Abstract
Crop agronomic parameters (leaf area index (LAI), nitrogen (N) uptake, total chlorophyll (Chl) content ) are very important for the prediction of crop growth. The objective of this experiment was to investigate whether the wheat LAI, N uptake, and total Chl content could be accurately predicted using spectral indices collected at different stages of wheat growth. Firstly, the product of the optimized soil-adjusted vegetation index and wheat biomass dry weight (OSAVI×BDW) were used to estimate LAI, N uptake, and total Chl content; secondly, BDW was replaced by spectral indices to establish new spectral indices (OSAVI×OSAVI, OSAVI×SIPI, OSAVI×CIred edge, OSAVI×CIgreen mode and OSAVI×EVI2); finally, we used the new spectral indices for estimating LAI, N uptake, and total Chl content. The results showed that the new spectral indices could be used to accurately estimate LAI, N uptake, and total Chl content. The highest R2 and the lowest RMSEs were 0.711 and 0.78 (OSAVI×EVI2), 0.785 and 3.98 g/m2 (OSAVI×CIred edge) and 0.846 and 0.65 g/m2 (OSAVI×CIred edge) for LAI, nitrogen uptake and total Chl content, respectively. The new spectral indices performed better than the OSAVI alone, and the problems of a lack of sensitivity at earlier growth stages and saturation at later growth stages, which are typically associated with the OSAVI, were improved. The overall results indicated that this new spectral indices provided the best approximation for the estimation of agronomic indices for all growth stages of wheat.
Introduction
The development of remote sensing has provided opportunities to quantitatively describe agronomic parameter changes across all growth stages of crops. The application of remote sensing to agronomic problems has created new methods to effectively improve field crop management. Many authors have provided detailed information about the relationships between spectral indices and agronomic parameters, including the leaf area index (LAI), nitrogen (N) uptake, total chlorophyll (Chl) content, and so on.
The LAI is a key variable for the diagnosis and prediction of crop growth and yield. This makes the LAI critical for effective understanding of the biophysical processes of plant canopies and the prediction of plant growth and productivity [1]–[6]. Rouse et al. suggested that the most well-known and widely used vegetation index was the normalized difference vegetation index (NDVI), and found that NDVI was linearly correlated with the leaf area index (LAI) in crop fields [7]. However, the NDVI does possess certain limitations related to soil background brightness, in that the NDVI tends to be affected by different soil color and moisture conditions [8]–[10]. To overcome this problem, Rondeaux et al. proposed using an optimized soil-adjustment factor, and obtained an optimized soil-adjusted vegetation index (OSAVI), which mitigated the effects of soil and moisture conditions [11].
Nitrogen is a critically important element that is monitored in an effort to maintain crop health, while there is a good relationship between nitrogen content and chlorophyll content [12]–[13], therefore chlorophyll content is an important indicator for nitrogen fertilizer applications. Stone et al. detected and predicted N uptake in winter wheat using hand-held sensors [14]–[15]. Osborne et al. identified the important reflectance wavelengths for the prediction of N concentration changes at different growth stages [16]. Gitelson et al. suggested that the green NDVI (GNDVI) was more sensitive than the NDVI for wheat N uptake over 100 kg/ha [17], but Moges et al. indicated that the NDVI was more robust than the GNDVI for prediction of crop N uptake [18]. Certain researchers have proposed many N indicators for the assessment of crop N change according to the spectral features of chlorophyll in the visible and red-edge bands [19]–[20]. A good relationship between a combined index (the ratio of modified chlorophyll absorption ratio index and modified triangular vegetation index2, MCARI/MTVI2) and leaf nitrogen concentration was found by Eitel et al. [21]. Fitzgerald et al. reported that a spectral index, the canopy chlorophyll content index (CCCI), could predict canopy N, and specifically found a good relationship between the CCCI and canopy N [22]. Vigneaua et al. used field hyperspectral imaging as a non-destructive method to assess leaf nitrogen content in wheat [23]. And recently, remote sensing methods have been developed and applied for the prediction of Chl content for field crop management [24]–[30]. Gitelson et al. identified the best vegetation indices for the estimation of Chl content [30]. A good correlation between total Chl content and (RNIR which is in the near infrared band reflectance/Rred edge which is in the red edge position reflectance)–1, (RNIR/Rgreen which is in the green band reflectance)–1 was found by Gitelson et al. [31], and Gitelson et al. applied the new vegetation index (RNIR/Rred edge–1 and RNIR/Rgreen–1) for the estimation of Chl content and indirectly estimated gross primary production (GPP) [32], [33].
However, spectral indices still eixst insensitivity at earlier growth stages, and saturation at later growth stages. Because biomass dry weight (BDW) was accumulated gradually as growth stages progressed, and insensitivity at earlier growth stages and saturation at later growth stages was not observed. Consequently, the objectives of this study were to (1) combine OSAVI and BDW for estimating LAI, N uptake, and total Chl content in wheat; (2) replace BDW with spectral indices; (3) and attempt to propose new spectral indices, in an effort to improve insensitivity at earlier growth stages and saturation at later growth stages.
Materials and Methods
2.1. Design of Experiment
Field experiments were conducted in 2009 and 2010 at the ShiHezi University experiment site (44°20′N, 86°3′E), Xinjiang Province, China. The experiment site had representative soil types and crop management practices for Xinjiang Province, China. The soil was fine-loamy with a total N content of 42.6 mg kg−1, Olsen P of 26.5 mg kg−1, exchangeable K of 139.4 mg kg−1, and organic matter content of 11.6 g kg−1 in the 0–30 cm layer. Three local wheat cultivars: Xinchun 6, Xinchun 17, and Xinchun 22, were planted on April 5th 2008 and April 8th 2009. Nitrogen fertilizer as urea was applied at four rates (0, 105, 225, and 345 kg N ha−1) before planting. The N application was distributed at three stages in the growth process in the following percentages: 50% at seeding, 25% at jointing, and 25% at booting. For all treatments, 99 kg ha−1 P2O5 (as monocalcium phosphate [Ca(H2PO4)2]) and 150 kg ha−1 K2O (as KCl) was applied prior to seeding. The experiment was a 2-way factorial arrangement of treatments in a randomized complete block design with three replications for each treatment. Other management practices followed local standard wheat production practices.
