Abstract
Objectives
Although blood purification improves outcomes in animal studies of sepsis, results of clinical trials have been mixed. We conducted a systematic review and meta-analysis of randomized trials to determine the association between various blood purification techniques and all-cause mortality in humans with sepsis.
Data Sources
We searched for relevant studies in MEDLINE, EMBASE, and the Cochrane Library database from January 1966 until May 2012.
Study Selection
Inclusion required a diagnosis of sepsis and comparison of blood purification techniques including hemofiltration, hemoperfusion, plasma exchange, or hemodialysis with no blood purification (control group).
Data Extraction
Two authors independently selected studies and extracted data. Summary statistics, risk ratios (RRs), and CIs were calculated using random-effects modeling. Study quality was assessed using Jadad score, and publication bias using funnel plots and Egger’s statistic.
Data Synthesis
Overall, blood purification decreased mortality compared to no blood purification (35.7% versus 50.1%; RR, 0.69; 95% CI, 0.56–0.84; p < 0.001; 16 trials, n=827). However, these results were driven mainly by hemoperfusion (RR, 0.63; 95% CI, 0.50–0.80; p < 0.001; 10 trials, n=557), and plasma exchange (RR, 0.63; 95% CI, 0.42–0.96; p = 0.03; 2 trials, n=128). Pooling of all trials of blood purification for treatment of sepsis was no longer associated with lower mortality (RR, 0.89; 95% CI, 0.71–1.13; p = 0.36; 8 trials, n=457) after excluding trials using polymyxin B hemoperfusion.
Conclusions
Blood purification techniques including hemoperfusion, plasma exchange, and hemofiltration with hemoperfusion were associated with lower mortality in patients with sepsis. These results were mainly influenced by studies using polymyxin B hemoperfusion from Japan.
Keywords: sepsis, blood purification, cytokines, inflammation, mortality, meta-analysis
INTRODUCTION
Severe sepsis, defined as sepsis with acute organ dysfunction, affects more than 750,000 people annually in the United States with a mortality rate ranging from 28% to 50% (1,2). With the recent removal of Xigris, the only FDA-approved treatment for sepsis, from the market due to failure to show a survival benefit for patients with septic shock (3), the medical community is urgently seeking a possible therapy. Source control and antibiotics remain the mainstays of therapy for infection (4) but no specific treatment is available for sepsis. Observations over more than 20 years have suggested a role for extracorporeal blood purification. However, no definitive trials have been published to date.
Sepsis involves complex interactions between endothelial cells, platelets, leukocytes, coagulation system, and multiple pro- and anti-inflammatory mediators, and often results in multiple organ dysfunction syndrome (MODS) leading to death (5,6). Since there are correlations between high concentrations of circulating inflammatory cytokines for patients with sepsis or septic shock (7–9) and since mortality is highest when both pro- and anti-inflammatory cytokine levels are high (7), extracorporeal blood purification is used by some centers in order to modulate the immune response. Unlike drugs targeting specific mediators, blood purification can influence a wide range of molecules.
Blood purification for sepsis has consisted of various techniques including high volume hemofiltration, high adsorption hemofiltration, high cut-off membrane hemofiltration, plasma exchange, and hybrid systems like coupled plasma filtration adsorption. Recently, the spectrum of techniques available for blood purification has been broadened further with technological advances particularly in the area of hemoperfusion. However, the use of blood purification is controversial and results vary among studies (9–17). No systematic reviews have pooled the available evidence from various types of blood purification compared to conventional therapy. Therefore we performed a systematic review and meta-analysis to attempt to determine whether blood purification decreases mortality in patients with sepsis so as to guide further research in this area.
MATERIALS AND METHODS
Selection of Studies
We reviewed MEDLINE and EMBASE citations between January 1, 1966, and May 1, 2012, and the Cochrane Central Register of Controlled Trials Library database through May 1, 2012. Search was performed using medical subject heading (MeSH) terms and text words with Boolean strategy, and cross-searching of the following 3 categories: (1) modality of blood purification (“hemofiltration” OR “renal replacement therapy” OR “blood purification” OR “dialysis” OR “hemoperfusion” OR “hemoadsorption” OR “plasmafiltration” OR “plasma exchange”); (2) disease (“sepsis” OR “infection” OR “septic shock” OR “systemic inflammatory response syndrome” OR “SIRS” OR “multiple organ dysfunction syndrome” OR “MODS”); and (3) others related (“outcome” OR “intensive care unit” OR “ICU” OR “critically ill patients” OR “mortality” OR “prognosis”).The limits were “human” and “English” language. We limited article types to randomized controlled trials, and because sepsis in children is different in terms of infectious etiology and host response, we only included adults more than 18 years of age. The bibliographies of all relevant studies and recent review articles were scanned to identify additional citations.
We categorized trials according to the type of blood purification technique used. Studies using continuous or intermittent veno-venous hemofiltration, regardless of filtration rate, duration and frequency, were classified as “hemofiltration”. Trials of a blood purification technique where a sorbent is placed in direct contact with blood in an extracorporeal circuit were considered to be “hemoperfusion”, and trials that removed and replaced plasma were grouped as “plasma exchange”. Conventional treatment was defined as the ordinary therapy (including fluid resuscitation, nutrition support, antibiotic therapy, and other organ support in the intensive care unit) but with no forms of extracorporeal treatment.
Quality Assessment
We assessed quality of each study included in the meta-analysis using the Jadad score (18), which assesses the conduct of randomization, concealment of treatment allocation, similarity of treatment groups at baseline, clinician blinding, and the description of withdrawals and dropouts. The Jadad score ranges from 1 (poor) to 5 (excellent) where RCT quality is high when scores are ≥3. The Jadad/Oxford quality scales require a double-blinded placebo for 2 of the 5 points. Due to the nature of the intervention and logistic reasons, none of the studies reported double-blinding. Thus we used “investigator blinding” for assessment of quality of studies included in this meta-analysis (18).
Data Abstraction and Clinical Outcome
Study selection and data abstraction was performed independently by two reviewers (FZ and ZP) according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement (19) and any discrepancies between reviewers were resolved by consensus. For each study raw data were extracted using a standard form, which included the first author, study design, year of publication, total number of patients, patient characteristics, details regarding the outcomes and types of sepsis. In addition, we also assessed the modality of blood purification, as well as the comparisons or related description of primary outcome between blood purification and conventional treatment, such as mortality or physiologic variables. The main endpoint was mortality as defined in the individual trials. If mortality was assessed at several time points in a study, we used data from the latest follow-up time for overall mortality assessment.
Statistical Analysis
For each trial, we derived the risk ratios (RRs) and 95% CIs of reported mortality in patients assigned to blood purification versus controls. Statistical heterogeneity among trials included in the meta-analysis was assessed and quantified using the I2 Statistic, which estimates the percentage of total variation across studies due to heterogeneity rather than chance (20). Because the random effects model incorporates statistical heterogeneity and provides a more conservative estimate of the pooled effect size compared to the fixed model, we present the results of all analyses according to a random effects model by using the method of DerSimonian and Laird that considers both within study and between-study variation (21).
To further ascertain what factors may have influenced treatment effects, we performed a variety of sensitivity analyses to determine the RR of death within particular groups: mean patient age ≥ 60 years vs. age < 60 years; APACHE score ≥ 28 vs. < 28; sepsis, severe sepsis vs. septic shock; publication year ≥ 2005 vs. < 2005; Jadad score ≥ 3 vs. < 3. We assessed publication bias by evaluating the funnel plots (i.e., plots of study results against precision) and with Egger’s statistic (22). Egger statistical analyses were performed using Stata version 10.0 (StataCorp, College Station,TX). Two-tailed p < 0.05 was considered statistically significant. All other statistical analyses were performed by using Review Manager, version 5.1.2 (RevMan, The Cochrane Collaboration, Oxford, United Kingdom).
