Abstract
Despite the large size of the Xenopus laevis egg (~1.2 mm diameter), a fertilized egg rapidly proceeds through mitosis in a spatially-coordinated fashion. Mitosis is initiated by a bistable system of regulatory proteins centered on Cdk11,2, raising the possibility that this spatial coordination could be achieved through trigger waves of Cdk1 activity3. Using an extract system that carries out cell cycles in vitro, we show that mitosis does spread through Xenopus cytoplasm via trigger waves, propagating at a linear speed of ~60 µm/min. Perturbing the feedback loops that give rise to Cdk1’s bistability changes the speed and dynamics of the waves. Time lapse imaging of intact eggs argues that trigger waves of Cdk1 activation are responsible for surface contraction waves, ripples in the cell cortex that precede cytokinesis4,5. These findings indicate that Cdk1 trigger waves help ensure the spatiotemporal coordination of mitosis in large eggs. Trigger waves may be an important general mechanism for coordinating biochemical events over large distances.
During mitosis, a cell undergoes dramatic, abrupt reorganization: the chromosomes condense, the Golgi and endoplasmic reticulum fragment, and the dynamics of the microtubules changes. These processes are driven by the activation of the protein kinase Cdk1. In a typical somatic cell, with a cellular radius on the order of 10 µm, it should be relatively easy to achieve a near-synchronous onset of mitosis in all parts of the cell. If Cdk1 were activated abruptly in the middle of the cell (current evidence suggests that Cdk1 is first activated on the centrosomes6), then it should take no more than a few seconds for the active complexes to spread via random walk diffusion to all parts of the cell (; assuming the diffusion coefficient D = 10 µm2/sec, t ≈ 2 sec). This would be sufficiently fast to account for the observed near-synchronicity of mitotic entry in somatic cells.
However, some eukaryotic cells, notably the well-studied Xenopus laevis egg, are much larger; a Xenopus egg is ~600 µm in radius, which corresponds to a diffusion time on the order of 2 h rather than 2 sec. This suggests that mitosis might be a sluggish process in these cells. But actually the cell cycle is even more rapid in early Xenopus embryos than in somatic cells7. Moreover, mitotic events occur within minutes of each other in distant parts of the cell. For example, nuclear envelope breakdown, which occurs in the interior of the fertilized egg, takes place only a few minutes before the mitotic surface contraction waves (SCWs; discussed in more detail below) sweep over the cortex of the egg8. Thus some mechanism other than simple diffusion of active Cdk1 must coordinate spatially distant mitotic events.
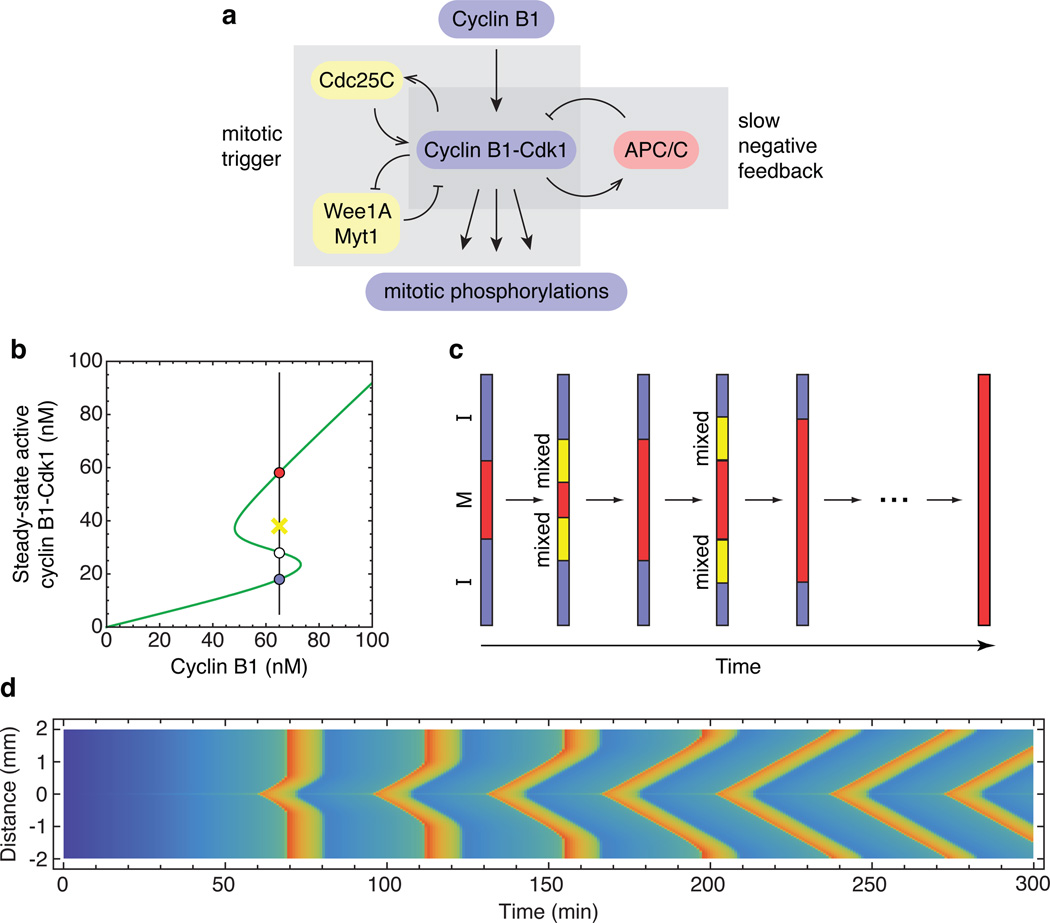
One possible mechanism is suggested by the systems-level organization of the mitotic trigger circuit, which includes interlinked positive and double-negative feedback loops (Fig. 1a). Circuits like this can exhibit bistability, and indeed experimental studies in Xenopus egg extracts have shown that the response of Cdk1 to non-degradable cyclin B1 is hysteretic, with two alternative stable levels of Cdk1 activity possible at intermediate cyclin B1 concentrations (Fig. 1b)1,2. Bistability could, in principle, allow Cdk1 activation to propagate rapidly through trigger waves3,9–11.
Figure 1. Trigger waves in Cdk1 activation.