2.2. Measurement of Canopy Reflectance
Spectral measurements were carried out at the following stages (2009, 2010): tillering (8th May, 6th May), jointing (20th May, 25th May), heading (8th June, 10th June), anthesis (18th June, 20th June), and filling (27th May, 25th May), respectively. All canopy spectral measurements were mounted on the tripod boom and held in a nadir orientation 1.0 m above the canopy. Measurements were taken under clear sky conditions between 10∶00 and 14∶00 (Beijing local time) using an ASD Field Spec Pro Spectrometer (Analytical Spectral Devices, Boulder, CO, USA). This spectrometer is fitted with a 25° field of view fiber optics, operating in the 350–2500 nm spectral region with a sampling interval of 1.4 nm between 350 and 1050 nm, and 2 nm between 1050 and 2500 nm, and with spectral resolution of 3 nm at 700 nm, and 10 nm at 1400 nm. A 40 cm × 40 cm BaSO4 calibration panel was used for calculating the black and baseline reflectance. To reduce the possible effect of sky and field conditions, spectral measurements were taken at four sites in each plot and were averaged to represent the canopy reflectance of each plot. Vegetation radiance measurement was taken by averaging 16 scans at an optimized integration time, with a dark current correction at every spectral measurement. A panel radiance measurement was taken before and after the vegetation measurement by two scans each time.
2.3. Agronomic Parameters Measurement
Immediately following spectral measurements, the leaf area index (LAI) was measured using the LAI-2000 Plant Canopy Analyzer (LI-COR Inc., Lincoln, NE, USA) with spectrometric measurements at the same position and gained by destructive sampling. The wheat was cut at the ground level and wet weights were recorded in 0.24 m2. Each plant was then dried at 70°C for 3 d and the dry weight was recorded. Dry plant material was then milled and analyzed in the laboratory. Total N content was estimated using a dry combustion method in a Dumas Elementary Analyser (Macro-N, Foss Heraeus, Hanau, Germany) [34].
The spectral measurements positions of the wheat leaves were collected using a hole puncher (diameter, 0.4 cm). Then, about 0.2 g wheat leaves of each sample was punched off in the laboratory. The selected samples were placed in 95% ethanol or acetone solution and allowed to stand for 24 hr in the dark. Following the 24-hr treatment, the leaves were white-green in color. Finally, leaf pigment density was measured using a colorimetric spectro-photometer. Absorbance of the supernatant was measured at 645, 652 and 663 nm, and chlorophyll a plus chlorophyll b content per unit leaf area was then calculated by the method of McKinney [35].
2.4. Selection of Spectral Indices
This study tested the spectral indices that were considered to be good candidates for estimation of plant chlorophyll content and LAI (Table 1) [11], [31], [36]–[37].
Table 1. Summary of spectral indices, wavebands and citations for LAI, nitrogen uptake and total chlorophyll content in this paper.
| Index | Name | Formula | Developer(s) |
| OSAVI | Optimized soil-adjusted vegetation index | 1.16×(R800−R670)/(R800+R670+0.16) | Rondeaux et al. (1996) [11] |
| SIPI | Structure insensitive pigment index | (R800−R445)/(R800−R680) | Penuelas et al. (1995) [36] |
| CIred edge | Red edge model | (R800/R700)−1 | Gitelson et al. (2005) [31] |
| CIgreen model | Green model | (R800/R550)−1 | Gitelson et al. (2005) [31] |
| EVI2 | Enhanced vegetation index 2 | 2.5×(R800−R660 )/(1+R800+2.4×R660) | Jiang et al. (2008) [37] |
Note: Ri denotes reflectance at band i (nanometer).
2.5. Statistical Analysis
Linear and nonlinear regression analysis was carried out using the biomass dry weight, OSAVI and OSAVI×biomass dry weight (the product of OSAVI and biomass dry weight) as independent variables, and the LAI, total Chl content, and N uptake (leaves yield N concentration of measured leaves) as dependent variables.
Statistically significant (p<0.05 or 0.01) coefficients of determination (R2) between three crop parameters (LAI, total Chl content, N uptake) and new vegetation indices or biomass dry weight were analyzed using SPSS software (16.0, SPSS, Chicago, IIIinois, USA). The R2 and root mean square error (RMSE) were used as metrics for quantifying the amount of variation explained by the relationships developed, as well as their accuracy. The performance of the model was evaluated through R2 and RMSE for the estimation of in-situ measured LAI, total Chl content and N uptake. Generally, the performance of the model was estimated by comparing the differences of the R2 and RMSE between the measured value and predicted value. The higher the R2 and the lower the RMSE were, the higher the precision and accuracy of model to predict agronomic parameters was considered to be.
