Abstract
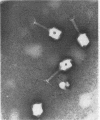
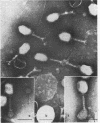
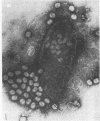
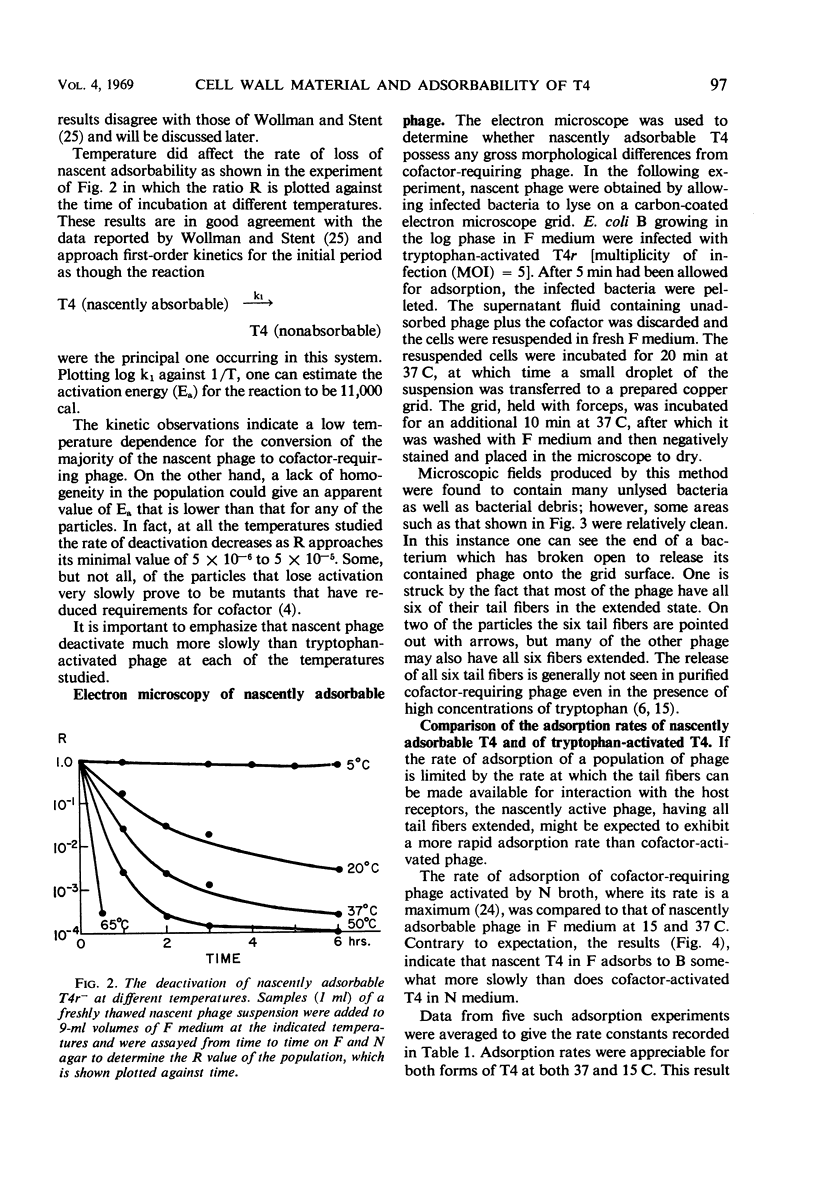
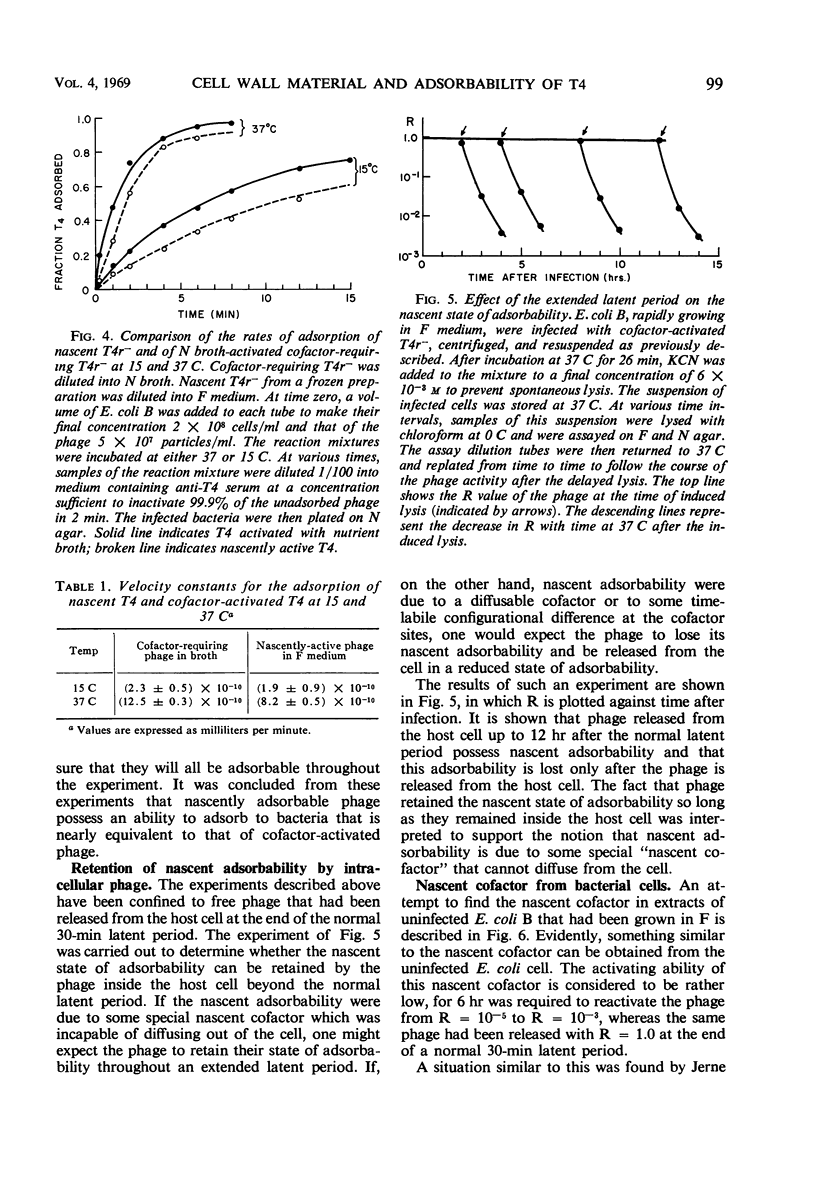
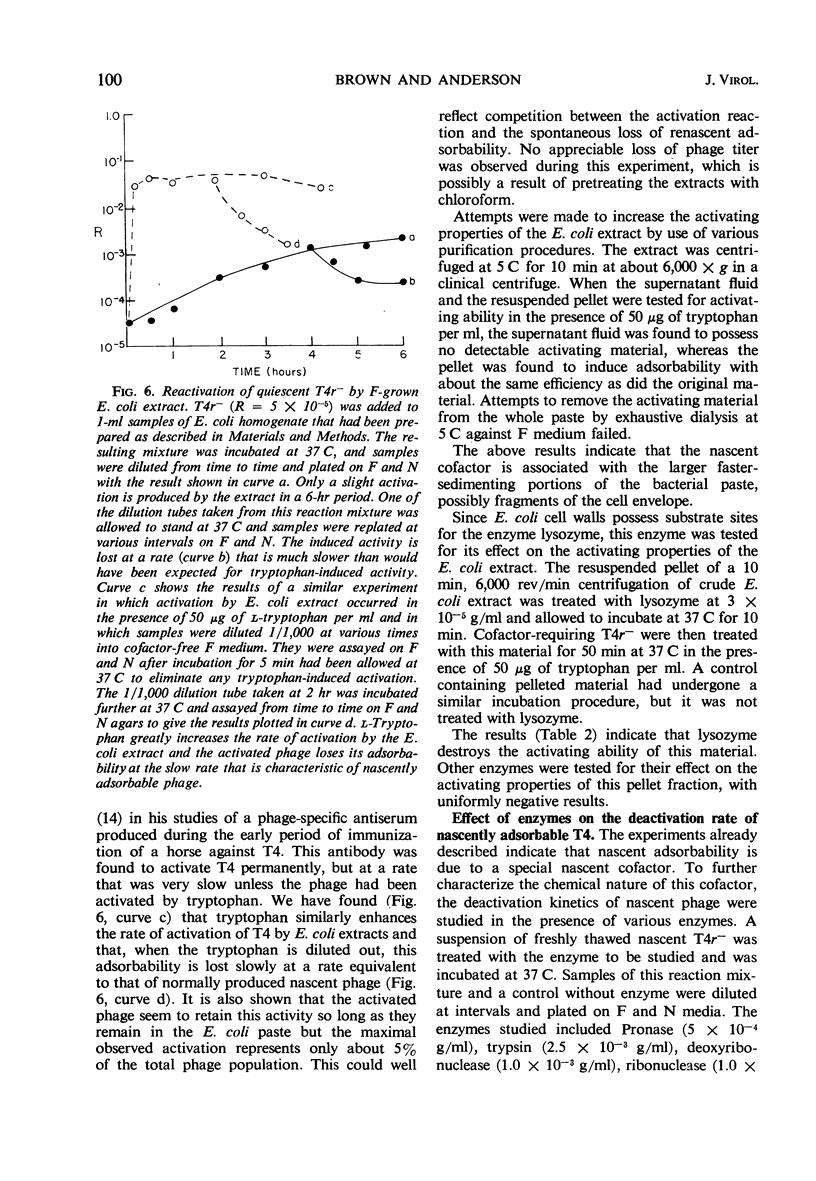
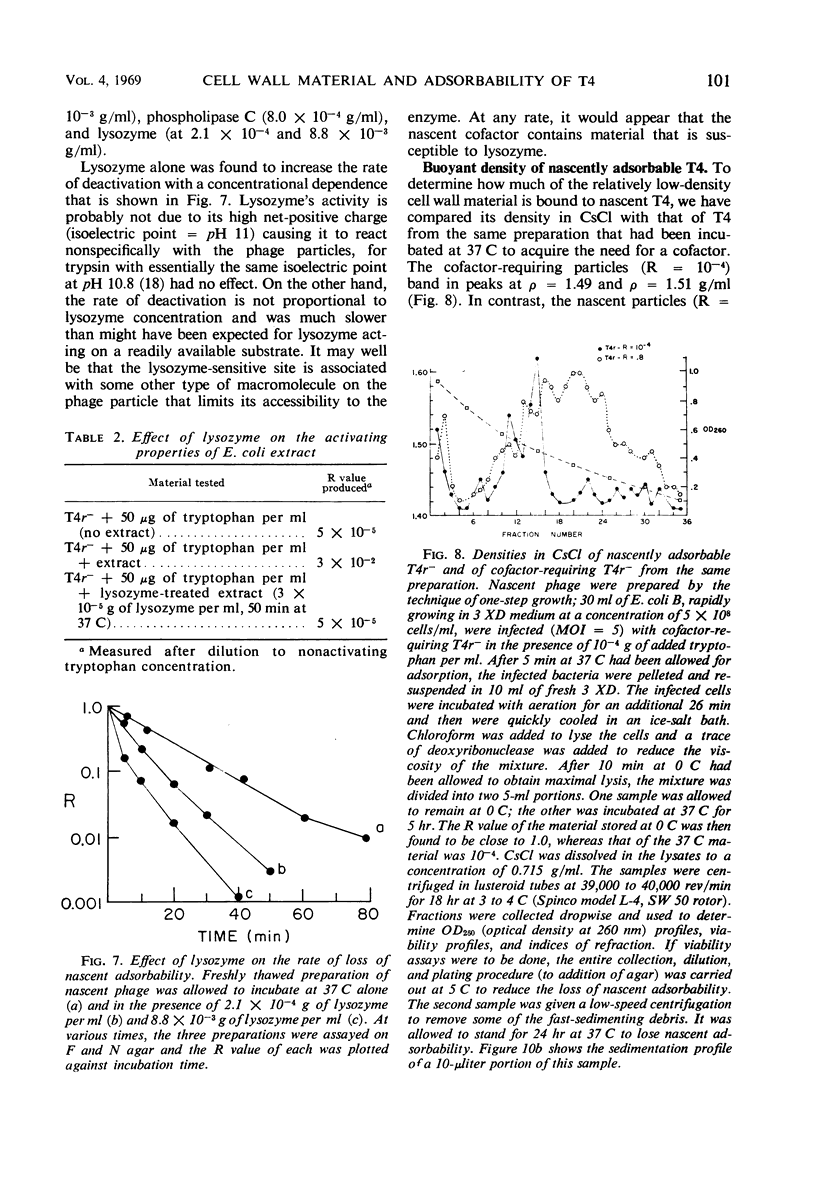
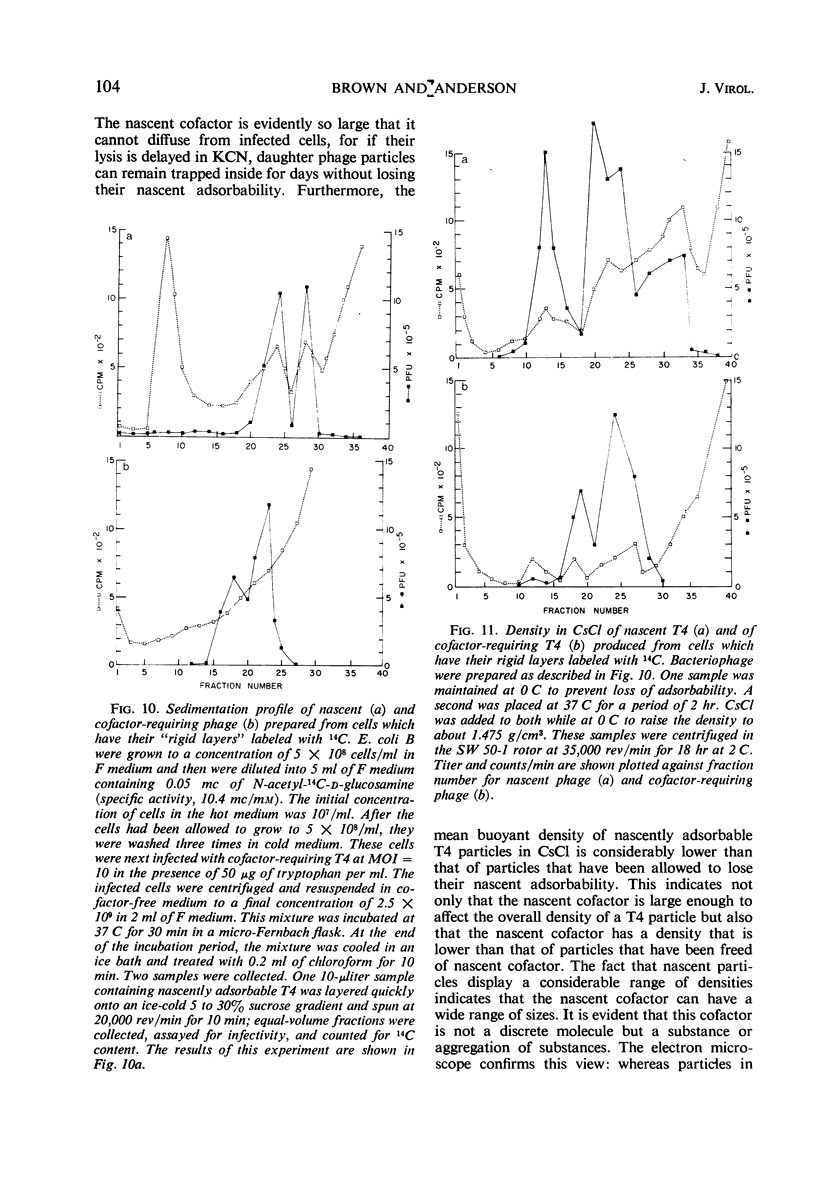
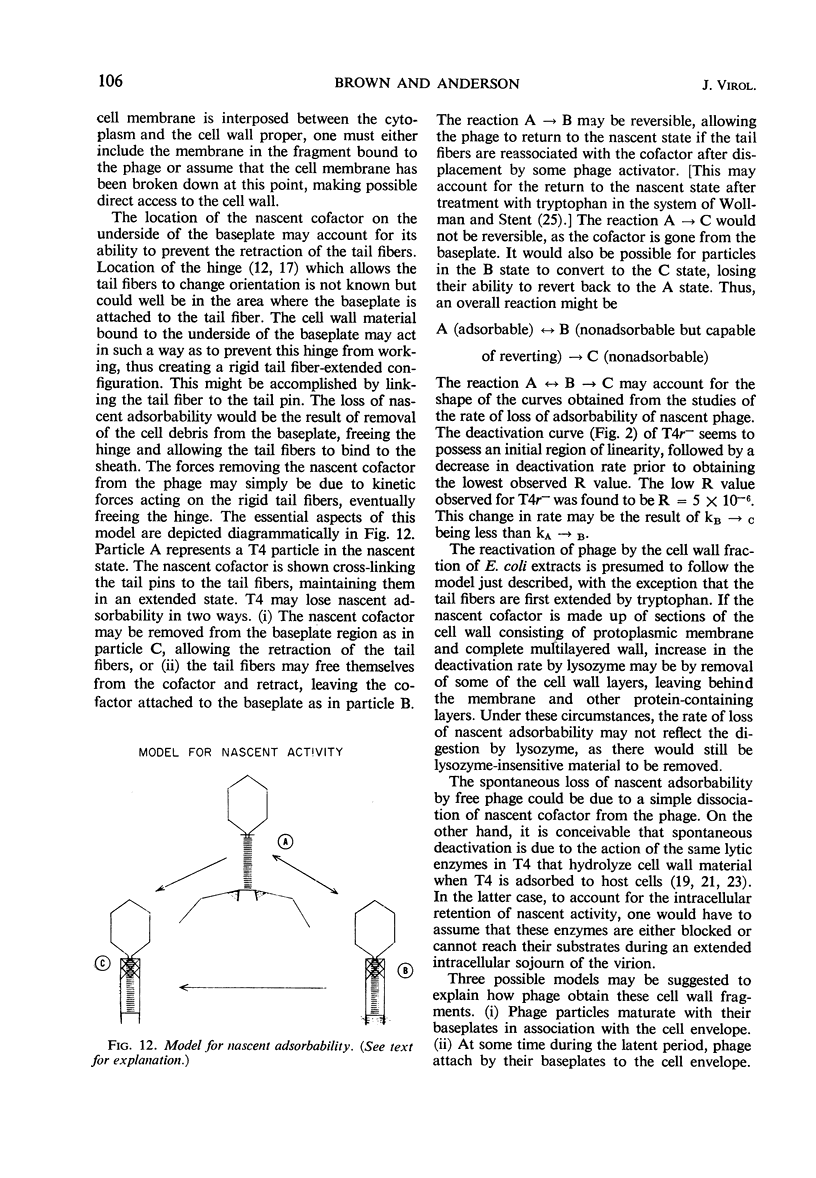
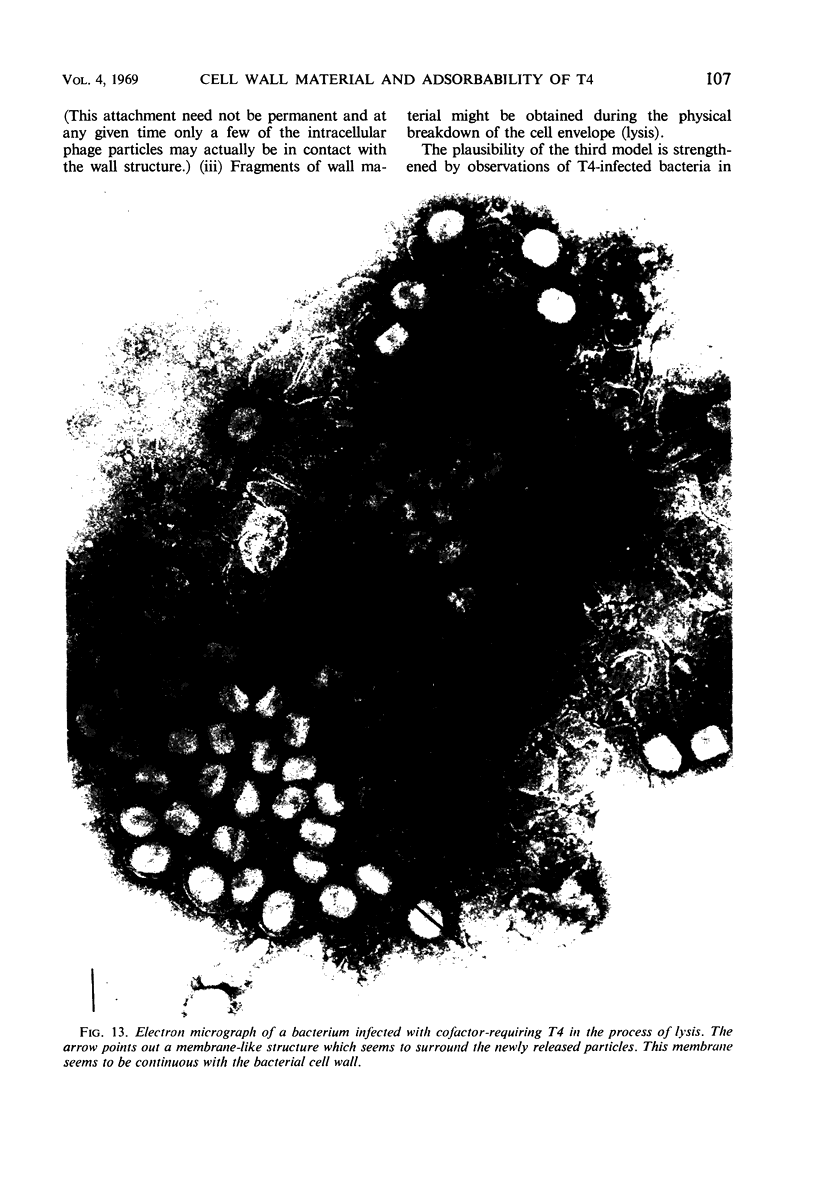
The adsorbability of T4 on host cells was determined as a function of time after their liberation from infected cells. Freshly liberated (nascent) particles are readily adsorbed but lose their adsorbability with a half-time of about 2 days at 5 C, but only about 20 min at 37 C. They can be made adsorbable again with an α-amino acid cofactor