Abstract
Angiogenesis is a fundamental part of the response to tissue injury, which is involved in the development of hepatic fibrosis. Vascular endothelial growth factor plays an important role in angiogenesis. The expression of VEGF is increased during hepatic fibrogenesis and correlates with the micro-vessel density. In this study, we investigated the effects of bevacizumab, an anti-angiogenetic drug, on the formation of hepatic fibrosis. We found that bevacizumab could attenuate the development of hepatic fibrosis and contribute to the protection of liver function. Bevacizumab was also found to downregulate the expression α-SMA and TGF-β1, which have been reported to be profibrogenic genes in vivo. We also observed that the expression of VEGF increased significantly during the development of hepatic fibrosis and CCl4 was found to induce hepatocytes to secrete VEGF, which led to the activation and proliferation of HSCs. Bevacizumab was also found to block the effects of the hepatocytes on the activation and proliferation of HSCs. Our results suggest that bevacizumab might alleviate liver fibrosis by blocking the effect of VEGF on HSCs. Bevacizumab might be suitable as a potential agent for hepatic fibrosis therapy.
Introduction
Hepatic fibrosis is characterized by excess production and deposition of extracellular matrix (ECM), which leads to loss of liver function and structure disruption of liver tissue[1], [2]. Angiogenesis is a complex process leading to generation of new blood vessels from pre-existing blood vessels[3], [4]. Angiogenesis is known to play a critical role in pathological settings like chronic inflammatory and tumor growth[5], [6], [7]. Vascular endothelial growth factor (VEGF) is considered to be the central angiogenic factor during chronic liver injury. The present study demonstrates that expression of VEGF-A is up-regulated during liver fibrosis, and its expression is increased in activated hepatic stellate cells (HSCs) [5], [6], [8], [9].
Hepatic stellate cells (HSCs) play an important role in the development of hepatic fibrosis. HSCs are considered as a key target in anti-fibrotic therapy because of their role in ECM accumulation[10], [11], [12], [13]. Evidence indicates that activated HSCs can express VEGF and VEGF receptors in the liver after carbon tetrachloride (CCl4) intoxication[14], [15]. Inflammatory mediators cause the HSCs to differentiate into myofibroblasts. They play a role in angiogenesis and act by releasing the proangiogenic mediators VEGF and angiopoietin-1 during the development of liver fibrosis[16], [17].
Bevacizumab, a full-length humanized monoclonal antibody, is a therapeutic candidate suitable for use as a direct inhibitor of angiogenesis. Its antiangiogenic efficacy is attributable to its ability to bind and neutralize all isoforms of VEGF-A [18]. Bevacizumab has been used to treat metastatic colorectal and metastatic breast cancer[19], [20]. Recent studies suggest that anti-angiogenic therapies can prevent liver fibrosis[5], [21], [22], [23], [24], [25]. Bevacizumab has a potent anti-fibrotic effect in human Tenon's fibrosis by inhibiting VEGF-A. However, to date, the effects of bevacizumab in liver fibrosis are largely unknown. These observations have led us to hypothesize that bevacizumab may inhibit the development of pathological angiogenesis in fibrotic tissue and influence the progress of hepatic fibrosis.
In this study, we investigated the effects of bevacizumab on liver fibrosis. Carbon tetrachloride was used to establish a hepatic fibrosis animal model suitable for observation of the effect of bevacizumab in vivo. We then, examined the role of bevacizumab in the proliferation and activation of HSCs in vitro. Our results demonstrated that bevacizumab administration could alleviate liver fibrosis by inhibiting activation and proliferation of HSCs.
