Abstract
The parietal cortex has been functionally divided into various subregions; however, very little is known about how these areas relate to each other. Two such regions are the transverse occipital sulcus (TOS) scene area and inferior intraparietal sulcus (IPS). TOS exhibits similar activation patterns to the scene selective parahippocampal place area (PPA), suggesting its role in scene perception. Inferior IPS, in contrast, has been shown to participate in object individuation and selection via location. Interestingly, both regions have been localized to the same general area of the brain. If these two were actually the same brain region, it would have important implications regarding these regions’ role in cognition. To explore this, we first localized TOS and inferior IPS in individual participants and examined the degree of overlap between these regions in each participant. We found that TOS showed only a minor degree of overlap with inferior IPS (∼10%). We then directly explored the role of TOS and inferior IPS in object individuation and scene perception by examining their responses to furnished rooms, empty rooms, isolated furniture, and multiple isolated objects. If TOS and inferior IPS were the same region, we would expect to see similar response patterns in both. Instead, the response of TOS was predominantly scene selective, while activity in inferior IPS was primarily driven by the number of objects present in the display, regardless of scene context. These results show that TOS and inferior IPS are nearby, but distinct regions, with different functional roles in visual cognition.
Keywords: Inferior intraparietal sulcus, transverse occipital sulcus, scene processing, multiple object individuation, functional MRI
Introduction
The parietal cortex has been shown to be a hub for a variety of cognitive processes, including attention (Behrmann et al., 2004; Colby & Goldberg, 1999; Corbetta & Shulman, 2002; Culham and Kanwisher, 2001; Kastner and Ungerleider, 2000; Yantis and Serences, 2003), visual short-term memory (Todd & Marois, 2004; Xu and Chun, 2006, 2009), numerical cognition (Dehaene et al., 2003; Hubbard et al., 2005), motor planning (Buneo and Andersen, 2006; Culham et al., 2006; Gottlieb & Goldberg 1999; Grefkes and Fink, 2005; Merriam and Colby, 2005; Orban et al., 2006), visual object individuation and identification (Xu and Chun, 2006, 2009), and scene processing (Grill-Spector, 2003; Hasson et al., 2003; Levy et al., 2004; Nakamura et al., 2000), with each of these cognitive operations being localized to specific parietal subregions. However, these regions have mostly been studied within isolated cognitive domains (e.g., activity in putative scene processing regions are only examined in tasks involving scenes), leaving it unclear whether the same parietal region is involved in different cognitive tasks. Research in other areas of the brain has given us numerous examples of singular regions that can be functionally localized using a variety of tasks and stimuli, such as the superior temporal sulcus (STS; Bonda et al., 1996; Grossman et al., 2000; Haxby et al., 2000). Thus, our understanding of the functional organization of the human parietal cortex remains rather disjointed, leaving us left to wonder what relationships, if any, exist between parietal regions identified via different cognitive tasks. The inferior intraparietal sulcus (IPS) and the transverse occipital sulcus (TOS) scene area are prime examples of this problem.
Inferior IPS has been shown to play a role in the individuation of multiple objects by their locations (Xu 2008, 2009; Xu and Chun, 2006, 2009). TOS, on the other hand, appears to be involved in scene processing (Dilks et al., 2013; Grill-Spector, 2003; Hasson et al., 2003; Levy et al., 2004; Nakamura et al., 2000), showing similar patterns of activity to PPA (Epstein et al., 2005, 2007; Epstein and Higgins, 2007; Hasson et al., 2003; Levy et al., 2004; Ward et al., 2010), and the retrosplenial complex (RSC) (Epstein et al., 2007; Epstein and Higgins, 2007; MacEvoy and Epstein, 2007). While PPA, RSC, and TOS have all been shown to be involved in scene processing, RSC does show some interesting differences from PPA and TOS. RSC shows stronger familiarity effects than TOS and PPA (Epstein et al., 2007), is insensitive to the retinotopic extent of objects (Troiani et al., 2012), and lacks the eye centered coding that is seen in TOS and PPA (Ward et al., 2010). Thus, it has been suggested that TOS and PPA are primarily involved in the visual analysis of stimuli, while RSC is more involved in either mnemonic processes related to scenes (Troiani et al., 2012) or to processing a scene relative to the broader environment (Ward et al, 2010). Little is known, however, about how processing in TOS differs from that of PPA. Only two studies, to our knowledge, have found significant differences between TOS and PPA. Troiani et al. (2012) found that while PPA (and RSC) showed responses to object-based properties (like size, distance, etc.) when the objects were placed both within a scene context and on a white background, TOS showed object-based responses only when the objects were placed on a white background. The authors suggest that this may indicate that processing in TOS is related to the spatial qualities of individual objects. Dilks et al (2011), on the other hand, found that while TOS was sensitive to mirror reversals of scene stimuli, PPA was more tolerant, suggesting a split between scene recognition in PPA and navigation in TOS. However, both TOS and RSC showed similar sensitivity to mirror reversals. Thus, the true nature of processing within TOS remains unclear.
Despite the different functions ascribed to inferior IPS and TOS, both have been described as lying within the same general area of the brain, in the region where the IPS meets the transverse occipital sulcus (See Table 1). The parietal cortex has also been shown to contain several topographic subregions, including V3A/B and IPS0 through IPS4 (Konen and Kastner, 2008a; Schluppeck et al., 2005; Silver et al., 2005; Swisher et al., 2007; Wandell et al., 2007; see also Sereno et al., 2001). In particular, V3A/B has been localized to the same general area of both TOS and inferior IPS, namely at the base of IPS, where it transects the transverse occipital sulcus (Orban et al., 2004). Moreover, when directly compared to these topographic subregions, both inferior IPS and TOS appear to co-localize with V3B (Bettencourt and Xu, in preparation; Nasr et al., 2011). All together, this suggests that inferior IPS and TOS may be the same brain region, but are labeled differently in different tasks due to the way in which each region was localized.
Table 1.