Results
3.1. Leaf Area Index (LAI)
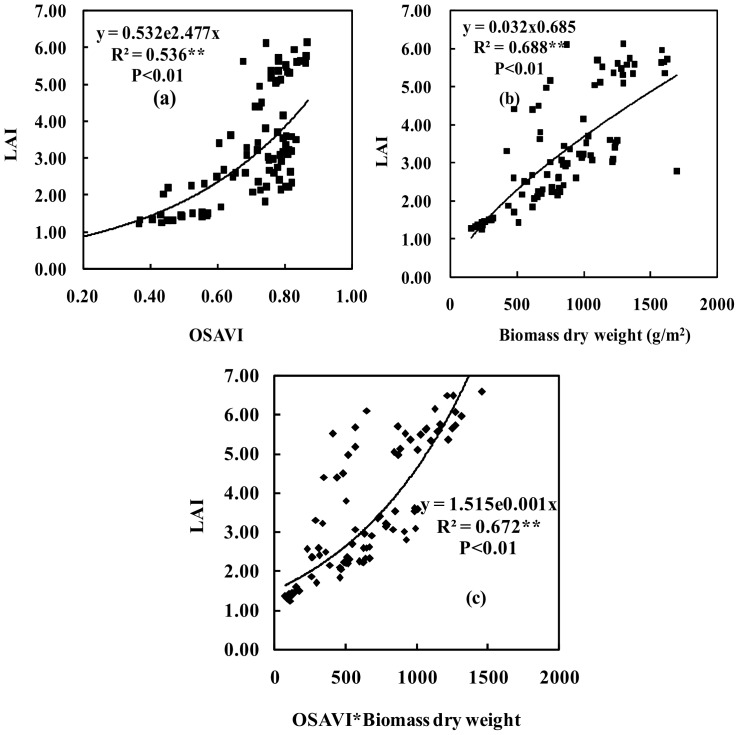
A significant relationship was found between the OSAVI and LAI for the selected data, ranging from all growth stages of wheat across 2009 (Figure 1a). As noted above, the OSAVI was calculated and measured for each stage by averaging ASD-2500 sensor readings from taken at the vertical height of the canopy at 1.0 m. Steven showed that the LAI has a strong relationship with the OSAVI [38]. Figure 1a has shown that for the OSAVI index, there was a problem with saturation at later growth stages in wheat, determination coefficient (R2) value was 0.536. It is mainly for this reason that the OSAVI was not sensitive to the LAI (ranges from 4 to 6) at later growth stages. Possibly, this was because the band combination only considered the visible and near-infrared bands and was thus affected by environmental conditions.
Figure 1. The coefficient of determination (R2) between OSAVI, biomass dry weight (BDW), BDW×OSAVI and LAI under the different nitrogen treatments for wheat (n = 90).
Note: Probability levels are indicated by n.s., * and ** for ‘not significant’, 0.05, and 0.01, respectively.
Biomass dry weight and LAI were highly correlated (R2 = 0.688, P<0.01), independent of the area the wheat occupied. At earlier growth stages, the correlation between biomass dry weight and LAI (Figure 1b) was better than that between OSAVI and LAI (Figure 1a), R2 value was 0.688. The improvement was even greater at later growth stages. This is important because it indicates that biomass dry weight can be used to estimate wheat LAI, and to compensate for the OSAVI’s lack of sensitivity to the later growth stages changes in LAI. So, we used the OSAVI×biomass dry weight (BDW) index to estimate LAI changes using the data from all growth stages in wheat. The results showed that the OSAVI×BDW index was a good predictor of LAI, though the biomass dry weight alone was a more accurate predictor (Figure 1c). We performed non-linear regression on the OSAVI×BDW and LAI at all growth stages, and the resulting R2 was 0.672. These results suggested that the OSAVI×biomass dry weight index was an improvement over the OSAVI index, because the OSAVI alone was not sensitive to LAI changes at later growth stages.
3.2. Nitrogen Uptake
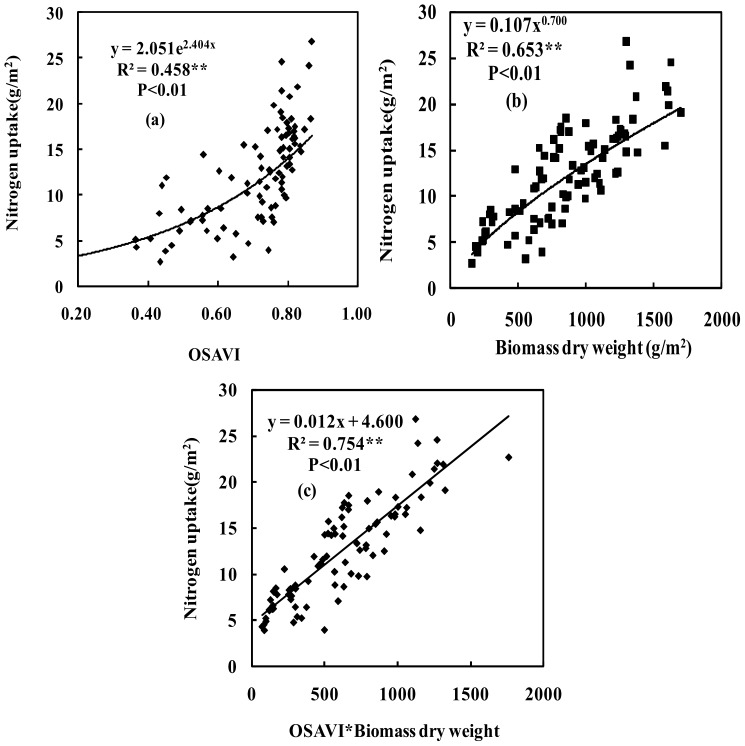
The amount of nitrogen (N) taken up in wheat was correlated with the OSAVI with an R2 value of 0.458 (Figure 2a). The results showed that the relationship between the OSAVI and N uptake was highly correlated (P<0.01) at all growth stages. However, at later growth stages when N uptake ranged from 15 to 27 g/m2, saturation was observed. A main reason for this problem was that the OSAVI was affected by other environmental factors [11]. Specifically, in this paper, the OSAVI saturation was mainly influenced by the structure of the wheat canopy. For example, the plant height of wheat canopy vary from 80 cm to 120 cm at the later growth stages, the reflectance at the top of wheat canopy (0–40 cm) can be detected by spectrometer (direct light), but the reflectance at the bottom of canopy (40–120 cm) cannot be detected by spectrometer (diffused light). The high-density wheat affected the spectral measurement and plant height. This caused the proportion of the diffused light to increase, thereby increasing the OSAVI saturation. The above problems led to a lack of sensitivity to the changes in N uptake at later growth stages. This suggested that with the increase in wheat biomass, the OSAVI became slightly affected by the surrounding environmental conditions, and the OSAVI and N uptake were correlated. The correlation between the OSAVI and N uptake could be explained by the ability of OSAVI to detect differences in red absorption. Moges et al. showed a similar finding, their research investigated the relationships of green, red, and near infrared bands to N uptake [16]. The results showed that the correlation between biomass dry weight and N uptake was better than that between OSAVI and N uptake, and the R2 value was 0.653 (Figure 2b).