Results
Effects of bevacizumab on hepatic fibrosis induced by CCl4 in rats
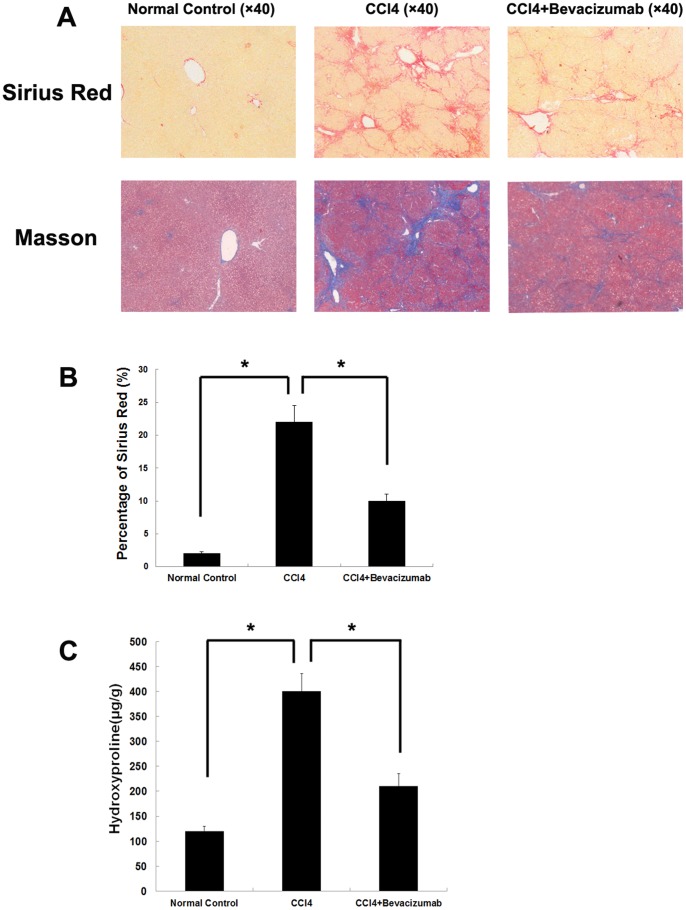
We assessed the effects of bevacizumab in a rat model of CCl4-induced hepatic fibrosis. As shown in Figure 1A, bevacizumab administration had significantly reduced in fibrosis deposition as demonstrated by Sirius red staining and Masson's trichrome staining relative to the control group. This confirmed that bevacizumab attenuated hepatic fibrosis induced by CCl4 in rats. Semiquantitative analysis of the ECM area revealed that bevacizumab significantly reduced the area of ECM (Sirius red staining) after injection in the CCl4-treated fibrotic livers(Figure 1B). In CCl4-induced models, quantitative estimation of hydroxyproline content in the fibrotic groups indicated that the hydroxyproline content in bevacizumab-treated rats was 202.78±38.56 µg/g, which was lower than that in the positive control group (404.13±37.1 µg/g, P<0.05) (Figure 1C).
Figure 1. Bevacizumab attenuates hepatic fibrosis induced by CCl4 in rats.
CCl4 was used to construct a hepatic fibrosis model to evaluate the therapeutic effects of bevacizumab(n = 8 for each group). (A) Sirius red and Masson's trichrome staining were used to determine the amount of ECM in the liver tissue of each groups. (B) Semiquantitative analysis of the ECM area was performed to evaluate the relative amount Sirius-red in fibrotic tissue using an image analysis system. (C) The amount of ECM was quantitated by quantitative estimation of hydroxyproline content. (*P<0.05).
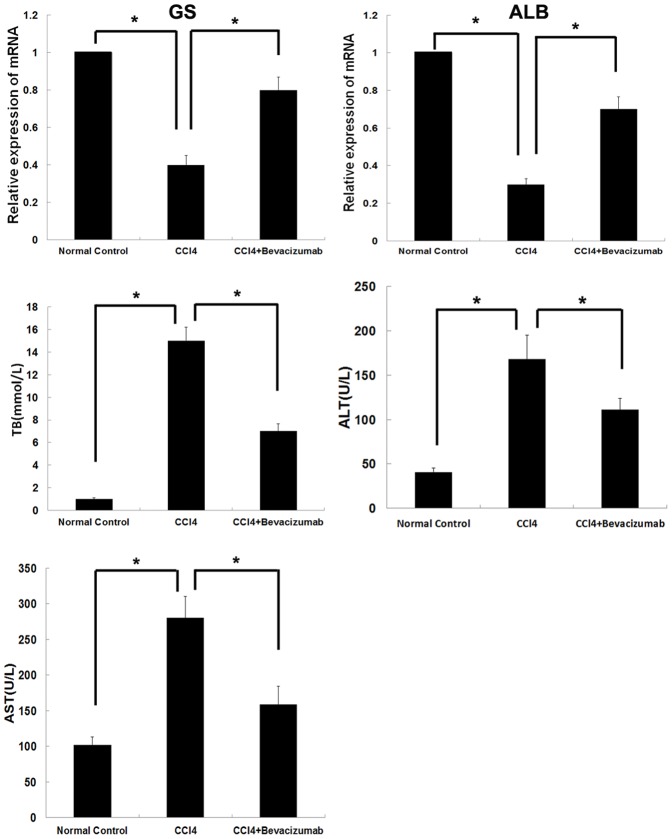
As shown in Figure 2, bevacizumab ameliorated liver function in the fibrotic rats. Bevacizumab delivery significantly improved albumin (ALB) and glutamine synthetase (GS) levels in rats with hepatic fibrosis. Total bilirubin (TB), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) concentration in bevacizumab-treated rats presented an obvious decrease relative to the control group.
Figure 2. Effects of bevacizumab on liver function in fibrotic rats.
The liver tissue and serum of the rats in each group was collected for assessment of liver function. GS, ALB, TB, AST and ALT were examined to assess hepatic function. Shown as the first two panels, GS and ALB levels were significantly lower of CCl4 group than that of normal control group. Bevacizumab administration significantly promoted synthesis of GS and ALB than that of CCl4 group. According to the left three panels, there were significantly differences of TB,ALT and AST levels among three groups. (*P<0.05).