Talairach coordinates for inferior IPS and TOS across a variety of studies.
| Inferior IPS | Xu & Chun, 2006 | +26/−21, −80/−85, +30/+26 (off-centre presentation) +26/−25, −65/−70, +34/+29 (centered presentation) |
| Xu, 2008 | +27/−21, −76/−77, +28/+25 | |
| Xu, 2009 | +29/−30, −78/−82, +28/+27 | |
| TOS | Hasson et al, 2003 | +33/−34, −77/−79, +12/+12 |
| Epstein & Higgins, 2007 | +32/−33, −75/−79, +34/+31 | |
| Epstein et al, 2005 | +40, −78, +22 (right only) | |
| Epstein et al, 2007 | +36/−42, −75/−77, +24/+26 Familiarity effect (Exp. 1) +31/−36, −82/−81, +20/+19 Viewpoint-specific adaptation (Exp. 1) −45, −75, +23 Familiarity effect, left only (Exp. 2) +26/−38, −80/−80, +26/+20 Viewpoint-specific adaptation (Exp. 2) −34, −85, +19 Viewpoint-specific adaptation, new vs. old, left only (Exp. 2) |
|
| Levy et al., 2004 | +32/−34, −79/−77, +14/+16 |
If these brain regions were actually a singular region, it would substantially alter our understanding of the role of these regions in visual cognition. Given the individuation processes ascribed to inferior IPS, and the recent finding of a preference for big, relative to small, objects within TOS (Konkle and Oliva, 2012), it would suggest that the role of TOS in scene processing might be to individuate objects within a scene for further processing. This would be consistent with the findings from Troiani et al. (2012) and Dilks et al. (2011) and would provide perhaps a key distinction between PPA and TOS. However, this theory cannot be fully supported by the current state of the literature, as these two areas have been studied only in isolation, in separate groups of participants, with very specialized task paradigms. Given the strong individual differences seen in parietal structure (e.g., topographic regions in IPS, see Swisher et al., 2007), overlap between functional regions defined by group-averaged data could be easily inflated and/or obscured. As such, the precise relationship between TOS and inferior IPS can only be understood when comparisons are made within the same participants.
Thus, here, we localized both TOS and inferior IPS in the same individual participants with functional localizers previously established in the literature (see Levy et al., 2004; Xu and Chun, 2006). We then determined the amount of overlap between TOS and inferior IPS. To further understand how these regions relate anatomically, we also examined the overlap between TOS and topographic IPS regions. Finally, to document the functional similarities and differences between these regions, we investigated the role of TOS and inferior IPS in scene perception and object individuation. While scenes often contain multiple objects, they can also be impoverished and contain relatively few objects (i.e., an empty room). Previous research has shown that PPA is relatively insensitive to the total number of objects present within a scene (Epstein and Kanwisher, 1998). However, if TOS co-localizes with inferior IPS and is involved in individuating objects in a scene, then its response should be high whenever multiple objects are present, regardless of whether or not a scene context is also present. On the other hand, if TOS is primarily a scene-processing region and is functionally distinct from inferior IPS, then it would show a high response to any type of scene stimuli, even when they contain very few objects, and a lower response to non-scene stimuli even when they contain many objects. The response of inferior IPS, on the other hand, should primarily reflect the number of objects present, regardless of the presence or absence of a scene context. Thus, by comparing activation patterns within TOS and inferior IPS to different types of stimuli, we should be able to see whether a functional distinction exists between them.
We found that despite of the close proximity seen in group-averaged studies, TOS and inferior IPS are both anatomically and functionally distinct regions. Even at a very liberal threshold of p < 0.05, the two regions showed a very small degree of overlap (∼10%). Functionally, TOS was primarily scene driven and showed a low response to non-scene stimuli even when they contained many objects, while inferior IPS was driven by the presence of multiple objects in the display, independent of the presence a scene context. Together these results show that TOS and inferior IPS are nearby, but distinct regions, with different roles in visual cognition.
Materials and Methods
Participants
Eight paid participants (5 female) from the Harvard University community were recruited to participate in this experiment. All participants gave informed consent in accordance with the Institutional Review Board of Harvard University. Participants were between 22 and 34 years old (mean age = 28.6). All had normal or corrected-to-normal visual acuity and all were right-handed.
Visual Stimuli and Experimental Paradigm
Stimuli were presented by a Macintosh MacBook Pro to a liquid crystal display projected onto a screen mounted at the rear end of the scanner bore. Topographic mapping stimuli were presented using VisionEgg software (Straw, 2008), while stimuli for the main experiment were presented using Matlab with Psychtoolbox extensions (Brainard, 1997).
For the main experiment, participants were shown blocks (16s each) of digitized black and white photographs with 20 pictures per block. Each photograph subtended 13.2 × 13.2° of visual angle. Each block contained images from one of eight object categories: faces, scenes (outdoor and indoor), single isolated everyday objects, multiple isolated objects, furnished rooms, empty rooms, isolated furniture, and noise images (see Figure 1). Interspersed amongst stimuli blocks were five fixation blocks in which only a fixation dot was present throughout the entire block.
Figure 1.
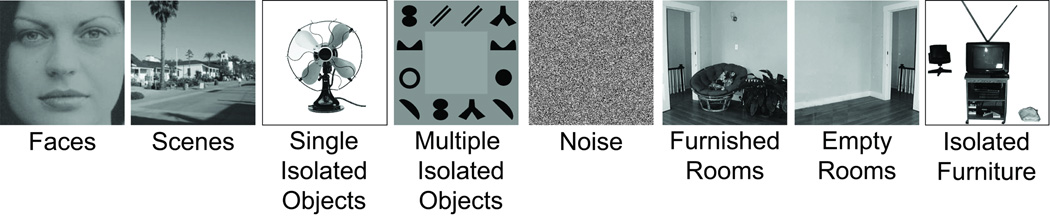
Example stimuli for each of the eight conditions used in the experiment. The furnished room, empty room and furniture conditions were the exact same ones used in Epstein and Kanwisher (1998).
Stimuli used in the furnished room, empty room, and isolated furniture conditions were the exact same ones used by Epstein and Kanwisher (1998). Specifically, the furnished room stimuli consisted of unfamiliar indoor scenes with furniture, plants, and room decorations; empty rooms were the same rooms as the furnished rooms but with all furniture, plants, and room decorations removed; and each isolated furniture image consisted of the furnishings from one of the furnished rooms cut out from the original background, rearranged, and placed on a blank white background.
The multiple isolated objects stimuli used here consisted of the same shapes used in Xu and Chun (2006), but with a slightly different placement of the shapes. Previously, it has been shown that scenes activate a more peripheral representation than faces (Levy et al., 2001, 2004). To ensure that this peripheral bias would not cause distortions in the localization of TOS relative to inferior IPS due to differences in the perceived eccentricity of the stimulus, the multiple object stimuli here, unlike in Xu and Chun (2006), were presented at the peripheral most extent of the stimulus area and the number of stimuli was increased to 12 to ensure adequate coverage of this region (see Figure 1).
