Abstract
In direct skeletal attachment (DSA) of limb prostheses, a construct is implanted into an amputee’s residuum bone and protrudes out of the residuum’s skin. This technology represents an alternative to traditional suspension of prostheses via various socket systems, with clear indications when the sockets cannot be properly fitted. Contemporary DSA was invented in the 1990s, and several implant systems have been introduced since then. The current review is intended to compare the design features of implants for DSA whose use in humans or in animal studies has been reported in the literature.
Keywords: design features, implants, direct skeletal attachment, osseointegration, limb prostheses
INTRODUCTION
A method of direct skeletal attachment (DSA) of the limb prosthesis is a way of improving quality of life for amputees, who could not be treated adequately with the socket suspension of their prostheses.1–4 Short residuum, skin and soft tissue problems, phantom pain could result in rejecting the use of prosthesis and relying on aids (e.g., crutches and wheelchair).5–8
The principles of direct and permanent attachment of artificial limbs were formulated in the 1970s, after which the topic became a subject of research and development4,9,10 (Fig. 1).
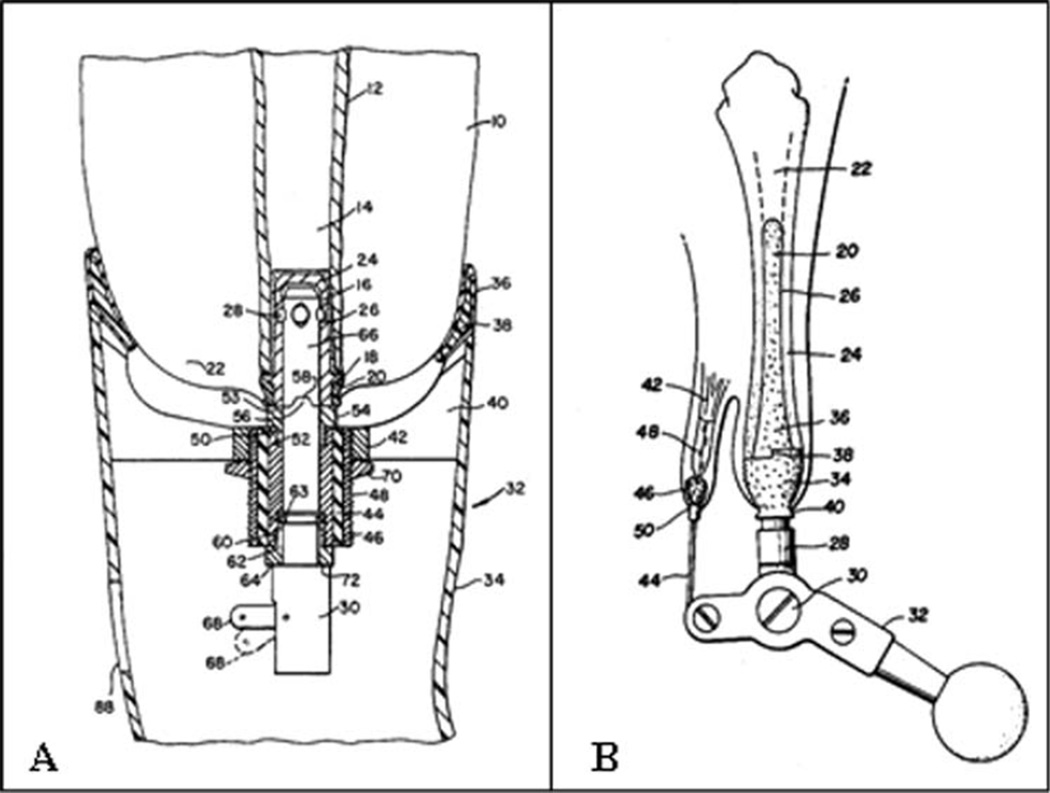
FIGURE 1.
(A) Apparatus for connecting a prosthesis to a bone—US Patent 3947897;10 (B) permanently attached artificial limb—US Patent 4143426.9
Fernie et al.11 tested a percutaneous implant in 14 pigs. The intramedullary metal stem was porous-surface layered, and Dacron velour was used at the soft tissue interface. The authors reported that some adhesion of bone cells to the porous stem was achieved, but the velour was unable to maintain adequate epithelial adhesion to form an anatomical seal and a barrier to bacteria.
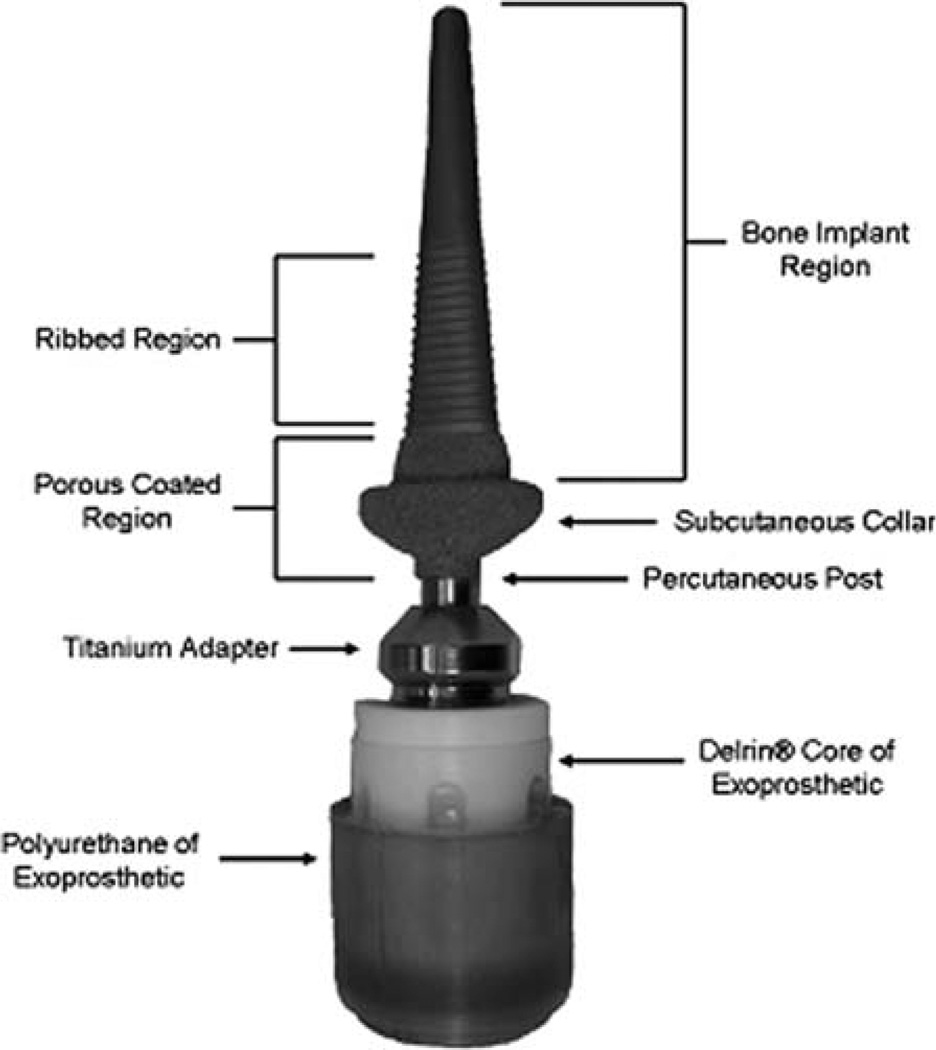
The first skeletal prosthesis attachment unit for application in humans was developed and tested in three patients—above-knee and above-elbow.amputees—at Rancho Los Amigos Hospital (RLAH, Downey, CA).12 The device had a stainless steel shaft for intramedullary implantation and a subcutaneous collar made of unpolished carbon [Fig. 2(A)]. The implant was cemented with methylmethacrylate to the intramedullary canal of the bone in the amputee’s stump and then passed through the skin [Fig. 2(B)]. Suspension of the prosthesis was made possible with a quick disconnect device that locks into the shaft of the implant. It was reported that all implants had to be removed within 6 months postimplantation due to chronic infection aggravated by mechanical irritation. A main catalyst of infection was assumed to be the relative motion between skin and the device.
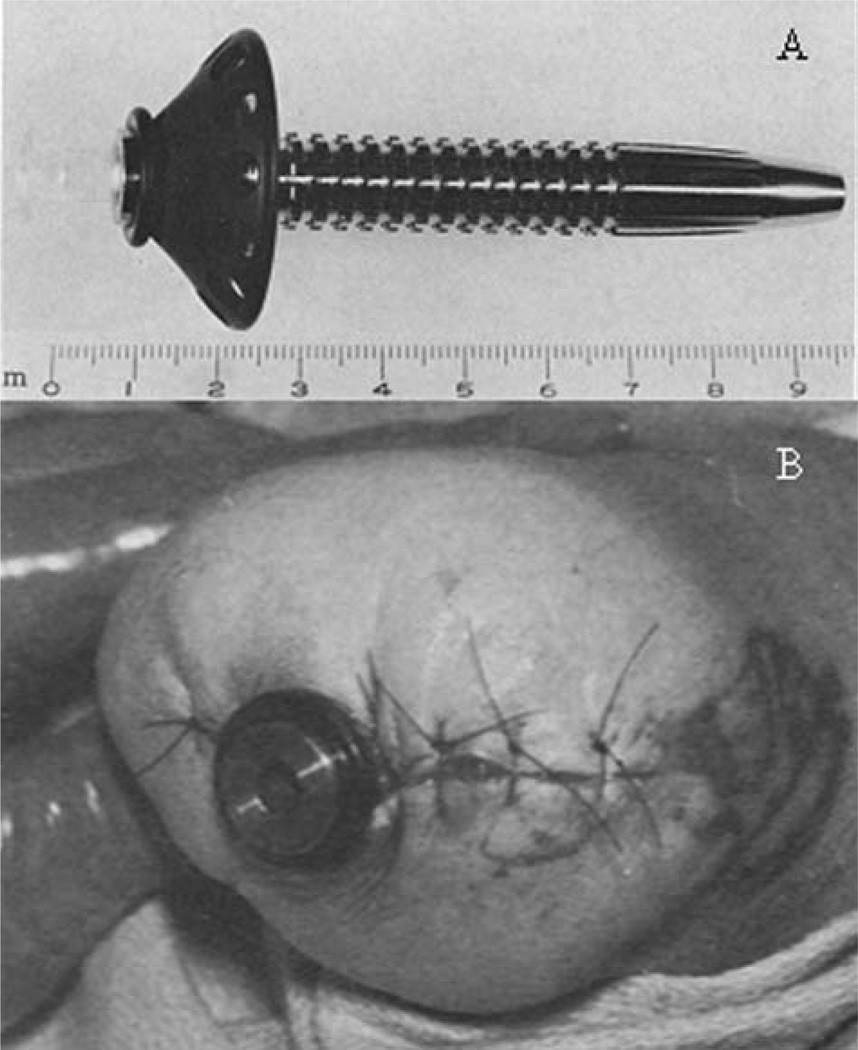
FIGURE 2.