Bevacizumab downregulated profibrogenic genes expression in vivo
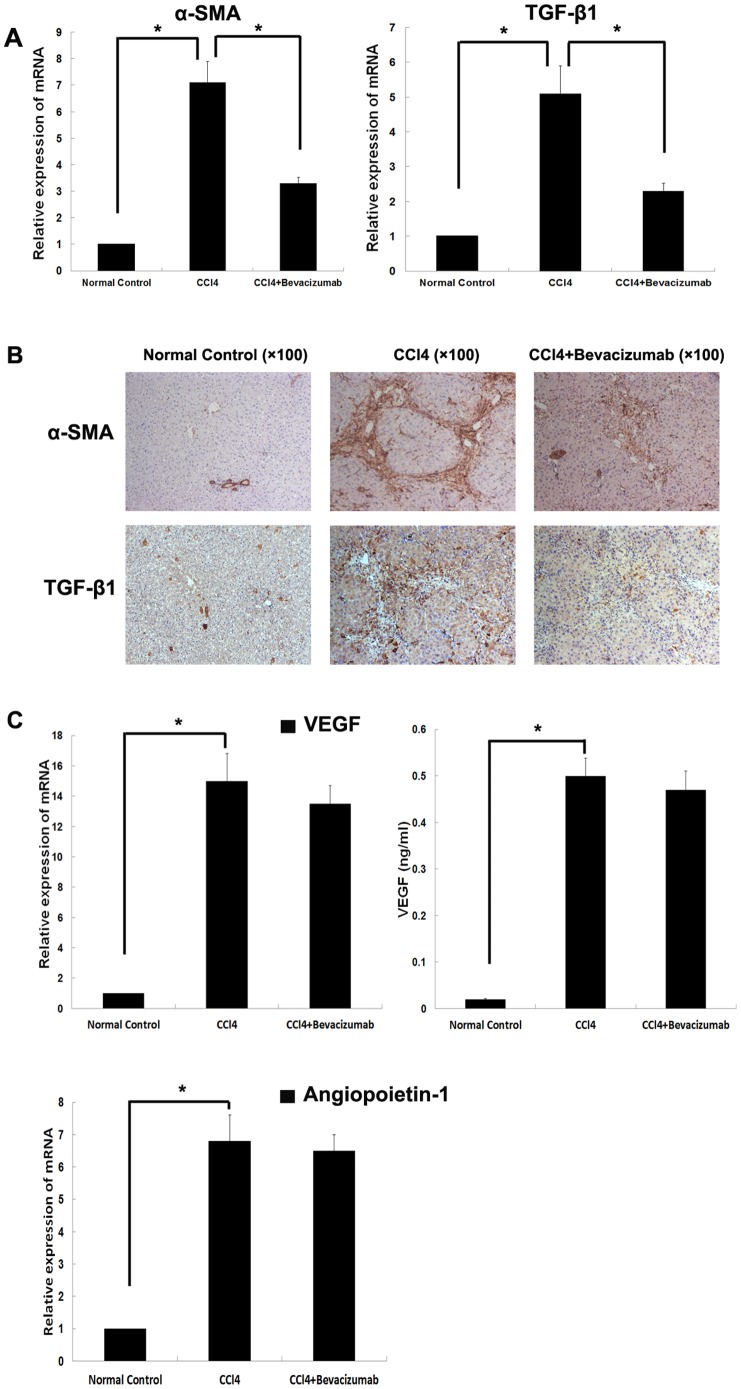
Real-time PCR and immunohistochemical staining assays (IHC) were used to detect the expression of α-smooth muscle actin (α-SMA) and transforming growth factor-β1 (TGF-β1) in the liver tissues. These have been reported to be important for the development of hepatic fibrosis. The results demonstrated that expression of α-SMA and TGF-β1 was very low in normal liver tissue. However, a significant increase in gene expression was observed with the development of hepatic fibrosis induced by CCl4. Bevacizumab injection was found to remarkably down-regulate the expression of the genes related to liver fibrosis relative to the control groups (Figures 3A and B).
Figure 3. Effects of bevacizumab on profibrogenic gene expression in vivo.
(A) Real-time PCR was used to assess the expression of α-SMA and TGF-β1, which have been reported to be important to the development of hepatic fibrosis. (B) Immunohistochemistry was used to examine α-SMA and TGF-β1 expression in liver tissues. (C) The expression of VEGF and angiopoietin-1 was examined by using real-time PCR and ELISA in the liver and serum. (*P<0.05).
