamper lesion regeneration as an energetically costly mechanism [58]. Chronic nutrient enrichment at Planch’Alizé reef flats favours particularly the development of benthic algae, especially during the hot rainy season [27], [59]. We suggest that this chronic disturbance contributed to the seasonal impairment of the lesion regenerative capacity of P. lutea [22] and may also have contributed to the slower lesion repair in A. muricata at this site during the same hot and warming seasons.
An understanding of key processes of corals is critical to appreciating the divergent population trajectories after exposure to disturbances [60]. Our results suggest that the path to recovery (growth, regeneration, etc.) may strongly differ according to local environmental conditions. Such conditions need to be taken into account when assuming the resilience capacities of coral reefs exposed to increasing levels of disturbances.
Acknowledgments
We thank members of ECOMAR laboratory and MIO for their help and comments on this manuscript, and staff members of the Réserve Naturelle Marine de la Réunion for assistance in sample collection. We are also grateful to Allen Chen (Academia Sinica) for his suggestions to improve this manuscript. Meteo France kindly provided solar radiation data.
Funding Statement
A PhD fellowship to VD and a research grant to JHB and MMMG (ITUE program) were provided by the Regional Council of Réunion Island. CNRS-INSU and MIO financially support this collaborative work. VD is currently the recipient of a postdoctoral fellowship from the National Science Council of Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bythell JC, Gladfelter EH, Bythell M (1993) Chronic and catastrophic natural mortality of three common Caribbean reef corals. Coral Reefs 12: 143–152. [Google Scholar]
- 2. Connell JH (1997) Disturbance and recovery of coral assemblages. Coral reefs 16: 101–113. [Google Scholar]
- 3. Bak RPM, Steward-Van Es Y (1980) Regeneration of superficial damage in the scleractinian corals Agaricia agaricites f. purpurea and Porites astreoides . B Mar Sci 30: 883–887. [Google Scholar]
- 4. Jones RJ (2008) Coral bleaching, bleaching-induced mortality, and the adaptive significance of the bleaching response. Mar Biol 154: 65–80 doi:10.1007/s00227-007-0900-0 [Google Scholar]
- 5. Diaz-Pulido G, McCook LJ, Dove S, Berkelmans R, Roff G, et al. (2009) Doom and boom on a resilient reef: climate change, algal overgrowth and coral recovery. PLoS One 4: e5239 doi:10.1371/jourbal.pone.0005239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crabbe MJC (2009) Scleractinian coral population size structures and growth rates indicate coral resilience on the fringing reefs of North Jamaica. Mar Environ Res 67: 189–198 doi:10.1016/j.marenvres.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 7. Guzman HM, Burns KA, Jackson JBC (1994) Injury, regeneration and growth of Caribbean reef corals after a major oil spill in Panama. Mar Ecol Prog Ser 105: 231–241. [Google Scholar]
- 8. Fisher EM, Fauth JE, Hallock P, Woodley CM (2007) Lesion regeneration rates in reef-building corals Montastraea spp. as indicators of colony condition. Mar Ecol Prog Ser 339: 61–71. [Google Scholar]
- 9. Lester RT, Bak RPM (1985) Effect of environment on regeneration of tissue lesions in the reef coral Montastrea annularis (Scleractinia). Mar Ecol Prog Ser 24: 183–185. [Google Scholar]
- 10. Oren U, Rinkevich B, Loya Y (1997) Oriented intra-colonial transport of 14C labeled materials during coral regeneration. Mar Ecol Prog Ser 161: 117–122. [Google Scholar]
- 11. Fine M, Oren U, Loya Y (2002) Bleaching effect on regeneration and resource translocation in the coral Oculina patagonica . Mar Ecol Prog Ser 234: 119–125. [Google Scholar]
- 12. Rinkevich B (1996) Do reproduction and regeneration in damaged corals compete for energy allocation? Mar Ecol Prog Ser 143: 297–302. [Google Scholar]
- 13. Brickner I, Oren U, Frank U, Loya Y (2006) Energy integration between the solitary polyps of the clonal coral Lobophyllia corymbosa . J Exp Biol 209: 1690–1695 doi:10.1242/jeb.02168 [DOI] [PubMed] [Google Scholar]
- 14. Bak RPM (1983) Neoplasia, regeneration and growth in the reef-building coral Acropora palmata . Mar Biol 77: 221–227. [Google Scholar]