(A) Intramedullary skeletal prosthesis attachment unit; stainless steel shaft with a carbon subcutaneous collar. (B) Skeletal prosthesis attachment unit (A) implanted in leg of amputee at Rancho Los Amigos Hospital (Modified from Ref. 12 with permission from Springer Science + Business Media).
The design features of the RLAH system are summarized in Table I. The most practical ones have been replicated in contemporary systems (see Table I). The RLAH system could not be successful because there was no bond between the stainless steel shaft and the bone. The required bond probably could have been achieved if the RLAH shaft had been made of titanium, whose ‘‘osseointegration’’ capacity was already reported by Dr. Per-Ingvar Branemark in 1959.13 He discovered that chambers made of titanium could be permanently incorporated (osseointegrated) into bone. Indeed, study14 found very close apposition of the bone to the titanium implant’s surface—50 Å—and tissue was found to respond to the tightly adherent titanium oxide layer on the surface of the implant similarly as to a ceramic material.
TABLE I.
Implant Systems for Direct Skeletal Attachment and Their Design Features
| Usage |
Surgery |
Material |
Medullary Part |
Percutaneous Part |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Systems | In Humans |
In Animals |
One- Stage |
Two- Stage |
Stainless Steel |
Carbon | Titanium Alloy Ti6AI4V |
Cobalt- Chrome Alloy |
Tantalum | Cylindrical | Conical | Double Tempered |
Permeable | Elastic Sleeve | Polished | Porous Surface |
Permeable | Perforated Flange |
Provision For Cable Passage |
Coated |
| RLAH | X | x | x | x | x | x | ||||||||||||||
| OPRA | X | x | x | x | x | x | ||||||||||||||
| EEFP | X | x | x | x | x | x | ||||||||||||||
| ITAP | X | x | x | x | x | x | x | x | x | |||||||||||
| POP | x | x | x | x | x | x | ||||||||||||||
| SBIP | x | x | x | x | x | x | x | x | x | |||||||||||
| AEAHBM | x | x | x | x | x | |||||||||||||||
| UA | x | x | x | |||||||||||||||||
The first contemporary system for DSA, called Osseointegrated Prostheses for the Rehabilitation of Amputees (OPRA), was introduced by Dr. Branemark himself in the 1990s15 following his experience in using titanium in dental implants.16–19
The success of the DSA technology generically rests on the long-lasting integrity of the interface of human tissues with a nonbiological implant.4,12,20,21 In contrast with freely suspended subcutaneous implants like pace makers, DSA implants must transmit high loads and moments to the bone22–24 and deal with infections at the skin-device barrier.25–28
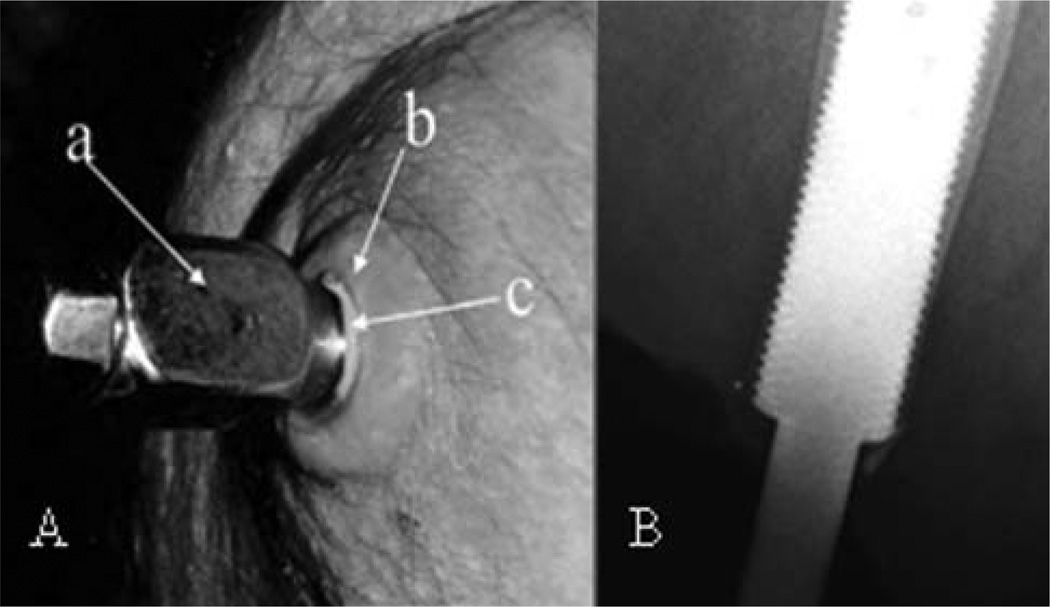
In addition to the challenges faced by DSA in skeletal fixation and transcutaneous interface, there are also issues of occasional infections29 and breakage of fixation components.24,30 To protect the integrity of the hosting bone in case of falls, different clutching systems have been considered to disintegrate the prosthesis from the residuum when the loads and moments exceed the safety threshold.31–33 The skin seal issue is illustrated in Figure 3(A), showing a pus layer (c) around the abutment (a) penetrating the skin stoma (b) of the residuum.34
FIGURE 3.
(A) Skin issues in DSA: (a) solid titanium abutment penetrating the residuum skin stoma. Adapted from Ref. 34. (B) Cortical thinning in the distal zones. Adapted from Ref. 35; copyright © 2012, Informa Healthcare. Reproduced with permission from Informa Healthcare. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The bone-device interface, even if there were no problems with infection, may be associated with the thinning of the cortical bone, as illustrated in Figure 3(B) from Ref. 35. The study found that 2 years after DSA procedure, 35% of patients experienced cortical thinning in the distal zones. That percentage increased to 55% by 5 years after the procedure.
Over 150 patients in Sweden, Germany, the Netherlands, and Australia have been operated on with DSA.36–39 Analysis of an in-depth interview with patients living with osseointegrated prostheses40–42 showed that participants described living with an osseointegrated prosthesis as a revolutionary change. The change went beyond the objectively confirmed functional improvements,43,44 but also impacted the broad concept of quality of life.43–48
Among the advantages of the method of DSA is how easy it is to attach the prosthesis when compared to the frequent difficulties from donning and doffing the socket.49 Since the prosthesis is not supported over the skin, all skin and soft tissue issues related to sockets are eliminated. Due to fusion of the prosthesis with the residual bone, the DSA allows also for more natural osseoperception, which is an important factor for functionality and safety35,41,50 for patients with upper-limb amputations as well.51,52
Amputees who use conventional sockets are aware of both the advantages and disadvantages of DSA, and are influenced by them. In a survey,53 33% of respondents with above knee amputation stated that they would consider undergoing the osseointegration procedure for prosthetic attachment. Forty-two percent of the participants responded that they would not consider having the procedure due to potential infection, implant failure, long rehabilitation course, and risk of a broken bone in the residual limb.
The DSA procedure is still not allowed by the Food and Drug Administration in the United States, even though there are a substantial number of amputees, including US Veterans, who would benefit and whose quality of life would improve from DSA. The agency requires a compelling response to the issues of skin seal and bone interface viability. After the first skeletal prosthesis attachment unit had been tested at Rancho Los Amigos12 several implant systems have been introduced in attempts to address the challenges of DSA.
In this article, we will present the design features of the systems whose use in humans or in animal trials was reported in the literature known to the author.
METHODS
In selecting which DSA implantation systems to review, we restrict ourselves to those which have already demonstrated their practicality by their application in human subjects/ patients or in animal studies. References are made to the published reports and to the patent information related to the systems selected. Keywords associated with DSA of limb prostheses were used to search the literature available on PubMed, ScienceDirect, Web of Science, and Google Scholar databases.
Eight systems were selected for further analysis according to the selection criteria (see Table I). One of them [Skin and Bone Integrated Pylon (SBIP)] was developed by the author. For this reason, it was considered not to be ethical to range the systems in terms of their effectiveness. We also do not critically compare the systems’ design features, because some of them, as with the RLAH system with the reported failure, were given a ‘‘second life,’’ with new features, in the current designs. Thus, we leave to the reader to make his/her own judgement after reviewing the references and the features of the selected systems presented in this article.