Figure 2. Relationship between OSAVI, biomass dry weight (BDW) and BDW×OSAVI and nitrogen uptake under the different nitrogen treatments for wheat (n = 90).
Note: Same as above.
The OSAVI×biomass dry weight index (BDW) could be used to estimate N uptake changes, and was a good predictor of N uptake. It performed better than either biomass dry weight or OSAVI alone in the prediction of N uptake for wheat. The results showed that a linear regression performed between the OSAVI×BDW and N uptake at all growth stages resulted in an R2 value of 0.754 (Figure 2c). This result suggested that the OSAVI×BDW index could be effectively used to improve the OSAVI’s prediction accuracy for N uptake.
3.3. Total Chlorophyll Content
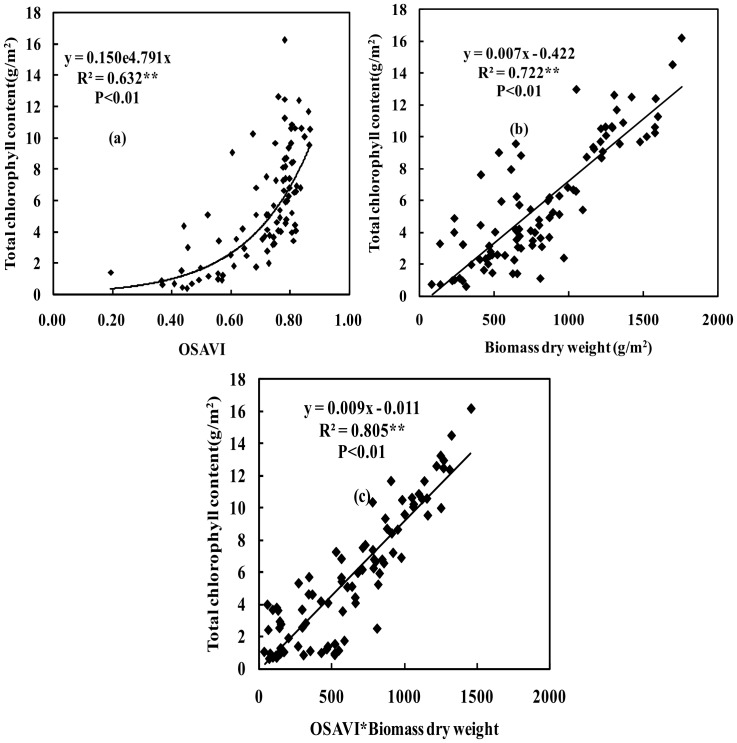
The total chlorophyll (Chl) content was correlated with OSAVI, with a coefficient of determination (R2) of 0.632. The results suggested that the total Chl content in the wheat canopy could be estimated using OSAVI (Figure 3a). Possible explanation could be the red edge absorption waveband (670 nm) is very sensitive to total Chl content [23]–[24], [26], [29]. Previous research also showed a good relationship between total Chl content and biomass [39], and biomass changes influenced the spectral reflectance in the near-infrared waveband (800 nm). Both red edge absorption waveband and near-infrared waveband are involved in OSAVI calculation. Therefore, variations in Chl content changes were detected by OSAVI.
Figure 3. Quantitative relationship between OSAVI, biomass dry weight (BDW) and BDW×OSAVI and total chlorophyll content under the different nitrogen treatments for wheat (n = 90).
Note: Same as above.
Figure 3b shows that biomass dry weight and total chlorophyll content were more highly correlated than were the OSAVI and total Chl content, with an R2 value of 0.722. Linear regression was performed between biomass dry weight and total Chl content, and between biomass dry weight and total Chl content at the different growth stages. The results showed that the relationship between the OSAVI and total chlorophyll content exhibited similar results to that between OSAVI and N uptake vis-à-vis a lower correlation at earlier growth stages.
The OSAVI×biomass dry weight index could be used to estimate total Chl content changes, and it was a good indicator of total Chl content for wheat. It improved upon the results of the biomass dry weight and OSAVI alone for estimation of the total Chl content for wheat, and the R2 was 0.805 (Figure 3c). This result indicated that the OSAVI×biomass dry weight index improved upon the prediction accuracy for total Chl content.
Taken together, these results indicated that the OSAVI×biomass dry weight index (BDW) could be used to improve the estimation accuracy of LAI, nitrogen (N) uptake, and total chlorophyll (Chl) content, respectively. The most accurate estimation was gained for total Chl content, the worst for LAI, and the median for N uptake, among three agronomic parameters.
The results showed that biomass dry weight and LAI were highly correlated (R2 = 0.688, P<0.01) (Fig. 1b). Most of results indicated that the R2 and RMSE of the curves fitting was better than the linear fitting [40]–[43]. But sometimes, the linear fitting was better than the curves fitting, it may be a high related to measurement data and crops physiological mechanism. In this paper, we try to find the best fitting lines by using the curves fitting or the linear fitting, the result indicated that the equations of curves fitting is more than the equations of linear fitting, our results was consistent with previous researches [40]–[43]. Therefore, we justify the curves fitting was better than the linear fitting according to the R2 and RMSE of regression model. The results showed that biomass dry weight and LAI were highly correlated (Fig. 1b). It indicated that the size of biomass dry weight represented the size of LAI. It was mainly because LAI growth is synchronized with biomass dry weight according to the certain proportion. The biomass dry weight and nitrogen content were also highly correlated (Fig. 2b). It suggested that the appropriate nitrogen was used to increase the biomass dry weight accumulation. Because the nitrogen was applied for improving crops photosynthesis, and there was a good relationships between photosynthesis and biomass dry weight, thereby biomass dry weight accumulation was increased. Similarly, the appropriate total chlorophyll content could be used to increase crops photosynthesis and biomass dry weight accumulation, thus there was a high correlation between biomass dry weight and total chlorophyll content (Fig. 3b).
