Abstract
Effective and safe drug administration in neonates should be based on integrated knowledge on the evolving physiological characteristics of the infant who will receive the drug, and the pharmacokinetics (PK) and pharmacodynamics (PD) of a given drug. Consequently, clinical pharmacology in neonates is as dynamic and diverse as the neonates we admit to our units while covariates explaining the variability are at least as relevant as median estimates.
The unique setting of neonatal clinical pharmacology will be highlighted based on the hazards of simple extrapolation of maturational drug clearance when only based on ‘adult’ metabolism (propofol, paracetamol). Secondly, maturational trends are not at the same pace for all maturational processes. This will be illustrated based on the differences between hepatic and renal maturation (tramadol, morphine, midazolam). Finally, pharmacogenetics should be tailored to neonates, not just mirror adult concepts.
Because of this diversity, clinical research in the field of neonatal clinical pharmacology is urgently needed, and facilitated through PK/PD modeling. In addition, irrespective of already available data to guide pharmacotherapy, pharmacovigilance is needed to recognize specific side effects. Consequently, paediatric anesthesiologists should consider to contribute to improved pharmacotherapy through clinical trial design and collaboration, as well as reporting on adverse effects of specific drugs.
Keywords: pharmacokinetics, pharmacodynamics, newborn, infant, anesthesia, ontogeny
Clinical pharmacology in neonates: limited size, extensive variability
When a drug is administered, it is with the intention to attain a proportional therapeutic effect (e.g. analgesia, sedation, muscular relaxation), preferably without disproportional adverse effects (e.g. prolonged sedation, hypotension, toxicity). Clinical pharmacology aims to predict such (adverse) effects based on drug-, population and patient-specific pharmacokinetics (PK) and –dynamics (PD). PK describe the relationship between a drug concentration at a given site (e.g. plasma, cerebrospinal fluid) and time (‘what the body does to the drug’). PD describe the relationship between a drug concentration at a given site and (adverse)-effects (‘what the drug does to the body’). These general principles of clinical pharmacology obviously also apply to neonates, but their specific characteristics warrant a population focused approach (1-5). These specific characteristics mainly relate to developmental physiology, and are also reflected in the different topics covered in this special issue on neonates, and include - but are not limited to - the neonatal cardiovascular system, lung and brain. Historical observations on the grey baby syndrome (chloramphenicol toxicity related due to impaired glucuronidation), the neonatal gasping syndrome (benzyl alcohol toxicity, co-administrated as excipient in the setting of impaired alcohol clearance capacity) or hexachlorophene bathing (increased transcutaneous absorption and limited clearance capacity, aimed to reduce impetigo) encephalopathy all illustrate the obvious clinical need to know more about neonatal pharmacology in order to avoid further tragedies (1-10).
Growth and development throughout pediatric human life comprises of a series of simultaneously ongoing physiologic events that result both in growth and maturation, commonly subdivided into subpopulations (newborn, infancy, childhood, and adolescence). Across this pediatric life span, organ size and function change as does body composition and ultimately, cellular function and metabolic activity. This will affect population specific pharmacokinetics (6-10). In addition, some tissues may be more sensitive to pharmacologic effects early in life, irrespective of a given concentration or exposure whereas others will be less. This will affect population specific pharmacodynamics (6-10). The issue on apoptosis following exposure to analgosedatives and anesthetics during infancy is a reflection of such population specific vulnerability (11), similar to dexamethasone (impaired cerebral growth, cerebral palsy) (12) or exposure to nephrotoxic compounds during nephrogenesis in preterm neonates (reduced number of glomeruli, up to 34-36 weeks postmenstrual age)(13).
There is already one order of magnitude difference in weight (0.5 to 5 kg) in the population currently hospitalized in neonatal intensive care units, similar to the full paediatric size spectrum (5 to 50 kg). The height velocity rate (10-20 cm/year) in the last trimester of intra-uterine life and the first months of extra-uterine life, the increase in body weight (50 % increase in the first 6 weeks, and 3 times in the first year of life) and the total energy requirements of infancy further illustrate the dynamics of an evolving biological system, characterized by growth and maturation as most crucial features (1-10). Since maturational physiological changes are most prominent in infancy, variability is the key feature of clinical pharmacology in infancy: developmental pharmacology is applied developmental physiology.