Implant systems for DSA
We will consider the features of seven implant systems for DSA:
RLAH system12;
OPRA (Integrum AB, Sweden, http://www.integrum.se)54;
Endo-exo femoral prosthesis (EEFP), Eska Orthodynamics GmbH (www.orthodynamics.de), formerly ESKA IMPLANTS AG, Lubeck, Germany37;
Intraosseous transcutaneous amputation prosthesis (ITAP), Stanmore group (University College, London, UK)55;
Percutaneous osseointegrated prostheses (POP), IMDS Co-Innovative, Logan, UT (http://www.imds.net)56;
SBIP, Poly-Orth International, Sharon, MA34;
The Alameda East Animal Hospital & BioMedtrix (AEAHBM) system, the Alameda East Animal Hospital, Denver, CO; the BioMedtrix, Boonton NJ; and the Colorado Limb Consultants, Denver Clinic for Extremities at Risk, Denver, CO.57
The University of Akron (UA) system, The University of Akron, Akron, OH.58
Summarized in Table I is the following information about the selected systems: in human patients or in animals only; structure of the surgery: one-stage or two-stage, materials, and some other design features.
As is presented the Table I, only four systems: RLAH, OPRA, EEEF, and ITAP have been applied to human patients. The POP, SBIP, and UA systems are currently being used in the preclinical studies.
OPRA system
Schematics of the design used in OPRA technology is depicted in Figure 4. The fixture is a threaded cylinder which is screwed into the marrow canal of the residuum bone.59 The abutment is a construct which is coupled with the fixture at the second stage of implantation 6 months after implanting the fixture (see Table I). The abutment penetrates the residuum skin, and its outer part, capped by the abutment screw, is used for attaching the leg prosthesis.39 The threads on the fixture increase the mechanical stability of the bone-device bond, and also increase the implant surface; such a threaded implant was originally used in dental implantation.60
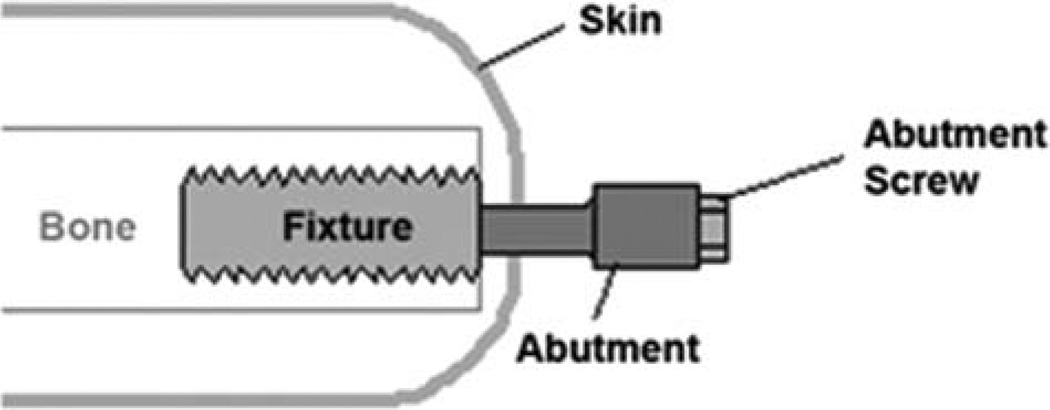
FIGURE 4.
Schematics of the design of the device for a two-stage technique of direct skeletal attachment. Fixture is a threaded cylinder which is screwed into the marrow canal of the residuum bone. Abutment is a construct which is coupled with the fixture at the second stage of implantation several months after implanting the fixture. The abutment penetrates the residuum skin, and its outer part, capped by the abutment screw, is used for attaching the leg prosthesis. Adapted from Ref. 39. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The OPRA is a system with continuously developing components for better device-bone connection.19,61,62 The system have been applied to more than 100 patients in Sweden39 and to 10 patients in Australia.38
The functionality of the OPRA system was confirmed in follow up gait studies.41,63 However, the risk of skin infection and bone thinning remain unresolved issues for the OPRA system.35,64
The OPRA approach influenced the development of bone-anchored hearing aids which implant a titanium threaded fixture into the skull.65,66
EEFP system
Application of the EEFP system was reported in 54 patients in Germany37 and 24 patients in the Netherlands.36 Similar to OPRA, the EEFP system follows a two-stage approach46,67,68 (see Table I). The intramedullar component is press fit, rather than screwed into the bone canal as in the OPRA technology (Fig. 5). The EEFP is a cobalt–chrome alloy device covered with spongiosa metal which creates a deep porous surface and favorable modulus for bone formation. The polished coupler exits distally through the skin for attachment of limb prosthesis.
FIGURE 5.
Endo-exo prosthetic system (EEFP). The device is a modular construct with a femoral stem with a porous surface, and a smoothly polished coupler exiting distally through the skin enabling for attachment of a limb prosthesis (Reproduced from Ref. 46 with kind permission from JBJS-A). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In the first operation, the femoral stem is implanted and the residuum is closed up again. In the following 4–6–8 weeks, a circular skin opening (stoma) is created. The dual cone adapter is connected to the internal femur stem through this exit hole (stoma). The silicone cover is used to protect the stoma.
The skin and soft tissues in the area where the coupler exits the stoma require constant careful care to deal with infection. Infection in the stoma region was observed in 14 of the 37 patients who underwent minor revisions because of problems around the stoma, with twelve of the 14 electing to exchange the coupler as a result of soft-tissue problems or irritation.46 It has been reported that the initially high rate of stoma-associated infections of the soft tissue coat could be dramatically reduced through a change of design of the skin-penetrating parts, such as using smoothly polished couplers instead of rough textured couplers.46
The special feature of the implant is the Spongiosa-Metal® II porous surface. Bone grows through this threedimensional grid structure, providing secure fixation of the prosthesis.
ITAP system
The ITAP system: http://www.itap-prosthetics.com/ was designed for a one-step implantation procedure (see Table I).69,70 It was reported to be applied to one transhumeral amputee,71 to the human thumb and index finger in one human patient and to four dogs 72. The approach for the system design is rooted in analysis of special biological structures such as horns, hair, feathers, fingernails, hooves, teeth, and antlers as examples where nature has solved the problems of percutaneous devices.73,74 As a result, the ITAP system emulates antlers: a flange caps the residuum, and is perforated by twenty-four 0.7 mm holes are bored immediately below the epithelium to increase dermal attachment.55
The ITAP flange (see Fig. 6) is the system’s key feature. It serves as a mediator for the bone and skin to integrate, and creates a stable interface among the implant, the bone, and the soft tissues.71 Interestingly, the ITAP flange resembles the flange in the RLAH system, except the latter was made of carbon (see Fig. 1).
FIGURE 6.
ITAP system (Adapted from Ref. 71 with kind permission from Elsevier). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Studies were conducted that demonstrated improved fibroblast adhesion to the silanized fibronectin (SiFn) titanium alloy, and on fibronectin adsorbed onto hydroxyapatite (HAFn),75 suggesting that SiFn and HAFn surfaces could be useful in optimizing the soft tissue seal around ITAP.
POP system
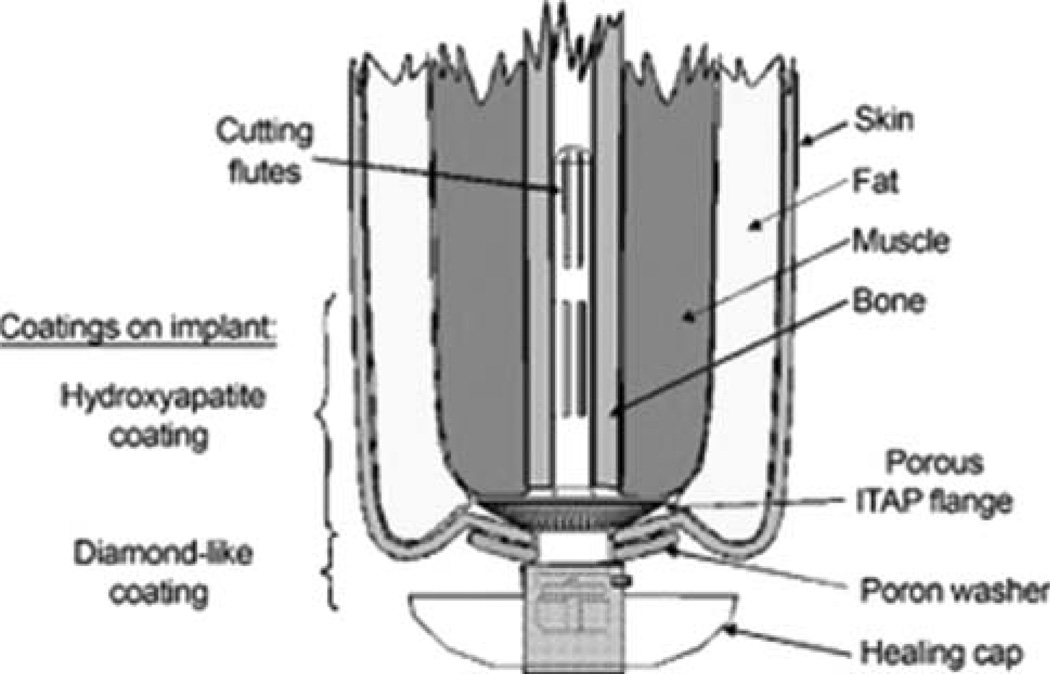
The POP system was developed for a one-stage implantation procedure (see Table I). The design features include a double tempered stem (Fig. 7), which supports at least 3-point fixation along the length of the implant, validating the surgical principle of 3-point fixation for implant survivorship. From the scanned images of the metatarsal II bones of 20 mature sheep carcasses, three implant sizes and surgical broaches, corresponding to the 25th, 50th, and 75th percentiles, were designed (Intelligent Implant Systems, LLC, Charlotte, NC) and fabricated (IMDS Co-Innovative) from medical grade Ti6Al4V titanium alloy.56 In a study with 14 sheep, the majority of the implants (n = 11) were in contact with the bone walls on at least two sides, which ensured the load transmission from the implant to the bone at the distal surface of the residual bone end.56 The device has a porouscoated subcutaneous collar (Fig. 4) intended to achieve skin-implant integration and to prevent periprosthetic infection. Clinical, microbiological, and histopathological data showed that the porous-coated Ti collar prevented superficial and deep tissue infections in all animals (14/14, 100%) at the 9-month endpoint, while animals with the smooth Ti implant construct had a 25% (2/8) infection rate.76
FIGURE 7.