a, Schematic view of the Cdk1-APC/C circuit. b, Modeled steady-state response of Cdk1 to cyclin B1, with parameters based on experimental studies25,26. At intermediate concentrations of cyclin, the response is bistable. The stable low (interphase) and high (mitotic) Cdk1 activity steady-states corresponding to one such cyclin concentration (65 nM) are shown as blue and red circles, respectively. The white circle denotes the unstable steady state, and the yellow X denotes an intermediate level of Cdk1 activity that would be attracted to the mitotic (red) steady state. c, Schematic view of the propagation of Cdk1 activity up and down a one-dimensional tube through successive rounds of mixing and conversion. d, Modeled propagation of Cdk1 activity in a one-dimensional tube. It is assumed that cyclin B1 is synthesized at a uniform rate everywhere in the tube, but in a 5 µm region in the middle of the tube, the concentration of Cdc25C is 50% higher than in the rest of the tube, allowing Cdk1 to become activated earlier. Cyclin B1-Cdk1 activity is denoted by the color scale (blue is low, red is high). Numerical solution of the PDEs was carried out using Mathematica 9.0 (Wolfram) as described in the Supplementary Materials. See also Supplementary Fig. 1.
To see why bistability can generate rapidly-propagating trigger waves, imagine we have a long thin tube containing cytoplasm with a uniform concentration of cyclin B1 and Cdk1, and assume that in some region of the tube the cytoplasm is in the mitotic, high Cdk1-activity state (Fig. 1c, red) while the rest of the cytoplasm is in the interphase, low Cdk1-activity state (Fig. 1c, blue). Within some small distance of the interface, the cytoplasm will rapidly mix by diffusion, resulting in an intermediate level of Cdk1 activity. If this activity is above the unstable steady state (Fig. 1b, white point), this slice of cytoplasm will flip to the mitotic state. The process of mixing and conversion repeats, and the mitotic state propagates down the tube at a constant linear velocity. The propagation speed can be estimated by Luther’s formula, , where D is the diffusion coefficient and τ is related to the flipping time for the bistable system3,12. If we assume D=10 µm2/sec and τ=10 to 100 sec, the expected propagation speed would be 40 to 120 µm/min, and Cdk1 activity could propagate from the congressed pronuclei to the animal pole in 2 to 5 min. This would be fast enough to account for the coordination of nuclear and cortical mitotic events. If Cdk1 activation is followed by APC/C activation and cyclin degradation, a wave of mitotic exit would follow the wave of mitotic entry, and if this is followed by new cyclin synthesis the whole process would repeat.
To explore this idea further, we created a simple partial differential equation model of Cdk1 activation and propagation (Supplementary Materials). We assumed that cyclin B1 was synthesized at a uniform, constant rate everywhere in the cytoplasm, and that the mitotic activator Cdc25C was 50% higher in concentration in one 5 µm section of the tube than in the rest of the tube. This inhomogeneity could represent the centrosome, which, in somatic cells, has a high concentration of Cdc25C13. As anticipated, the modeled activation of Cdk1 was found to first occur in this high Cdc25C region, and then to spread linearly up and down the tube, resulting in a V-shaped front of Cdk1 activation in the plot of activity as a function of time and position (Fig. 1d; also see Supplementary Fig. 1). The propagation rate, which is the slope of the diagonal wave fronts, was approximately 60 µm/min, compatible with the estimates from Luther’s formula. Farther from the centrosomal region, cyclin synthesis reached the threshold for Cdk1 activation before the trigger wave arrived, resulting in a vertical front of Cdk1 activity. However, with successive cycles, the trigger waves came to occupy more and more of the tube. These results support the plausibility of trigger waves as a mechanism for allowing Cdk1 activation to spread through an egg in a rapid and orderly manner.