RESULTS
Selection and Characteristics of Trials
Our initial search yielded 1717 studies (Fig. 1). After excluding 128 studies due to duplicate publication, we considered the abstracts of 1589 studies. After evaluating the abstract of each study, 1553 studies were excluded as they did not meet the inclusion criteria. Subsequently, we carefully read the full-text of each of the remaining 36 trials and excluded 20 trials: as they did not report comparison between blood purification and conventional treatment (n=15); enrolled patients without a diagnosis of sepsis (n=3) or did not report mortality (n=2).
Figure 1.
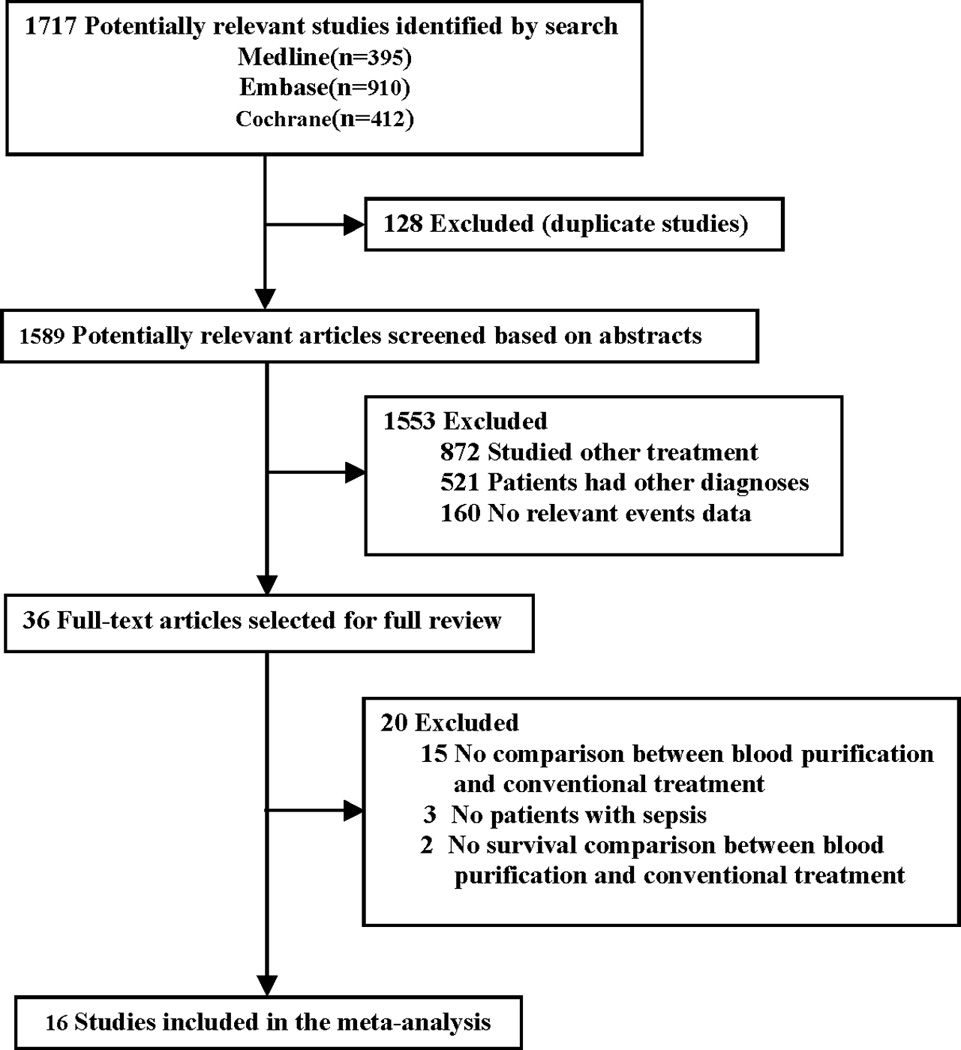
Quorum Chart of Study Cohort.
Table 1 shows the characteristics of randomized trials. Ten single-center (9,17,23, 26,29–34) and six multicenter studies (24,25,27,28,35,36) were identified. These trials were reported between 1999 and 2010. The Country of origin in six studies is Japan (29,30,31,33,34,36), all of which reported on hemoperfusion (Table 1, Table 2). The mean age of the study participants ranged from 33 to 75 years; 637 (77%) patients were admitted to the ICU (17,24–29,31–33,35); and the mean Acute Physiology and Chronic Health Evaluation (APACHE) II score was 24.2 (9,17,23,24,27–36). Patients with sepsis, severe sepsis or septic shock were diagnosed mainly according to the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference criteria (37).
Table 1.
Baseline characteristics of selected trials of blood purification in sepsis
| Source | Country of origin |
No. of Patients |
Mean Age (years) |
Male (%) |
Center | Mean APACHE II/III/ SAPS II/ SOFA score |
Diagnosis | Jadad Score* |
|---|---|---|---|---|---|---|---|---|
| Huang, 2010(23) | China | 44 | 74.9 | 45.5 | S | APACHE II: 28.8 SOFA: 7.6 | Severe sepsis or septic shock† | 2 |
| Peng, 2010(9) | China | 22 | 53.4 | 59.1 | S | APACHE II:18.6 | Severe sepsis† | 2 |
| Cruz, 2009(24) | Italy | 64 | 64 | 65.5 | M | APACHE II: 20.5 SOFA: 10 | Severe sepsis or septic shock† | 5 |
| Payen, 2009(25) | France | 76 | 58.1 | 74.4 | M | SAPS II: 53.4 SOFA:11 | Severe sepsis or septic shock† | 2 |
| Peng, 2005(26) | China | 20 | 33.2 | 95 | S | N/A | Sepsis† | 1 |
| Vincent, 2005(27) | Belgium | 35 | 57.5 | 63 | M | APACHE II: 17.7 SOFA:10.1 | Severe sepsis or septic shock† | 4 |
| Reinhart, 2004(28) | Germany | 143 | 61.2 | 62.2 | M | APACHE II:28 SOFA:11.8 | Severe sepsis or septic shock† | 4 |
| Nakamura, 2004(29) | Japan | 25 | 60 | 75 | S | APACHE II:28.2 | Severe sepsis† | 3 |
| Nakamura, 2003(30) | Japan | 20 | 63.7 | 60 | S | APACHE II:27.3 | Sepsis† | 2 |
| Nakamura, 2003(31) | Japan | 60 | 55.5 | 66.7 | S | APACHE II:23.5 | Sepsis† | 4 |
| Busund, 2002(32) | Norway | 106 | 44 | 56.6 | S | APACHE III: 54.9 | Severe sepsis or septic shock† | 2 |
| Nakamura, 2002(33) | Japan | 18 | 40 | 66.7 | S | APACHE II: 28 | Sepsis† | 3 |
| Cole, 2002(17) | Australia | 24 | 66.8 | 58.3 | S | APACHE II: 22 SAPS II: 45 | Septic shock or septic organ dysfunction† | 5 |
| Nemoto, 2001(34) | Japan | 98 | 62 | 61.2 | S | APACHE II: 22.5 | Sepsis, severe sepsis or septic shock† | 2 |
| Reeves, 1999(35) | Australia | 22 | 59.4 | 63.6 | M | APACHE II: 25.2 | Sepsis†† | 2 |
| Nakamura, 1999(36) | Japan | 50 | 53.8 | 60 | M | APACHE II: 24.8 | Septic shock†† | 1 |
APACHE, acute physiology and chronic health evaluation; SAPS, simplified acute physiology score; SOFA, sequential organ failure assessment; S, single center trial; M, multicenter trial; N/A, not applicable.