This collection of nine conditions (eight stimulus conditions plus one fixation condition) allowed us to localize TOS and inferior IPS, as well as examine each region’s response to the presence of multiple objects both within and outside of a scene context. Trial and block design was based on Epstein and Kanwisher (1998). Each image was presented for 300ms, followed by a 500ms blank interval. To equate attention across conditions, participants were asked to detect a slight spatial jitter that would occur randomly throughout each block. A block of trials from each stimulus condition was presented twice during a run and block order was counterbalanced across runs (ABCD-EFGH-HGFE-DCBA for version 1 and HGFE-DCBA-ABCD-EFGH for version 2). Each participant completed two runs (each 5 min, 36 s).
Topographic visual field representations of polar angle were also mapped for each participant, using between 4–6 runs (each 11 min, 5.6s). Cortical representations of polar angle were mapped with flashing checkerboard stimuli using standard techniques (DeYoe et al., 1996; Engel et al., 1994; Sereno et al., 1995; Swisher et al., 2007) with parameters optimized to reveal maps in the parietal cortex (Swisher et al., 2007). The polar angle wedge swept across the entire screen (23.4 × 17.5° of visual angle), had an arc of 72°, flashed at 4Hz, a sweep period of 55.467s, and swept out 12 cycles per run (for more details, see Swisher et al., 2007). The task varied slightly across participants. All participants were asked to detect a dimming in the visual display, for some participants the dimming occurred only at fixation, for others it occurred only within the polar angle wedge, and for some it could occur in both locations, commiserate with the various methodologies used in the literature (Bressler and Silver, 2010; Swisher et al. 2007). No differences were seen in the maps obtained through each of these methods. We were able to identify in each participant areas within IPS including V3A, V3B, IPS0, IPS1, IPS2, IPS3, and IPS4. Other visual areas such as V1, V2, V3, V4, and MT could also be identified, but since our focus was on parietal areas, these other areas were not considered further in the present analysis.
fMRI Methods
The data was acquired on a Siemens Tim Trio 3T scanner with a 32 channel head coil at the Center for Brain Science at Harvard University (Cambridge, MA). Participants participated in two or three sessions of MRI scanning. In one session, a high resolution (1.0 × 1.0 × 1.3 mm) anatomical image was collected for surface reconstruction. Before functional imaging in each session, T1-weighted echo-planar images were collected in the same slice prescription as the functional scans to allow each session to be registered to the participant’s high-resolution anatomical scan. Functional data were acquired using T2*-weighted gradient-echo, echo-planar sequences. Each volume of the topographic data contained 42 slices (3mm thick, 3.125 × 3.125mm in plane, no skip) oriented just off parallel from the AC-PC line to cover the full brain (TR = 2.6s, TE = 30ms, flip angle = 90°). Each volume of the main experimental data contained 24 slices (5mm thick, 3.75 × 3.75mm in plane, no skip) parallel to the AC-PC line (TR = 2s, TE = 30ms, flip angle = 90°).
Data Analysis
fMRI data were analyzed using the Freesurfer software package (Dale et al., 1999; Fischl et al., 1999, 2001). Data preprocessing included motion correction and intensity normalization. Computer representations of each cortical hemispheric surface were unfolded and inflated.
Parietal topographic maps were obtained by followings the steps described in detail in Swisher et al. (2007). Scene selective PPA, RSC, and TOS regions of interest (ROIs) were identified as areas that showed higher activity for scenes relative to both faces and single objects, as in Epstein and Kanwisher (1998). Inferior IPS was selected using the same procedure as Xu and Chun (2006) and consisted of the region that showed higher activation for multiple isolated objects relative to noise and that was located around the intersection of IPS and the transverse occipital sulcus and around the Talairach coordinates previously reported (Xu, 2009; Xu and Chun, 2006). All ROIs were defined independently of the data used for functional comparisons within and between ROIs in terms of object and scene processing. That is, contrasts used to define the ROIs were not analyzed further in the main analysis.
The significance thresholds for inferior IPS and TOS were initially set to a lenient p < 0.05 (uncorrected) in order to ensure that the totality of these regions would be selected and that any lack of overlap between them would not be due to an overly strict ROI definition. Additionally, a second set of inferior IPS and TOS ROIs were created using a stricter threshold (p < 0.001, uncorrected) to examine whether the center of these two regions would overlap. The percentage of overlap between inferior IPS and TOS was calculated as the intersection of the two regions divided by the averaged size of these two regions (Kung et al., 2007). In other words, the area (in mm2) of overlap between inferior IPS and TOS divided by the average size of the whole TOS and whole inferior IPS regions, multiplied by 100.
The amount of overlap between TOS and topographic regions was calculated as the percentage of TOS that overlapped with each topographic region. In other words, the area (in mm2) of overlap between TOS and each topographic region divided by the total area of TOS and then multiplied by 100. This percentage of overlap for each topographic region was then averaged across participants to get an average percent overlap between TOS and each topographic region. To obtain the total percentage of overlap between TOS and all of topographic regions, the average percent per topographic region was summed together. The same procedure was used to calculate the overlap between inferior IPS and topographic regions. The overlap analyses between the topographic regions and inferior IPS and between the topographic regions and TOS were both done using the inferior IPS and TOS ROIs defined using both the lenient p < 0.05 and stricter p < 0.001 thresholds.
To compare the functional relationship between TOS and inferior IPS, fMRI response amplitudes in the furnished rooms, empty rooms, isolated furniture, and multiple isolated objects conditions were extracted within each ROI for each participant. By using data from these four conditions only, we were able to examine object and scene processing in our ROIs, while retaining independence from the data used for ROI definition. fMRI response amplitudes for each stimulus condition were measured in percent signal change, calculated by taking the difference in average signal intensity between each stimulus condition and the fixation condition, then dividing this difference by that of the fixation condition and multiplying it by 100. Left and right hemisphere ROIs were combined in our analysis as no response pattern difference was found between the two hemispheres. Data was analyzed in an individual subjects analysis approach. To account for response amplitude differences when comparing across ROIs, data were also normalized within each participant, by dividing the response amplitude of each condition by that of the furnished room condition.