POP system (Adapted from Ref. 56 with kind permission from Elsevier).
SBIP system
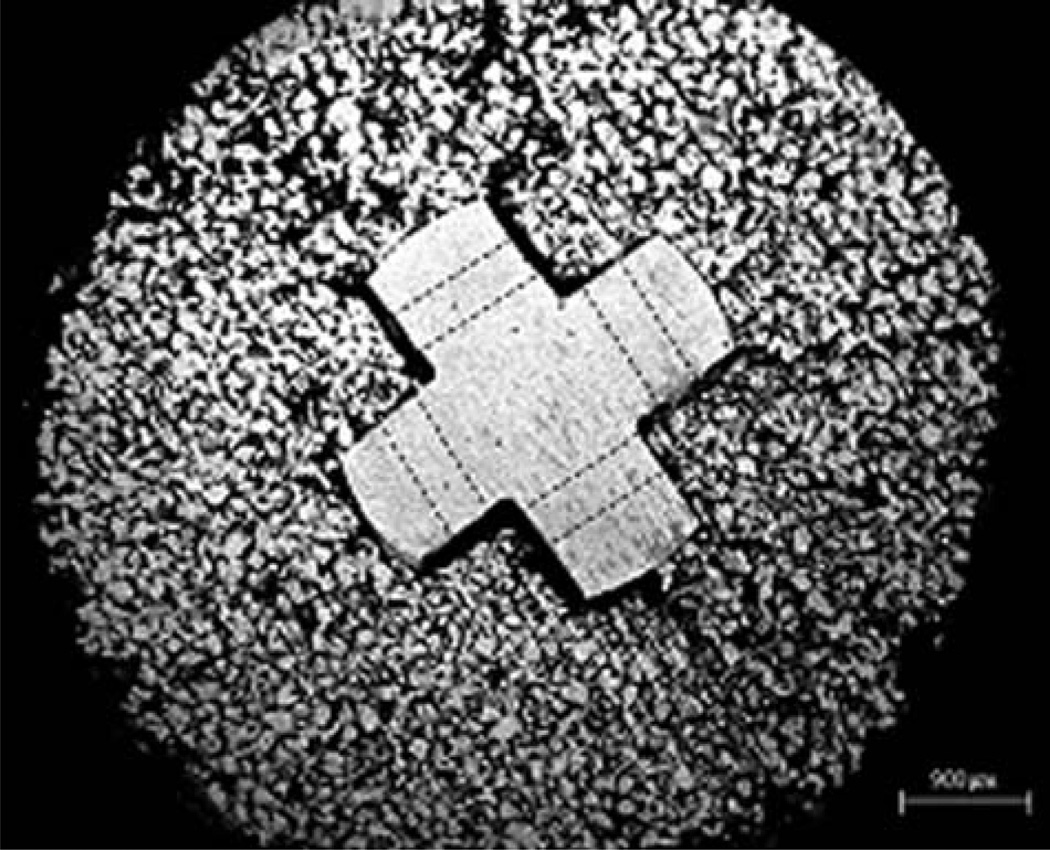
The SBIP system was developed by Poly-Orth International in several modifications77 both for one-step and two-stage implantation procedure (see Table I). A key feature of the system is the total permeability of the composite structure consisting of porous cladding and an enforcing frame with the holes in the web (Fig. 8). The frame maintains the F8 needed strength, and is selectively perforated to allow for complete ingrowth of bone and soft tissues.78 Total porosity distinguishes the SBIP system from other systems, which use porous metal in a limited fashion as a relatively thin layer of coating.11
FIGURE 8.
Cross section of the pylon with a solid cross-shape insert and the surrounding porous cladding. Dashed lines indicate projections of the holes in the web (Reproduced from Ref. 78 with kind permission from John Wiley and Sons).
The system has been applied to rats, rabbits, and cats.34,79–85
A modification without the enforcing frame (SBIP-1) constitutes a totally porous cylinder of cone and was used only for in vitro studies34,86 due to its low strength.
The SBIP-2 modification is a composite of porous cladding and different enforcing frames like wires and bars with a cross-shaped cross-section (see Fig. 8).78
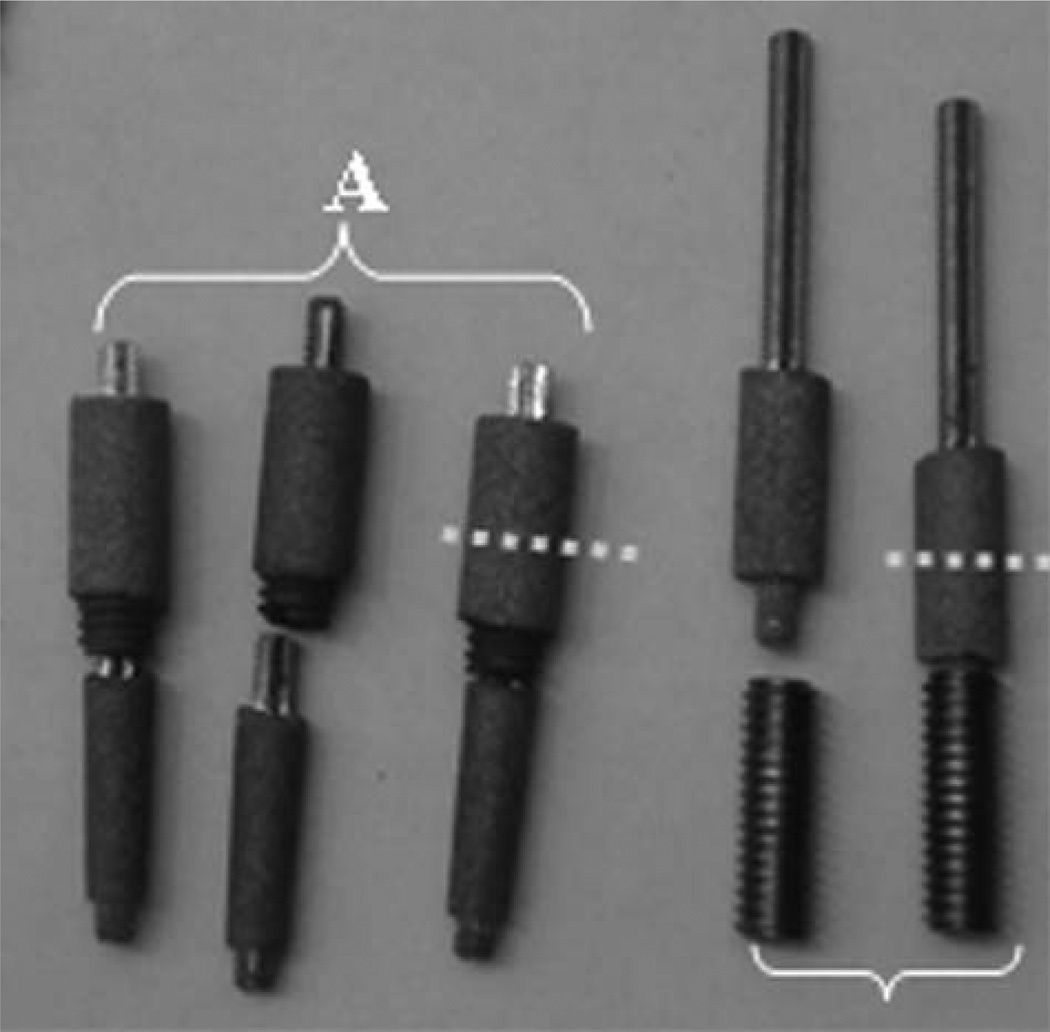
Similar to SBIP-2, the SBIP-3 is also a composite, but includes side elements (fins) that are placed in the precut slots in the cortical bone walls.84,87,115
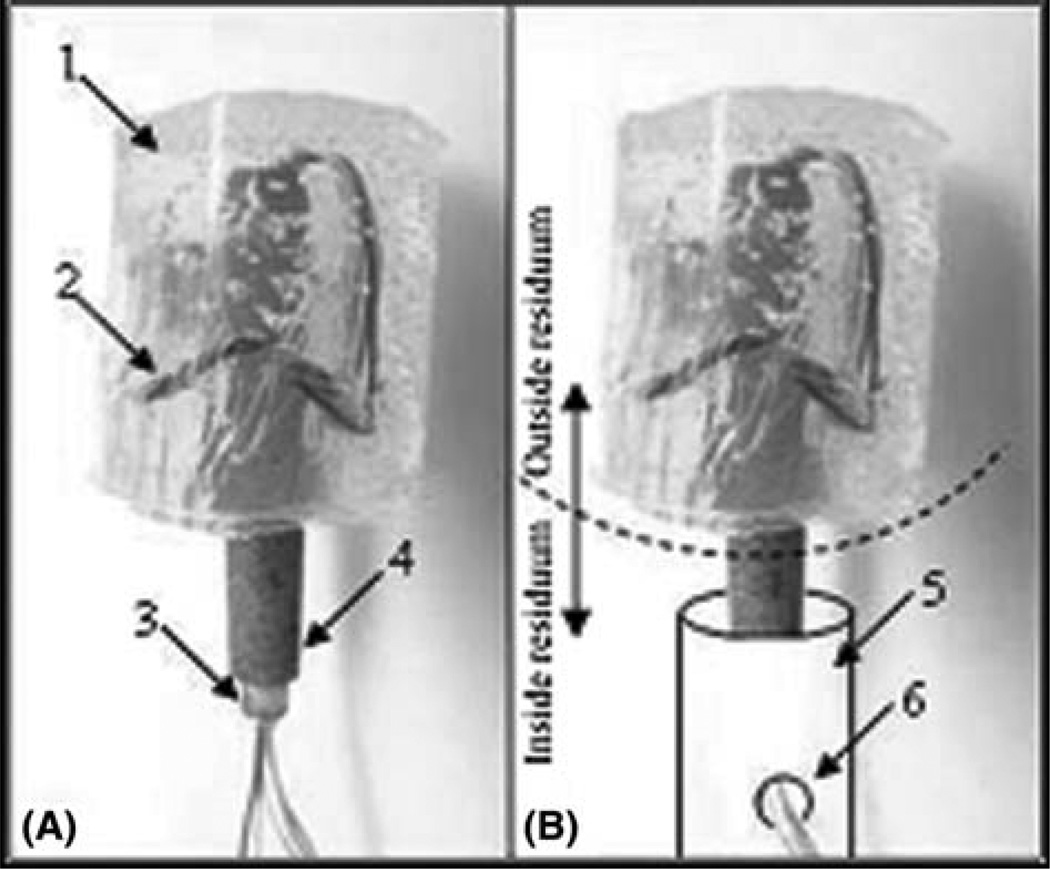
A modification with a peripheral nerve interface (SBIP-PNI) is a wired device for transcutaneous transmission of electric signals from a residuum’s muscle or nerve to an outside receiver for neurocontrol over a powered prosthesis.79 The SBIP-PNI is depicted in Figure 9, with a silicon shield (1) for wire electrodes (2) passing through the tube (3) surrounded by porous cladding (4).