From a clinical pharmacology perspective, the consequence of such a rapidly changing system is that there is extensive within and between subject variability in drug disposition and effects in early life. This maturation related variability is further aggravated by interfering pathological processes (e.g. growth restriction, sepsis, associated cardiopathy, organ failure) or treatment modalities (e.g. co-medication, extracorporeal membrane oxygenation, surgical intervention). Consequently, there is an obvious need to focus on the covariates contributing to this variability within this population as well as quantification of median estimates. Maturational pharmacokinetics consider maturational changes in either drug absorption, distribution, metabolism and elimination (ADME), while maturational pharmacodynamics consider maturational changes in the concentration-effect profile e.g. differences in receptor expression, function or specific tissue/organ maturational sensitivity (either more or less vulnerable) (1-10).
Absorption relates to physicochemical characteristics and patient factors that influence the translocation of a given compound from its exposure site (e.g. enteral, pulmonary, cutaneous) to the bloodstream or another effect compartment. Distribution describes the transfer of a specific drug from one location to another (e.g. tissues, organs, fluid spaces) within the body, and is commonly quantified by the distribution volume [Vd = total amount of a given drug/concentration]. Although this volume of distribution not necessary represents a physiological volume or space, it is commonly affected by maturational physiological changes like e.g. body composition, regional blood flow, organ size, barriers or plasma protein concentrations. Metabolism and elimination clearance (i.e. excretion) together reflect the clearance capacity, i.e. the volume of blood or plasma from which a drug is completely removed per unit of time. Table 1 provides some illustrations on the impact of population specific characteristics on the pharmacokinetics of compounds also administered in the perioperative period (1,9,14-24).
Table 1.
Illustrations of the impact of neonatal physiology on the pharmacokinetics (absorption, distribution, metabolism, elimination) of specific drugs commonly administered to neonates (1,9,14-24)[NMBD = neuromuscular blocking drugs].
| compound | pharmacokinetics | relevance |
|---|---|---|
| iodine disinfectant (14) | skin more permeable, skin surface/kg higher (a) | higher absorption may suppress thyroid function |
| inhalational agents (1,9) | higher alveolar ventilation/functional residual capacity ratio (a) | faster wash in |
| cefazolin (15) | lower protein binding capacity results in higher distribution volume (d), and higher free plasma fraction (e) lower glomerular filtration rate (e) |
peak concentration is lower bactericid effect relates to free concentration lower clearance, prolonged duration of bactericid effect |
| bupivacaine (16) | lower protein binding capacity (d) lower clearance (e) |
free concentration related to adverse effects accumulation during continuous administration |
| propofol (17,18,19) | lipophilic compound, lower distribution volume (d) glucuronidation for metabolic clearance (m) |
peak concentration is lower, redistribution more limited. accumulation during continuous or repeated administration more profound hypotension due to immature (para)sympathetic balance |
| paracetamol (20,21) | water soluble compound, higher distribution volume (d) glucuronidation for metabolic clearance (m) |
peak concentration is lower, less effective analgesia likely accumulation during repeated administration possible |
| midazolam (22) | clearance to metabolite (1-hydroxy) is low (m) elimination clearance of (1-hydroxy) midazolam is low (e) |
metabolite is also sedative, less prolonged sedation. lower clearance results in prolonged sedation. |
| EMLA cream (23) | skin more permeable, skin surface/kg higher (a) | higher absorption, may induce local anesthetics related seizures. Increased risk for methemoglobinemia |
| Codeine (24) | clearance to metabolite (morphine) is low (m) elimination of codeine and metabolite is low (e) |
shorter or reduced analgesic effect accumulation of codeine or metabolite more likely, prolonged or more pronounced analgesia |
| NMBD (1,3) | increased distribution volume (d) lower clearance (m) |
lower concentration at the endplate, compensated by lower acetylcholine. prolonged effect |
For compounds commonly used in perioperative medicine [e.g. cefazolin (15), paracetamol (20) compared to midazolam (22), morphine (25)], the newborn displays a higher distribution volume (l/kg) for water soluble drugs, necessitating a higher loading dose (/kg) to reach a given plasma concentration (12,16,18,20). In contrast to this higher initial dose, the lower clearance capacity (l/kg/h) results in the need for lower maintenance doses (lower dose, or prolonged dosing interval) to avoid accumulation (15,20,22,25). It is only once maturational PK have been taken into account, that maturational differences in PD can be explored. The perception that the effects of a given drug are different in the newborn is often due to the fact that PK have not yet been adequately studied in this population. The same dose (e.g. kg−1) in a newborn may result in a different concentration/time profile and subsequent different effects.