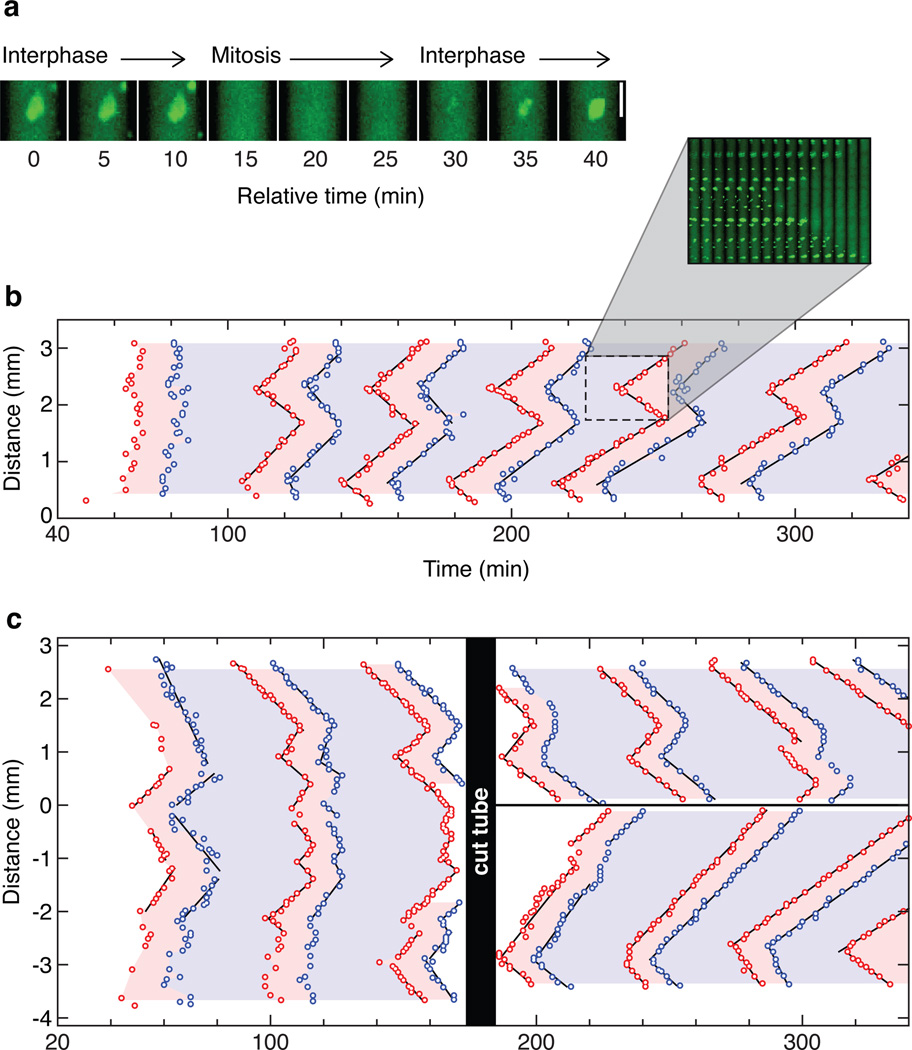
We therefore set out to experimentally determine whether trigger waves do occur, using cycling Xenopus laevis egg extracts14. Demembranated sperm chromatin and purified GFP-NLS protein were added to the extracts as reporters of mitotic entry. In interphase extracts, the sperm chromatin forms nuclei, complete with nuclear pores that import the GFP-NLS protein. As the cell cycle progresses and the extract enters mitosis, the nuclear envelopes break down and GFP-NLS quickly disperses (Fig. 2a). About 15 min later the nuclei begin to reform (Fig. 2a), indicating the end of mitosis. We loaded extract into a Teflon tube, submerged the tube in mineral oil, and cut the tube into sections 2–3 mm long. These sections were then imaged by time-lapse epifluorescence microscopy. Care was taken to keep the temperature of the tube constant to avoid convective flow. Raw microscopy images were stitched together, and the time and location of nuclear envelope breakdown and re-formation were recorded.