Jadad score was calculated using investigator-blinding in place of double-blind design.
Patients were diagnosed according to the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference criteria;
Patients were diagnosed according to the other criteria.
Table 2.
Blood purification modality and outcome in selected trials
| Source | Blood purification | Outcome measures | |||||
|---|---|---|---|---|---|---|---|
| Modality | Intensity | Hemofilter | Mediators cleared |
Improved hemodynamics |
Improved APACHE II /SAPS II /SOFA score |
Improved survival |
|
| Huang, 2010(23) | HP | 2h per treatment (blood flow rate, 100–200ml/min) | HA330 resin cartridge | IL-6,8 | Yes:CI, MAP, SVRI | Yes:SOFA | No:Hospital/28-d survival Yes:ICU survival |
| Peng, 2010(9) | PHVHF | 85ml/kg/h for 6h followed by 35ml/kg/h for 18h, at least 72h | AN69 filter | TNF, IL-1,4,6,10 | Yes:SBP, DBP, MAP | Yes:APACHE II/SAPS II /SOFA | No:28-d survival |
| Cruz, 2009(24) | HP | 2h first, and then the second HP for 24h | PMX-B | N/A | Yes:MAP | Yes:SOFA | Yes:Hospital/28-d survival |
| Payen, 2009(25) | CVVH | 2000ml/h for at least 96h | HPM | No:IL-6,IL-1α | N/A | Yes:SOFA | No:28-d survival |
| Peng, 2005(26) | CVVHDF | 1500 –1900 ml /h | AN69 filter | IL-1β,6,8, TNF | N/A | N/A | No: No detail survival days reported |
| Vincent, 2005(27) | HP | 2h per time (blood flow rate,100–200ml/min) | PMX-B | No:Endotoxin,IL-6 | Yes:CI, LVSW | No:APACHE II /SOFA | No:28-d survival |
| Reinhart, 2004(28) | HP | First 4days | Endotoxin adsorber | No:IL-6, TNF-α | N/A | No:APACHE II | No:28-d survival |
| Nakamura, 2004(29) | HP | Twice within a 24h interval, for 2h at a flow rate of 80 to 100 ml/min | PMX-B | Endotoxin | N/A | N/A | Yes:No detail survival days reported |
| Nakamura, 2003(30) | HP | Twice within a 24h interval, for 2h at a flow rate of 80 to 100 ml/min | PMX-B | Endotoxin | N/A | N/A | Yes:Hospital survival |
| Nakamura, 2003(31) | HP | Twice within a 24h interval, for 2h at a flow rate of 80 to 100 ml/min | PMX-B | Endotoxin | N/A | N/A | N/A:Hospital survival |
| Busund, 2002(32) | Plasma-pheresis | Two treatments: 1820±402 ml first and then 1763±312ml | PF-0.5 | N/A | N/A | Yes:APACHE III | No:28-d survival |
| Nakamura, 2002(33) | HP | Twice within a 24h interval, a flow rate of 100 ml/min | PMX-B | Endotoxin | N/A | No:APACHE II | Yes:No detail survival days reported |
| Cole, 2002(17) | CVVH | 2 L/h for 48h | AN69 filter | No:TNF-α, IL-6, IL-8, IL-10 | N/A | N/A | No:Hospital survival |
| Nemoto, 2001(34) | HP | 4h at a flow rate of 80–100ml/min for once or twice | PMX-B | Endotoxin | Yes:MAP | N/A | Yes:28-d survival |
| Reeves, 1999(35) | Plasma-filtration | Twice during the first 4–6h and then a lower rate of exchange for another 28–30h | PF1000 | No:IL-6 | N/A | N/A | No:14-d survival |
| Nakamura, 1999(36) | HP | Twice within a 24h interval, for 2h at a flow rate of 80–100 ml/min | PMX-B | Endotoxin | Yes:SBP | Yes:APACHE II | Yes:Hospital survival |
APACHE, acute physiology and chronic health evaluation; SAPS, simplified acute physiology score; SOFA, sequential organ failure assessment; HP, hemoperfusion; PHVHF, pulse high-volume hemofiltration; HVHF, high volume hemofiltration; CVVH, continuous venovenous hemofiltration; CVVHDF, continuous venovenous hemodiafiltration; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; CI, cardiac index; LVHF, low volume hemofiltration; LVSW, left ventricular stroke work index; IL, interleukin; PMX-B, polymyxin B immobilized fiber ; HPM, Heparin-coated polysulfone membrane; PF, plasma filter; N/A, not applicable.
Ten trials reported patients with either severe sepsis or septic shock, while five trials reported only patients with a diagnosis of sepsis. One trial included patients with sepsis, severe sepsis or septic shock (Table 1). The blood purification techniques used included hemoperfusion (10 trials), hemofiltration (4 trials), and plasma exchange (2 trials) (Table 2). Six trials included in our analysis reported the results of 28-day mortality and 4 trials reported results of hospital mortality. Two trials reported 28-day, hospital, and/or ICU mortality, and one trial reported 14-day mortality. There still had three trials in which mortality was reported but length of follow-up was not clearly stated (Table 2). All studies evaluated the effects between blood purification and conventional treatment in patients with sepsis using some primary clinical outcome such as survival, hemodynamics, or change in organ function (APACHE II /III score /SAPS II score/SOFA score) (Table 2).
Association of Blood Purification with Mortality
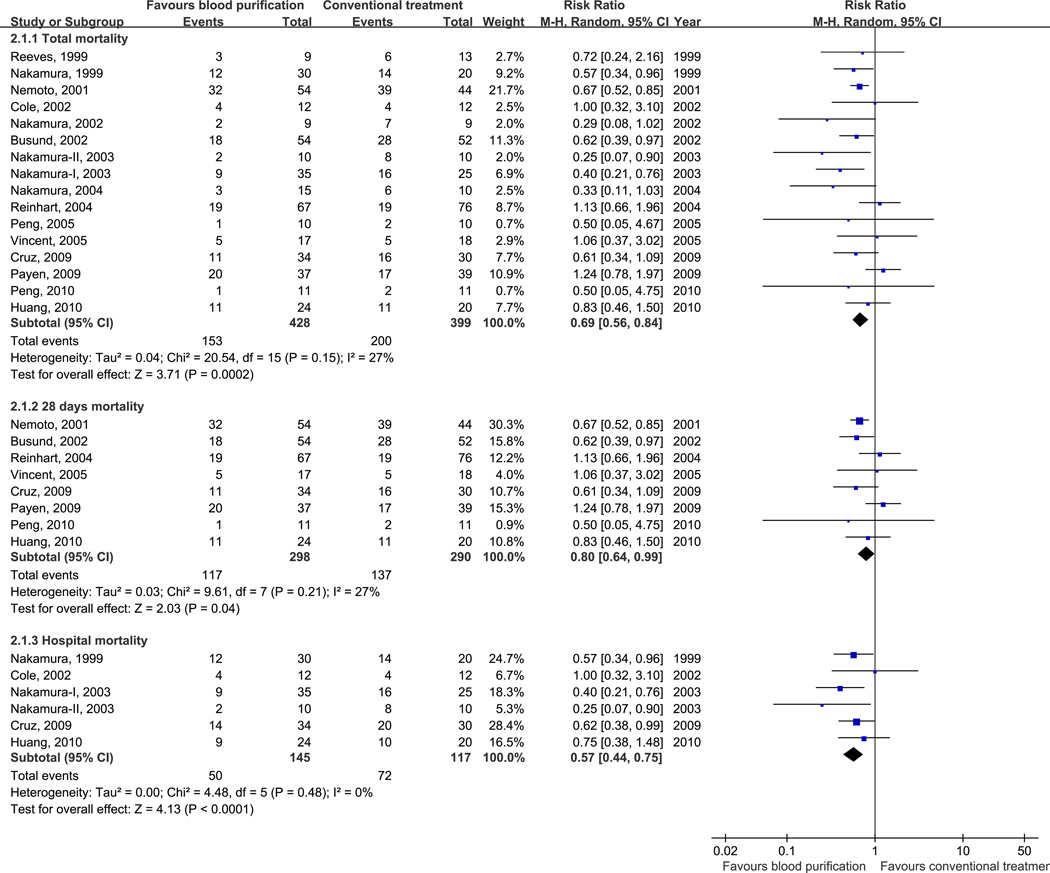
Overall mortality in 16 trials was 42.7%. Of the blood purification group, 35.7% of patients died compared to 50.1% in the conventional treatment group. Overall, blood purification techniques decreased mortality in patients with sepsis, severe sepsis or septic shock (RR, 0.69; 95% CI, 0.56–0.84; p < 0.001), including 28-day mortality (RR, 0.80; 95% CI, 0.64–0.99; p = 0.04) and hospital mortality (RR, 0.57; 95% CI, 0.44–0.75; p < 0.001) (Fig. 2). No significant heterogeneity was found (Chi2 = 20.54, df = 15, p = 0.15; I2 = 27%) (Fig. 2).