A straight linear relationships between biomass and LAI before heading stages, but this relationship between biomass and LAI have changed after heading stages. The rate of increase in the LAI is less than in the biomass (stem and spike weights increase more than leaf weight) before and after flowering. The LAI arrived at the highest values at flag leaf fully expanded, but the biomass dry weight (BDW) is still increased (it is mainly increased from spike weight). Therefore, a curvilinear relationship between wheat BDW and LAI should be better after heading stages. Metabolic physiology exists different before and after flowering. The wheat metabolic physiology was dominated by nitrogen and supplemented by carbon before flowering, canopy leaf nitrogen content change was relatively small, canopy leaf nitrogen content was accurately estimated by more accurately monitoring LAI. However, the wheat metabolic physiology was dominated by carbon and supplemented by nitrogen after flowering, the differences are significant in canopy leaf nitrogen content, nitrogen transfers from lower leaves to the upper leaves and spike in wheat. The nitrogen was not estimated accurately by monitoring LAI because of the lower leaves nitrogen was moved away by nitrogen transfer, resulting in a relative large error will be generated. If biomass factors are taken into account, thereby the transfer of nitrogen will also be included in the monitoring results, therefore it could be used to improve the nitrogen estimation accuracy. These results indicated that the biomass dry weight was highly related to LAI, nitrogen content and total chlorophyll content because biomass dry weight had a close relationship with LAI, nitrogen content and total chlorophyll content in crops physiological mechanism. These results also suggested that the biomass dry weight could be used to estimate the LAI, nitrogen content and total chlorophyll content for wheat. The results showed that a good relationships among the LAI, nitrogen content, total chlorophyll content and OSAVI×BDW (Figs. 1c, 2c and 3c). It provided a basis for BDW was replaced by spectral indices to establish new spectral indices.
3.4. New Spectral Indices
The new spectral index (OSAVI×BDW) had a good relationship with LAI, N uptake and total Chl content, but BDW was gained by destructive sampling, and required significant time investment for data acquisition. Thus, we attempted to build new spectral indices by replacing the BDW with others spectral indices. Tables 2, 3 and 4 showed that the new spectral indices: OSAVI×OSAVI, OSAVI×SIPI, OSAVI×CIred edge, OSAVI×CIgreen model and OSAVI×EVI2, could be used to improve LAI, N uptake and total Chl content estimation accuracy. For LAI, the lowest and highest determination coefficient (R2) observed were OSAVI×SIPI and OSAVI×EVI2 with R2 values of 0.563 and 0.711, respectively (Table 2); similarly, the lowest and highest R2 were OSAVI×CIgreen model and OSAVI×OSAVI, for which R2 were 0.542 and 0.785 for nitrogen uptake, respectively (Table 3); the lowest and highest R2 were OSAVI×CIred edge and OSAVI×OSAVI, with R2 values of 0.652 and 0.846 for total Chl content, respectively (Table 4).
Table 2. Relationship between the new spectral indices and LAI under the different nitrogen treatments for wheat (n = 90).
| Spectral indices | Simulated equations | Determination coefficient (R2) |
| OSAVI×OSAVI | y = 0.253ln(x)+0.221 | 0.634** |
| OSAVI×SIPI | y = 0.174ln(x)+0.507 | 0.563** |
| OSAVI×CIred edge | y = 3.756ln(x)+0.337 | 0.692** |
| OSAVI×CIgreen model | y = 3.534ln(x)+0.527 | 0.706** |
| OSAVI×EVI2 | y = 0.269ln(x)+0.151 | 0.711** |
Note: n = number of pairs of data. x represents spectral indices, y represents LAI.
Probability levels are indicated by n.s.,
and ** for ‘not significant’, 0.05, and 0.01, respectively.
Table 3. Relationship between the new spectral indices and nitrogen uptake under the different nitrogen treatments for wheat (n = 90).
| Spectral indices | Simulated equations | Determinationcoefficient (R2) |
| OSAVI×OSAVI | y = 0.246ln(x) − 0.095 | 0.542** |
| OSAVI×SIPI | y = 0.164ln(x) + 0.302 | 0.641** |
| OSAVI×CIred edge | y = 0.145x1.331 | 0.785** |
| OSAVI×CIgreen model | y = 0.203x1.200 | 0.776** |
| OSAVI×EVI2 | y = 0.274ln(x) − 0.212 | 0.783** |
Note: n = number of pairs of data. x represents spectral indices, y represents nitrogen uptake.
Probability levels are indicated by n.s.,
and ** for ‘not significant’, 0.05, and 0.01, respectively.
Table 4. Relationship between the new spectral indices and total chlorophyll content under the different nitrogen treatments for wheat (n = 90).
| Spectral indices | Simulated equations | Determinationcoefficient (R2) |
| OSAVI×OSAVI | y = 0.264x0.400 | 0.652** |
| OSAVI×SIPI | y = 0.504x0.279 | 0.683** |
| OSAVI×CIred edge | y = 0.137x0.769 | 0.846** |
| OSAVI×CIgreen model | y = 1.307x0.742 | 0.824** |
| OSAVI×EVI2 | y = 0.208x0.482 | 0.798** |
Note: n = number of pairs of data. x represents spectral indices, y represents total chlorophyll content.
Probability levels are indicated by n.s.,
and ** for ‘not significant’, 0.05, and 0.01, respectively.