Figure 2. Rapid, linear propagation of mitotic entry and exit through Xenopus cytoplasm.
a, An example of nuclear envelope breakdown and nuclear envelope reformation in an extract with added sperm chromatin and GFP-NLS. b, The timing of mitotic entrance and exit in a 3 mm section of a Teflon tube submerged in mineral oil. Each data point represents the time and position at which an individual nucleus underwent nuclear envelope breakdown (red points) or nuclear envelope re-formation (blue points). The pink and blue regions of the plot denote mitosis and interphase, respectively. Time is measured relative to when the extract was warmed to room temperature. The inset shows frames from the video in montage form. c, Trigger waves vs. phase waves. The tube was cut under mineral oil at 160 min. See also Supplementary Fig. 2.
Supplementary video 1 and Fig. 2b show the behavior of a typical cycling extract. The nuclei in the extract initially entered mitosis about 60 min after the extract was warmed to room temperature, and then continued to undergo cycles of interphase and mitosis every ~40 min over the next several hours. During the first cycle, the mitoses occurred nearly synchronously, with some apparently stochastic variation in timing from nucleus to nucleus. From the second cycle on, both mitotic entry and mitotic exit swept through the tube in a linear, wave-like fashion (Fig. 2b), with the waves initiating near the same locations during every cycle. Initially the wave speed was 54 ± 20 µm/min (mean ± S.D.), in good agreement with the expected speeds of trigger waves based on either Luther’s formula or our PDE simulations (Fig. 1). The waves gradually slowed to about half that speed by the end of the experiment (Fig. 2b and Supplementary Fig. 2). Generally the waves became better organized and spanned longer lengths of the channel as the experiment proceeded (Figs. 2b, c, and 3a). These findings suggest that waves of mitosis emanating from the earliest or strongest foci eventually dominate the dynamics of the whole system.
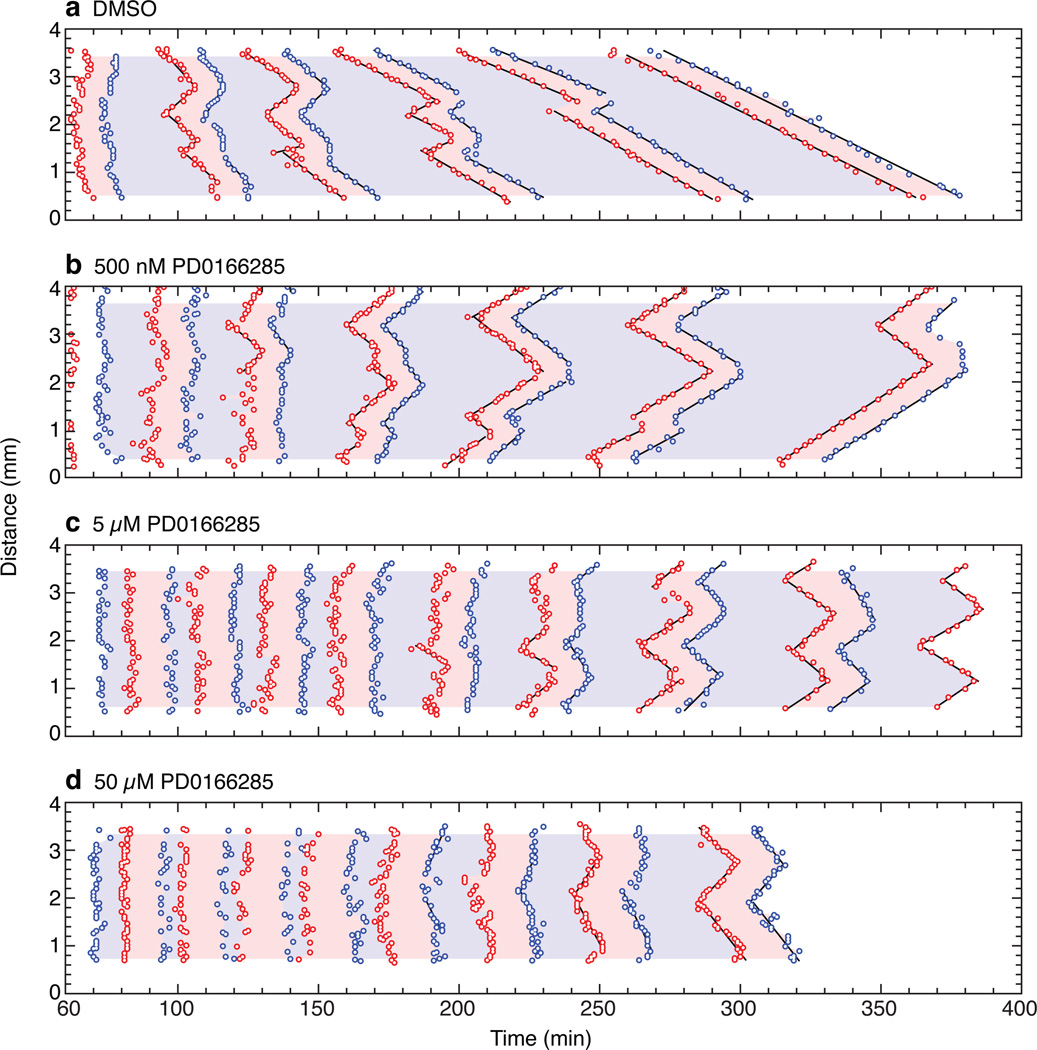
Figure 3. The Wee1/Myt1 inhibitor PD0166285 accelerates the trigger waves.