We also examined the expression of VEGF in liver tissue and serum. As shown in Figure 3C, the expression of VEGF was absent in normal liver tissue and serum, but up-regulation of VEGF was observed in hepatic fibrosis liver tissue and serum. Furthermore, we observed the VEGF level in liver and serum of hepatic fibrosis rats which have been administrated with bevacizumab. However, bevacizumab did not lead to a significant down-regulation of VEGF in the fibrosis liver. Beside that, we detected the expression of angiopoietin-1, another important angiogenesis associated factor, in liver. We could observe a obvious up-regulation of angiopoietin-1 in hepatic fibrosis group compared with control group and bevacizumab did not lead to a down-regulation of angiopoietin-1 in fibrosis liver. These results suggest that hepatocytes might produce VEGF during the formation of hepatic fibrosis.
CCl4 lead to up-regulation of VEGF in hepatocytes
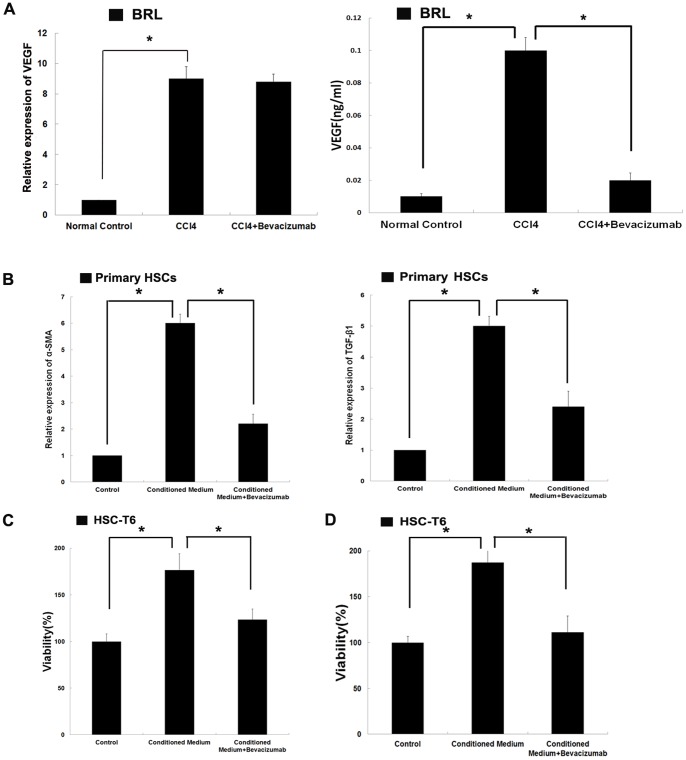
The expression of VEGF was higher in fibrotic hepatic tissue than in healthy tissue. Real-time PCR and ELISA were used to examine VEGF expression in hepatocytes after exposure to CCl4. As shown in Figure 4A, there was a significant up-regulation of VEGF relative to the control group.
Figure 4. Effects of CCl4 on VEGF expression in hepatocytes and effects of conditioned medium collected from hepatocytes on the activation and proliferation of hepatic stellate cells.
(A)BRL cells were exposed to CCl4 for 12 hours and then the culture medium was replaced with fresh DMEM. After another 24 hours of culture, the conditioned medium was collected. Real-time PCR and ELISA assays were used to assess VEGF expression in hepatocytes after exposure to CCl4. (B) BRL cells were exposed to CCl4 for 12 hours and then the culture medium was replaced with fresh DMEM. After another 24 hours of culture, the conditioned medium was collected. The primary HSCs were plated in 6-well plates (1×106 cells/well) and treated by conditioned medium with or without bevacizumab (100 µg/ml) for 72 hours. Then the cells were harvested and real-time PCR was performed to assess the expression of α-SMA and TGF-β1 in HSCs. (C) CCK-8 assay was used to assess the effects of conditioned medium with or without bevacizumab (100 µg/ml) on the proliferation of the HSC-T6 cell line. (D) MTT assay was employed to examine the effects of conditioned medium with or without bevacizumab (100 µg/ml) on the proliferation of the HSC-T6 cell line. (*P<0.05).
Effects of conditioned medium collected from hepatocytes on the activation and proliferation of hepatic stellate cells
We have demonstrated that the expression of VEGF increased significantly during the formation of hepatic fibrosis and that bevacizumab could effectively attenuate hepatic fibrosis. HSCs have been shown to play a central role in the development of hepatic fibrosis. HSCs are considered a key target in anti-fibrotic therapy because of their role in ECM accumulation. The conditioned medium was collected from hepatocytes which were treated with CCl4. We then detected the gene expression of fibrotic markers in HSCs treated with the conditioned medium. As shown in Figure 4B, the expression of α-SMA and TGF-β1 in HSCs treated with conditioned medium was significantly higher than in self-activated HSCs. The HSC-T6 cell line was used to examine the effects of conditioned medium on the proliferation of HSCs. As shown in Figure 4C and D, conditioned medium promoted the proliferation of HSCs. These results indicated that the hepatocytes in fibrotic livers might play an important role in the activation of HSCs.