FIGURE 9.
(A) SBIP-PNI: silicon shield (1) electrodes, (2) tube, and (3) porous cladding (4). (B) Bone of the residuum (5) with the hole (6) for releasing the electrodes for implanting to a muscle or nerve (Adapted from Ref. 79). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
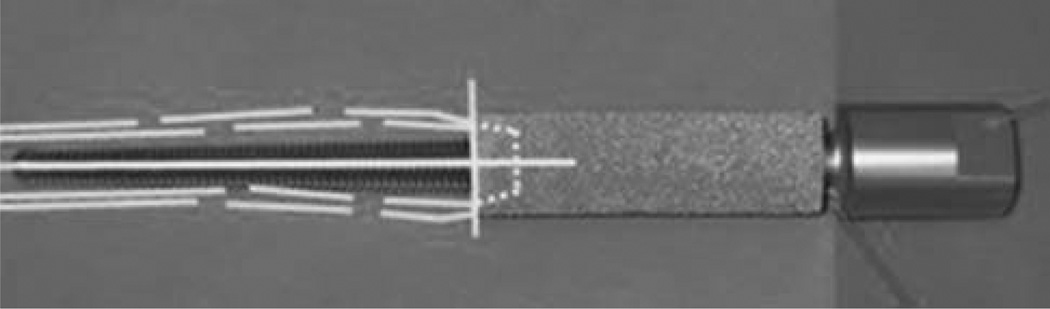
A two-stage procedure can be performed with the SBIP-F10 TS kits. As shown in Figure 10, a part to be implanted to the bone can be porous (A) or solid (B), similar to the OPRA system.
FIGURE 10.
SBIP-TS kits. (A) A kit with porous in-bone implant; (B) with solid in-bone implant. Dashed line indicates the skin-device line of interface. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
AEAHBM system
Two implants were designed, custom fabricated and applied to a dog with bilateral transtibial amputation.57 They were composed of a tapered, threaded titanium stem with a preassembled porous tantalum outer 14-mm-diameter sleeve and a 35mm distal segment with a Morse taper fitting (Fig. 11). The tantalum pores were dodecahedral in shape F11 with an average diameter of 500 mm. The tantalum collar was 70–80% porous by volume.
FIGURE 11.
AEAHBM system. Yellow lines represent the patient’s tibial shaft. Upper arrow identifies the Morse taper fitting. Lower arrow identifies the base of the tantalum metal (Reproduced from Ref. 57 with kind permission from John Wiley and Sons). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
In 14 months, the modified devices were reimplanted due to loosening. Positive outcomes were reported following 27 months after the first procedure.
UA system
The UA system for DSA was evaluated in one Yucatan micropig.58 The key feature of the system is a carbon sleeve (ring) threaded over a titanium rod for the device-skin interface (see Table I; Fig. 12). Unlike the carbon flange in the RLAH system12 (see Fig. 1), the device was incorporated with 99.9% pure silver to induce silver ion release into the amputation site, which is meant to increase the chance of successful, antibacterial interfacing.88 Silver wire was wrapped around a vitreous carbon ring and soldered to a 1.5 V battery and a 10 MX resistor to release a constant ionic charge of 150 nA.
FIGURE 12.
The UA system for the skin-interfacing stage. The carbon ring (upper left) (Adapted from Ref. 58; copyright © 2012, Informa Healthcare, with permission from Informa Healthcare).
The authors report the trial’s failure 10 weeks following implantation. However, they believe that the Ti6Al4V/ carbon/silver combination should be further investigated.
DISCUSSION
The safety of the skin-device interface constitutes the obvious challenge for a wider acceptance of the method of DSA.29,71,89–91 The longevity of the bone-device bond is also an important issue considering the relatively short hosting bone of the residuum and the high loads associated with operating limb prostheses.22,24,87,92 An additional concern for longevity of the bone-device bond is that after replacement surgery, the porosity of the cortical bone hosting the femur implant increases compared to the ipsilateral bone, potentially reducing cortical bone strength.93,94
To meet the existing challenges faced by DSA, the development of new designs and methodologies for reducing the associated risks95,96 continues. New challenges may arise with the promising use of the transcutaneous implants for neuromuscular control of the limb prostheses.79,97 These systems have an additional vulnerable interface between the tissues and the electrodes implanted to the muscles or nerves.98,99,116
There are reports on positive effects of different chemical, mechanical and biological treatments of the implant surface.100–102
Positive results were reported following treatment of the implant surface with fibronectin,103 laminin,104 and fibroblast growth factor FGF18.105 Bone morphogenic protein 2 (BMP-2) was suggested to enhance peri-implant osteogenesis in patients whose bone-healing capacity is compromised. That was confirmed in an animal study in which implants had a coating-incorporated depot of BMP-2.106
The osteoconductive properties of impacted titanium particles with a calcium–phosphate coating were found comparable to impacted allograft bone and impacted biphasic ceramics in a study analyzing the restoration of bone defects in hip arthroplasty.107 Osseointegration of poroussurfaced ceramic implants made of an alumina matrix composite (AMC) was assessed on maximum shear strength and histomorphometric bone ongrowth.108 Despite the low percentage of bone ingrowth, the AMC test implants demonstrated good mechanical stability due to bone ingrowth into the pores with subsequent interlocking.
Bone tissue response to implantation was investigated in the whole cavity, close to the implant and in its vicinity. Porous titanium coatings, a thickened titanium dioxide layer, amorphous microporous silica and bioactive glass were implanted into the tibia of 20 rabbits.109 A new finding was that bioactive glass was highly osteogenic, even at some distance from the implant.
It has been shown that the roughness of the metal surface increases cell apposition.110 Microroughened and nanosurface superimposed featured implants improved osteogenesis and the inflammatory/immune responses.111–114
CONCLUSIONS
Various implantation systems for DSA of limb prostheses have been developed for clinical and for preclinical applications. These systems possess their specific features and are at different levels of development in terms of number of patients treated worldwide and evidence provided in literature.
The high risks associated with DSA could be reduced with specific design features, including means for safer interface of the implanted device with the hosting tissues.
ACKNOWLEDGEMENTS
This material was based on work supported in part by the National Institutes of Health Grant HD057492.
