Abstract
It has been known since the 1970’s that the suprachiasmatic nucleus (SCN) is the brain’s main biological clock, and since the 1990’s that it uses a genetic clock based on transcriptional-translational loops to tell time. However, the recent demonstration that many other cells in the brain and the body also make use of the same genetic clock raises the question of how the SCN synchronizes all of the other clocks to arrive at a coherent circadian profile of physiology and behavior. In this review, we re-examine the evidence that the SCN clock is necessary for bringing order to the body’s biological rhythms, and the circuitry of the circadian timing system by which it accomplishes this goal. Finally, we review the evidence that under conditions of restricted food availability, other clocks may be able to take over from the SCN to determine rhythms of behavior and physiology.
Introduction
The discovery in 1970’s that lesions of the suprachiasmatic nucleus (SCN) caused loss of circadian rhythms of locomotion, corticosteroid secretion, and other physiological and behavioral activities initially seemed to identify the SCN as the key source of circadian rhythms1, 2. However, with the discovery of that the same biological genetic clock that drives the SCN is also present in many, if not most other cells in the body, attention has shifted to the mechanism by which the SCN keeps the potential cacophony of independent clocks in synchrony3, 4. In this review, we will cover recent studies that address some of the fundamental questions about how the SCN exerts its influence over circadian rhythms.
Is the SCN necessary or sufficient for central circadian rhythms?
This first question may seem simple to answer, given the results of SCN lesion studies, but in fact the problem is deceptively complex. Nearly all SCN lesion studies that have been done to establish the role of the SCN in circadian rhythms have used electrolytic lesions. Electrolytic lesions are relatively simple to do, and the SCN neurons have proven to be remarkably resistant to various cell-specific toxins, such as glutamate analogues (ibotenic acid, kainic acid) or saporin conjugate5. However, electrolytic lesions big enough to reliably kill most or all of the SCN neurons virtually always involve the adjacent optic chiasm and the subparaventricular zone (SPZ) just dorsal to the SCN (sometimes called the peri-SCN region). Because the retinohypothalamic component of the optic chiasm also transmits inputs to other hypothalamic nuclei and the intergeniculate leaflet6, and the subparaventricular zone itself is necessary for many circadian rhythms5, nearly all of the accumulated literature on the role of the SCN is confounded by including these additional targets in the lesions.
Cell-specific lesions of just the SCN have been surprisingly difficult to achieve. Genetic approaches have focused on eliminating SCN neurotransmission. Many dorsomedial (shell) SCN neurons use arginine vasopression (AVP) as a neurotransmitter, and many ventrolateral (core) SCN neurons use vasoactive intestinal peptide (VIP). Animals that lack either the AVP receptor2 (VP2) or the VIP receptor 2 (VIPR2) have been reported to maintain a typical circadian rhythm of locomotion in a light-dark (LD) environment, but to lose this rhythm in continuous darkness (DD)7, 8. However, the SCN is not the only cell group to use these receptors for neurotransmission, and many of the SCN neurons are not affected in these animals because they use different neurotransmitters.
Another approach has been to try to eliminate neurotransmission from the SCN using genetic targeting. However, no gene product has yet been found that is expressed by all SCN neurons, and not by other targets in the brain. By placing Cre under the neuromedinS promoter, it has been possible to kill or to disable GABA neurotransmission from about half of the SCN neurons, but this did not affect circadian rhythms9. Methods to kill or disable the SCN neurons selectively are sorely needed to test whether the SCN is indeed necessary for the maintenance of circadian rhythms.
Another approach to assessing the role of the SCN has been to delete clock genes. Because each of the other core components of the mammalian circadian clock (Period, Cryptochrome, and Clock) have either multiple alleles or paralogues that can constitute a functional clock, the simplest approach for this has been to delete Bmal1, which has no paralogue. Deletion of the Bmal1 clock gene from the entire brain does cause loss of circadian rhythms10 so it is clear that a central clock is needed to keep the circadian timing system intact (i.e., the brain does not just integrate inputs from peripheral clocks, which would be an alternative hypothesis 11). In Bmal1 null mice, restoring the Bmal1 gene only to the SCN is able to completely reconstitute circadian rhythms of locomotor activity and body temperature 12. In these mice, the SCN contains the only clock in the body, so it is clear that the SCN clock is sufficient for circadian rhythms, at least of these two functions.
What is the mechanism of SCN influence over the central circadian timing system?