Figure 2.
Risk Ratios (RRs) for Blood Purification versus Conventional Treatment.
Pooled risk ratios are from a random effects model; CI indicates confidence interval; Size of the data markers indicates weight of the study.
Association of Blood Purification Modality with Mortality
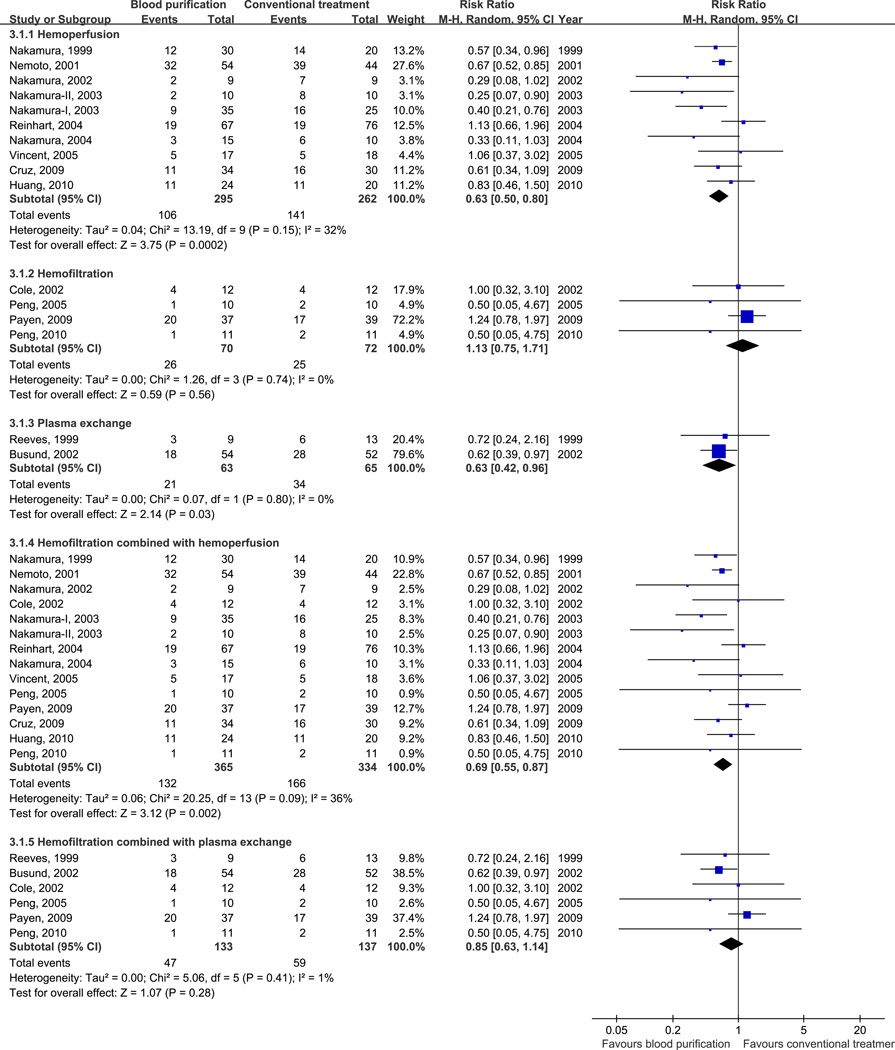
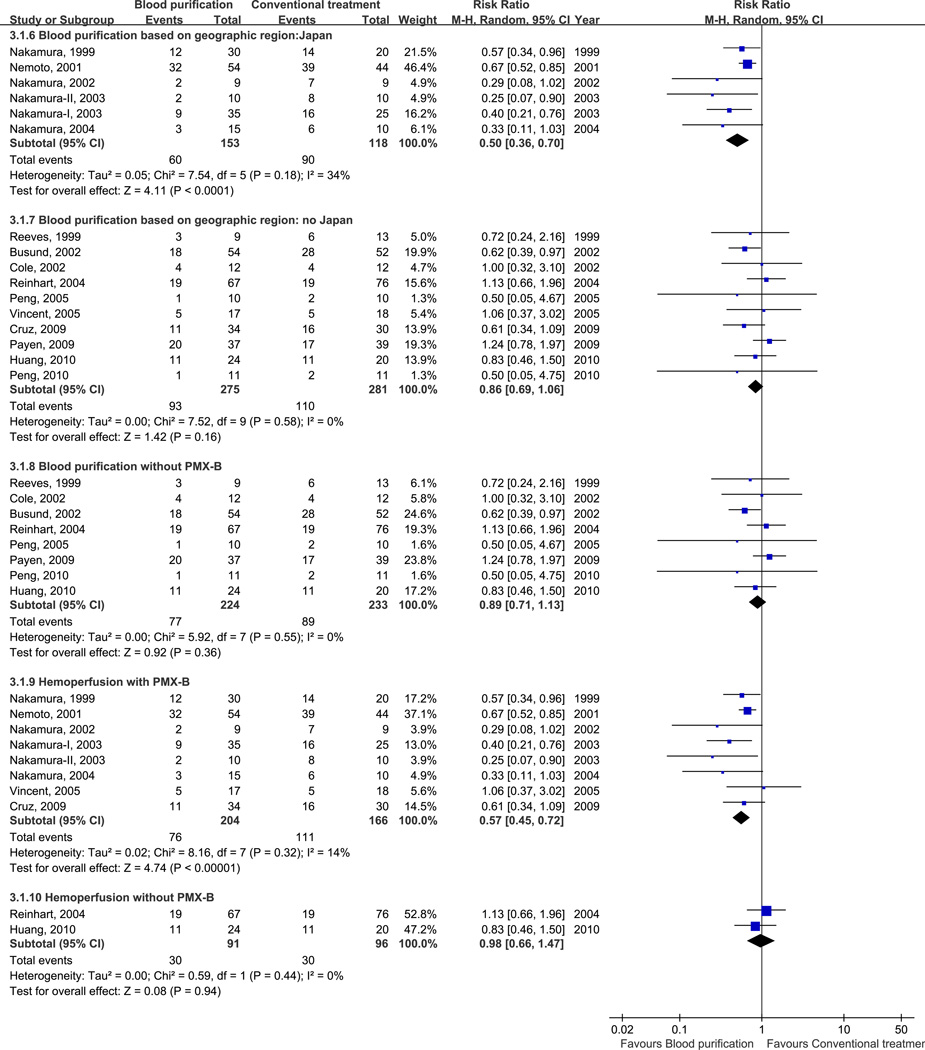
We found that hemoperfusion (RR, 0.63; 95% CI, 0.50–0.80; p < 0.001; 10 trials, n=557; heterogeneity, p = 0.15) or plasma exchange (RR, 0.63; 95% CI, 0.42–0.96; p = 0.03; 2 trials, n=128; heterogeneity, p = 0.80) decreased mortality in patients with sepsis. However, we could not find a similar effect with hemofiltration alone (RR, 1.13; 95% CI, 0.75–1.71; p = 0.56; 4 trials; n = 142; heterogeneity, p = 0.74) (Fig. 3A). We also found that hemoperfusion with polymyxin B (PMX-B) decreased mortality in patients with sepsis (RR, 0.57; 95% CI, 0.45–0.72; p < 0.001; 8 trials, n=370; heterogeneity, p = 0.32) while hemoperfusion without PMX-B (RR, 0.98; 95% CI, 0.66–1.47 ; p = 0.94; 2 trials, n=187; heterogeneity, p = 0.44), or pooling all blood purification studies without PMX-B (RR, 0.89; 95% CI, 0.71–1.13 ; p = 0.36; 8 trials, n=457; heterogeneity, p = 0.55) did not (Fig. 3B). When combined with hemoperfusion, hemofiltration was associated with greater benefit (RR, 0.69; 95% CI, 0.55–0.87; p = 0.002; 14 trials; n = 699; heterogeneity, p = 0.09) than hemofiltration alone. On the other hand, hemofiltration combined with plasma exchange did not affect the mortality (RR, 0.85; 95% CI, 0.63–1.14; p = 0.28; 6 trials; n = 270; heterogeneity, p = 0.41) (Fig. 3A). Studies conducted in Japan showed that blood purification decreased mortality in patients with sepsis (RR, 0.50; 95% CI, 0.36–0.70; p < 0.001; 6 trials; n = 271; heterogeneity, p = 0.18) while pool results from studies conducted in other countries were not significant (RR, 0.86; 95% CI, 0.69–1.06; p = 0.16; 10 trials; n = 556; heterogeneity, p = 0.58) (Fig. 3B).
Figure 3.
Risk Ratios (RRs) for Different Modality of Blood Purification versus Conventional Treatment.
- Different modalities of blood purification versus conventional treatment.
- Different geographic region and hemoperfusion analysis of blood purification versus conventional treatment.
Sensitivity Analyses of Association between Blood Purification and Mortality
We conducted sensitivity analyses by stratifying our analysis by various subgroups known to influence outcome from sepsis (Table 3). We found no significant differences in effect when trials were stratified by mean age (≥ 60 versus < 60 years) and mean APACHE II score (≥ 28 versus < 28) at enrollment. However, blood purification appeared to have a greater effect on mortality in trials enrolling patients with sepsis (RR, 0.40; 95% CI, 0.26–0.64; p < 0.001; 5 trials, n=140) compared to those enrolling patients with severe sepsis or septic shock (RR, 0.79; 95% CI, 0.62–1.00; p = 0.05; 10 trials, n=589), and the p value for interaction between these two groups was 0.01 (Table 3). Similar results could be seen in effect when trials were stratified by publication year (p = 0.04) (Table 3). Study quality (Jadad score ≥ 3 or < 3) did not affect the results (p = 0.64). We also conducted sensitivity analyses restricted to hemoperfusion studies by stratifying mean age (≥ 60 versus < 60 years), mean APACHE II score (≥ 28 versus < 28) at enrollment, or publication year (before 2005 versus 2005 and later). The results were consistent with the findings with all "purification techniques" except for publication year (p = 0.28) (Table 3).