- 15. Veghel MLJ, Bak RPM (1994) Reproductive characteristics of the polymorphic Caribbean reef building coral, Montastrea annularis. III. Reproduction in damaged and regenerating colonies. Mar Ecol Prog Ser 109: 229–233. [Google Scholar]
- 16. Bak RPM, Criens SR (1981) Survival after fragmentation of colonies of Madracis mirabilis, Acropora palmata and A. cervicornis (Scleractinia) and the subsequent impact of a coral disease. Proc 4th Int Coral Reef Symp 2: 221–227. [Google Scholar]
- 17. Meesters EH, Noordeloos M, Bak RPM (1994) Damage and regeneration: links to growth in the reef-building coral Montastrea annularis . Mar Ecol Prog Ser 112: 119–128. [Google Scholar]
- 18. Meesters EH, Wesseling I, Bak RPM (1997) Coral colony tissue damage in six species of reef-building corals: partial mortality in relation with depth and surface area. J Sea Res 37: 131–144. [Google Scholar]
- 19. Oren U, Benayahu Y, Loya Y (1997) Effect of lesion size and shape on regeneration of the Red Sea coral Favia favus . Mar Ecol Prog Ser 146: 101–107. [Google Scholar]
- 20. van Woesik R (1998) Lesion healing on massive Porites spp. corals. Mar Ecol Prog Ser 164: 213–220. [Google Scholar]
- 21. Henry LA, Hart M (2005) Regeneration from injury and resource allocation in sponges and corals–a review. Int Rev Hydrobiol 90: 125–158 doi:10.1002/iroh.200410759 [Google Scholar]
- 22. Denis V, Debreuil J, de Palmas S, Richard J, Guillaume MMM, et al. (2011) Lesion regeneration capacities in populations of the massive coral Porites lutea at Réunion Island: environmental correlates. Mar Ecol Prog Ser 428: 105–117 doi:10.3354/meps09060 [Google Scholar]
- 23. Scoffin TP, Tudhope AW, Brown BE, Chansang H, Cheeney RF (1992) Patterns and possible environmental control of skeletogenesis of Porites lutea, South Thailand. Coral Reefs 11: 1–11. [Google Scholar]
- 24. Harriott (1999) Coral growth in subtropical eastern Australia. Coral Reefs 18: 281–291. [Google Scholar]
- 25. Lough JM, Barnes DJ (2000) Environmental controls on growth of the massive coral Porites . J Exp Mar Biol Ecol 254: 225–243. [DOI] [PubMed] [Google Scholar]
- 26. Hall VR (1997) Interspecific differences in the regeneration of artificial injuries on scleractinian corals. J Exp Mar Biol Ecol 212: 9–23. [Google Scholar]
- 27. Okubo N (2008) Size-independent investment allocation to regeneration and growth of the branching coral Acropora muricata . Galaxea 10: 83–87. [Google Scholar]
- 28.Wallace C (1999) Staghorn corals of the world: a revision of the coral genus Acropora. Collingwood: CSIRO. 421 p. [Google Scholar]
- 29. Mioche D, Cuet P (1999) Métabolisme du carbone, des carbonates et des sels nutritifs en saison chaude, sur un récif frangeant soumis à une pression anthropique (île de la Réunion, océan Indien). CR Acad Sci II a 329: 53–59. [Google Scholar]
- 30.Bruggemann H, Guillaume M, Bigot L, Chabanet P, Denis V, et al.. (2008) Mise en oeuvre de l’effet réserve: développement des protocoles et établissement de l’état initial de la Réserve Naturelle Nationale Marine de la Réunion (secteurs de la Saline–Souris Blanche et de Saint-Leu). 74 pp, Annexes 42 pp.
- 31. Naim O (2006) The structure of coral reef benthic communities at Saint-Gilles la Saline in 1987 (Réunion, Mascarene Archipelago, SW Indian Ocean). J Nat 18: 13–31. [Google Scholar]
- 32. Kohler KE, Gill SM (2006) Coral Point Count with Excel extensions (CPCe): A Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comp Geosci 32: 1259–1269 doi:10.1016/j.cageo.2005.11.009 [Google Scholar]
- 33. Bucher DJ, Harriott VJ, Roberts LG (1998) Skeletal micro-density, porosity and bulk density of acroporid corals. J Exp Mar Biol Ecol 228: 117–136. [Google Scholar]
- 34. Maxwell K, Johnson GN (2000) Chlorophyll fluorescence–a practical guide. J Exp Bot 51: 659. [DOI] [PubMed] [Google Scholar]
- 35. LaJeunesse TC (2001) Investigating the biodiversity, ecology, and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the ITS region: in search of a “species” level marker. J Phycol 37: 866–880 doi:10.1046/j.1529-8817.2001.01031.x [Google Scholar]
- 36. LaJeunesse TC (2002) Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar Biol 141: 387–400. [Google Scholar]
- 37. Johannes RE, Wiebe WJ (1970) Method for determination of coral tissue biomass and composition. Limnol Oceanogr 15: 822–824. [Google Scholar]
- 38. Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pfl 167: 1–194. [Google Scholar]