REFERENCES
- 1.Kapp SL. Transfemoral socket design and suspension options. Phys Med Rehabil Clin N Am. 2000;11(3):569–583. vi. [PubMed] [Google Scholar]
- 2.Kapp S. Suspension systems for prostheses. Clin Orthop Relat Res. 1999;(361):55–62. doi: 10.1097/00003086-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Mak AF, Zhang M, Boone DA. State-of-the-art research in lowerlimb prosthetic biomechanics-socket interface: A review. J Rehabil Res Dev. 2001;38(2):161–174. [PubMed] [Google Scholar]
- 4.Mooney V, Predecki PK, Renning J, Gray J. Skeletal extension of limb prosthetic attachment—problems in tissue reaction. J Biomed Mater Res. 1971;5(6):143–159. [Google Scholar]
- 5.Horgan O, MacLachlan M. Psychosocial adjustment to lowerlimb amputation: A review. Disabil Rehabil. 2004;26(14–15):837–850. doi: 10.1080/09638280410001708869. [DOI] [PubMed] [Google Scholar]
- 6.Bosmans JC, Suurmeijer TPBM, Hulsink M, van der Schans CP, Geertzen JHB, Dijkstra PU. Amputation, phantom pain and subjective well-being: A qualitative study. Int J Rehabil Res. 2007;30(1):1–8. doi: 10.1097/MRR.0b013e328012c953. [DOI] [PubMed] [Google Scholar]
- 7.Hanley MA, Ehde DM, Jensen M, Czerniecki J, Smith DG, Robinson LR. Chronic pain associated with upper-limb loss. Am J Phys Med Rehabil. 2009;88(9):742. doi: 10.1097/PHM.0b013e3181b306ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geertzen JHB, Martina JD, Rietman HS. Lower limb amputation Part 2: Rehabilitation—A 10 year literature review. Prosthet Orthot Int. 2001;25(1):14–20. doi: 10.1080/03093640108726563. [DOI] [PubMed] [Google Scholar]
- 9.Hall CW. Developing a permanently attached artificial limb. Bull Prosthet Res. 1974;22:144–157. [PubMed] [Google Scholar]
- 10.Owens LJ. Apparatus for connecting a prosthesis to a bone. 3, 947,897. US Patent. 1976
- 11.Fernie GR, Kostuik JP, Lobb RJ. A percutaneous implant using a porous metal surface coating for adhesion to bone and a velour covering for soft tissue attachment: Results of trials in pigs. J Biomed Mater Res. 1977;11(6):883–891. doi: 10.1002/jbm.820110608. [DOI] [PubMed] [Google Scholar]
- 12.Mooney V, Schwartz SA, Roth AM, Gorniowsky MJ. Percutaneous implant devices. Ann Biomed Eng. 1977;5(1):34–46. doi: 10.1007/BF02409337. [DOI] [PubMed] [Google Scholar]
- 13.Brånemark P-I. Vital microscopy of bone marrow in rabbit. Scand J Clin Lab Invest. 1959;11(Suppl 38):1–82. [PubMed] [Google Scholar]
- 14.Albrektsson T, Brånemark PI, Hansson HA, Kasemo B, Larsson K, Lundström I, McQueen DH, Skalak R. The interface zone of inorganic implantsIn vivo: Titanium implants in bone. Ann Biomed Eng. 1983;11(1):1–27. [Google Scholar]
- 15.Eriksson E, Branemark PI. Osseointegration from the perspective of the plastic surgeon. Plast Reconstr Surg. 1994;93(3):626–637. [PubMed] [Google Scholar]
- 16.Brånemark P-I. Osseointegration and its experimental studies. J Prosthet Dent. 1983;50:399–410. doi: 10.1016/s0022-3913(83)80101-2. [DOI] [PubMed] [Google Scholar]
- 17.Branemark PI, af Ekenstam BT. Element for implantation in body tissue, particularly bone tissue. 4,330,891. US Patent. 1982
- 18.Branemark P. Implant fixture for tooth prosthesis. 4,988,299. US Patent #. 1991
- 19.Branemark PI. Fixture for anchoring in bone tissue. 5,324,199. US Patent #. 1994
- 20.Kasemo B, Lausmaa J. Material–tissue interfaces: The role of surface properties and processes. Environ Health Perspect. 1994;102(Suppl 5):41. doi: 10.1289/ehp.94102s541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleckman P, Olerud JE. Models for the histologic study of the skin interface with percutaneous biomaterials. Biomed Mater. 2008;3(3):034006. doi: 10.1088/1748-6041/3/3/034006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frossard L, Lee Gow D, Contoyannis B, Ewins D, Sullivan J, Tranberg R, Haggstrom E, Branemark R. Proceedings: International Society of Biomechanics XIXth Congress. The Human Body in Motion. Dunedin, New Zealand: University of Otago; 2003. Loading applied to the implant of transfemoral amputees fitted with a direct skeletal fixation during walking in a straight line and around a circle; pp. 114–114. [Google Scholar]
- 23.Helgason B, Pálsson H, Rúnarsson TP, Frossard L, Viceconti M. Risk of failure during gait for direct skeletal attachment of a femoral prosthesis: A finite element study. Med Eng Phys. 2009;31(5):595–600. doi: 10.1016/j.medengphy.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Frossard LA, Tranberg R, Haggstrom E, Pearcy M, Brånemark R. Load on osseointegrated fixation of a transfemoral amputee during a fall: Loading, descent, impact and recovery analysis. Prosthet Orthot Int. 2010;34(1):85. doi: 10.3109/03093640903585024. [DOI] [PubMed] [Google Scholar]
- 25.Isackson D, McGill LD, Bachus KN. Percutaneous implants with porous titanium dermal barriers: An in vivo evaluation of infection risk. Med Eng Phys. 2011;33(4):418–426. doi: 10.1016/j.medengphy.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pendegrass CJ, Tucker B, Patel S, Dowling R, Blunn GW. The effect of adherens junction components on keratinocyte adhesion in vitro: Potential implications for sealing the skin-implant interface of intraosseous transcutaneous amputation prostheses. J Biomed Mater Res A. 2012;100:3463–3471. doi: 10.1002/jbm.a.34290. [DOI] [PubMed] [Google Scholar]
- 27.Recum AFV, Shannon CE, Cannon CE, Long KJ, Kooten TGV, Meyle J. Surface roughness, porosity, and texture as modifiers of cellular adhesion. Tissue Eng. 1996;2(4):241–253. doi: 10.1089/ten.1996.2.241. [DOI] [PubMed] [Google Scholar]
- 28.von Recum AF. Applications and failure modes of percutaneous devices: A review. J Biomed Mater Res. 1984;18(4):323–336. doi: 10.1002/jbm.820180403. [DOI] [PubMed] [Google Scholar]
- 29.Tillander J, Hagberg K, Hagberg L, Branemark R. Osseointegrated titanium implants for limb prostheses attachments: Infectious complications. Clin Orthop Relat Res. 2010;468(10):2781–2788. doi: 10.1007/s11999-010-1370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson ML, Backman D, Branemark R, Mechefske CK. Evaluating the bending response of two osseointegrated transfemoral implant systems using 3D digital image correlation. J Biomech Eng. 2011;133(5):051006. doi: 10.1115/1.4003871. [DOI] [PubMed] [Google Scholar]
- 31.Thompson ML, Mechefske CK. Investigating the tightening torque of an osseointegrated transfemoral implant system. 25th Southern Biomedical Engineering Conference 2009; Springer; 15–17 May 2009; Miami, FL, USA. 2009. pp. 15–17. [Google Scholar]
- 32.Bachus K, Triplett DJ, Lewis TK. Releasble attachment systems for a prosthetic limb. 2012/0310371. US Patent Application #. 2012
- 33.Lee WCC, Frossard LA, Hagberg K, Haggstrom E, BrÃ¥nemark R, Evans JH, Pearcy MJ. Kinetics of transfemoral amputees with osseointegrated fixation performing common activities of daily living. Clin Biomech. 2007;22(6):665–673. doi: 10.1016/j.clinbiomech.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Pitkin M, Raykhtsaum G, Galibin OV, Protasov MV, Chihovskaya JV, Belyaeva IG. Skin and bone integrated prosthetic pylon: A pilot animal study. J Rehabil Res Dev. 2006;43(4):573–580. doi: 10.1682/jrrd.2005.05.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nebergall A, Bragdon C, Antonellis A, Kärrholm J, Brånemark R, Malchau H. Stable fixation of an osseointegated implant system for above-the-knee amputees. Acta Orthop. 2012;83(2):121–128. doi: 10.3109/17453674.2012.678799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van de Meent H, Frölke JP, Palm B. 4th Int. Conf. Advances in Orthopaedic Osseointegration. San Francisco, CA: UCSF; 2012. Feb 10–12, Preliminary results of the first 24 patients in The Netherlands; p. 31. [Google Scholar]
- 37.Aschoff HH, Juhnke DL. Evaluation of 10 years experience with endo-exo femur prostheses—Background, data and results. Z Orthop Unfall. 2012;150:607–614. doi: 10.1055/s-0032-1327932. [DOI] [PubMed] [Google Scholar]
- 38.Al Muderis M, Bosley B, Kumar A, Laux S. 4th Int. ConfAdvances in Orthopaedic Osseointegration. San Francisco, CA: UCSF; 2012. Feb 10–12, Early results of the osseointegration group of Australia accelerated protocol; p. 32. [Google Scholar]
- 39.Hagberg K, Brånemark R. One hundred patients treated with osseointegrated transfemoral amputation prostheses— Rehabilitation perspective. J Rehabil Res Dev. 2009;46(3):331. [PubMed] [Google Scholar]
- 40.Lundberg M, Hagberg K, Bullington J. My prosthesis as a part of me: A qualitative analysis of living with an osseointegrated prosthetic limb. Prosthet Orthot Int. 2011;35(2):207–214. doi: 10.1177/0309364611409795. [DOI] [PubMed] [Google Scholar]
- 41.Berlin Ö, Brånemark R, Hagberg K, Rydevik B, Bergh P, Gunterberg B. 4th Int. Conf. Advances in Orthopaedic Osseointegration. San Francisco, CA: UCSF; 2012. Feb 10–12, Osseointegrated prosthesis for the rehabilitation of amputees: Two-year results of the prospective OPRA study; pp. 23–24. [Google Scholar]
- 42.Hagberg K, Branemark R, Gunterberg B, Rydevik B. Osseointegrated trans-femoral amputation prostheses: Prospective results of general and condition-specific quality of life in 18 patients at 2-year follow-up. Prosthet Orthot Int. 2008;32(1):29–41. doi: 10.1080/03093640701553922. [DOI] [PubMed] [Google Scholar]