If the SCN is sufficient to maintain many if not all important circadian rhythms of physiology and behavior, how does it accomplish this task? Early studies in which SCN tissue was transplanted back into animals that had electrolytic lesions of the SCN (and presumably the retinohypothalamic tract and parts of the subparaventricular zone as well) showed that the transplants could restore at least a partial circadian rhythm of locomotor activity, if not other functions13, 14. This phenomenon persisted even if the SCN transplant was encapsulated in a way that prevented it from re-establishing axonal connections to the brain15. These experiments suggested that the SCN may secrete a paracrine signal that can regulate nearby neurons, thereby causing circadian rhythms. On the other hand, if even if SCN neurons normally release these chemical signals at synpases, they could continue to release them from terminals within the capsules, from which they could diffuse to their targets in the recipient brain, even if that is not their normal mode of communication. Several potential paracrine signaling molecules have been proposed over the years, including prokineticin and transforming growth factor alpha (TGFa) 16, 17, but it is still not clear to what extent the signaling by these molecules is paracrine rather than synaptic.
On the other hand, the relatively modest rhythms recovered with transplants suggest that the axonal outflow from the SCN is likely to be the major avenue for its influence over the rest of the brain. The SCN contacts a variety of targets in the nearby hypothalamus and thalamus 18–20, and in some cases, such as the secretion of melatonin, there are direct outputs from the SCN to target neurons that control the function5, 21. However, for most circadian functions, the SCN maintains control by its dense projection into the subparaventricular zone (SPZ), a region of the anterior hypothalamic area along the wall of the third ventricle, between the SCN and the paraventricular hypothalamic nucleus5, 19, 22. Recordings of SPZ neurons in mice show that they fire in anti-phase to those in the SCN23, as might be expected give that SCN output neurons are GABAergic. Cell-specific lesions of the ventral SPZ (just dorsal to the SCN) cause loss of circadian rhythms of locomotor activity and wake-sleep, but have only small effects on body temperature5. On the other hand, lesions of the dorsal SPZ, just ventral to the paraventricular hypothalamic nucleus, cause more profound loss of the rhythm of body temperature, but have little effect on the rhythms of locomotor activity or wake-sleep.
The ventral SPZ contains many neurons that project to targets similar to those of the SCN22, 24. Particularly profuse projections have been found to the dorsomedial and ventromedial hypothalamic nuclei. These observations led to studies which tested the effects of cell-specific lesions of the dorsomedial nucleus on circadian rhythms24. Such lesions dramatically reduce the circadian rhythms of wake-sleep, locomotor activity, feeding, and corticosteroid secretion, but also reduce the overall levels of locomotor activity and corticosteroid secretion to that seen during the inactive part of the day. Body temperature was also about 0.3°C lower, but its circadian rhythm was not disturbed. Predominantly glutamatergic projections from the dorsomedial nucleus were traced to the lateral hypothalamus, which contains neurons that are involved in driving wakefulness, and to the arcuate nucleus, where they may drive feeding24, 25. Predominantly GABAergic projections were traced to the ventrolateral preoptic nucleus, where they may inhibit sleep. These observations suggest that the predominant role of the dorsomedial nucleus is to drive the waking, active state.
The mechanisms by which the SCN drives body temperature rhythm remains elusive. However, there is some evidence that the body temperature rhythm may play a key role in the SCN’s ability to synchronize the other bodily clocks26. Recent studies indicate that the clock gene cycles in isolated tissue (including pituitary, lung, liver, kidney, and olfactory bulb) are reset by shifts in temperature, but that the SCN clock gene cycle is not. Although clocks in peripheral tissues clearly receive other signals (including both parasympathetic and sympathetic innervation and hormonal signals, as well as metabolic consequences of SCN control of behaviors, such as eating or exercise)27–29, the SCN regulation of body temperature may be an important signal that keeps peripheral clocks in alignment.
How can other oscillators take over in the absence of the SCN?
One of the most contentious areas in circadian research in recent years has been the question of how other oscillators can take over in the absence of the SCN. One such paradigm is that of food anticipation during restricted feeding, in which animals are typically given access to food for only a few hours per day, generally during the normally inactive cycle. Such animals rapidly (in 1–2 days) begin to show anticipation of the food arrival, with increased locomotor activity for 2–3 hours before the food presentation. Other biological rhythms such as wake-sleep, body temperature, and corticosteroid secretion also rapidly switch to the new feeding schedule30. There is evidence that this regimen activates a “food entrainable oscillator,” rather than the food stimulus itself providing a new timing cue for the response, because the anticipation continues for at least 2–3 days in DD after the animals are no longer fed31. Interestingly, the food anticipatory rhythms emerge whether the SCN is intact or has been ablated, indicating that the food entrainable oscillator does not depend on the SCN, but actually takes precedence over the SCN rhythm30.