3.5. Validation Model and Comparison
To validate the model accuracy, we compared the predicted values (the predicted values were gained by the LAI, N uptake and total Chl content of regression equations in 2009) with the actual values (the actual values were gained by the LAI, N uptake and total Chl content field measurement data in 2010). A good correlation between the predicted values and the actual values was observed for the following indices: OSAVI, biomass dry weight, and OSAVI×biomass dry weight (BDW). The corresponding root mean square errors (RMSEs) were 1.42, 1.02, and 1.12 for LAI; 7.93 g/m2, 4.45 g/m2, and 4.23 g/m2 for nitrogen uptake; 3.42 g/m2, 1.85 g/m2, and 2.23 g/m2 for leaf chlorophyll content, respectively (Table 5). These data indicated that OSAVI×biomass dry weight could be used to improve the estimation accuracy of LAI, nitrogen uptake, and leaf chlorophyll content. The new spectral indices were proposed by replacing BDW with spectral indices, and then obtained OSAVI×OSAVI, OSAVI×SIPI, OSAVI×CIred edge, OSAVI×CIgreen model and OSAVI×EVI2. The results showed that the new spectral indices were better than OSAVI alone for estimating LAI, nitrogen uptake and total chlorophyll content (Tables 2, 3, 4 and 5 and Figs. 1a, 2a and 3a). The results indicated that the products of spectral indices and OSAVI could be used to improve the LAI, N uptake and total Chl content estimation accuracy.
Table 5. The relationships the measured value and predicted value for LAI, nitrogen uptake and total chlorophyll content under the different nitrogen treatments for wheat.
| LAI | Nitrogen uptake | Leaf chlorophyll content | |||
| RMSE (g/m2) | RMSE (g/m2) | RMSE (g/m2) | |||
| OSAVI | 1.41 | OSAVI | 7.93 | OSAVI | 3.42 |
| biomass dry weight | 1.02 | biomass dry weight | 4.45 | biomass dry weight | 3.12 |
| OSAVI×BDW | 1.12 | OSAVI×BDW | 4.23 | OSAVI×BDW | 2.11 |
| OSAVI×OSAVI | 1.18 | OSAVI×OSAVI | 6.46 | OSAVI×OSAVI | 3.36 |
| OSAVI×SIPI | 1.36 | OSAVI×SIPI | 4.85 | OSAVI×SIPI | 3.02 |
| OSAVI×CIred edge | 0.98 | OSAVI×CIred edge | 3.98 | OSAVI×CIred edge | 0.65 |
| OSAVI×CIgreen model | 0.84 | OSAVI×CIgreen model | 4.12 | OSAVI×CIgreen model | 0.79 |
| OSAVI×EVI2 | 0.78 | OSAVI×EVI2 | 4.03 | OSAVI×EVI2 | 1.02 |
Discussion
Crop leaf area index (LAI), total chlorophyll (Chl) content and nitrogen (N) uptake was estimated using new spectral indices. The results showed that the new spectral indices could be used to improve the LAI, total Chl content and N uptake estimation accuracy. We used the OSAVI×biomass dry weight (BDW) index (the product of OSAVI and biomass dry weight) to improve the relationship between the LAI and spectral indices. The results indicated that the OSAVI×BDW index was better than the OSAVI at estimating wheat LAI. The OSAVI×BDW index was more sensitive to LAI due to the further decreased effects of soil at earlier growth stages. For example, the spectrometer was sensitive to the soil color and moisture, thus reducing the wheat canopy spectral information detection at earlier growth stages, and leading decreased sensitive to canopy spectral reflectance changes. The OSAVI×BDW ameliorated the saturation at later growth stages because of the addition of biomass dry weight, which was increased gradually with progression through the wheat growth stages. Overall, the saturation problem observed for the spectral indices was mainly influenced by the structure of the wheat canopy at later growth stages (see section 3.2). The OSAVI×BDW index was used to improve the estimation of nitrogen, and the results demonstrated that it was better than OSAVI alone for nitrogen assessment. The reason for this was similar to that explaining the reasonable estimation of LAI, the results were similar to those of previous research [14]–[19]. We proposed that the OSAVI×BDW index could be used to effectively improve the estimation accuracy of total Chl content indirectly, and the results demonstrated that it could accomplish the proposed tasks. Again, the reasons for such are similar to those explaining the reasonable estimation of N uptake.
The new spectral indices in Tables 2, 3 and 4 are derived from BDW×OSAVI. Biomass dry weight (BDW) was gained by destructive sampling, but required a greater time investment for data acquisition. To quickly obtain data and estimate leaf area index (LAI), total chlorophyll (Chl) content and nitrogen (N) uptake, we attempted to build new spectral indices by replacing BDW with other spectral indices. We obtained the OSAVI×OSAVI, OSAVI×SIPI, OSAVI×CIred edge, OSAVI×CIgreen model and OSAVI×EVI2. The results suggested the new spectral indices were better than OSAVI alone for estimating LAI, total Chl content and N uptake (Tables 2, 3, 4 and 5 and Figs. 1a, 2a and 3a). The main reason is that the SIPI, CIred edge, CIgreen model and EVI2 include the 800 nm band is sensitive to LAI changes. The products of OSAVI and SIPI, CIred edge, CIgreen model and EVI2 will further improve the OSAVI sensitivity in LAI changes. Therefore, these new spectral indices could be used for improving the LAI. For N uptake, the products of OSAVI and SIPI, CIred edge, CIgreen model and EVI2, making OSAVI increase the sensitive bands of the chlorophyll content changes (445 nm, 550 nm, 660 nm and 680 nm), these new spectral indices are sensitive to detect chlorophyll content changes. Thus, the N uptake estimation was improved by using the new spectral indices. For total Chl content, it was similar to those explained reasonable estimation of N uptake.
Taken together, the results indicated that it was feasible to use new methods to improve agronomic parameters (LAI, N uptake and total Chl content) assessment accuracy. This paper evaluated the estimation accuracy of agronomy parameters by multiplying the spectral indices in OSAVI, it showed the OSAVI is unnecessary and able to be replaced by others spectral indices. For example, if you want to better estimate total Chl content or N uptake, you could be selected to the product of two spectral indices are highly related to chlorophyll. Further, we used these indices to improve the accuracy of these predictions for all crop growth stages. In future studies, we will try to multiply two spectral indices that are highly related to LAI, total Chl content or N uptake to estimate agronomic parameters of different crops.