In order to confirm the contribution of VEGF to the activation of HSCs, bevacizumab was added in the conditioned medium, which was collected from hepatocytes exposed to CCl4. We found that the up-regulation of α-SMA and TGF-β1 in conditioned medium-treated HSCs was cancelled by bevacizumab (Figure 4B). In addition, bevacizumab blocked the enhancement of HSC-T6 cells proliferation caused by conditioned medium (Figure 4C and D). These results suggested that bevacizumab might be useful in preventing the activation and proliferation of HSCs during the development of hepatic fibrosis.
Discussion
The hepatic fibrosis caused by many etiologies is an essential pathological process in chronic liver diseases and leads to loss of liver function and disrupts the structure of liver tissue[1], [2]. Angiogenesis is the main process of new vessel formation, accompanies with liver fibrosis and cirrhosis[5], [6], [24], [26], [27]. It can lead to generation of the new vessels from pre-existing blood vessels[3], [4]. Recently several studies reveal that angiogenesis plays a key role in fibrogenic progression of chronic liver diseases and the inhibition of pathological angiogenesis could regress or reverse liver fibrosis in experimental and clinical studies[4], [5], [6], [24], [26], [27]. The development of hepatic fibrosis was always associated with hypoxia and angiogenesis in hepatocytes[28], [29].
VEGF is the central angiogenic factor during chronic liver injury. The present study demonstrates that expression of VEGF-A is up-regulated in liver fibrosis, and its expression is increased in activated HSCs [5], [6], [8], [9]. Rosmorduc et al showed that biliary cirrhosis is associated with hepatocellular hypoxia in experimental models[8]. The expression of VEGF can be acticated by some hypoxic factor, such as hypoxia-inducible factor-1α (HIF-1α)[30]. Diethylnitrosamine induced chemical cirrhosis in rat demonstrates progressive hepatic fibrosis accompanied by up-regulation of VEGF and VEGF receptor and angiogenesis[29]. VEGF is produced by hepatocytes and induces hepatocellular growth by autocrine action[31]. Beside that, VEGF generated by hepatocytes also stimulates the proliferation of endothelial cells(ECs) in a paracrine fashion[32].
Bevacizumab has been proved to be a useful angiogenesis inhibitor. Its antiangiogenic efficacy is attributable to binding and neutralization of all isoforms of VEGF-A [18]. In this study, we investigated the effect of bevacizumab on the formation of hepatic fibrosis. We demonstrated that bevacizumab could effectively attenuate the development of hepatic fibrosis and contribute to the protection of liver function. Bevacizumab was also found to downregulate the expression α-SMA and TGF-β1, which have been reported to be profibrogenic genes in vivo. Furthermore, we observed the VEGF level in liver and serum of hepatic fibrosis rats which have been administrated with bevacizumab. However, bevacizumab did not lead to a significant down-regulation of VEGF. This result implied that bevacizumab may work by neutralizing VEGF rather than directly inhibiting the expression of VEGF in the fibrosis liver. These observations indicated that bevacizumab might inhibit the development of pathological angiogenesis in fibrosis tissue and influence the progress of liver fibrosis.
The activation of resident HSCs has been shown to play a central role in the development of hepatic fibrosis. HSCs are key target in anti-fibrotic therapy because of their function in ECM accumulation. HSCs underwent activation and conversion to myofibroblast-like cells capable of producing collagens and aggravating the deposition of ECM. Inflammatory mediators cause HSCs to differentiate into myofibroblasts and play a role in angiogenesis by releasing proangiogenic mediators, VEGF, and angiopoietin-1 during the development of liver fibrosis [16], [17]. On one hand, HSCs can behave as proangiogenic cells able to react to conditions of hypoxia by up-regulating transcription and synthesis of VEGF and its receptors[15], [17], [33]. On the other hand, HSCs are target cells for the action of VEGF. Hypoxia-dependent up-regulation of VEGF produced by HSCs demonstrates a paracrine and/or autocrine manner[17].
We showed that the expression of VEGF increased significantly during the development of hepatic fibrosis. The conditioned medium collected from hepatocytes exposed to CCl4 was found to activate the expression of fibrotic markers in HSCs and promote the proliferation of HSCs. We also observed that the expression of VEGF increased significantly during the development of hepatic fibrosis and that CCl4 could cause hepatocytes to produce VEGF. Bevacizumab neutralized VEGF significantly, which led to the blockage of the effect of VEGF produced by hepatocytes.
In conclusion, the results of our study suggested that bevacizumab might alleviate liver fibrosis by neutralizing VEGF produced by hepatocytes and block their effects on the activation of HSCs. Bevacizumab might be suitable as a potential agent for hepatic fibrosis therapy.