To illustrate this, we would like to summarize the available PK/PD data on paracetamol in neonates (20,21,26-32). Because of slower gastric emptying, absorption at the duodenal level will be slower after oral administration while rectal administration does not result in predictable exposure (26). Because of differences in body composition (e.g. higher body water content) and because of the fact that paracetamol is a water soluble compound, the distribution volume (0.6 l.kg-1) is higher in the newborn: the peripheral volume of distribution decreases from 27 weeks postmenstrual age (45 l .70 kg−1) to reach 110% of its mature value by 6 months of age (20,27). The delayed absorption and the more extensive distribution will result in a delayed, blunted peak paracetamol concentration when compared to children or adults. Subsequent metabolic clearance of paracetamol through sulphation matures more rapidly than glucuronidation which is still poor in neonates. This results in lower clearance and slower decrease after the peak paracetamol concentration has been reached. Because of this lower clearance, accumulation is more likely (20,21,27). Subsequent toxicity related to paracetamol accumulation will depend on both the capacity to produce highly reactive electrophilic arylating metabolites [e.g. N-acetyl-p-benzoquinone-imine (NAPQI)] by the hepatic cytochrome P-450-dependent (CYP) E1 mixed function oxidase enzyme system, as well as NAPQI detoxification capacity through glutathione conjugation (21,28).
As opposed to PK, PD is only poorly described in neonates (29). Paracetamol PD is no exception. To further address potential maturational differences in analgesia (PD outcome variable), pain scores were collected in neonates and effect were estimated when an effect compartment paracetamol concentration of 10 mg/l was attained (30). The pain relief in neonates at this concentration was similar to that described in children following adenotonsillectomy (30,31). Although preliminary, these data suggest that population related differences in paracetamol analgesia mainly relate to PK and not to PD differences (30,31). Similarly, the opioid sparing effect of intravenous paracetamol in non-cardiac neonates has recently been described (32).
However, some paracetamol PD aspects may be specific to the neonatal population. There are reports of the association – not necessary causal - between paracetamol exposure during infancy or even during fetal life and the subsequent risk of developing atopy-related syndromes (33,34). Even more intriguing, reports on an association between paracetamol exposure and patent ductus arteriosus closure in a limited number of extreme preterm neonates have been published (35,36). However, causality cannot yet be taken for granted because a link between the physiology of ductal closure and the pharmacology of paracetamol is unknown. This is because it is assumed that paracetamol has only modest effects at peripheral sites and is a poor anti-inflammatory and anti-thrombotic compound (29,35,36).
There are several recent reviews on the impact of maturation of absorption, distribution, metabolism and elimination on PK in neonates (1-10,37). Consequently, the unique setting of neonatal clinical pharmacology will be highlighted based on the hazards of simple extrapolation of maturational drug clearance when only based on ‘adult’ metabolism (propofol, paracetamol). Secondly, maturational trends are not at the same pace for all maturational processes. This will be illustrated based on the differences between hepatic and renal maturation (tramadol, morphine, midazolam). Finally, pharmacogenetics should be tailored to neonates and not just mirror adult concepts.
Neonatal drug metabolism is not just a ‘miniaturized’ adult pattern
Drug metabolizing enzymes are crucial in drug biotransformation and elimination. Consequently, drug metabolizing iso-enzyme specific ontogeny will affect neonatal drug clearance. Profound changes in drug metabolizing enzyme expression occurs during development that impacts on drug effect and the risk of adverse events in the neonate (7,8,10). The current knowledge suggests that individual drug metabolizing iso-enzymes can be categorized into one of three classes based on developmental trajectories. A first group of enzymes (e.g. CYP3A7, SULT1A3/1A4) are expressed at their highest level in fetal life. Their activity will decrease and disappear over the first two years of life. The second group consists of enzymes (e.g. CYP3A5, CYP2C19, SULT1A1) that only display a moderate increase after birth and become more active in later pediatric life. The third group (e.g. CYP2D6, CYP3A4, CYP2C9, CYP1A2) displays modest ontogeny in the second or third trimester of pregnancy with another relevant increase in phenotypic activity throughout infancy (7,8,10).