Conclusions
Building upon previous studies, wheat leaf area index (LAI), nitrogen uptake, and total chlorophyll content were predicted using the products spectral indices and spectral indices methods, and the main results and conclusions follow. The results suggested that the LAI, nitrogen uptake and total Chl content could be accurately predicted using the products of OSAVI and biomass dry weight (OSAVI×biomass dry weight (BDW) index), the corresponding determination coefficient (R2) and root mean square errors (RMSEs) were 0.672 and 1.12, 0.754 and 4.23 g/m2, 0.805 and 2.11 g/m2, respectively. We obtained the new spectral indices by using spectral indices replace BDW. The relationships between LAI, nitrogen uptake and total Chl content and new spectral indices, OSAVI×EVI2, OSAVI×CIred edge and OSAVI×CIred edge had the highest R2 and the lowest RMSEs for LAI, nitrogen uptake and total Chl content, respectively, R2 and RMSEs were 0.711 and 0.78, 0.785 and 3.98 g/m2, 0.846 and 0.65 g/m2, respectively.
Funding Statement
The study is supported by the National Natural Science Foundation of China (grant numbers 31071371, 30760109, 41161068) and the Key Projects in the National Science & Technology Pillar Program during the Twelfth Five-Year Plan Period (grant number 2012BAH27B04). The National Natural Science Foundation of China had role in study design, data collection and analysis, the Key Projects in the National Science & Technology Pillar Program during the Twelfth Five-Year Plan Period had role in decision to publish,or preparation of the manuscript.
References
- 1. Daughtry CST, Gallo KP, Goward SN, Prince SD, Kustas WD (1992) Spectral estimates of absorbed radiation and phytomass production in corn and soybean canopies. Remote Sensing of Environment 39: 141–152. [Google Scholar]
- 2. Goetz SJ, Prince SD (1996) Remote sensing of net primary production in boreal forest stands. Agricultural and Forest Meteorology 78: 149–179. [Google Scholar]
- 3. Liu J, Chen J, Cihlar J, Park WM (1997) A process-based boreal ecosystem productivity simulator using remote sensing inputs. Remote Sensing of Environment 62: 158–175. [Google Scholar]
- 4. Moran MS, Inoue Y, Barnes EM (1997) Opportunities and limitations for image-based remote sensing in precision crop management. Remote Sensing of Environment 61: 319–346. [Google Scholar]
- 5. Moran MS, Maas SJ, Pinter JPJ (1995) Combining remote sensing and modeling for estimating surface evaporation and biomass production. Remote Sensing of Environment 12: 335–353. [Google Scholar]
- 6. Tucker CJ, Holben BN, Elgin JJH, McMurtrey JE (1980) Relationship of spectral data to grain yield variations. Photogrammetric Engineering & Remote Sensing 46: 657–666. [Google Scholar]
- 7.Rouse JW, Haas RH, Schell JA, Deering DW, Harlan JC (1974) Monitoring the vernal advancements and retrogradation of natural vegetation. In: NASA/GSFC, Final Report, Greenbelt, MD, USA, 1974, pp.1–137.
- 8. Bausch WC (1993) Soil background effects on reflectance-based crop coefficients for corn. Remote Sensing of Environment 46: 213–222. [Google Scholar]
- 9. Elvidge CD, Lyon RJP (1985) Influence of rock-soil spectral variation on the assessment of green biomass. Remote Sensing of Environment 17: 265–279. [Google Scholar]
- 10. Huete AR, Jackson RD, Post DF (1985) Spectral response of a plant canopy with different soil backgrounds. Remote Sensing of Environment 17: 37–53. [Google Scholar]
- 11. Rondeaux G, Steven M, Baret F (1996) Optimization of soil adjusted vegetation indices, Remote Sensing of Environment. 55: 95–107. [Google Scholar]
- 12. Yang JC, Wang ZQ, Zhu QS (1996) Effects of nitrogen nutrition on rice yield and its physiology mechanism under the different status of soil moisture, Chinese Agriculture Science. 29: 58–66 (in Chinese).. [Google Scholar]
- 13.Rhykerd CL, Noller CH (1974). The role of nitrogen in forage production, In D.A. Mays (ed.) Forage fertilization, American Society of Agronomy, Madison, WI, 416–424.
- 14. Stone ML, Solie JB, Raun WR, Whitney RW, Taylor SL, et al. (1996a) Use of spectral radiance for correcting in-season fertilizer nitrogen deficiencies in winter wheat. Transactions of the American Society Agriculture Engineers 39: 1623–1631. [Google Scholar]
- 15.Stone ML, Solie JB, Whitney RW, Raun WR, Lees HL (1996b) Sensors for the detection of nitrogen in winter wheat. Tech. Paper Series No. 961757. SAE, Warrendale, PA.