An increase in cFos expression in the dorsomedial nucleus at the onset of the food anticipatory responses suggested that this cell group may play a role in food anticipation similar to its role in SCN-directed circadian rhythms 31–33. Subsequent studies showed that large dorsomedial nucleus lesions reduced the amplitude of food anticipatory increases in wakefulness, locomotor activity, and body temperature by about 80% in both rats and mice, even after accounting for the lower level of overall activity in those animals 31, 34. In addition, daytime restricted feeding causes a dramatic (approximately three-fold) increase in transcription of clock genes Per1and Per2 in anticipation of the new active period and Bmal1 during the new inactive period in the compact zone of the dorsomedial nucleus12, 35. The compact zone is a cluster of small, darkly staining cells that resemble the SCN, and which generally have a low amplitude of clock gene expression during ad libitum feeding.
These findings indicate that the dorsomedial nucleus contain a food-entrained genetic oscillator. On the other hand, the question has been raised whether the food anticipatory activity rhythm that is observed behaviorally depends upon clock gene cycles. Two groups reported that Bmal1 null mice given food for only during the light cycle still showed food anticipatory wheel-running36, 37. To test whether this was due to an oscillator, Pendergast and colleagues then placed Bmal1 null and wildtype mice in continuous darkness. After adapting to restricted feeding, which caused an anticipatory increase in activity in both groups, the mice were then placed on ad libitum feeding for three days, followed by two days of food deprivation37. The wildtype mice returned during the food deprivation to an activity pattern that anticipated the timing of previous restricted feeding, but the Bmal1 null mice did not. Hence the authors could not rule out that “in the absence of Bmal1, the mechanism that controls the expression of FAA [food anticipatory activity] becomes an interval timer”37 (i.e., that food deprived mice gradually increase their wheel running as they become hungrier, which of course becomes maximal at the end of the daily 20 hr period of food deprivation).
Fuller and colleagues examined both locomotor activity and body temperature by telemetry, in mice that were in cages without running wheels. They reported that Bmal1 null mice lacked food anticipatory increases in both locomotor activity and body temperature during restricted feeding12. However, the anticipatory increases in both body temperature and locomotor activity were rescued by restoring the gene for Bmal1 and normal clock gene cycling in the dorsomedial nucleus, but not the SCN. These observations suggest that the genetic clock in the dorsomedial nucleus may be sufficient to drive food anticipatory biological rhythms. Studies addressing whether the dorosmedial nucleus clock is necessary for food anticipation, or whether other clock mechanisms may take over in its absence, would be of interest.
Conclusions
Despite the fact that clock genes are expressed by neurons throughout the brain, and by cells in many other tissues, the SCN serves as the primary biological clock in mammalian brains, and all of the other clocks are normally synchronized to its signals4, 26. This influence of the SCN relies upon paracrine secretion and neural connections to drive local hypothalamic circuitry that regulates specific biological functions. Autonomic and endocrine control of peripheral tissues, as well as timing of functions such as the daily cycles of body temperature and feeding, provide important signals to keep other clocks in synchrony with the SCN.
The largest SCN output is to the adjacent subparaventricular zone, which relays timing signals to the dorsomedial nucleus, which controls circadian cycles of wake-sleep, feeding, locomotor activity, and corticosteroid secretion5, 24. However, circadian control of body temperature apparently takes a different route, which has not yet been characterized.
Surprisingly, even in the absence of the SCN clock, a genetic clock in the dorsomedial nucleus can be dramatically upregulated by restricted feeding35, and this clock can then reconstitute circadian rhythms of body temperature and locomotion12. Future work to establish the circuity used to drive the individual biological responses, and how these are altered during restricted feeding, will be of great interest.