Although the concept is very helpful to explain and even predict maturational drug disposition, this should be used cautiously. Clearance maturation for two drugs that share common elimination pathways in adults may differ. We cannot simply miniaturize ‘major’ and ‘minor’ routes of elimination as documented in adults to neonates. In the absence of an adult ‘major’ route, an ‘minor’ route, either metabolic or primary elimination may turn to be a more relevant route of elimination clearance in neonates (e.g. caffeine elimination is through renal clearance in neonates and is metabolized by CYP1A2 in adults). To illustrate this further, we refer to observations on propofol metabolism in adults compared to neonates and put these into perspective based on similar paracetamol data (17,18,38,39).
Propofol is highly lipophilic. Consequently, clearance depends on hepatic metabolism and is perfusion limited, with subsequent urinary elimination of conjugated metabolites. Different human cytochrome isoforms (hydroxylation, mainly CYP2B6, 22 %) are involved in propofol metabolism, while primary glucuronidation (77 %) is the most relevant metabolic pathway in adults after single intravenous bolus administration (3 mg.kg−1). In contrast, hydroxylation contributed 65 % and glucuronidation only 34 % in neonates (18,38). These proportional differences in the absence of other routes of elimination are of clinical relevance because this explains the impact of both postmenstrual and postnatal age (covariates of phenotypic glucuronidation capacity) on propofol clearance in neonates [0.029 × (postmenstrual age.38−1)11.5 + 0.03 when > 10 days postnatal life] l.min−1. This equation results in a similar propofol clearance capacity in a 38 postmenstrual weeks newborn at birth (0.029 l.min−1) when compared to a former 27 weeks postmenstrual age with a postnatal age of 10 days (0.035 l.min−1)(39).
Despite the fact that the main route of elimination of paracetamol in adults is also through glucuronidation, the same ‘magic of 10 days postnatal age’ does not apply to paracetamol pharmacokinetics in neonates and size is the main covariate of paracetamol clearance in neonates. This is because in the setting of poor glucuronidation capacity, sulphation capacity and even primary renal elimination become more relevant routes of paracetamol elimination in early life until glucuronidation capacity improves (20,21).
Renal and hepatic maturation: the need for an integrated interpretation
The main routes of clearance of drugs and metabolites are the kidneys and hepatobiliary system. Primary elimination clearance is mainly through the kidneys while metabolic clearance is mainly through the liver. The renal elimination capacity is reflected by diuresis (free water clearance only), glomerular filtration rate (GFR) and renal tubular activity (reabsorption and secretion). The extensive variability in early infancy relates to maturation (e.g. age, birth weight) and disease characteristics (e.g. peripartal asphyxia, renal congenital malformations, co-medication or growth restriction) (2,5). Similar to renal elimination, the phenotypic variation in hepatic elimination relates to constitutional, disease related and genetic factors. In infancy, the main driver is age-dependent phenotypic enzymatic activity. Obviously, drug metabolizing enzymes play a crucial role in the extent of drug biotransformation to elimination, and ontogeny of hepatic drug metabolizing enzymes can significantly alter drug clearance throughout infancy (8,9,10). However, the maturation pattern of the individual renal or hepatic elimination processes differs substantially. This implies that it is important to integrate all ontogeny-related knowledge of the different elimination routes to predict compound specific, phenotypic in vivo observations in neonates: there is no such thing as an isolated neonatal kidney or liver, but only newborns in need of improved predictability.
Besides aspects related to the distribution volume, the phenotypic concentration of a metabolite will depend on both metabolite formation as well as subsequent metabolite elimination. We shall illustrate the clinical relevance of such an integrated approach based on observations on morphine (glucuronidation, subsequent renal elimination)(25,40), midazolam (oxidation and glucuronidation, subsequent renal elimination)(22) and tramadol (O- and N-demethylation, subsequent renal elimination)(41,42) disposition in neonates. Many of these observations were published in this journal.