- 16. Osborne SL, Schepers JS, Francis DD, Schlemmer MR (2002) Detection of phosphorous and nitrogen deficiencies in corn using spectral radiance measurement. Agronomy Journal 94: 1215–1221. [Google Scholar]
- 17. Gitelson AA, Merzlyak MN (1996) Signature analysis of leaf reflectance spectra: algorithm development for remote sensing of chlorophyll. Journal of Plant Physiology 148: 494–500. [Google Scholar]
- 18. Moges SM, Raun WR, Mullen RW, Freeman KW, Johnson GV, et al. (2004) Estimating of green, red, and near infrared bands for predicting winter wheat biomass, nitrogen uptake, and final grain yield. Journal of Plant Nutrition 27: 1431–1441. [Google Scholar]
- 19. Reyniers M, Walvoort DJJ, De Baardemaaker J (2006) A linear model to predict with a multi-spectral radiometer the amount of nitrogen in winter wheat. International Journal of Remote Sensing 27: 4159–4179. [Google Scholar]
- 20. Zhu Y, Yao X, Tian YC, Liu XJ, Cao WX (2008) Analysis of common canopy vegetation indices for indicating leaf nitrogen accumulations in wheat and rice. International Journal of Appllied earth Obsevation and Geoinformation 10: 1–10. [Google Scholar]
- 21. Eitel JUH, Long DS, Gessler PE, Smith AMS (2007) Using in-situ measurements to evaluate the new RapidEye satellite series for prediction of wheat nitrogen status. International Journal of Remote Sensing 28: 4183–4190. [Google Scholar]
- 22. Fitzgerald G, Rodriguez D, O’Leary G (2010) Measuring and predicting canopy nitrogen nutrition in wheat using a spectral index-The canopy chlorophyll content index (CCCI). Field Crops Research 116: 318–324. [Google Scholar]
- 23. Vigneaua N, Ecarnotb M, Rabatel G, Roumetb P (2011) Potential of field hyperspectral imaging as a non destructive method to assess leaf nitrogen content in wheat. Field Crops Research 122: 25–31. [Google Scholar]
- 24. Buschmann C, Nagel E (1993) In vivo spectroscopy and internal optics of leaves as basis for remote sensing of vegetation. International Journal of Remote Sensing 14: 711–722. [Google Scholar]
- 25. Gitelson AA, Merzlyak MN (1994a) Quantitative estimation of chlorophyll a using reflectance spectra: Experiments with autumn chestnut and maple leaves. Journal of Photochemistry and Photobiology Biology 22: 247–252. [Google Scholar]
- 26. Gitelson AA, Merzlyak MN (1994b) Spectral reflectance changes associated with autumn senescence of Aesculus hippocastanum andAcer platanoides leaves. Spectral features and relation to chlorophyll estimation. Journal of Plant Physiology 143: 286–292. [Google Scholar]
- 27. Markwell J, Osterman JC, Mitchell JL (1995) Calibration of the Minolta SPAD-502 leaf chlorophyll meter. Photosynthesis Research 46: 467–472. [DOI] [PubMed] [Google Scholar]
- 28. Gamon JA, Surfus JS (1999) Assessing leaf pigment content and activity with a reflectometer. New Phytologist 143: 105–117. [Google Scholar]
- 29. Gitelson AA, Merzlyak MN, Chivkunova OB (2001) Optical properties and non-destructive estimation of anthocyanin content in plant leaves, Journal of Photochemistry Photobiology. 74: 38–45. [DOI] [PubMed] [Google Scholar]
- 30. Gitelson AA, Zur Y, Chivkunova OB, Merzlyak MN (2002) Assessing carotenoid content in plant leaves with reflectance spectroscopy. Journal of Photochemistry Photobiology 75: 272–281. [DOI] [PubMed] [Google Scholar]
- 31. Gitelson AA, Vina A, Ciganda V, Rundquist DC, Arkebauer TJ (2005) Remote estimation of canopy chlorophyll content in crops. Geophysical Research Letters 32: L08403 doi:10.1029/2005 GL022688 [Google Scholar]
- 32. Gitelson AA, Vina A, Verma SB, Rundquist DC, Arkebauer TJ, et al. (2006) Relationship between gross primary production and chlorophyll content in crops: Implications for the synoptic monitoring of vegetation productivity. Geophysical Research Letters 111: D08S11 doi:10.1029/2006JD006017 [Google Scholar]
- 33. Peng Y, Gitelson AA, Keydan G, Rundquist DC, Moses W (2011) Remote estimation of gross primary production in maize and support for a new paradigm based on total crop chlorophyll content. Remote Sensing of Environment 115: 978–989. [Google Scholar]
- 34. Schepers JS, Francis DD, Thompson MT (1989) Simultaneous determination of total C, total N and 15N on soil and plant material. Communications in Soil Science and Plant Analysis 20: 949–959. [Google Scholar]
- 35. McKinney G (1941) Absorption of light by chlorophyll solutions. Journal of Biology Chemistry 140: 315–322. [Google Scholar]
- 36. Penuelas J, Baret F, Filella I (1995) Semiempirical indexes to assess carotenoids chlorophyll-a ratio from leaf spectral reflectance. Photosynthetica 31: 221–230. [Google Scholar]
- 37. Jiang Z, Huete AR, Didan K, Miura T (2008) Development of a two-band enhanced vegetation index without a blue band. Remote Sensing of Environment 112: 3833–3845. [Google Scholar]
- 38. Steven MD (1998) The densitivity of the OSAVI vegetation Index to observational parameters, Remote Sensing of Environment. 63: 49–60. [Google Scholar]
- 39. Cheng JP, Cao CG, Cai ML, Yuan BZ, Zhai J (2008) Effect of different nitrogen nutrition and soil water potential on the physiology parameters and yield of hybrid rice. Plant Nutrition and Fertilizer Science 14: 199–206 (in Chinese).. [Google Scholar]
- 40.Gitelson AA, Gurlin D, Moses WJ, Barrow T (2009) A bio-optical algorithm for the remote estimation of the chlorophyll-a concentration in case 2 waters. Environment Research Letters 045003, doi:10.1088/1748-9326/4/4/045003.
- 41. Houborg R, Anderson MC, Daughtry CST, Kustas WP, Rodell M (2011) Using leaf chlorophyll to parameterize light-use-efficiency within a thermal-based carbon, water and energy exchange model. Remote Sensing of Environment 115: 1694–1705. [Google Scholar]
- 42. Sakamoto T, Gitelson AA, Wardlow BD, Arkebauer TJ, Verma SB, et al. (2012) Application of day and night digital photographs for estimating maize biophysical characteristics. Precision Agriculture 13: 285–301. [Google Scholar]
- 43. Gurlin D, Gitelson AA, Moses WJ (2011) Remote estimation of chl-a concentration in turbid productive waters-Return to a simple two-band NIR-red model? Remote Sensing of Environment 115: 3479–3490. [Google Scholar]