like l-tryptophan, and this state of adsorbability can be stabilized by cell wall material from Escherichia coli. Such stabilized particles lose their adsorbability at a rate similar to that at which nascent particles lose theirs. Most freshly liberated particles are observed by means of electron microscopy to have “debris” attached to their baseplates and to have most of their six, long tail fibers free, whereas “old” particles that have lost their adsorbability appear relatively “clean” with most of their tail fibers wrapped around their sheaths. Nascent particles have densities that are lower than those of old particles. The material responsible for nascent adsorbability seems to be a fragment of the host's cell wall, for nascent adsorbability is destroyed by lysozyme. Furthermore, nascent T4 particles liberated from host cells with radioactively labeled walls carry the label in density gradients but lose it as they lose adsorbability. In addition, only a small proportion of particles liberated from infected spheroplasts are nascently adsorbable, whereas most particles liberated from intact cells are adsorbable.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson T. F. The Activation of the Bacterial Virus T4 by l-Tryptophan. J Bacteriol. 1948 May;55(5):637–649. doi: 10.1128/jb.55.5.637-649.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T. F. The Inheritance of Requirements for Adsorption Cofactors in the Bacterial Virus T4. J Bacteriol. 1948 May;55(5):651–658. doi: 10.1128/jb.55.5.651-658.