Results
Anatomical overlap
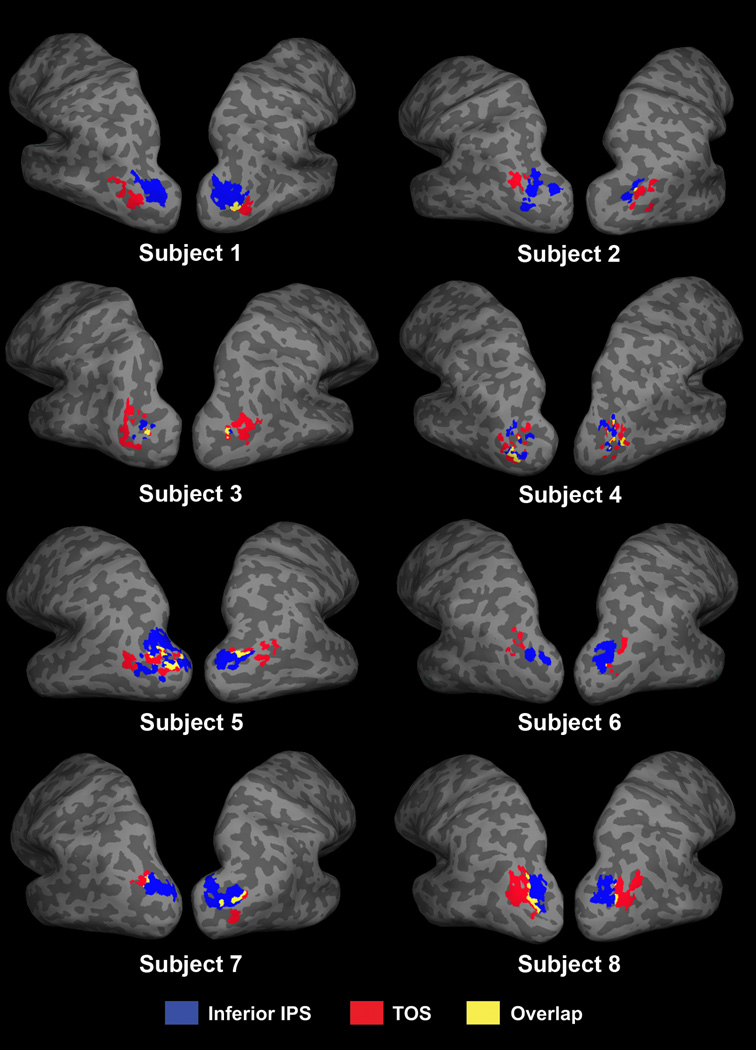
Overall, at the p < 0.05 (uncorrected) threshold, we found that there was a significant, but very small, overlap between the TOS and inferior IPS regions within individual participants (see Figure 2). On average, the two regions showed a 10% (SE = 2.9) overlap, which was significantly greater than zero (t(7) = 4.0, p < 0.01). We also examined the overlap between these two brain regions at the stricter threshold of p < 0.001 (uncorrected). At this threshold level, inferior IPS could not be localized in one participant in both hemispheres, and in the left hemisphere only for another participant. Excluding these two participants, in the remaining six participants, TOS and inferior IPS showed no overlap, indicating that the peak voxels for TOS and inferior IPS are in separate brain regions. There were no significant differences between the amount overlap between the left and right hemispheres (p = 0.45). Overall, this indicates that while Talairach coordinates from other studies have suggested that these two regions may co-localize, TOS and inferior IPS are actually anatomically distinct regions.
Figure 2.
TOS (red), inferior IPS (blue), and their overlap (yellow) in all eight participants.
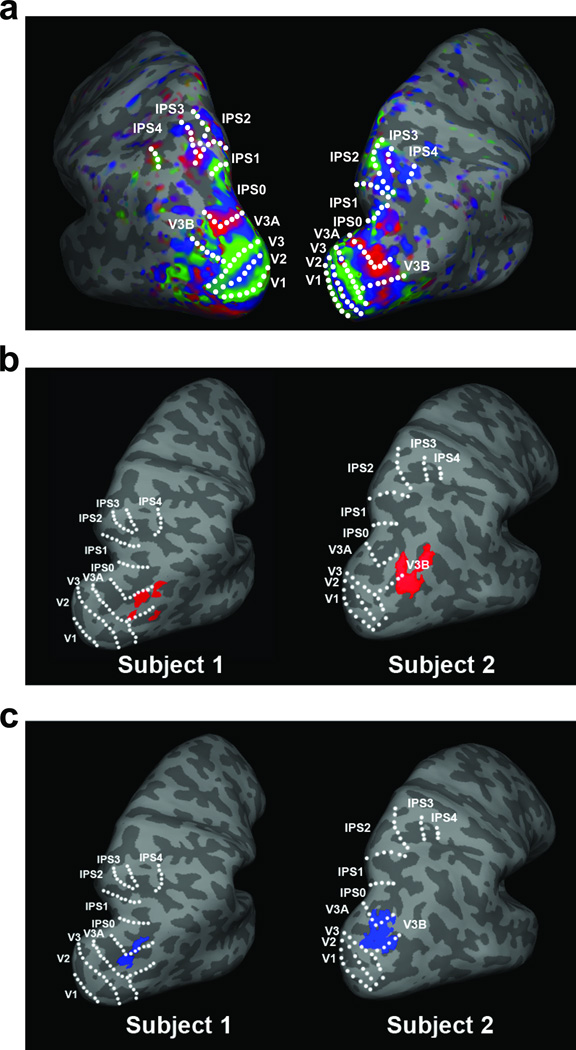
We then examined the overlap between both TOS and inferior IPS, separately, with the topographic regions at the p < 0.05 (uncorrected) threshold level. TOS showed, on average, a 49.8 ± 10.0% of overlap with all parietal topographic areas, with 38.9 ± 7.3% of TOS located within V3B, 2.6 ± 1.8% in V3A, and 8.2 ± 4.4% in IPS0 (see Figure 3b). The difference in percentage of overlap between the different parietal topographic regions and TOS reached significance (F(2,14) = 18.1, p < 0.001), with more overlap seen between TOS and V3B than V3A and IPS0, (ts > 4.5, ps < 0.01) and no differences between the latter two (p > 0.29). Replicating our previous work (Bettencourt & Xu, in preparation), inferior IPS showed, on average, a 87.2 ± 2.9% of overlap with all parietal topographic areas, with 40.0 ± 7.6% of inferior IPS located within V3B, 28.8 ± 6.9% in V3A, and 18.5 ± 4.2% in IPS0 (see Figure 3c). The difference in percentage of overlap between inferior IPS and the different parietal topographic regions did not reach significance (p = 0.18). These findings suggest that TOS overlaps with the same topographic regions in IPS as suggested by Nasr et al. (2011), and the same topographic regions as inferior IPS. The latter confirms that TOS and inferior IPS are indeed located in the same general region of the brain. However, over half of TOS is located outside of topographic regions, while 87% of inferior IPS is within these same topographic regions (this difference is statistically significant across our group of participants, t(7) = 3.78, p < 0.01). This stark difference in the degree of overlap between these regions and topographic IPS again suggests that, while they are nominally in the same topographic area (V3A/B-IPS0 region), TOS and inferior IPS cannot be the same brain region. Decreasing the significance threshold for TOS and inferior IPS ROIs to p < 0.001 (uncorrected) did not affect the degree of overlap between these regions and topographic IPS. Excluding the two participants whose inferior IPS could not be localized in both hemispheres at this threshold (see earlier description), inferior IPS showed a 89.4 ± 3.5% overlap with all topographic regions, with 36.2 ± 6.9% of inferior IPS in V3B, 39.2 ± 9.0% in V3A, and 13.9 ± 3.4% in IPS0. TOS could be localized in all participants at this threshold level and showed a 40.4 ± 10.8% overlap with topographic regions, with 32.0 ± 7.8% of TOS in V3B, 0.6 ± 0.6% in V3A, and 7.9 ± 6.8% in IPS0.