a–d, Mitotic entrance and exit waves in extracts treated with DMSO or one of three concentrations of PD0166285. See also Supplementary Fig. 3.
In principle, the observed waves could actually be phase waves (also called pseudowaves or kinematic waves3,15), which could occur if different parts of the cytoplasm initiated oscillations at different times and this temporal difference was then faithfully maintained in the subsequent waves. To test this possibility, we placed a tube of extract in mineral oil, monitored three cycles of mitotic entry and exit, and then cut the tube into two pieces with a scalpel. As shown in Fig. 2c, the nuclei on either side of the cut quickly lost coordination, and appeared to follow the rhythm established by the fastest-initiating region of cytoplasm on their side of the cut. This rules out the possibility that the observed waves are simply phase waves.
If the Cdk1 network generates trigger waves, then changing the dynamics of the feedback loops that regulate Cdk1 should affect wave dynamics. To test this idea, we made use of PD0166285, a small molecule inhibitor of Wee1A and Myt116. Experiments in bulk extracts showed that inhibition of cyclin B1-induced Cdk1 tyrosine phosphorylation was half-maximal at ~1 µM (Supplementary Fig. 3). We treated extracts with 0.5, 5, or 50 µM PD0166285 or just DMSO, and monitored the effects on the timing of mitotic onset and the speed of mitotic propagation.
As shown in Fig. 3, Supplementary Fig. 3 and Supplementary video 2, PD0166285 advanced the onset of mitosis by ~30 min and increased the speed of the mitotic waves to as much as 120 µm/min. Interphase durations were shortened by as much as 50%, and there was also an increase in mitotic duration (Supplementary Fig. 3), which underscores the importance of Wee1A/Myt1 activation in mitotic exit. At the highest concentrations of PD0166285, waves were not evident until late in the experiment (Fig. 3d). These findings support the hypothesis that the waves of mitotic entry and exit arise from the bistable mitotic trigger.
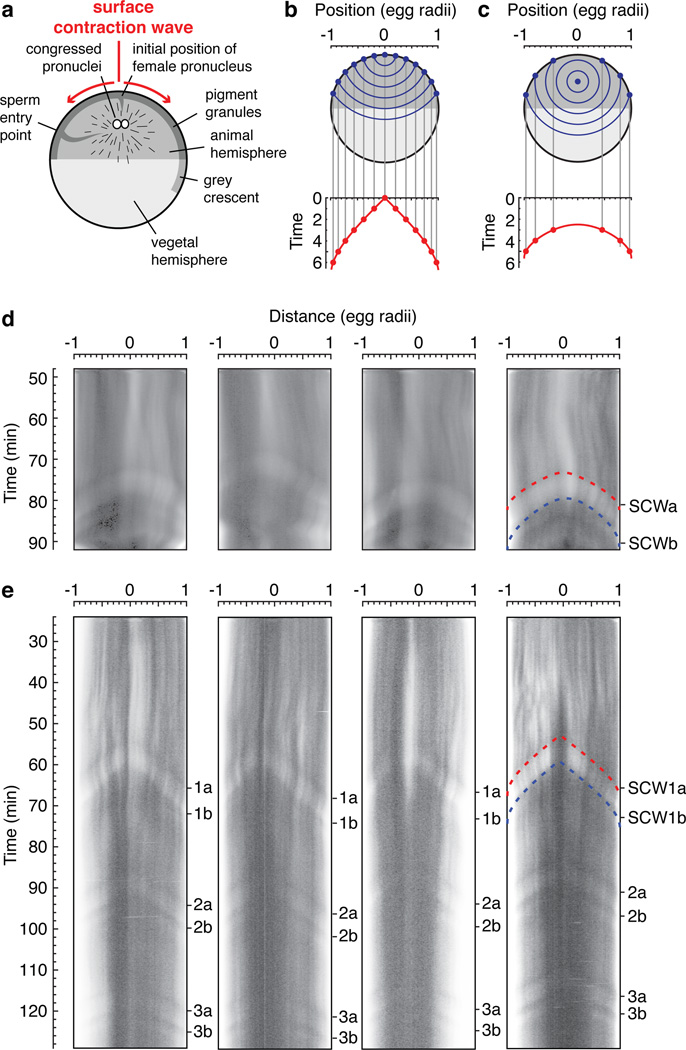
Finally, we turned to the question of whether the trigger waves seen in extracts also occur in intact fertilized eggs. We hypothesized that waves of Cdk1 activation could be initiated at the centrosome, spread outward via the cyclin B1-Cdk1 complexes diffusely present throughout the interphase cytoplasm17, and then cause surface contraction waves (SCWs) when they reach the cortical cytoskeleton. SCWs are waves of pigmentation change at the cell cortex that proceed from the animal pole to the vegetal pole just prior to cytokinesis4,18,19 (Supplementary video 3 and Fig. 4a). The first wave (SCWa) is linked to Cdk1 activation, and the second wave (SCWb) requires Cdk1 inactivation18. It has been speculated that the change in pigmentation is controlled by the phosphorylation of microtubule associated proteins by Cdk120.