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUMMINGS D. J. SEDIMENTATION AND BIOLOGICAL PROPERTIES OF T-PHAGES OF ESCHERICHIA COLI. Virology. 1964 Jul;23:408–418. doi: 10.1016/0042-6822(64)90264-8. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Chapman V. A., DeLong S. S. The sedimentation and conformational variance among T-even bacteriophages. Virology. 1969 Jan;37(1):94–108. doi: 10.1016/0042-6822(69)90310-9. [DOI] [PubMed] [Google Scholar]
- Darlington R. W., Moss L. H., 3rd Herpesvirus envelopment. J Virol. 1968 Jan;2(1):48–55. doi: 10.1128/jvi.2.1.48-55.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- Fraser D., Mahler H. R., Shug A. L., Thomas C. A. THE INFECTION OF SUB-CELLULAR ESCHERICHIA COLI, STRAIN B, WITH A DNA PREPARATION FROM T2 BACTERIOPHAGE. Proc Natl Acad Sci U S A. 1957 Nov 15;43(11):939–947. doi: 10.1073/pnas.43.11.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTHRIE G. D., SINSHEIMER R. L. Observations on the infection of bacterial protoplasts with the deoxyribonucleic acid of bacteriophage phi X174. Biochim Biophys Acta. 1963 Jun 25;72:290–297. [PubMed] [Google Scholar]
- Gamow R. I., Kozloff L. M. Chemically induced cofactor requirement for bacteriopage T4D. J Virol. 1968 May;2(5):480–487. doi: 10.1128/jvi.2.5.480-487.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JERNE N. K. The presence in normal serum of specific antibody against bacteriophage T4 and its increase during the earliest stages of immunization. J Immunol. 1956 Mar;76(3):209–216. [PubMed] [Google Scholar]