Figure 3.
(a) Topographic activation in a representative participant; (b) the location of TOS (p < 0.05) and (c) inferior IPS (p < 0.05) relative to topographic IPS regions in two representative participants.
These findings demonstrate the importance of analyzing data within the same participants when comparing the precise locations of different brain regions. Using either Talairach coordinates or proximity to topographic regions, one would be led to assume that inferior IPS and TOS are highly co-localized brain regions; however, our individual subject approach, which better accounts for individual variations in the location of brain regions, clearly shows that these are two spatially separable regions.
Functional differences
While our results suggest that TOS and inferior IPS are anatomically separable, this may be due to the way in which these areas are defined, and may not actually represent a functional difference in the types of processing handled by these two regions. Thus, in order to determine whether there are functional differences between inferior IPS and TOS in how they process visual stimuli, we examined their responses to furnished rooms, empty rooms, isolated furniture, and multiple isolated objects. These four types of stimuli differ in the number of objects present, as well as the presence or absence of scene context, allowing us to examine the two processes that have been attributed to these regions in the literature, that of scene and object perception.
Behaviorally, performance on the motion detection task was high (84% correct or higher) in each of these four stimulus conditions. There were no significant differences between conditions, though there was a slight trend towards higher performance in the multiple isolated objects condition (89.8%) when compared to the empty rooms condition (84.4%)(t(7) = 2.11, p = 0.07).
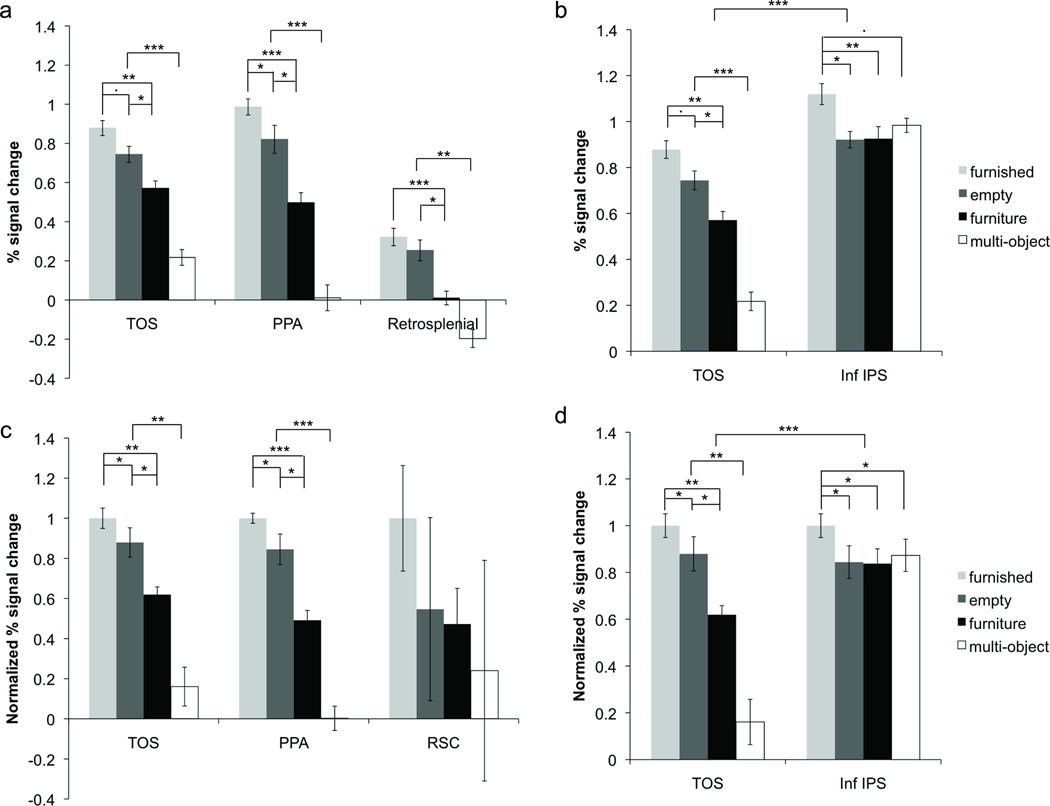
As previous research has suggested that TOS plays a role in scene perception, we first compared activation in TOS with that of other scene areas, PPA and RSC (see Figure 4a and c). First, we examined the non-normalized results within each ROI. All three scene areas showed significantly higher activation for scene stimuli (both furnished and empty) relative to both isolated furniture and multiple isolated objects (all ts > 2.9, all ps < 0.05). All three also showed higher activation for isolated furniture relative to multiple isolated objects (ts > 4.0, ps < 0.01). PPA showed significantly higher activation for furnished rooms relative to empty rooms (t(7) = 2.5, p < 0.05), and TOS showed a similar trend (t(7) = 2.2, p = 0.07). There was no significant difference between empty and furnished rooms in RSC (p > 0.3). Thus, for each of our scene areas, we observed higher activation for empty rooms compared with isolated furniture, showing a strong preference for scene stimuli within these regions. Our PPA results are consistent with those reported by Epstein and Kanwisher (1998) who also found a difference between the furnished and empty rooms and the isolated furniture conditions; although they did not find a significant difference between the furnished rooms and the empty rooms conditions, a trend indicating this difference was present in their results.
Figure 4.