Figure 4. Surface contraction waves in intact Xenopus eggs.
a, Schematic view of the anatomy of a fertilized egg just before the onset of mitosis. Adapted from ref. 27. b, c, Expected propagation of surface contraction waves if they were due to a spherical wave of Cdk1 activation spreading from a point source. An equation for the spreading of the waves (Eq S1) is derived in the Supplementary Materials. Panel B assumes the point source is at the animal pole. Panel C assumes the point source is halfway between the animal pole and the center of the cell. d–e, Kymographs depicting surface contraction waves, indicated by transitions from light to dark or dark to light, in four fertilized eggs (d) and four parthenogenetically-activated eggs (e). The red and blue dashed curves are fits of the experimental data to the Eq S1. See also Supplementary Fig. 4.
We derived a formula for the time at which such a constant velocity, spherical wave would reach various points on the surface of the egg as a function of the position of origin of the wave and the wave speed (Supplementary Materials, Eq S1 and Supplementary Fig. 4). If the trigger wave were to begin in the middle of the cell, we would expect it to arrive at all points on the cortex simultaneously. If it were to originate from the animal pole, we would expect a plot of the wave’s cortical position as a function of time to be V-shaped (Fig. 4b). If it originated in the middle of the animal hemisphere, we would expect the plot to be U-shaped (Fig. 4c).
We then imaged SCWs traveling across the animal hemispheres of fertilized eggs and expressed the data as kymographs (Fig. 4d). The position vs. time curves were U-shaped and agreed well with Eq S1 (Fig. 4d). By fitting the observed SCWa curves to Eq S1, we inferred a trigger wave speed of 66 ± 17 µm/min (mean ± S.D., n = 4). This speed is similar to that observed in extracts (Figs. 2 and 3). The inferred origin of the waves was at a location 85% ± 5% (mean ± S.D., n = 4) up from the vegetal pole on the animal-vegetal axis. This origin is near where the congressed pronuclei reside (Fig. 4a), and the 99% confidence intervals for the location of the origin in each embryo (87–93%, 76–91%, 71–85%, and 75–88%) essentially rule out the possibility that the trigger waves originated at the animal pole. The inferred time at which the mitotic trigger wave originated was ~70 min after fertilization and 3 ± 1 min (mean ± S.D., n = 3) prior to the beginning of SCWa.
Parthenogenetically-activated eggs do not undergo cleavages, but do exhibit a succession of SCWs5. Since pronuclear congression does not occur in parthenogenetically-activated eggs, it seemed plausible that the spherical mitotic trigger waves hypothesized to give rise to these SCWs might have a different point of origination. As shown in Fig. 4e and Supplementary video 4, parthenogenetically-activated eggs exhibited a very regular succession of SCWs whose position vs. time curves were V-shaped rather than U-shaped. Fitting these curves to Eq S1 yielded an inferred wave speed of 58 ± 3 µm/min (mean ± S.D., n = 4) and a point of origination immediately under the animal pole. The 99% confidence intervals for the locations were 99–101%, 98–100%, 99–102%, and 98–102% up from the vegetal pole. Thus, the quantitative characteristics of the SCWs in both fertilized and parthenogeneticaly-activated eggs are consistent with the hypothesis that they occur when trigger waves of Cdk1 activation and inactivation arrive at the cell cortex.