- KANNER L. C., KOZLOFF L. M. THE REACTION OF INDOLE AND T2 BACTERIOPHAGE. Biochemistry. 1964 Feb;3:215–223. doi: 10.1021/bi00890a013. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., BOLLE A., BOYDELATOUR E., EPSTEIN R. H., FRANKLIN N. C., JERNE N. K., REALE SCAFATI A., SECHAUD J. FUNCTIONS AND PROPERTIES RELATED TO THE TAIL FIBERS OF BACTERIOPHAGE T4. Virology. 1965 Jul;26:419–440. doi: 10.1016/0042-6822(65)90006-1. [DOI] [PubMed] [Google Scholar]
- Martin H. H. Biochemistry of bacterial cell walls. Annu Rev Biochem. 1966;35:457–484. doi: 10.1146/annurev.bi.35.070166.002325. [DOI] [PubMed] [Google Scholar]
- Mukai F., Streisinger G., Miller B. The mechanism of lysis in phage T4-infected cells. Virology. 1967 Nov;33(3):398–404. doi: 10.1016/0042-6822(67)90115-8. [DOI] [PubMed] [Google Scholar]
- Simon L. D., Anderson T. F. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. II. Structure and function of the baseplate. Virology. 1967 Jun;32(2):298–305. doi: 10.1016/0042-6822(67)90278-4. [DOI] [PubMed] [Google Scholar]
- WOLLMAN E. L., STENT G. S. Studies on activation of T4 bacteriophage by cofactor. I. The degree of activity. Biochim Biophys Acta. 1950 Nov;6(2):292–306. doi: 10.1016/0006-3002(50)90103-x. [DOI] [PubMed] [Google Scholar]
- WOLLMAN E. L., STENT G. S. Studies on activation of T4 bacteriophage by cofactor. IV. Nascent activity. Biochim Biophys Acta. 1952 Nov;9(5):538–550. doi: 10.1016/0006-3002(52)90204-7. [DOI] [PubMed] [Google Scholar]