Non-normalized functional activity for furnished rooms, empty rooms, isolated furniture, and multiple isolated objects in (a) the three scene areas and (b) TOS and inferior IPS. To account for response amplitude differences when comparing across ROIs and to facilitate between ROI comparisons, the data were also normalized within each participant by dividing the response amplitude for each condition by the furnished room condition. Normalized data is shown below for (c) the three scene areas and (d) TOS and inferior IPS. All three scene areas exhibited virtually the same response pattern, showing the lowest response to multiple isolated objects and preferring empty rooms to isolated furniture. Inferior IPS, on the other hand, showed similar responses to multiple isolated objects, empty rooms, and isolated furniture. A significant difference was found between TOS and inferior IPS in their responses to multiple isolated objects and in their response differences to empty rooms and isolated furniture.
We then normalized our data to account for any magnitude differences across the ROIs and compared response patterns across the three scene areas. The data was normalized by dividing the response amplitude for each condition by the furnished room condition. We found a main effect of stimulus condition (F(3,21) = 5.9, p < 0.01), but no effect of ROI or interaction between ROI and condition (ps > 0.89). This suggests that the pattern of responses across TOS, PPA, and RSC were similar, a finding that was supported in the non-normalized detailed pairwise comparisons.
We then examined the response within inferior IPS (see Figure 4b and d). As in the scene areas, inferior IPS activation was higher for furnished rooms than for both isolated furniture and empty rooms (ts > 2.6, ps < 0.05). However, unlike the scene areas, inferior IPS showed no difference for empty rooms and isolated furniture (p > 0.9). We also found a high response in inferior IPS for multiple objects (as expected), and while this response was trending towards significantly less than that of furnished rooms (t(7) = 2.1, p = 0.07), it was no different to that of empty rooms or isolated furniture (ps > 0.2). The trend towards higher activation for furnished rooms compared to multiple isolated objects was likely due to the fact that the former was more interesting and engaging than the latter. Moreover, the frequent repetition of the same set of objects within each block in the multiple isolated objects condition may have also caused a lowering of response amplitude due to fMRI adaptation, which would not have occurred in the furnished room condition.
Most importantly for the purpose of the present study, we compared activity between inferior IPS and TOS in the four stimulus conditions, using normalized data to account for response magnitude differences between ROIs. We found a main effect of ROI, a main effect of condition and a significant interaction between the two (all Fs > 17.2, ps < 0.01). The latter indicates that the response patterns for the four stimulus conditions differed in these two brain regions (see Figure 4d). Specifically, the response differences between isolated furniture and both empty and furnished room conditions, as well as the differences between the multiple isolated objects condition and all other conditions, were greater in TOS than inferior IPS (ts > 3.3, ps < 0.05). These results were robust and were seen in each of the eight participants tested. This suggests that TOS and inferior IPS differ significantly in how they represent scenes and objects.
Lastly, we examined whether the pattern of activation differed between regions of TOS that overlapped with topographic IPS regions and regions that did not. For one participant there was no overlap between TOS and any of the topographic regions in one hemisphere and so, he was excluded from these analyses. Again, we used normalized data to account for response magnitude differences between ROIs. Here we found a main effect of stimulus condition (F(3,18) = 11.7, p < 0.001), but no main effect of ROI (overlap vs. no overlap) or interaction between the two (p > 0.4), and the overall result pattern mirrored that of the TOS results reported in Figure 4. Thus, whether or not TOS overlaps with topographic regions in IPS does not seem to affect the nature of its visual representation.
Discussion
Previous research has placed both inferior IPS (Xu and Chun, 2006, 2009) and TOS (Grill-Spector, 2003; Hasson et al., 2003; Levy et al., 2004; Nakamura et al., 2000) in the same general region of the brain, suggesting that these two regions may be one singular region. However, the work presented here clearly shows that while these two regions are located within close proximity to each other, they are separate regions, both anatomically and functionally. In spite of our very liberal statistical threshold for defining each ROI (p < 0.05, uncorrected), we found only a small percentage of overlap between the anatomical locations of these regions (∼10%). Moreover, while inferior IPS was shown to be highly co-localized with topographic cortex (around 87%), we found that over half of TOS is located outside of topographic regions. This suggests that these are distinct brain regions that have only appeared to be co-localized across previous studies due to blurring caused by group-averaging and Talairach transformations.
Most importantly, TOS and inferior IPS showed functional differences in the processing of furnished rooms, empty rooms, isolated furniture, and multiple isolated objects. This set of conditions were chosen because they involved different amount of scene- and object-related processing, the two operations that have been associated with TOS and inferior IPS, respectively, in the literature. If TOS and inferior IPS were the same functional region, we would expect to see very similar response patterns in both. Instead, we saw distinct patterns of responses for processing scenes and multiple isolated objects in these two brain regions. Specifically, TOS showed a high response to any type of scene stimuli, even when they contain very few objects, and a much lower response to non-scene stimuli even when they contain many objects. This response pattern replicated a previous finding by Epstein and Kanwisher (1998) in PPA (another scene selective region) and is considered a hallmark of scene-selective processing in the brain. Inferior IPS, on the other hand, had a very different response pattern, showing a high level of activation whenever multiple objects were present, regardless of whether or not a scene context was also present. This indicates that TOS and inferior IPS are functionally distinctive in how they represent scenes and objects.
While it may seem obvious that different functional contrasts would activate anatomically and functionally distinct regions, there are many counter examples. For instance, STS has been shown to be activated by both faces and biological motion (Bonda et al., 1996; Grossman et al., 2000; Haxby et al., 2000) and lower visual areas such as V1 can be activated by a variety of visual stimuli. Similarly, PPA is usually defined by contrasting scene and non-scene objects; however, this region also highly overlaps with regions defined using ensemble stimuli (Cant and Xu, 2012). It is by understanding the plurality of stimuli and tasks that can activate a region that we understand the role of that region in cognition. Thus, this experiment provides a valuable first step towards understanding the relationship between TOS and inferior IPS.
Previous research has shown that inferior IPS participates in visual object individuation. This would predict that inferior IPS activation for isolated furniture should be higher than that for empty rooms, as by definition, the former would contain more objects than the latter. However, we found that inferior IPS activation did not differ between these two conditions. In our stimuli (which we took from Epstein and Kanwisher, 1998), empty rooms were not simply blank walls and floors, but many also contained doorways, windows, outlets, etc. (see examples shown in Figure 5). Because the individuation and identification of these objects is likely important in scene recognition and navigation, they may be individuated in a manner similar to more standard objects (such as furniture or plants) in inferior IPS. This could explain the similarity in activity levels for empty rooms and multiple isolated objects, as well as the higher response to furnished rooms, which contained furniture as well as doorways, etc.