Table 3.
Sensitivity analyses of association between blood purification on mortality
| No. of Studies |
No. of Patients (Death/Total) |
RR (95% CI) |
Heterogeneity I2(p value) |
Test for effect (p value†) |
p value for interaction between subgroups† |
||
|---|---|---|---|---|---|---|---|
| Blood purification |
Conventional treatment |
||||||
| APACHE II score, mean | Total | Conventional treatment | |||||
| ≥28 | 4 | 35/115 | 43/115 | 0.67(0.38–1.21) | 53% (.09) | .19 | .81 |
| < 28 | 9 | 79/212 | 110/183 | 0.62(0.52–0.75) | 0% (.63) | < .001 | |
| Hemoperfusion only | Conventional treatment | ||||||
| ≥28 | 4 | 35/115 | 43/115 | 0.67(0.38–1.21) | 53% (.09) | .19 | .73 |
| < 28 | 6 | 71/180 | 98/147 | 0.60(0.49–0.75) | 8% (.36) | < .001 | |
| The severity of sepsis †† | Total | Conventional treatment | |||||
| Sepsis | 5 | 17/73 | 39/67 | 0.40(0.26–0.64) | 0% (.74) | < .001 | .01 |
| Severe sepsis or septic shock | 10 | 104/301 | 122/288 | 0.79(0.62–1.00) | 22% (.24) | .05 | |
| Hemoperfusion only | Conventional treatment | ||||||
| Sepsis | 3 | 7/54 | 31/44 | 0.20(0.10–0.41) | 0% (.63) | < .001 | .001 |
| Severe sepsis or septic shock | 6 | 61/187 | 71/174 | 0.74(0.54–0.99) | 21% (.28) | .05 | |
| Publication year | Total | Conventional treatment | |||||
| < 2005 | 10 | 104/295 | 147/271 | 0.62(0.49–0.78) | 26% (.20) | < .001 | .04 |
| ≥ 2005 | 6 | 49/133 | 53/128 | 0.90(0.67–1.21) | 0% (.51) | .50 | |
| Hemoperfusion only | Conventional treatment | ||||||
| < 2005 | 7 | 79/220 | 109/194 | 0.57(0.40–0.79) | 48% (.07) | .0009 | .28 |
| ≥ 2005 | 3 | 27/75 | 32/68 | 0.75(0.51–1.11) | 0% (.60) | .15 | |
| Age, mean, yrs | Total | Conventional treatment | |||||
| ≥ 60 | 7 | 82/216 | 103/202 | 0.70(0.54–0.92) | 27% (.22) | .01 | .76 |
| < 60 | 9 | 71/212 | 97/197 | 0.66(0.48–0.91) | 35% (.14) | .01 | |
| Jadad score, mean | Total | Conventional treatment | |||||
| ≥3 | 7 | 53/189 | 73/180 | 0.64(0.42–0.96) | 43% (.10) | .03 | .64 |
| <3 | 9 | 100/239 | 127/219 | 0.71(0.57–0.88) | 19% (.28) | .0002 | |
CI, confidence interval; RR, risk ratio;
Based on the X2 test;
Based on the report of the studies included.
Adverse Effects
There were few clinically important adverse effects related to blood purification. Two trials reported immediate adverse events, which were considered to be possibly device related (fever) during hemoperfusion treatment (23,27). Cruz et al (24) reported some adverse reactions, including cartridge clotting (4 cases, 6%), hypotension (1 case, 1.5%) and tachycardia (2 cases, 3%). Busund et al (32) reported that six patients had episodes of hypotension during the plasmapheresis procedure, and one patient had a reaction to fresh-frozen plasma.