- 39. Markwell MAK, Haas SM, Bieber LL, Tolbert NE (1978) A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem 87: 206–210. [DOI] [PubMed] [Google Scholar]
- 40. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275. [PubMed] [Google Scholar]
- 41. Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Phys 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 42. Chauvin A, Denis V, Cuet P (2011) Is the response of coral calcification to seawater calcification related to nutrient loading? Coral Reefs 30: 911–923 doi: 10.1007/s00338-011-0786-7 [Google Scholar]
- 43. Muscatine L, Cernichiari E (1969) Assimilation of photosynthetic products of zooxanthellae by a reef coral. Biol Bull 137: 506–523. [DOI] [PubMed] [Google Scholar]
- 44. Grottoli AG, Rodrigues LJ, Palardy JE (2006) Heterotrophic plasticity and resilience in bleached corals. Nature 440: 1186–1189 doi:10.1038/nature04565 [DOI] [PubMed] [Google Scholar]
- 45. Wooldridge SA (2009) A new conceptual model for the enhanced release of mucus in symbiotic reef corals during «bleaching» conditions. Mar Ecol Prog Ser 396: 145–152 doi:10.3354/meps08310 [Google Scholar]
- 46. Houlbrèque F, Ferrier-Pagès C (2009) Heterotrophy in tropical scleractinian corals. Biol Rev 84: 1–17 doi:10.1111/j.1469-185X.2008.00058.x [DOI] [PubMed] [Google Scholar]
- 47. Middlebrook R, Anthony KR, Hoegh-Guldberg O, Dove S (2010) Heating rate and symbiont productivity are key factors determining thermal stress in the reef-building coral Acropora formosa . J Exp Biol 213: 1026–1034 doi:10.1242/jeb.031633 [DOI] [PubMed] [Google Scholar]
- 48.Fisher P (2006) Investigating the photo-physiology of Symbiodinium sub-clades and its relationship to coral bleaching. PhD thesis, Centre for Marine Studies, University of Queensland, Brisbane, Australia.
- 49. Yang SY, Keshavmurthy S, Obura D, Sheppard CRC, Visram S, et al. (2012) Diversity and distribution of Symbiodinium associated with seven common coral species in the Chagos Archipelago, central Indian Ocean. PLoS ONE 7: e35836 doi:10.1371/journal.pone.0035836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. LaJeunesse TC, Loh WKW, van Woesik R, Hoegh-Guldberg O, Schmidt GW, et al. (2003) Low symbiont diversity in southern Great Barrier Reef corals, relative to those of the Caribbean. Limnol Oceanogr 48: 2046–2054 doi: 10.4319/lo.2003.48.5.2046 [Google Scholar]
- 51. LaJeunesse TC, Bhagooli R, Hidaka M, deVantier L, Done T, et al. (2004) Closely related Symbiodinium spp. differ in relative dominance across environmental, latitudinal and biogeographic gradients. Mar Ecol Prog Ser 284: 147–161. [Google Scholar]
- 52. Lirman D (2000) Lesion regeneration in the branching coral Acropora palmata: effects of colonization, colony size, lesion size, and lesion shape. Mar Ecol Prog Ser 197: 209–215. [Google Scholar]
- 53. Meesters EH, Bos A (1992) Gast (1992) Effects of sedimentation and lesion position on coral tissue regeneration. Proc 7th Int Coral Reef Symp 2: 671–678. [Google Scholar]
- 54. Meesters EH, Wesseling I, Bak RP (1996) Partial mortality in three species of reef-building corals and the relation with colony morphology. Bull Mar Sci 58: 838–852. [Google Scholar]
- 55. Pratchett MS, Pisapia C, Sheppard CRC (2013) Background mortality rates for recovering populations of Acropora cytherea in the Chagos Archipelago, central Indian Ocean. Mar Environ Res 86: 29–34 doi: 10.1016/j.marenvres.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 56. Kramarsky-Winter E, Loya Y (2000) Tissue regeneration in the coral Fungia granulosa: the effect of extrinsic and intrinsic factors. Mar Biol 137: 867–873. [Google Scholar]
- 57. Wooldridge SA (2010) Is the coral algae symbiosis really ‘mutually beneficial’ for the partners? BioEssays 32: 615–625 doi:10.1002/bies.200900182 [DOI] [PubMed] [Google Scholar]
- 58. Titlyanov EA, Titlyanova TV (2008) Coral-algal competition on damaged reefs. Russ J Mar Biol 34: 199–219 doi: 10.1134/S1063074008040019 [Google Scholar]
- 59. Naim O (1993) Seasonal responses of a fringing reef community to eutrophication (Reunion Island, Western Indian Ocean). Mar Ecol Prog Ser 99: 137–151. [Google Scholar]
- 60. van Woesik R, Jordán-Garza AG (2011) Coral populations in a rapidly changing environment. J Exp Mar Biol Ecol 408: 11–20 doi:10.1016/j.jembe.2011.07.022 [Google Scholar]