In summary, we have shown through modeling studies that the Cdk1/Wee1A/Cdc25C system could plausibly generate trigger waves of sufficient speed to help spatially coordinate mitosis in the large, fertilized Xenopus laevis egg, and have shown experimentally that linear waves of mitotic entry and exit do occur in unstirred cycling Xenopus egg extracts and are likely to occur in intact eggs. Note that the early cell cycles of the syncytial Drosophila embryo also exhibit mitotic waves. By cycles 10–13, mitosis can been seen to begin at the two poles and spread towards the middle of the embryo, at a rate of ~50–250 µm/min21. Mitotic waves have also been detected in some mulitnucleate fungi (e.g., Stemonitis flavogenita22 and Aspergillus nidulans23, but not in Ashbya gossypii24). We suspect that all of these mitotic waves are essentially the same phenomenon we are observing here.
Random walk diffusion is rapid enough to allow proteins to communicate between different parts of a cell on a time scale of seconds, and communication in large organisms often takes place by flow. Trigger waves provide another mechanism for communicating rapidly over millimeter to meter distance scales. Trigger waves are the basis for the propagation of action potentials down an axon, for the propagation of calcium waves through excitable cells and tissues, and for cAMP waves in Dictyostelium discoideum9. Mitotic waves appear to be another example of this phenomenon. The proteins, biochemical processes, and time scales involved in action potentials and mitotic waves are different, but the basic systems-level logic is the same. This suggests that trigger waves may be an important general mechanism for communication within tissues, organs, and organisms.
Materials and Methods
Time-lapse epifluorescence microscopy of Xenopus cycling extracts
Cycling extracts were prepared14 and, while kept on ice, supplemented with demembranated sperm chromatin (to ~400/µL extract) and GFP-NLS (to ~10 µM). Extracts were then taken off ice and a portion was loaded into Teflon tubing (Cole-Parmer #YO-06417-72), submerged in mineral oil on a glass slide, and cut into 2–3 mm sections.
The tube was imaged at room temperature (22–25°C) on an inverted epifluorescence microscope (Leica DMI6000 B). Samples from extract not loaded into the tubes were frozen down and later assayed for histone H1 kinase activity.
For studies using PD0166285, extracts were supplemented with a fixed volume of DMSO or inhibitor along with the sperm chromatin and GFP-NLS.
Image analysis
Images of the channels were cropped, stitched together, and contrast-adjusted using a custom MATLAB (Mathworks) script. The locations and times at which nuclei underwent envelope breakdown or formation were recorded manually.
Supplementary Material
Acknowledgements
We thank Hiro Funabiki and Mary Dasso for providing GFP-NLS protein and constructs, Eduardo Sontag and Liming Wang for discussions and calculations, Tony Tsai for sharing his unpublished data on the effects of PD0166285 on Xenopus embryos, Qiong Yang for helping to build the ODE model upon which our PDE model is based, Gautam Dey, Howard Stone, and Bill Sullivan for discussions, Steve Quake and the Quake lab for advice, James Chen and the Chen lab for the use of their microscope and discussions, Joe Pomerening for discussions and technical advice, the Stanford Cell Sciences Imaging Facility for technical assistance, and members of the Ferrell lab for discussions. This work was supported by grants from the National Institutes of Health (GM046383 and GM077544) and by an NSF Graduate Research Fellowship and a Lieberman Fellowship (to J.B.C.).
Footnotes
Supplementary Information is provided in an accompanying document.
Author Contributions J.B.C. carried out experiments and calculations, analysed data, and helped write the paper. J.E.F. carried out calculations, analysed data and helped write the paper.
The authors declare no competing financial interests.
References
- 1.Pomerening JR, Sontag ED, Ferrell JE., Jr Building a cell cycle oscillator: hysteresis and bistability in the activation of Cdc2. Nature Cell Biol. 2003;5:346–351. doi: 10.1038/ncb954. [DOI] [PubMed] [Google Scholar]
- 2.Sha W, et al. Hysteresis drives cell-cycle transitions in Xenopus laevis egg extracts. Proc Natl Acad Sci U S A. 2003;100:975–980. doi: 10.1073/pnas.0235349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novak B, Tyson JJ. Numerical analysis of a comprehensive model of M-phase control in Xenopus oocyte extracts and intact embryos. J Cell Sci. 1993;106:1153–1168. doi: 10.1242/jcs.106.4.1153. [DOI] [PubMed] [Google Scholar]