- 43.Tranberg R, Zügner R, Kärrholm J. Improvements in hip-and pelvic motion for patients with osseointegrated trans-femoral prostheses. Gait Posture. 2011;33(2):165–168. doi: 10.1016/j.gaitpost.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Hagberg K, Haggstrom E, Uden M, Branemark R. Socket versus bone-anchored trans-femoral prostheses: Hip range of motion and sitting comfort. Prosthet Orthot Int. 2005;29(2):153–163. doi: 10.1080/03093640500238014. [DOI] [PubMed] [Google Scholar]
- 45.Hagberg K, Branemark R. Consequences of non-vascular transfemoral amputation: A survey of quality of life, prosthetic use and problems. Prosthet Orthot Int. 2001;25(3):186–194. doi: 10.1080/03093640108726601. [DOI] [PubMed] [Google Scholar]
- 46.Aschoff HH, Kennon RE, Keggi JM, Rubin LE. Transcutaneous, distal femoral, intramedullary attachment for above-the-knee prostheses: An endo-exo device. J Bone Joint Surg. 2010;92(Suppl 2):180–186. doi: 10.2106/JBJS.J.00806. [DOI] [PubMed] [Google Scholar]
- 47.Rochminski R, Sibnski M, Synder M. Osseointegration as a method of direct stabilization of amputation prostheses to the bone. Chir Narzadow Ruchu Ortop Pol. 2011;76(1):36–40. [PubMed] [Google Scholar]
- 48.Frossard L, Hagberg K, Häggström E, Gow DL, Brånemark R, Pearcy M. Functional outcome of transfemoral amputees fitted with an osseointegrated fixation: Temporal gait characteristics. J Prosthet Orthot. 2010;22(1):11. [Google Scholar]
- 49.Ax E. The personal dimension of innovation: Making a personal choice to improve quality of life with innovation; International Society for Prosthetics and Orthotics 11th World Congress Hong Kong; 2004. [Google Scholar]
- 50.Haggstrom E, Branemark M. Proc. 11th World Congress of ISPO2004. Hong Kong: 2004. The Osseointegrated Transfemoral Prosthesis. [Google Scholar]
- 51.Palmquist A, Jarmar T, Emanuelsson L, Branemark R, Engqvist H, Thomsen P. Forearm bone-anchored amputation prosthesis: A case study on the osseointegration. Acta Orthop. 2008;79(1):78–85. doi: 10.1080/17453670710014806. [DOI] [PubMed] [Google Scholar]
- 52.Jönsson S, Caine-Winterberger K, Brånemark R. Proceedings of the 2011 MyoElectric Controls/Powered Prosthetics Symposium Fredericton. New Brunswick, Canada: 2011. Osseointegration on upper limb amputee. Prosthetic treatment. [Google Scholar]
- 53.Webster JB, Chou T, Kenly M, English M, Roberts TL, Bloebaum RD. Perceptions and acceptance of osseointegration among individuals with lower limb amputations: A prospective survey study. J Prosthet Orthot. 2009;21(4):215. [Google Scholar]
- 54.Branemark R, Branemark PI, Rydevik B, Myers RR. Osseointegration in skeletal reconstruction and rehabilitation: A review. J Rehabil Res Dev. 2001;38(2):175–181. [PubMed] [Google Scholar]
- 55.Pendegrass CJ, Goodship AE, Blunn GW. Development of a soft tissue seal around bone-anchored transcutaneous amputation prostheses. Biomaterials. 2006;27(23):4183–4191. doi: 10.1016/j.biomaterials.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 56.Shelton TJ, Peter Beck J, Bloebaum RD, Bachus KN. Percutaneous osseointegrated prostheses for amputees: Limb compensation in a 12-month ovine model. J Biomech. 2011;44:2601–2606. doi: 10.1016/j.jbiomech.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drygas KA, Taylor R, Sidebotham CG, Hugate RR, McAlexander H. Transcutaneous tibial implants: A surgical procedure for restoring ambulation after amputation of the distal aspect of the tibia in a dog. Vet Surg. 2008;37(4):322–327. doi: 10.1111/j.1532-950X.2008.00384.x. [DOI] [PubMed] [Google Scholar]
- 58.Saunders MM, Brecht JS, Verstraete MC, Kay DB, Njus GO. Lower limb direct skeletal attachment. A Yucatan micropig pilot study. J Invest Surg. 2012;25(6):387–397. doi: 10.3109/08941939.2012.670366. [DOI] [PubMed] [Google Scholar]
- 59.Brånemark PI. Anchoring element for implantation in tissue, for holding prosthesis, artificial joint components or the like. 5,702,445. US Patent. 1997
- 60.Orsini E, Giavaresi G, Trirà A, Ottani V, Salgarello S. Dental implant thread pitch and its influence on the osseointegration process: An in vivo comparison study. Int J Oral Maxillofac Implants. 2012;27(2):383. [PubMed] [Google Scholar]
- 61.Brånemark PI. Fixture. 7,753,942. US Patent #. 2010
- 62.Branemark R, Thomsen P. Implant and an implant member. 2008/0125868. US Patent App #. 2008
- 63.Tranberg R, Zugner R, Karrholm J. Improvements in hip- and pelvic motion for patients with osseointegrated trans-femoral prostheses. Gait Posture. 2011;33(2):165–168. doi: 10.1016/j.gaitpost.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 64.Xu W, Robinson K. X-ray image review of the bone remodeling around an osseointegrated trans-femoral implant and a finite element simulation case study. Ann Biomed Eng. 2008;36(3):435–443. doi: 10.1007/s10439-007-9430-7. [DOI] [PubMed] [Google Scholar]
- 65.Larsson A, Wigren S, Andersson M, Ekeroth G, Flynn M, Nannmark U. Histologic evaluation of soft tissue integration of experimental abutments for bone anchored hearing implants using surgery without soft tissue reduction. Otol Neurotol. 2012;33(8):1445–1451. doi: 10.1097/MAO.0b013e318268d4e0. [DOI] [PubMed] [Google Scholar]
- 66.Tjellstrom A, Hakansson B. The bone-anchored hearing aid. Design principles, indications, and long-term clinical results. Otolaryngol Clin North Am. 1995;28(1):53–72. [PubMed] [Google Scholar]
- 67.Aschoff HH, Clausen A, Hoffmeister T. The endo-exo femur prosthesis-a new concept of bone-guided, prosthetic rehabilitation following above-knee amputation. Zeitschrift fur Orthopadie und Unfallchirurgie. 2009;147(5):610. doi: 10.1055/s-0029-1185893. [DOI] [PubMed] [Google Scholar]
- 68.Aschoff HH, McGough RL. The endo-exo femoral prosthesis: A new rehabilitation concept following above knee amputation. J Bone Joint Surg Br. 2012;94(Suppl XXXIX):77–77. [Google Scholar]
- 69.Blunn G, Cobb J, Goodship A, Unwin P. Transcutaneous prosthesis. 7,014,661. US Patent #. 2006
- 70.Blunn G, Cobb J, Goodship A, Unwin P. Method of installing a transcutaneous prosthesis. 8,137,409. US Patent #. 2012
- 71.Kang NV, Pendegrass C, Marks L, Blunn G. Osseocutaneous integration of an intraosseous transcutaneous amputation prosthesis implant used for reconstruction of a transhumeral amputee: Case report. J Hand Surg Am. 2010;35(7):1130–1134. doi: 10.1016/j.jhsa.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 72.Fitzpatrick N, Smith TJ, Pendegrass CJ, Yeadon R, Ring M, Goodship AE, Blunn GW. Intraosseous transcutaneous amputation prosthesis (ITAP) for limb salvage in 4 dogs. Vet Surg. 2011;40(8):909–925. doi: 10.1111/j.1532-950X.2011.00891.x. [DOI] [PubMed] [Google Scholar]
- 73.Banks WJ, Newbrey JW. Antler Development in Cervidae. First International Symposium of the Caesar Kleberg Wildlife Research Institute. Kingsville, TX: College of Agriculture, Texas A&I University; 1983. Antler development as a unique modification of mammalian endochondral ossification; pp. 279–306. [Google Scholar]
- 74.Grosse-Siestrup C, Affeld K. Design criteria for percutaneous devices. J Biomed Mater Res. 1984;18(4):357–382. doi: 10.1002/jbm.820180405. [DOI] [PubMed] [Google Scholar]
- 75.Chimutengwende-Gordon M, Pendegrass C, Blunn G. Enhancing the soft tissue seal around intraosseous transcutaneous amputation prostheses using silanized fibronectin titanium alloy. Biomed Mater. 2011;6(2):025008. doi: 10.1088/1748-6041/6/2/025008. [DOI] [PubMed] [Google Scholar]
- 76.Jeyapalina S, Beck JP, Bachus KN, Williams DL, Bloebaum RD. Efficacy of a porous-structured titanium subdermal barrier for preventing infection in percutaneous osseointegrated prostheses. J Orthop Res. 2012;30(8):1304–1311. doi: 10.1002/jor.22081. [DOI] [PubMed] [Google Scholar]
- 77.Pitkin M, Raykhtsaum G. Skin Integrated Device. 8,257,435. US Patent #. 2012
- 78.Pitkin M, Pilling J, Raykhtsaum G. Mechanical properties of totally permeable titanium composite pylon for direct skeletal attachment. J Biomed Mater Res Part B: Appl Biomater. 2012;100B(4):993–999. doi: 10.1002/jbm.b.32663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pitkin M, Cassidy C, Muppavarapu R, Edell D. Recording of electric signal passing through a pylon in direct skeletal attachment of leg prostheses with neuromuscular control. IEEE Trans Biomed Eng. 2012;59(5):1349–1353. doi: 10.1109/TBME.2012.2187784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pitkin M, Cassidy C, Muppavarapu R, Raymond J. 4th Int. Conf. Advances in Orthopaedic Osseointegration. San Francisco, CA: UCSF; 2012. Feb 10–12, New method of fixation of in-bone implanted prosthesis; p. 12. [Google Scholar]
- 81.Farrell B, Kistenberg R, Dalton J, Strong A, Pitkin M, Prilutsky B. 4th Int. Conf. Advances in Orthopaedic Osseointegration. San Francisco, CA: UCSF; 2012. Feb 10–12, Evaluation of skin and bone integration with a porous titanium pylon after prosthetic gait rehabilitation in the cat; p. 14. [Google Scholar]
- 82.Farrell B, Pitkin M, Popat K, Prilutsky B. 4th Int. Conf. Advances in Orthopaedic Osseointegration. San Francisco, CA: UCSF; 2012. Feb 10–12, Effect of pore size, implantation time and nano-surface properties on rat skin ingrowth into porous titanium; p. 13. [Google Scholar]
- 83.Shevtsov M, Galibin O, Yudintseva N, Blinova M, Pinaev G, Scherbina K, Shvedovchenko I, Pitkin M. Osseointegration in reconstructive surgery: Contemporary state and perspectives of further development. Traumatol Orthop Russia. Forthcoming. [Google Scholar]