Materials and Methods
Cells and animals
Bevacizumab was obtained from Roche Ltd. (Shanghai, China). A rat normal liver cell line-BRL and an activated HSCs cell line-HSC-T6 were obtained from the cell bank of the Chinese Academy of Sciences (Shanghai, China).
Male Sprague-Dawley rats (190±15 g) were housed under standard animal laboratory conditions in the specific-pathogen-free-grade animal room at the Experimental Animal Center of the Second Military Medical University. The rats had free access to standard rat chow and water. This study was approved by the Local Ethical Committee of the Second Military Medical University.
Hepatic fibrosis model
The hepatic fibrosis model of SD rats was induced by subcutaneous injection of 40% CCl4 at a dose of 2.4 ml/kg twice per week for 8 weeks[34]. Twenty-four male Sprague-Dawley (SD) rats were randomly divided into 3 groups. The first group (n = 8) served as a normal control group. The rats in next two groups (n = 16) were hepatic fibrosis models. The second group served as positive control group, and the rats in the third group were given 200 µg/kg bevacizumab. Infusions were given via the tail vein twice a week for 4 weeks starting from the 5th week in group 3. The rats in groups 1 and 2 were infused equal volumes of saline. One week after the last infusion, the animals were sacrificed by CO2 exposure and liver tissues were harvested.
Histological examination and immunohistological staining
All paraffin-embedded liver tissues were HE stained for histopathological examination. Sirius red staining and Masson's trichrome staining were used to assess collagen levels. The red-stained areas in the Sirius Red stained sections were assessed with an image analyzer (Image-Pro Plus, MediaCybernetics) for semiquantitative analysis. The percentage of the Sirius Red was used to demonstrate the differences in each groups. Immunohistochemical examinations were used to detect the expression of α-SMA (Sigma Chemicals, St. Louis, MO, U.S.), and TGF-β1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, U.S.).
Measurement of hepatic hydroxyproline content
Total hepatic hydoxyproline levels were determined in the hydrolysates of liver samples as described previously [35].
Serum biochemical analysis
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin(TB)were assessed by the kits from Sigma-Aldrich.
HSCs isolation and culture
Primary HSCs were freshly isolated as described previously [36]. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and incubated at 37°C, 5% CO2 in a humidified incubator.
Conditioned medium
BRL cells were stimulated with CCl4(5 mmol/L) for 12 hours. Then the culture medium was replaced with fresh DMEM. After another 24 hours of culture, the conditioned medium was obtained by collection and 0.22 µm filtration of the supernatant medium from BRL cells.
Enzyme linked immunosorbent assay
ELISA assays were performed using a commercial VEGF ELISA kit (R&D Systems, Minneapolis, MN, U.S.). Samples were diluted 10-fold in deionized water before the assay. Assays were performed in duplicate, and readings were compared using standard curves obtained with standard protein provided with the kit. Samples were collected in triplicate, and means and standard deviations were compared using the t-test.
Cell counting kit-8 assay
The measurement of viable cell mass was assessed by Cell Counting Kit-8 (Dojindo, Japan). Cells (5×103 cells/well) were seeded in 96-well plates for overnight, we changed the medium with conditioned medium and continued to culture these cells for 24 hours. When the treatment was completed, 10 µl solution of Cell Counting Kit-8 was added in each well. The plate was continuously incubated for 2 hours. Finally, the absorbance of sample taken from each well was measured by microplate reader (Synergy HT, Bio-Tek) at 450 nm.
MTT colorimetric assay
In order to measure the effects of conditioned medium on the viability of HSC-T6 cells, the cells were seeded in 96-well plates at a density of 5×103 cells/well for overnight. Then we changed the culture medium with conditioned medium and continued to culture these cells for 24 hours. Twenty microlitres of MTT (5 mg/ml) were added to each well and the plate was continuously incubated for 4 hours. The formazan crystals were dissolved in 200 µl of DMSO. Finally, the absorbance of sample taken from each well was measured by microplate reader (Synergy HT, Bio-Tek) at 490 nm.
Real-time polymerase chain reaction
Total RNA was extracted from the cells using Trizol reagent (Invitrogen, Carlsbad, CA, U.S.). The cDNA was synthesized using MMLV reverse transcriptase (Promega, WI, U.S.) and 2 µg total RNA and oligo dT18-primers. Real-time PCR was performed in triplicate using a SYBR PrimeScript RT-PCR Kit (Takara, Dalian, China). Total RNA was normalized by endogenous β-actin mRNA. The level of mRNA expression is presented as fold change relative to an untreated control. The primer sequences used in realtime-PCR are shown in Table 1.