Quality of Studies and Publication Bias
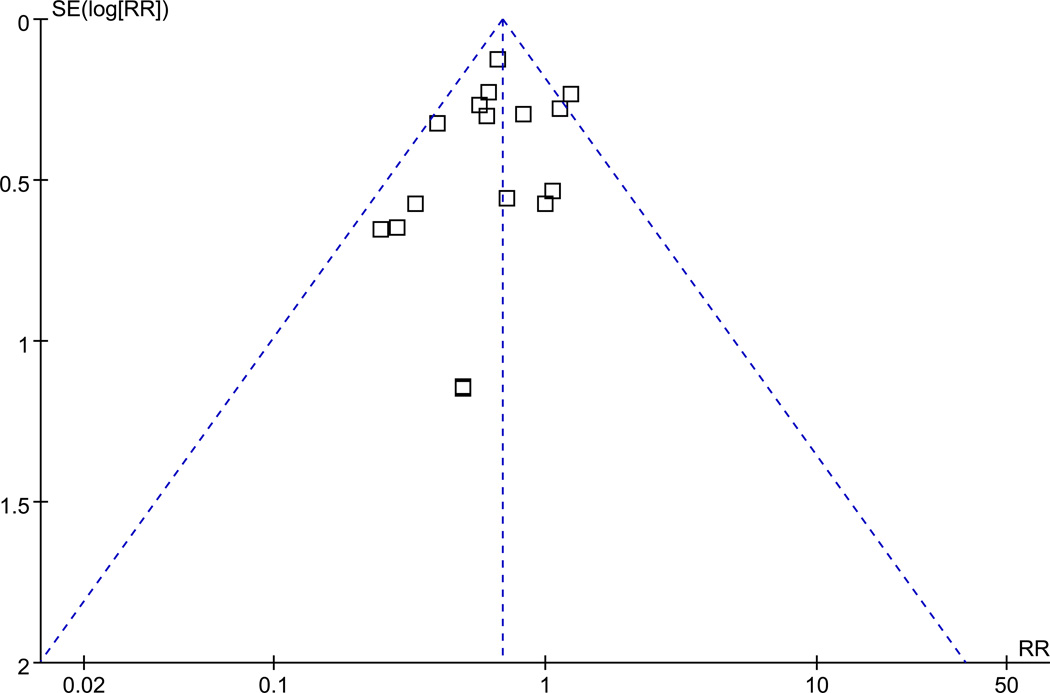
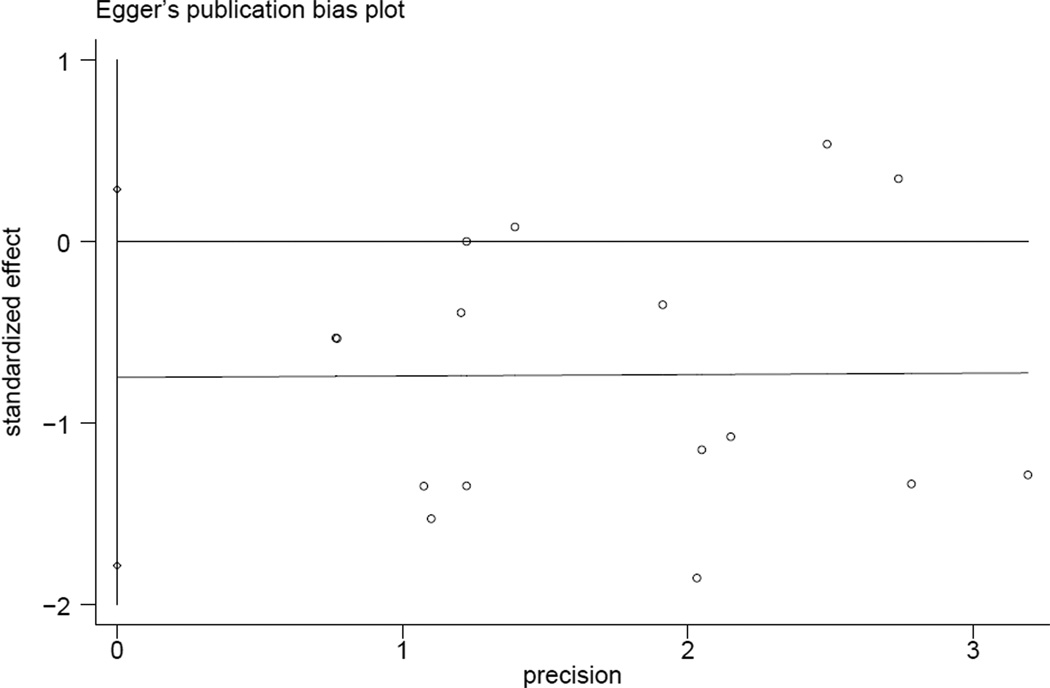
All trials included in the meta-analysis were randomized and have been published in full manuscript form. The mean Jadad score was 2.75 for studies included in our analysis (using investigator-blinding for double-blinding). Seven trials had a Jadad score ≥ 3, while 9 trials received a score of 2 or less (Table 1). No evidence of publication bias was detected for RR of death by either funnel plots or Egger test (p = 0.14) (Fig. 4A, 4B).
Figure 4.
- A funnel plot.
- Egger’s test
DISCUSSION
We found evidence that blood purification using hemoperfusion, plasma exchange, and hemofiltration combined with hemoperfusion was significantly associated with a decrease in mortality among patients with sepsis, severe sepsis or septic shock. Thus, further development of blood purification strategies for management of sepsis would seem warranted.
Early clinical and experimental studies in blood purification for sepsis focused on methods used for treatment of renal failure, especially continuous veno-venous hemofiltration (CVVH) (15,38,39). Often these trials used standard “renal dose” intensities although more recently, so-called high-volume hemofiltration has been advocated (9–11,15–16). Meanwhile, large multi-centered clinical trials have revealed that increasing intensity of renal replacement therapy beyond conventionally recommended doses does not improve patient survival (40,41). Subgroup analysis in these trials also does not support an advantage for higher intensity in patients with renal failure and sepsis. This may be because conventional renal replacement therapy is not able to affect changes in soluble inflammatory mediators (17,25) and thus alternative techniques are needed if blood purification is to result in improved survival for patients with sepsis.
Importantly however, the exact targets for blood purification in sepsis are unknown. We recently demonstrated in rodents that acute changes in the usual sepsis mediators were not necessary to impact survival using hemoperfusion (42). Indeed, it is increasingly recognized that death from sepsis (or perhaps critical illness in general) may be more a function of immune suppression than of cytotoxic inflammation (43). Therefore, the targets of immune modulation may be immune suppressive factors, immune effector cells, or perhaps, chemokine gradients.
Alternatives to standard hemofiltration such as high-adsorption CVVH appear more effective for reducing plasma cytokine concentrations in patients with septic shock as well as for impacting physiologic outcomes such as decreasing norepinephrine requirements (12). However, other modalities such as hemoperfusion and plasma exchange are now being examined more closely. For example, hemoperfusion with a Polymyxin B fiber column appears to improve survival compared with conventional treatment (24,29,30,33,34,36). Trials included in this meta-analysis varied in terms of blood purification modality, and reflected the diversity of clinical practice informing trial methodology. Interestingly, our results were reasonably consistent across various forms of blood purification without significant heterogeneity. Likewise, the risk of publication bias was low, though not impossible given limitations of the Eggers statistic.
A surprising finding of our analysis shown in table 3 was the fact that the impact of blood purification on survival was not attenuated in subgroups with lower risk of death (age < 60, APACHE II score < 28, non-severe sepsis). This finding may be of particular importance because many sepsis trials have focused on patients with severe disease (9,17,23–25,27–29,32,36). One consequence of this approach is that patients tend to be enrolled late in the course of sepsis, perhaps when therapies are less likely to be effective. Concern over this strategy is further heightened when one appreciates that preclinical models are often based on early treatment or even pretreatment in animals (39,42). Future trials of blood purification may need to consider this aspect more carefully.