- 4.Hara K. Cinematographic observation of "surface contraction waves" (SCW) during the early cleavage of axolotl eggs. Wilhelm Roux' Archiv. 1971;167:183–186. doi: 10.1007/BF00577039. [DOI] [PubMed] [Google Scholar]
- 5.Hara K, Tydeman P, Kirschner M. A cytoplasmic clock with the same period as the division cycle in Xenopus eggs. Proc Natl Acad Sci U S A. 1980;77:462–466. doi: 10.1073/pnas.77.1.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackman M, Lindon C, Nigg EA, Pines J. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat Cell Biol. 2003;5:143–148. doi: 10.1038/ncb918. [DOI] [PubMed] [Google Scholar]
- 7.Newport J, Kirschner M. A major developmental transition in early Xenopus embryos: I characterization and timing of cellular changes at the midblastula stage. Cell. 1982;30:675–686. doi: 10.1016/0092-8674(82)90272-0. [DOI] [PubMed] [Google Scholar]
- 8.Gerhart JC. In: Biological Regulation and Development. Goldberger RF, editor. Vol. 2. Plenum Press; 1980. pp. 133–316. [Google Scholar]
- 9.Tyson JJ, Keener JP. Singular perturbation theory of traveling waves in excitable media (a review) Physica D. 1988;32:327–361. [Google Scholar]
- 10.Markevich NI, Tsyganov MA, Hoek JB, Kholodenko BN. Long-range signaling by phosphoprotein waves arising from bistability in protein kinase cascades. Mol Syst Biol. 2006;2:61. doi: 10.1038/msb4100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds AR, Tischer C, Verveer PJ, Rocks O, Bastiaens PI. EGFR activation coupled to inhibition of tyrosine phosphatases causes lateral signal propagation. Nat. Cell Biol. 2003;5:447–453. doi: 10.1038/ncb981. [DOI] [PubMed] [Google Scholar]
- 12.Luther R. Raumliche fortplanzung chimischer reaktionen. Z. Elektrochemie. 1906;12:596–600. [Google Scholar]
- 13.Bonnet J, Coopman P, Morris MC. Characterization of centrosomal localization and dynamics of Cdc25C phosphatase in mitosis. Cell Cycle. 2008;7:1991–1998. doi: 10.4161/cc.7.13.6095. doi:6095 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Murray AW. Cell cycle extracts. Meth Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- 15.Winfree AT. Two kinds of wave in an oscillating chemical sollution. Faraday Symp Chem Soc. 1974;9:38–46. [Google Scholar]
- 16.Wang Y, et al. Radiosensitization of p53 mutant cells by PD0166285, a novel G(2) checkpoint abrogator. Cancer Res. 2001;61:8211–8217. [PubMed] [Google Scholar]
- 17.Nakamura N, Tokumoto T, Ueno S, Iwao Y. The cytoskeleton-dependent localization of cdc2/cyclin B in blastomere cortex during Xenopus embryonic cell cycle. Molecular reproduction and development. 2005;72:336–345. doi: 10.1002/mrd.20348. [DOI] [PubMed] [Google Scholar]
- 18.Rankin S, Kirschner MW. The surface contraction waves of Xenopus eggs reflect the metachronous cell-cycle state of the cytoplasm. Curr Biol. 1997;7:451–454. doi: 10.1016/s0960-9822(06)00192-8. [DOI] [PubMed] [Google Scholar]
- 19.Shinagawa A, Konno S, Yoshimoto Y, Hiramoto Y. Nuclear involvement in localization of the initiation site of surface contraction waves in Xenopus eggs. Develop Growth & Differ. 1989;31:249–255. doi: 10.1111/j.1440-169X.1989.00249.x. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Mongiovi D, Chang P, Houliston E. A propagated wave of MPF activation accompanies surface contraction waves at first mitosis in Xenopus. J Cell Sci. 1998;111(Pt 3):385–393. doi: 10.1242/jcs.111.3.385. [DOI] [PubMed] [Google Scholar]
- 21.Foe VE, Alberts BM. Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J Cell Sci. 1983;61:31–70. doi: 10.1242/jcs.61.1.31. [DOI] [PubMed] [Google Scholar]
- 22.Haskins EF. Stemonitis flavogenita (Myxomycetes). Plasmodial phase (Aphanoplasmodium) Institut Wissenschaftliche Film. 1974;E 2000 [Google Scholar]
- 23.Clutterbuck AJ. Synchronous nuclear division and septation in Aspergillus nidulans. J Gen Microbiol. 1970;60:133–135. doi: 10.1099/00221287-60-1-133. [DOI] [PubMed] [Google Scholar]
- 24.Gladfelter AS, Hungerbuehler AK, Philippsen P. Asynchronous nuclear division cycles in multinucleated cells. J Cell Biol. 2006;172:347–362. doi: 10.1083/jcb.200507003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trunnell NB, Poon AC, Kim SY, Ferrell JE., Jr Ultrasensitivity in the regulation of Cdc25C by Cdk1. Mol Cell. 2011;41:263–274. doi: 10.1016/j.molcel.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Q, Ferrell JE., Jr The Cdk1-APC/C cell cycle oscillator circuit functions as a time-delayed ultrasensitive switch. Nat Cell Biol. 2013;15:519–525. doi: 10.1038/ncb2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hausen P, Riebesell M. The early development of Xenopus laevis: an atlas of the histology. Springer-Verlag: 1991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.