Figure 5.
Examples of the furnished room, empty room, and isolated furniture conditions showing the variety of stimuli used in each condition.
Although inferior IPS was localized by contrasting its response to multiple isolated objects to that of noise images, this brain region did not show the highest response to multiple isolated objects (the stimulus condition that defined it). But rather, it showed the highest response to furnished rooms. This was likely due to two reasons. First, the furnished room condition was the most interesting, enriched, and engaging stimulus condition, which could have caused an increase in attention, and thus an increase in BOLD response. Second, in the multiple isolated objects condition, the same set of shapes were used repeatedly, albeit in different placements, in each trial. This likely resulted in fMRI adaptation effects and decreased the response for this condition compared to the other conditions. Note that these two effects were largely independent of the processing specificity of a brain region and would modulate responses in TOS as well in a similar manner. Despite the influence of these two effects, the pattern of response across all conditions varies significantly between inferior IPS and TOS, providing strong evidence that these are functionally distinctive brain regions.
One could argue that inferior IPS may only individuate objects in isolation, while TOS may individuate objects in a scene context, as this account would predict a pattern of TOS response similar to what we observed here (i.e., the highest response for the most number of objects present in a scene context (furnished rooms), the lowest response to isolated objects with no scene context (multiple isolated objects), and a moderately low response to object that have an implied scene context (isolated furniture)). However, it is unlikely that inferior IPS evolved to only individuate isolated objects, which are rarely encountered in the real world, and not also objects in a scene, which are seen everyday. Thus, it seems unnecessary for our brain to dedicate a separate brain region to individuate objects in a scene. Comparing the response of TOS in scene processing to other scene areas, we found that while PPA, RSC, and TOS differed in their overall response amplitudes, they exhibited very similar response patterns (see Figure 4a and c), suggesting that all three regions participate in a similar manner in this aspect of scene perception. As such, it is insufficient to argue that TOS is involved in individuating objects in a scene but not PPA or RSC. Although the exact role of TOS in scene representation is unknown, the close proximity of TOS to inferior IPS and its minor overlap with parietal topographic areas as found in the present study, together with its position invariance related to the hemifield in which the stimuli are presented (MacEvoy and Epstein, 2007) suggests that TOS is likely involved in higher order spatial processing related to scenes, such as encoding the spatial relationships between objects within a scene.
More work is needed to explore the role of inferior IPS in individuation and to pinpoint the precise and unique role of TOS in scene processing relative to those of the other scene areas. Nevertheless, by more clearly localizing TOS in relationship to both inferior IPS and parietal topographic regions, the present findings represent an important step towards understanding the role of these brain regions in visual cognition.
Acknowledgments
We would like to thank Jonathan S. Cant and Sonia Poltoratski for their help in data collection, Jascha Swisher for his retinotopy code, and Nancy Kanwisher and Russell Epstein for sharing the scene stimuli from their 1998 publication. This research was supported by National Science Foundation grant 0855112 to YX and National Institutes of Health grant F32-EY022874 to KCB.
Contributor Information
Katherine C. Bettencourt, Harvard University
Yaoda Xu, Harvard University.
References
- Behrmann M, Geng JJ, Shomstein S. Parietal cortex and attention. Current Opinion in Neurobiology. 2004;14:212–217. doi: 10.1016/j.conb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Bettencourt KC, Xu Y. The role of parietal topographic maps in visual object individuation and identification. Journal of Neuroscience. in preparation. [Google Scholar]
- Bonda E, Petrides M, Ostry D, Evans A. Specific involvement of human parietal systems and the amygdala in the perception of biological motion. Journal of Neuroscience. 1996;16(11):3737–3744. doi: 10.1523/JNEUROSCI.16-11-03737.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Bressler DW, Silver MA. Spatial attention improves reliability of fMRI retinotopic mapping signals in occipital and parietal cortex. NeuroImage. 2010;53:526–533. doi: 10.1016/j.neuroimage.2010.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buneo CA, Andersen RA. The posterior parietal cortex: sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia. 2006;44:2594–2606. doi: 10.1016/j.neuropsychologia.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Cant JS, Xu Y. Object ensemble processing in human anterior -medial ventral visual cortex. Journal of Neuroscience. 2012;32:7885–7700. doi: 10.1523/JNEUROSCI.3325-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CL, Goldberg ME. Space and attention in parietal cortex. Annual Review of Neuroscience. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Current Opinion in Neurobiology. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Culham JC, Cavina-Pratesi C, Singhal A. The role of parietal cortex in visuomotor control: What have we learned from neuroimaging? Neuropsychologia. 2006;44(13):2668–2684. doi: 10.1016/j.neuropsychologia.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Piazza M, Pinel P, Cohen L. Three parietal circuits for number processing. Cognitive Neuropsychology. 2003;20(3–6):487–506. doi: 10.1080/02643290244000239. [DOI] [PubMed] [Google Scholar]
- DeYoe EA, Carman GJ, Bandettini P, Glickman S, Wieser J, Cox R, Miller D, Neitz J. Mapping striate and extrastriate visual areas in human cerebral cortex. Proceeding of the National Academy of Science USA. 1996;93:2382–2386. doi: 10.1073/pnas.93.6.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilks DD, Julian JB, Kubilius J, Spelke ES, Kanwisher N. Mirror-image sensitivity and invariance in object and scene processing pathways. The Journal of Neuroscience. 2011;31(31):11305–11312. doi: 10.1523/JNEUROSCI.1935-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilks DD, Julian JB, Paunov AM, Kanwisher N. The occipital place area is causally and selectively involved in scene perception. The Journal of Neuroscience. 2013;33(4):1331–1336. doi: 10.1523/JNEUROSCI.4081-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cerebral Cortex. 1997;7:181–192. doi: 10.1093/cercor/7.2.181. [DOI] [PubMed] [Google Scholar]