Similarly, older patients have an increased risk of death and shorter survival time in studies of sepsis (44). However, we could not demonstrate any difference in the effect of blood purification in patients < 60 years of age compared to older patients. In a cohort study, Brar et al reported that individuals with acute renal failure over 50 who were treated with continuous renal replacement therapy had a lower mortality (22%) than their younger counterparts (50%) (45).
Some investigators have sought to examine combination therapy using different blood purification techniques in patients with sepsis or septic shock (14,46). For example, Yonekawa et al (47) reported that patients with severe sepsis responded to treatment combining continuous endotoxin apheresis and hemodiafiltration. Too few trials are available to examine this approach. However, given the inherent differences in the various blood purification techniques on specific variables of interest in sepsis (e.g. endotoxin, cytokines, cells), combined therapy does seem appealing.
We found no evidence that study quality of the trials included affected our results. Although there were significant differences in effect when trials were stratified by publication year (p = 0.04), we did not find evidence for this effect when the analysis was restricted to hemoperfusion (p = 0.28). However, there are still important limitations to this report. First, and foremost, studies were small (most less than 80 subjects and none greater than 150) and overall quality was modest (mean Jadad score 2.75). The risk of false attribution of positive effect from pooling small trials is well known (48). Thus, we do not believe that these results constitute a reason to change clinical practice but rather support the need for further research, particularly given the dismal state of affairs in the area of sepsis therapeutics (3). However, we also note significant regional differences in the management of sepsis and the reality that blood purification is commonly used in some and unknown in other places around the world (49). Second, there was no standard reporting for survival and different authors chose different endpoints. Therefore it was not possible to use a single mortality endpoint (hospital, 28 day etc.) across trials. Patient-level data were not available for the majority of trials so we did not attempt to perform a patient-level analysis. Third, due to the nature of the intervention and for logistic reasons, studies were not double-blinded. Although we used “investigator blinding” for assessment of quality of studies included in this meta-analysis (18), there is still potential for bias. Similarly, underreporting of the adverse effects associated with blood purification is possible, especially since there are no standards for adverse effect reporting, and none of the studies included in the meta-analysis had a systematic approach to safety data collection and reporting.
Finally, we acknowledge that sepsis is a complex disease and blood purification is a complex intervention. The effectiveness of blood purification might be influenced by the unique constellation of treatments that are used for and epidemiology of sepsis at individual centers and may not be generalizable. For example, blood purification has the potential to impact plasma drug concentrations including antibiotics (50). It is possible therefore that blood purification might have different effects when used in conjunction with antibiotics that depend on time-dependent kinetics compared to peak concentration-dependent kinetics (50). Since selection of antibiotics is at least partially influenced by treating center, it is reasonable to hypothesize variable effects of blood purification across centers, all other factors aside. Similarly, our results suggest that the main drivers for the beneficial effects of blood purification in this analysis come from studies of hemoperfusion with PMX-B, and were performed in a single country (29,30,31,33,34,36). Although the overall effects of blood purification without PMX-B were consistent with PMX-B studies (p = 0.15; I2 = 27%) the effect size is considerably smaller (RR 0.89 vs. 0.57) and fails to reach statistical significance. Thus, much additional work is needed. However, our results suggest a likely role for this form a treatment in a disease that has, so far, eluded effective therapy.
CONCLUSION
In conclusion, pooled results of multiple small studies of moderate study quality show that blood purification (including hemoperfusion or plasma exchange alone, hemofiltration combined with hemoperfusion) is associated with lower mortality in patients with sepsis. These results were mainly influenced by studies using hemoperfusion with PMX-B.
Acknowledgments
Funding: This study was partly supported by a grant award (R01HL080926) from the National Institute of Health’s (NIH) National Heart Lung and Blood Institute (NHLBI) and a career development award (2KL2RR024154-06) from the National Center for Research Resources (NCRR). The content is solely the responsibility of the authors and does not necessarily represent the official views of NHLBI, NCRR, or the NIH.
JK has received grant support and consulting fees from Baxter, CytoSorbents, Gambro and Spectral Diagnostics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests: No other authors report any competing interests.
Contributor Information
Feihu Zhou, Email: fryhoo@yahoo.com.cn.
Zhiyong Peng, Email: pengz@upmc.edu.
Raghavan Murugan, Email: muruganr@ccm.upmc.edu.
John A. Kellum, Email: kellumja@ccm.upmc.edu.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;48:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Xigris [drotrecogin alfa (activated)]: Market Withdrawal -Failure to Show Survival Benefit. [Accessed January 15, 2013]; http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm277143.htm.
- 4.Dellinger RP, Levy MM, Carlet JM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;34:17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joannidis M. Continuous renal replacement therapy in sepsis and multisystem organ failure. Semin Dial. 2009;22:160–164. doi: 10.1111/j.1525-139X.2008.00552.x. [DOI] [PubMed] [Google Scholar]
- 6.Graziani G, Bordone G, Bellato V, et al. Role of the kidney in plasma cytokine removal in sepsis syndrome: a pilot study. J Nephrol. 2006;19:176–182. [PubMed] [Google Scholar]
- 7.Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gogos CA, Drosou E, Bassaris HP, et al. Pro-versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis. 2000;181:176–180. doi: 10.1086/315214. [DOI] [PubMed] [Google Scholar]
- 9.Peng Z, Pai P, Han-Min W, et al. Evaluation of the effects of pulse high-volume hemofiltration in patients with severe sepsis: a preliminary study. Int J Artif Organs. 2010;33:505–511. doi: 10.1177/039139881003300801. [DOI] [PubMed] [Google Scholar]
- 10.Honore PM, Joannes-Boyau O, Boer W, Collin V. High-volume hemofiltration in sepsis and SIRS: current concepts and future prospects. Blood Purif. 2009;28:1–11. doi: 10.1159/000210031. [DOI] [PubMed] [Google Scholar]
- 11.Boussekey N, Chiche A, Faure K, et al. A pilot randomized study comparing high and low volume hemofiltration on vasopressor use in septic shock. Intensive Care Med. 2008;34:1646–1653. doi: 10.1007/s00134-008-1127-3. [DOI] [PubMed] [Google Scholar]
- 12.Haase M, Silvester W, Uchino S, et al. A pilot study of high-adsorption hemofiltration in human septic shock. Int J Artif Organs. 2007;30:108–117. doi: 10.1177/039139880703000205. [DOI] [PubMed] [Google Scholar]
- 13.Morgera S, Haase M, Kuss T, et al. Pilot study on the effects of high cut off hemofiltration on the need for norepinephrine in septic patients with acute renal failure. Crit Care Med. 2006;34:2099–2104. doi: 10.1097/01.CCM.0000229147.50592.F9. [DOI] [PubMed] [Google Scholar]