Table 1. Oligonucleotide sequences of primers used in real-time PCR.
| Gene | Sequence (5′→3′) |
| α-SMA | F: 5′-CCGAGATCTCACCGACTACC-3′ |
| R: 5′-TCCAGAGCGACATAGCACAG-3′ | |
| TGF-β1 | F: 5′-ACCGCAACAACGCAATCTATG -3′ |
| R: 5′- ATTCCGTCTCCTTGGTTCAGC-3′ | |
| VEGF | F: 5′- CAGGGTTTCGGGAACTAG-3′ |
| R: 5′- GTGTATGTGGGTGGGTGT -3′ | |
| Angiopoietin-1 | F: 5′-TACAACACCGTGAGGATGGA-3′ |
| R: 5′-TATCAGCGTCCTTTGTGCTG -3′ | |
| GS | F: 5′-AAGAGGGCATAGCCCAGACT-3′ |
| R: 5′-TTGGAAGCTTCGTTGGTCTT-3′ | |
| ALB | F: 5′-TGCAGGCTTGCTGTGATAAG-3′ |
| R: 5′-AGTAATCGGGGTGCCTTCTT-3′ | |
| β-actin | F: 5′-ACCCACACTGTGCCCATCTATG-3′ |
| R: 5′-AGAGTACTTGCGCTCAGGAGGA-3′ |
Statistical Analysis
Data sets were analyzed by analysis of variance (ANOVA) with a posteriori contrast by least significant difference for comparisons among multiple groups and by Student t-test for comparison between two groups using the Microsoft Excel Analysis Tool Pak (Microsoft, Redmond, WA). The data, collected from at least three separate experiments, was showed as mean ±SEM. P<0.05 was considered to be statistically significant.
Funding Statement
Funded by Shanghai Municipal Health Bureau (Grant NO: 20114220); Foundation of Second Military Medical University for youth (Grant NO: 2010qn24); and Project of the Department of Science and Technology of Hebei Province (No. 11276156). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wynn TA (2007) Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest 117: 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Friedman SL (2000) Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem 275: 2247–2250. [DOI] [PubMed] [Google Scholar]
- 3. Carmeliet P (2005) Angiogenesis in life, disease and medicine. Nature 438: 932–936. [DOI] [PubMed] [Google Scholar]
- 4. Lee JS, Semela D, Iredale J, Shah VH (2007) Sinusoidal remodeling and angiogenesis: a new function for the liver-specific pericyte? Hepatology 45: 817–825. [DOI] [PubMed] [Google Scholar]
- 5. Medina J, Arroyo AG, Sanchez-Madrid F, Moreno-Otero R (2004) Angiogenesis in chronic inflammatory liver disease. Hepatology 39: 1185–1195. [DOI] [PubMed] [Google Scholar]
- 6. Tugues S, Fernandez-Varo G, Munoz-Luque J, Ros J, Arroyo V, et al. (2007) Antiangiogenic treatment with sunitinib ameliorates inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology 46: 1919–1926. [DOI] [PubMed] [Google Scholar]
- 7. Taura K, De Minicis S, Seki E, Hatano E, Iwaisako K, et al. (2008) Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology 135: 1729–1738. [DOI] [PubMed] [Google Scholar]
- 8. Rosmorduc O, Wendum D, Corpechot C, Galy B, Sebbagh N, et al. (1999) Hepatocellular hypoxia-induced vascular endothelial growth factor expression and angiogenesis in experimental biliary cirrhosis. Am J Pathol 155: 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maria De Souza M, Tolentino M Jr, Assis BC, Cristina De Oliveira Gonzalez A, Maria Correia Silva T, et al. (2006) Pathogenesis of septal fibrosis of the liver. (An experimental study with a new model.). Pathol Res Pract 202: 883–889. [DOI] [PubMed] [Google Scholar]
- 10. Bataller R, Brenner DA (2001) Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis 21: 437–451. [DOI] [PubMed] [Google Scholar]
- 11. Kinoshita K, Iimuro Y, Fujimoto J, Inagaki Y, Namikawa K, et al. (2007) Targeted and regulable expression of transgenes in hepatic stellate cells and myofibroblasts in culture and in vivo using an adenoviral Cre/loxP system to antagonise hepatic fibrosis. Gut 56: 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bataller R, Brenner DA (2005) Liver fibrosis. J Clin Invest 115: 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen SW, Chen YX, Zhang XR, Qian H, Chen WZ, et al. (2008) Targeted inhibition of platelet-derived growth factor receptor-beta subunit in hepatic stellate cells ameliorates hepatic fibrosis in rats. Gene Ther 15: 1424–1435. [DOI] [PubMed] [Google Scholar]
- 14. Ishikawa K, Mochida S, Mashiba S, Inao M, Matsui A, et al. (1999) Expressions of vascular endothelial growth factor in nonparenchymal as well as parenchymal cells in rat liver after necrosis. Biochem Biophys Res Commun 254: 587–593. [DOI] [PubMed] [Google Scholar]