- 84.Pitkin M, Raykhtsaum G, Pilling J, Shukeylo Y, Moxson V, Duz V, Lewandowski J, Connolly R, Kistenberg RS, Dalton JF, Prilutsky B, Jacobson S. Mathematical modeling and mechanical and histopathological testing of porous prosthetic pylon for direct skeletal attachment. J Rehabil Res Dev. 2009;46(3):315–330. doi: 10.1682/jrrd.2008.09.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pitkin M, Raykhtsaum G, Pilling J, Galibin O, Protasov M, Chihovskaya J, Belyaeva I, Blinova M, Yudintseva M, Potokin I, Pinaev G, Moxson V, Duz V. Porous composite prosthetic pylon for integration with skin and bone. J Rehabil Res Dev. 2007;44(5):723–738. doi: 10.1682/jrrd.2006.12.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pitkin M, Blinova MI, Yudintseva NV, Potokin IL, Raykhtsaum G, Pinaev GP. Skin and bone integrated prosthetic technology. I. Characterization and morphology of human cells cultivated on titanium implants of different structures; 9th Russian National Congress "People and Health"; 22–26 November 2004; St. Petersburg. 2004. [Google Scholar]
- 87.Pitkin M. One lesson from arthroplasty to osseointegration in a search for better fixation of in-bone implanted prosthesis. J Rehabil Res Dev. 2008;45(4):vii–xiv. [PMC free article] [PubMed] [Google Scholar]
- 88.Kloth LC, McCulloch JM. Promotion of wound healing with electrical stimulation. Adv Wound Care. 1996;9(5):42–45. [PubMed] [Google Scholar]
- 89.Albrektsson T, Branemark PI, Hansson HA, Lindstrom J. Osseointegrated titanium implants. Requirements for ensuring a long-asting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981;52(2):155–170. doi: 10.3109/17453678108991776. [DOI] [PubMed] [Google Scholar]
- 90.Holgers KM, Branemark PI. Immunohistochemical study of clinical skin-penetrating titanium implants for orthopaedic prostheses compared with implants in the craniofacial area. Scand J Plast Reconstr Surg Hand Surg. 2001;35(2):141–148. doi: 10.1080/028443101300165273. [DOI] [PubMed] [Google Scholar]
- 91.Holgers KM, Thomsen P, Tjellstrom A. Persistent irritation of the soft tissue around an osseointegrated titanium implant. Case report. Scand J Plast Reconstr Surg Hand Surg. 1994;28(3):225–230. doi: 10.3109/02844319409015984. [DOI] [PubMed] [Google Scholar]
- 92.Helgason B, Pálsson H, Rúnarsson TP, Frossard L, Viceconti M. Risk of failure during gait for direct skeletal attachment of a femoral prosthesis: A finite element study. Med Eng Phys. 2009;31(5):595–600. doi: 10.1016/j.medengphy.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 93.Rosenbaum Chou TG, Child JR, Naughtin RJ, Rigdon RR, Schumann C, Bloebaum RD. The relationship between femoral periprosthetic cortical bone geometry and porosity after total hip arthroplasty. J Biomed Mater Res Part A. 2008;87(1):107–115. doi: 10.1002/jbm.a.31702. [DOI] [PubMed] [Google Scholar]
- 94.Tomaszewski PK, van Diest M, Bulstra SK, Verdonschot N, Verkerke GJ. Numerical analysis of an osseointegrated prosthesis fixation with reduced bone failure risk and periprosthetic bone loss. J Biomech. 2012;45(11):1875–1880. doi: 10.1016/j.jbiomech.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 95.Tomaszewski PK, Verdonschot N, Bulstra SK, Rietman JS, Verkerke GJ. Simulated bone remodeling around two types of osseointegrated implants for direct fixation of upper-leg prostheses. J Mech Behav Biomed Mater. 2012;15:167–175. doi: 10.1016/j.jmbbm.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 96.Tomaszewski PK, Verdonschot N, Bulstra SK, Verkerke GJ. A comparative finite-element analysis of bone failure and load transfer of osseointegrated prostheses fixations. Ann Biomed Eng. 2010;38(7):2418–2427. doi: 10.1007/s10439-010-9966-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pantall A, Durham S, Ewins D. Surface electromyographic activity of five residual limb muscles recorded during isometric contraction in transfemoral amputees with osseointegrated prostheses. Clin Biomech. 2011;26(7):760–765. doi: 10.1016/j.clinbiomech.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 98.Rossini PM, Micera S, Benvenuto A, Carpaneto J, Cavallo G, Citi L, Cipriani C, Denaro L, Denaro V, Di Pino G. Double nerve intraneural interface implant on a human amputee for robotic hand control. Clin Neurophysiol. 2010;121(5):777–783. doi: 10.1016/j.clinph.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 99.Jia X, Koenig MA, Zhang X, Zhang J, Chen T, Chen Z. Residual motor signal in long-term human severed peripheral nerves and feasibility of neural signal-controlled artificial limb. J Hand Surg. 2007;32(5):657–666. doi: 10.1016/j.jhsa.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 100.Dohan Ehrenfest DM, Coelho PG, Kang BS, Sul YT, Albrektsson T. Classification of osseointegrated implant surfaces: Materials, chemistry and topography. Trends Biotechnol. 2010;28(4):198–206. doi: 10.1016/j.tibtech.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 101.Wang M, Li J, Keidar M, Zhang LG. MRS Proceedings. Cambridge Univ Press; 2012. Design a biologically inspired nanostructured coating for better osseointegration. [Google Scholar]
- 102.Chen J, Rungsiyakull C, Li W, Chen Y, Swain M, Li Q. Multiscale design of surface morphological gradient for osseointegration. J Mech Behav Biomed Mater. 2012 doi: 10.1016/j.jmbbm.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 103.Pendegrass CJ, El-Husseiny M, Blunn GW. The development of fibronectin-functionalised hydroxyapatite coatings to improve dermal fibroblast attachment in vitro. J Bone Joint Surg Br. 2012;94(4):564–569. doi: 10.1302/0301-620X.94B4.27698. [DOI] [PubMed] [Google Scholar]
- 104.Gordon DJ, Bhagawati DD, Pendegrass CJ, Middleton CA, Blunn GW. Modification of titanium alloy surfaces for percutaneous implants by covalently attaching laminin. J Biomed Mater Res A. 2010;94(2):586–593. doi: 10.1002/jbm.a.32735. [DOI] [PubMed] [Google Scholar]
- 105.Carli A, Gao C, Khayyat-Kholghi M, Li A, Wang H, Ladel C, Harvey EJ, Henderson JE. FGF18 augments osseointegration of intra-medullary implants in osteopenic FGFR3(−/−) mice. Eur Cell Mater. 2012;24:107–116. doi: 10.22203/ecm.v024a08. discussion 116–117. [DOI] [PubMed] [Google Scholar]
- 106.Hunziker EB, Enggist L, Kuffer A, Buser D, Liu Y. Osseointegration: The slow delivery of BMP-2 enhances osteoinductivity. Bone. 2012;51(1):98–106. doi: 10.1016/j.bone.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 107.Walschot LH, Aquarius R, Schreurs BW, Verdonschot N, Buma P. Osteoconduction of impacted porous titanium particles with a calcium-phosphate coating is comparable to osteoconduction of impacted allograft bone particles: In vivo study in a nonloaded goat model. J Biomed Mater Res B Appl Biomater. 2012;100(6):1483–1489. doi: 10.1002/jbm.b.32716. [DOI] [PubMed] [Google Scholar]
- 108.Schreiner U, Koester H, Pott P, Scheller G, Schwarz M. Osteointegration of an alumina matrix composite ceramic with a porous surface: Mechanical and histological results of an animal experiment. Z Orthop Unfall. 2009;147(5):603–609. doi: 10.1055/s-0029-1185623. [DOI] [PubMed] [Google Scholar]
- 109.Chaudhari A, Braem A, Vleugels J, Martens JA, Naert I, Cardoso MV, Duyck J. Bone tissue response to porous and functionalized titanium and silica based coatings. PLoS One. 2011;6(9):e24186. doi: 10.1371/journal.pone.0024186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hacking SA, Boyraz P, Powers BM, Sen-Gupta E, Kucharski W, Brown CA, Cook EP. Surface roughness enhances the osseointegration of titanium headposts in non-human primates. J Neurosci Methods. 2012;211(2):237–244. doi: 10.1016/j.jneumeth.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 111.Thalji G, Gretzer C, Cooper LF. Comparative molecular assessment of early osseointegration in implant-adherent cells. Bone. 2013;52(1):444–453. doi: 10.1016/j.bone.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 112.Wu X, Liu X, Wei J, Ma J, Deng F, Wei S. Nano-TiO2/PEEK bioactive composite as a bone substitute material: In vitro and in vivo studies. Int J Nanomed. 2012;7:1215–1225. doi: 10.2147/IJN.S28101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Balla VK, DeVasConCellos PD, Xue W, Bose S, Bandyopadhyay A. Fabrication of compositionally and structurally graded Ti–TiO2 structures using laser engineered net shaping (LENS) Acta Biomater. 2009;5(5):1831–1837. doi: 10.1016/j.actbio.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 114.Devasconcellos P, Balla VK, Bose S, Fugazzi R, Dernell WS, Bandyopadhyay A. Patient specific implants for amputation prostheses: Design, manufacture and analysis. Vet Comp Orthop Traumatol. 2012;25(4):286–296. doi: 10.3415/VCOT-11-03-0043. [DOI] [PubMed] [Google Scholar]
- 115.Pitkin MC, Cassidy R, Muppavarapu J, Raymond M, Shevtsov O, Galibin SD. New method of fixation of in-bone implanted prosthesis. J. Rehabil Res Dev. 2013 doi: 10.1682/jrrd.2012.11.0202. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Al-Ajam YH, Lancashire C, Pendegrass N, Kang R, Dowling S, Taylor BG. The Use of a Bone-Anchored Device as a Hard-Wired Conduit for Transmitting EMG Signals from Implanted Muscle Electrodes. IEEE Trans Biomed Eng. 2013 doi: 10.1109/TBME.2013.2241060. in print. [DOI] [PubMed] [Google Scholar]