- 14.Ronco C, Brendolan A, Lonnemann G, et al. A pilot study of coupled plasma filtration with adsorption in septic shock. Crit Care Med. 2002;30:1250–1255. doi: 10.1097/00003246-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Honore PM, Jamez J, Wauthier M, et al. Prospective evaluation of short-term, high-volume isovolemic hemofiltration on the hemodynamic course and outcome in patients with intractable circulatory failure resulting from septic shock. Crit Care Med. 2000;28:3581–3587. doi: 10.1097/00003246-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Ghani RA, Zainudin S, Ctkong N, et al. Serum IL-6 and IL-1-ra with sequential organ failure assessment scores in septic patients receiving high-volume haemofiltration and continuous venovenous haemofiltration. Nephrology (Carlton) 2006;11:386–393. doi: 10.1111/j.1440-1797.2006.00600.x. [DOI] [PubMed] [Google Scholar]
- 17.Cole L, Bellomo R, Hart G, et al. A phase II randomized, controlled trial of continuous hemofiltration in sepsis. Crit Care Med. 2002;30:100–106. doi: 10.1097/00003246-200201000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Z, Wang SR, Su W, et al. Removal of humoral mediators and the effect on the survival of septic patients by hemoperfusion with neutral microporous resin column. Ther Apher Dial. 2010;14:596–602. doi: 10.1111/j.1744-9987.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 24.Cruz DN, Antonelli M, Fumagalli R, et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009;301:2445–2452. doi: 10.1001/jama.2009.856. [DOI] [PubMed] [Google Scholar]
- 25.Payen D, Mateo J, Cavaillon JM, et al. Impact of continuous venovenous hemofiltration on organ failure during the early phase of severe sepsis: a randomized controlled trial . Crit Care Med. 2009;37:803–810. doi: 10.1097/CCM.0b013e3181962316. [DOI] [PubMed] [Google Scholar]
- 26.Peng Y, Yuan Z, Li H. Removal of inflammatory cytokines and endotoxin by veno-venous continuous renal replacement therapy for burned patients with sepsis. Burns. 2005;31:623–628. doi: 10.1016/j.burns.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Vincent JL, Laterre PF, Cohen J, et al. A pilot-controlled study of a polymyxin B-immobilized hemoperfusion cartridge in patients with severe sepsis secondary to intra-abdominal infection. Shock. 2005;23:400–405. doi: 10.1097/01.shk.0000159930.87737.8a. [DOI] [PubMed] [Google Scholar]
- 28.Reinhart K, Meier-Hellmann A, Beale R, et al. Open randomized phase II trial of an extracorporeal endotoxin adsorber in suspected Gram-negative sepsis. Crit Care Med. 2004;32:1662–1668. doi: 10.1097/01.ccm.0000132902.54925.b5. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura T, Kawagoe Y, Matsuda T, et al. Effect of polymyxin B-immobilized fiber on bone resorption in patients with sepsis. Intensive Care Med. 2004;30:1838–1841. doi: 10.1007/s00134-004-2357-7. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura T, Ushiyama C, Suzuki Y, et al. Hemoperfusion with polymyxin B-immobilized fiber in septic patients with methicillin-resistant Staphylococcus aureus-associated glomerulonephritis. Nephron Clin Pract. 2003;94:33–39. doi: 10.1159/000071279. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura T, Ushiyama C, Suzuki Y, et al. Combination therapy with polymyxin B-immobilized fibre haemoperfusion and teicoplanin for sepsis due to methicillin-resistant Staphylococcus aureus. J Hosp Infect. 2003;53:58–63. doi: 10.1053/jhin.2002.1332. [DOI] [PubMed] [Google Scholar]
- 32.Busund R, Koukline V, Utrobin U, et al. Plasmapheresis in severe sepsis and septic shock: a prospective, randomised, controlled trial. Intensive Care Med. 2002;28:1434–1439. doi: 10.1007/s00134-002-1410-7. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura T, Ushiyama C, Suzuki Y, et al. Hemoperfusion with polymyxin B immobilized fibers for urinary albumin excretion in septic patients with trauma. ASAIO J. 2002;48:244–248. doi: 10.1097/00002480-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Nemoto H, Nakamoto H, Okada H, et al. Newly developed immobilized polymyxin B fibers improve the survival of patients with sepsis. Blood Purif. 2001;19:361–369. doi: 10.1159/000046966. [DOI] [PubMed] [Google Scholar]
- 35.Reeves JH, Butt WW, Shann F, et al. Continuous plasmafiltration in sepsis syndrome. Plasmafiltration in Sepsis Study Group. Crit Care Med. 1999;27:2096–2104. doi: 10.1097/00003246-199910000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura T, Ebihara I, Shoji H, et al. Treatment with polymyxin B-immobilized fiber reduces platelet activation in septic shock patients: decrease in plasma levels of soluble P-selectin, platelet factor 4 and beta-thromboglobulin. Inflamm Res. 1999;48:171–175. doi: 10.1007/s000110050442. [DOI] [PubMed] [Google Scholar]
- 37.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 38.Cornejo R, Downey P, Castro R, et al. High-volume hemofiltration as salvage therapy in severe hyperdynamic septic shock. Intensive Care Med. 2006;32:713–722. doi: 10.1007/s00134-006-0118-5. [DOI] [PubMed] [Google Scholar]
- 39.Bellomo R, Kellum JA, Gandhi CR, et al. The effect of intensive plasma water exchange by hemofiltration on hemodynamics and soluble mediators in canine endotoxemia. Am J Respir Crit Care Med. 2000;161:1429–1436. doi: 10.1164/ajrccm.161.5.9809127. [DOI] [PubMed] [Google Scholar]
- 40.Jun M, Heerspink HJ, Ninomiya T, et al. Intensities of renal replacement therapy in acute kidney injury: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2010;5:956–963. doi: 10.2215/CJN.09111209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Wert R, Friedrich JO, Scales DC, et al. High-dose renal replacement therapy for acute kidney injury: Systematic review and meta-analysis. Crit Care Med. 2010;38:1360–1369. doi: 10.1097/CCM.0b013e3181d9d912. [DOI] [PubMed] [Google Scholar]
- 42.Peng ZY, Wang HZ, Carter MJ, et al. Acute removal of common sepsis mediators does not explain the effects of extracorporeal blood purification in experimental sepsis. Kidney Int. 2012;81:363–369. doi: 10.1038/ki.2011.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin YF, Ko WJ, Chu TS, et al. The 90-day mortality and the subsequent renal recovery in critically ill surgical patients requiring acute renal replacement therapy. Am J Surg. 2009;198:325–332. doi: 10.1016/j.amjsurg.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 45.Brar H, Olivier J, Lebrun C, et al. Predictors of mortality in a cohort of intensive care unit patients with acute renal failure receiving continuous renal replacement therapy. Am J Med Sci. 2008;335:342–347. doi: 10.1097/MAJ.0b013e3181571f56. [DOI] [PubMed] [Google Scholar]
- 46.Sakamoto Y, Mashiko K, Obata T, et al. Effectiveness of continuous hemodiafiltration using a polymethylmethacrylate membrane hemofilter after polymyxin B-immobilized fiber column therapy of septic shock. ASAIO J. 2008;54:129–132. doi: 10.1097/MAT.0b013e31815d2f01. [DOI] [PubMed] [Google Scholar]
- 47.Yonekawa C, Nakae H, Tajimi K, et al. Combining continuous endotoxin apheresis and continuous hemodiafiltration in the treatment of patients with septic multiple organ dysfunction syndrome. Ther Apher Dial. 2006;10:19–24. doi: 10.1111/j.1744-9987.2006.00341.x. [DOI] [PubMed] [Google Scholar]
- 48.LeLorier J, Grégoire G, Benhaddad A, et al. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med. 1997;337:536–542. doi: 10.1056/NEJM199708213370806. [DOI] [PubMed] [Google Scholar]
- 49.Kellum JA, Uchino S. International differences in the treatment of sepsis: are they justified? JAMA. 2009;301:2496–2497. doi: 10.1001/jama.2009.850. [DOI] [PubMed] [Google Scholar]
- 50.Langgartner J, Vasold A, Glück T, et al. Pharmacokinetics of meropenem during intermittent and continuous intravenous application in patients treated by continuous renal replacement therapy. Intensive Care Med. 2008;34:1091–1096. doi: 10.1007/s00134-008-1034-7. [DOI] [PubMed] [Google Scholar]