- 15. Ankoma-Sey V, Wang Y, Dai Z (2000) Hypoxic stimulation of vascular endothelial growth factor expression in activated rat hepatic stellate cells. Hepatology 31: 141–148. [DOI] [PubMed] [Google Scholar]
- 16. Aleffi S, Petrai I, Bertolani C, Parola M, Colombatto S, et al. (2005) Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology 42: 1339–1348. [DOI] [PubMed] [Google Scholar]
- 17. Novo E, Cannito S, Zamara E, Valfre di Bonzo L, Caligiuri A, et al. (2007) Proangiogenic cytokines as hypoxia-dependent factors stimulating migration of human hepatic stellate cells. Am J Pathol 170: 1942–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferrara N, Hillan KJ, Gerber HP, Novotny W (2004) Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 3: 391–400. [DOI] [PubMed] [Google Scholar]
- 19. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, et al. (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335–2342. [DOI] [PubMed] [Google Scholar]
- 20. Valachis A, Polyzos NP, Patsopoulos NA, Georgoulias V, Mavroudis D, et al. (2010) Bevacizumab in metastatic breast cancer: a meta-analysis of randomized controlled trials. Breast Cancer Res Treat 122: 1–7. [DOI] [PubMed] [Google Scholar]
- 21. Chaparro M, Sanz-Cameno P, Trapero-Marugan M, Garcia-Buey L, Moreno-Otero R (2007) Mechanisms of angiogenesis in chronic inflammatory liver disease. Ann Hepatol 6: 208–213. [PubMed] [Google Scholar]
- 22. Coulon S, Heindryckx F, Geerts A, Van Steenkiste C, Colle I, et al. (2011) Angiogenesis in chronic liver disease and its complications. Liver Int 31: 146–162. [DOI] [PubMed] [Google Scholar]
- 23. Lai WK, Adams DH (2005) Angiogenesis and chronic inflammation; the potential for novel therapeutic approaches in chronic liver disease. J Hepatol 42: 7–11. [DOI] [PubMed] [Google Scholar]
- 24. Fernandez M, Semela D, Bruix J, Colle I, Pinzani M, et al. (2009) Angiogenesis in liver disease. J Hepatol 50: 604–620. [DOI] [PubMed] [Google Scholar]
- 25.Van Bergen T, Stalmans I (2010) The effect of bevacizumab on human Tenon fibroblasts in ocular wound healing. Invest Ophthalmol Vis Sci 51: : 6903–6904; author reply 6904. [DOI] [PubMed] [Google Scholar]
- 26. Thabut D, Shah V (2010) Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? J Hepatol 53: 976–980. [DOI] [PubMed] [Google Scholar]
- 27. Mejias M, Garcia-Pras E, Tiani C, Miquel R, Bosch J, et al. (2009) Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology 49: 1245–1256. [DOI] [PubMed] [Google Scholar]
- 28. Marti HJ, Bernaudin M, Bellail A, Schoch H, Euler M, et al. (2000) Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol 156: 965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Corpechot C, Barbu V, Wendum D, Kinnman N, Rey C, et al. (2002) Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology 35: 1010–1021. [DOI] [PubMed] [Google Scholar]
- 30. Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, et al. (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16: 4604–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taniguchi E, Sakisaka S, Matsuo K, Tanikawa K, Sata M (2001) Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. J Histochem Cytochem 49: 121–130. [DOI] [PubMed] [Google Scholar]
- 32. Shimizu H, Miyazaki M, Wakabayashi Y, Mitsuhashi N, Kato A, et al. (2001) Vascular endothelial growth factor secreted by replicating hepatocytes induces sinusoidal endothelial cell proliferation during regeneration after partial hepatectomy in rats. J Hepatol 34: 683–689. [DOI] [PubMed] [Google Scholar]
- 33. Wang YQ, Luk JM, Ikeda K, Man K, Chu AC, et al. (2004) Regulatory role of vHL/HIF-1alpha in hypoxia-induced VEGF production in hepatic stellate cells. Biochem Biophys Res Commun 317: 358–362. [DOI] [PubMed] [Google Scholar]
- 34. Issa R, Zhou X, Constandinou CM, Fallowfield J, Millward-Sadler H, et al. (2004) Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology 126: 1795–1808. [DOI] [PubMed] [Google Scholar]
- 35. Zhong W, Shen WF, Ning BF, Hu PF, Lin Y, et al. (2009) Inhibition of extracellular signal-regulated kinase 1 by adenovirus mediated small interfering RNA attenuates hepatic fibrosis in rats. Hepatology 50: 1524–1536. [DOI] [PubMed] [Google Scholar]
- 36. Marra F, Efsen E, Romanelli RG, Caligiuri A, Pastacaldi S, et al. (2000) Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology 119: 466–478. [DOI] [PubMed] [Google Scholar]