- Epstein RA. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends in Cognitive Sciences. 2008;12(10):388–396. doi: 10.1016/j.tics.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA, Higgins S, Thomson-Schill SL. Learning places from views: variation in scene processing as a function of experience and navigational ability. Journal of Cognitive Neuroscience. 2005;17(1):73–83. doi: 10.1162/0898929052879987. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Higgins S, Jablonski K, Feiler AM. Visual scene processing in familiar and unfamiliar environments. Journal of Neurophysiology. 2007;97(5):3670–3683. doi: 10.1152/jn.00003.2007. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Higgins S. Differential parahippocampal and retrosplenial involvement in three types of visual scene recognition. Cerebral Cortex. 2007;17(7):1680–1693. doi: 10.1093/cercor/bhl079. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions on Medical Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Gottlieb J, Goldberg ME. Activity of neurons in the lateral intraparietal area of the monkey during an antisaccade task. Nature Neuroscience. 1999;2:906–912. doi: 10.1038/13209. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. The functional organization of the intraparietal sulcus in humans and monkeys. Journal of Anatomy. 2005;207:3–17. doi: 10.1111/j.1469-7580.2005.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K. The neural basis of object perception. Current Opinion in Neurobiology. 2003;13:159–166. doi: 10.1016/s0959-4388(03)00040-0. [DOI] [PubMed] [Google Scholar]
- Grossman E, Donnelly M, Price R, Pickens D, Morgan V, Neighbor G, Blake R. Brain areas involved in perception of biological motion. Journal of Cognitive Neuroscience. 2000;12(5):711–720. doi: 10.1162/089892900562417. [DOI] [PubMed] [Google Scholar]
- Hasson U, Harel M, Levy I, Malach R. Large-scale mirror symmetry organization of human occipito-temporal object areas. Neuron. 2003;37:1027–1041. doi: 10.1016/s0896-6273(03)00144-2. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4(6):223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hubbard EM, Piazza M, Pinel P, Dehaene S. Interactions between number and space in parietal cortex. Nature Reviews Neuroscience. 2005;6:435–448. doi: 10.1038/nrn1684. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Konen CS, Kastner S. Representation of eye movements and stimulus motion in topographically organized areas of human posterior parietal cortex. Journal of Neuroscience. 2008;28(33):8361–8375. doi: 10.1523/JNEUROSCI.1930-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konen CS, Kastner S. Two hierarchically organized neural systems for object information in human visual cortex. Nature Neuroscience. 2008;11:224–231. doi: 10.1038/nn2036. [DOI] [PubMed] [Google Scholar]
- Konkle T, Oliva A. A real-world size organization of object responses in occipitotemporal cortex. Neuron. 2012;74:1114–1124. doi: 10.1016/j.neuron.2012.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung CC, Peissig JJ, Tarr MJ. Is region-of-interest overlap comparison a reliable measure of category specificity? Journal of Cognitive Neuroscience. 2007;19(12):2019–2034. doi: 10.1162/jocn.2007.19.12.2019. [DOI] [PubMed] [Google Scholar]
- Levy I, Hasson U, Avidan G, Hendler T, Malach R. Center-periphery organization of human object areas. Nature Neuroscience. 2001;4(5):533–539. doi: 10.1038/87490. [DOI] [PubMed] [Google Scholar]
- Levy I, Hasson U, Harel M, Malach R. Functional analysis of the periphery effect in human building related areas. Human Brain Mapping. 2004;22:15–26. doi: 10.1002/hbm.20010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEvoy SP, Epstein RA. Position selectivity in scene- and object-responsive occipitotemporal regions. Journal of Neurophysiology. 2007;98(4):2089–2098. doi: 10.1152/jn.00438.2007. [DOI] [PubMed] [Google Scholar]
- Merriam EP, Colby CL. Active vision in parietal and extrastriate cortex. Neuroscientist. 2005;11:484–493. doi: 10.1177/1073858405276871. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kawashima R, Sato N, Nakamura A, Sugiura M, Kato T, Hatano K, Ito K, Fukuda H, Schormann T, Zilles K. Functional delineation of the human occipito-temporal areas related to face and scene processing - a PET study. Brain. 2000;123:1903–1912. doi: 10.1093/brain/123.9.1903. [DOI] [PubMed] [Google Scholar]
- Nasr S, Liu N, Devaney KJ, Yue X, Rajimehr R, Ungerleider LG, Tootell RBH. Scene-selective cortical regions in human and nonhuman primates. Journal of Neuroscience. 2011;31(39):13771–13785. doi: 10.1523/JNEUROSCI.2792-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban GA, Claeys K, Nelissen K, Smans R, Sunaert S, Todd JT, Wardak C, Durand JB, Vanduffel W. Mapping the parietal cortex of human and non-human primates. Neuropsychologia. 2006;44:2647–2667. doi: 10.1016/j.neuropsychologia.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Orban GA, Van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends in Cognitive Sciences. 2004;8(7):315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Schluppeck D, Glimcher P, Heeger DJ. Topographic organization for delayed saccades in human posterior parietal cortex. Journal of Neurophysiology. 2005;94:1372–1384. doi: 10.1152/jn.01290.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 2001;294:1350–1354. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ. Topographic maps of visual spatial attention in human parietal cortex. Journal of Neurophysiology. 2005;94:1358–1371. doi: 10.1152/jn.01316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straw AD. Vision Egg: An open-source library for realtime visual stimulus generation. Frontiers in Neuroinformatics. 2008;2:4. doi: 10.3389/neuro.11.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swisher JD, Halko MA, Merabet LB, McMains SA, Somers DC. Visual topography of human intraparietal sulcus. Journal of Neuroscience. 2007;27(20):5326–5337. doi: 10.1523/JNEUROSCI.0991-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Troiani V, Stigliani A, Smith ME, Epstein RA. Multiple object properties drive scene-selective regions. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs364. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell BA, Dumoulin SO, Brewer AA. Visual field maps in human cortex. Neuron. 2007;56(2):366–383. doi: 10.1016/j.neuron.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Ward EJ, MacEvoy SP, Epstein RA. Eye-centered encoding of visual space in scene-selective regions. Journal of Vision. 2010;10(14) doi: 10.1167/10.14.6. article 6. [DOI] [PubMed] [Google Scholar]
- Xu Y. Representing connected and disconnected shapes in human inferior intraparietal sulcus. NeuroImage. 2008;40:1849–1856. doi: 10.1016/j.neuroimage.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Xu Y. Distinctive neural mechanisms supporting visual object individuation and identification. Journal of Cognitive Neuroscience. 2009;21(3):511–518. doi: 10.1162/jocn.2008.21024. [DOI] [PubMed] [Google Scholar]
- Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]
- Xu Y, Chun MM. Selecting and perceiving multiple visual objects. Trends in Cognitive Sciences. 2009;13(4):167–174. doi: 10.1016/j.tics.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S, Serences JT. Cortical mechanisms of space-based and object-based attentional control. Current Opinion in Neurobiology. 2003;13:187–193. doi: 10.1016/s0959-4388(03)00033-3. [DOI] [PubMed] [Google Scholar]