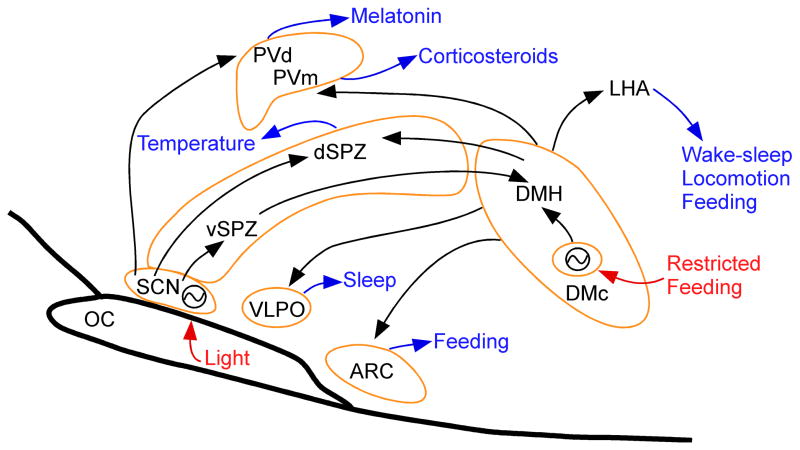
Figure 1.
A schematic drawing showing the main components of the circadian timing system in the mammalian brain. Arrows show neural pathways that convey these influences, but do not imply whether they are excitatory or inhibitory, which in some cases is not known. The neurons of the suprachiasmatic nucleus (SCN) form a genetically based clock which is reset daily by the light cycle. The SCN drives some circadian rhythms, such as that of melatonin, by direct outputs to target cell groups such as the parventricular nucleus (PV), but most circadian rhythms are mediated by relays through the subparaventricular zone (SPZ). Body temperature is regulated by the dorsal SPZ via an unknown pathway. The ventral SPZ (vSPZ) drives the dorsomedial nucleus of the hypothalamus (DMH), which in turn is responsible for circadian rhythms of wake-sleep, locomotion, feeding, and corticosteroid secretion, and can also reset the body temperature cycle. During periods of restricted feeding (food available for only a few hours a day during the normal inactive period), a second genetic clock is activated in the compact part of the DMH (DMc) that is set to the time of the food availability and can drive cycles of activity and body temperature that anticipate the feeding time. ARC, arcuate hypothalamic nucleus; OC, optic chiasm; PV, paraventricular hypothalamic nucleus; PVd, dorsal parvicellular PV; PVm, medial parvicellular PV; nucleusVLPO, ventrolateral preoptic nucleus. Zeitgebers are in red; cell groups outlined in tan; circadian functions in blue.
Highlights.
Clock gene expression solely in the suprachiasmatic nucleus (SCN) is sufficient to drive circadian rhythms of physiology and behavior.
The output from the SCN is relayed through the subparaventricular zone to control circadian rhythms of physiology and behavior.
In the absence of the suprachiasmatic nucleus, circadian rhythms of physiology and behavior can be restored by restricting animals to four hours per day of feeding time.
Clock gene expression in the dorsomedial nucleus during restricted feeding is sufficient for driving circadian rhythms of physiology and behavior.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 2.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoo SH, Yamazaki S, Lowrey PL, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 5.Lu J, Zhang YH, Chou TC, et al. Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. J Neurosci. 2001;21:4864–4874. doi: 10.1523/JNEUROSCI.21-13-04864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23:7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harmar AJ, Marston HM, Shen S, et al. The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109:497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- 8.Li JD, Burton KJ, Zhang C, Hu SB, Zhou QY. Vasopressin receptor V1a regulates circadian rhythms of locomotor activity and expression of clock-controlled genes in the suprachiasmatic nuclei. Am J Physiol Regul Integr Comp Physiol. 2009;296:R824–R830. doi: 10.1152/ajpregu.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang A, Matsuki T, Skach A, Yanagisawa M. Analyusis of circadian rhythms using a novel SCN-specific Cre transgenic mouse line. Neurosci Abstracts. 733.3 1–1–2010. [Google Scholar]
- *10.Mieda M, Sakurai T. Bmal1 in the nervous system is essential for normal adaptation of circadian locomotor activity and food intake to periodic feeding. J Neurosci. 2011;31:15391–15396. doi: 10.1523/JNEUROSCI.2801-11.2011. Demonstration that a central, genetically based clock is required for circadian rhythms of behavior and physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeSauter J, Hoque N, Weintraub M, Pfaff DW, Silver R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc Natl Acad Sci U S A. 2009;106:13582–13587. doi: 10.1073/pnas.0906426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuller PM, Lu J, Saper CB. Differential rescue of light- and food-entrainable circadian rhythms. Science. 2008;320:1074–1077. doi: 10.1126/science.1153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 14.Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, Bittman EL. Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology. 1999;140:207–218. doi: 10.1210/endo.140.1.6428. [DOI] [PubMed] [Google Scholar]
- 15.Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature. 1996;382:810–813. doi: 10.1038/382810a0. [DOI] [PubMed] [Google Scholar]
- 16.Cheng MY, Bullock CM, Li C, et al. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- 17.Kramer A, Yang FC, Snodgrass P, et al. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science. 2001;294:2511–2515. doi: 10.1126/science.1067716. [DOI] [PubMed] [Google Scholar]
- 18.Swanson LW, Cowan WM. The efferent connections of the suprachiasmatic nucleus of the hypothalamus. J Comp Neurol. 1975;160:1–12. doi: 10.1002/cne.901600102. [DOI] [PubMed] [Google Scholar]
- 19.Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916:172–191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- 20.Leak RK, Moore RY. Topographic organization of suprachiasmatic nucleus projection neurons. J Comp Neurol. 2001;433:312–334. doi: 10.1002/cne.1142. [DOI] [PubMed] [Google Scholar]
- 21.Vrang N, Larsen PJ, Moller M, Mikkelsen JD. Topographical organization of the rat suprachiasmatic- paraventricular projection. J Comp Neurol. 1995;353:585–603. doi: 10.1002/cne.903530409. [DOI] [PubMed] [Google Scholar]
- 22.Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I.Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol. 1987;258:204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura W, Yamazaki S, Nakamura TJ, Shirakawa T, Block GD, Takumi T. In vivo monitoring of circadian timing in freely moving mice. Curr Biol. 2008;18:381–385. doi: 10.1016/j.cub.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci. 2003;23:10691–10702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *25.Krashes MJ, Koda S, Ye C, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. Demonstration that genetic targeging of activation of the agouti-related peptide neurons in the arcuate nucleus of the hypothalamus can cause active feeding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **26.Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330:379–385. doi: 10.1126/science.1195262. Demonstration that genetic clocks of cells in peripheral tissues and other brain areas can be reset by ambient temperature shifts, but that cells from the suprachiasmatic nucleus cannot be rest by temperature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroeder AM, Truong D, Loh DH, Jordan MC, Roos KP, Colwell CS. Voluntary scheduled exercise alters diurnal rhythms of behaviour, physiology and gene expression in wild-type and vasoactive intestinal peptide-deficient mice. J Physiol. 2012;590:6213–6226. doi: 10.1113/jphysiol.2012.233676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sujino M, Furukawa K, Koinuma S, et al. Differential entrainment of peripheral clocks in the rat by glucocorticoid and feeding. Endocrinology. 2012;153:2277–2286. doi: 10.1210/en.2011-1794. [DOI] [PubMed] [Google Scholar]
- 29.Itokawa M, Hirao A, Nagahama H, et al. Time-restricted feeding of rapidly digested starches causes stronger entrainment of the liver clock in PER2::LUCIFERASE knock-in mice. Nutr Res. 2013;33:109–119. doi: 10.1016/j.nutres.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Stephan FK. The “other” circadian system: food as a Zeitgeber. J Biol Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- 31.Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- 32.Verhagen LA, Luijendijk MC, de Groot JW, et al. Anticipation of meals during restricted feeding increases activity in the hypothalamus in rats. Eur J Neurosci. 2011;34:1485–1491. doi: 10.1111/j.1460-9568.2011.07880.x. [DOI] [PubMed] [Google Scholar]
- 33.Angeles-Castellanos M, Aguilar-Roblero R, Escobar C. c-Fos expression in hypothalamic nuclei of food-entrained rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R158–R165. doi: 10.1152/ajpregu.00216.2003. [DOI] [PubMed] [Google Scholar]
- 34.Tahara Y, Hirao A, Moriya T, Kudo T, Shibata S. Effects of medial hypothalamic lesions on feeding-induced entrainment of locomotor activity and liver Per2 expression in Per2::luc mice. J Biol Rhythms. 2010;25:9–18. doi: 10.1177/0748730409352782. [DOI] [PubMed] [Google Scholar]
- 35.Mieda M, Williams SC, Richardson JA, Tanaka K, Yanagisawa M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc Natl Acad Sci U S A. 2006;103:12150–12155. doi: 10.1073/pnas.0604189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Storch KF, Weitz CJ. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proc Natl Acad Sci U S A. 2009;106:6808–6813. doi: 10.1073/pnas.0902063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pendergast JS, Nakamura W, Friday RC, Hatanaka F, Takumi T, Yamazaki S. Robust food anticipatory activity in BMAL1-deficient mice. PLoS ONE. 2009;4:e4860. doi: 10.1371/journal.pone.0004860. [DOI] [PMC free article] [PubMed] [Google Scholar]