Allometric scaling (kg0.75) combined with a sigmoidal maturation function provided a sound basis to describe clearance and consequently, predict morphine doses in humans throughout pediatric life (25). Similarly and assuming a midazolam sedation target concentration of 0.1 mg/l, steady-state infusion rates of 0.014 mg/kg/h in neonates, 0.05 mg/kg/h in a 1-year-old, 0.06 mg/kg/h in a 5-year-old and 0.05 mg/kg/h in a 12-year-old child have been suggested (22). Tramadol clearance was explained by size and postmenstrual age, while tramadol to O-demethyl tramadol clearance (CL2M1) was explained by size, postmenstrual age and polymorphisms [CYP2D6]. CL2M1 was very low in preterm neonates, irrespective of the CYP2D6 polymorphism with subsequent rapid maturation. The subsequent slope of the increase was depending on the individual CYP2D6 activity score (41). However, these studies mainly focussed on the total clearance, and only marginally considered the potential impact of the metabolite formation maturation compared to subsequent metabolite elimination clearance.
This is of clinical relevance, since morphine-glucuronides (analgosedation), 1-hydroxy-midazolam (sedation), and O-demethyl tramadol (analgesia) all have potential significant effects. Children with renal failure can display respiratory depression form morphine 6 glucuronide despite clearance of the parent morphine (25,40). A model for additive morphine and morphine-6-glucuronide respiratory effects described the respiratory rate and respiratory depression better than models describing either alone (25,40). Impaired morphine-6-glucuronide renal elimination resulted in respiratory depression episodes occurring later than those predicted by modelling morphine levels only. The same phenomenon can be anticipated for 1 hydroxy-midazolam (prolonged and deeper sedation in the setting of impaired glucuronidation), and has been described for tramadol metabolite disposition (41,42).
A recent exploration of the tramadol- and O-demethyl tramadol time-concentration profiles that also took the renal maturation into account described that O-demethyl tramadol the phenotypic concentration were already in the therapeutic range in near term neonates. This is not because of the high phenotypic CYP2D6 activity, but because CYP2D6 activity maturation is faster when compared to metabolite elimination clearance (through glomerular filtration rate)(42). The highest metabolite concentrations occur in the 52-week infant, where formation clearance (hepatic, CYP2D6) to O-demethyl tramadol is already mature but metabolite elimination clearance (through glomerular filtration rate) still is immature. Since different routes (CYP2D6, CYP3A and primary renal clearance) are involved in overall tramadol clearance, differences in CYP2D6 activity do not necessary affect overall tramadol, but only the contribution of the different routes.
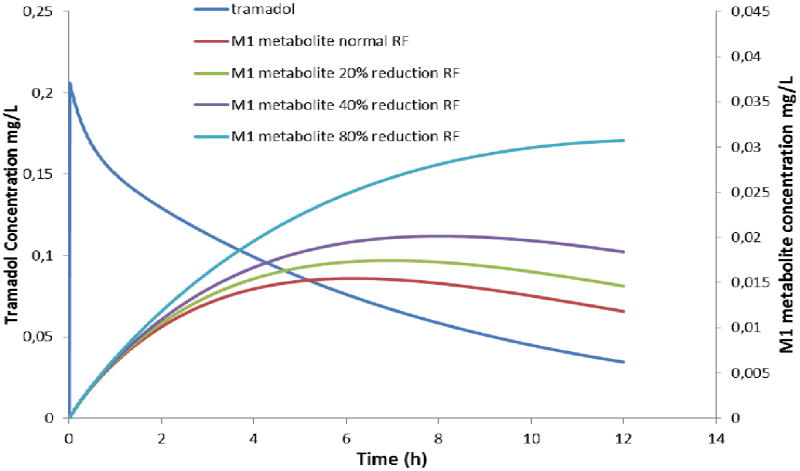
Figure 1 illustrates the impact of renal impairment on the tramadol and O-demethyl tramadol profiles in a full term neonate (3.2 kg, 1 mg.kg−1 intravenous bolus of tramadol). The thresholds in renal impairment used (− 20 % and − 40 % respectively, compared to − 80 %) in these models refer to the decrease in renal function during exposure to ibuprofen or indomethacin in neonates (42). Neonates can be considered as having renal impairment as one of their basic physiological characteristics (2,7).
Figure 1.
The impact of renal impairment on the O-demethyl tramadol (M1) profile following single intravenous bolus administration (1 mg.kg−1) in a term newborn (3.2 kg). A newborn with a normal glomerular filtration rate (GFR) is compared with a newborn with a GFR reduction of 20 % and 40 % in GFR (equal to the reduction during ibuprofen or indomethacin exposure respectively) or 80 % (41,42).
Pharmacogenetics should be tailored to neonates, not just mirror adult observations
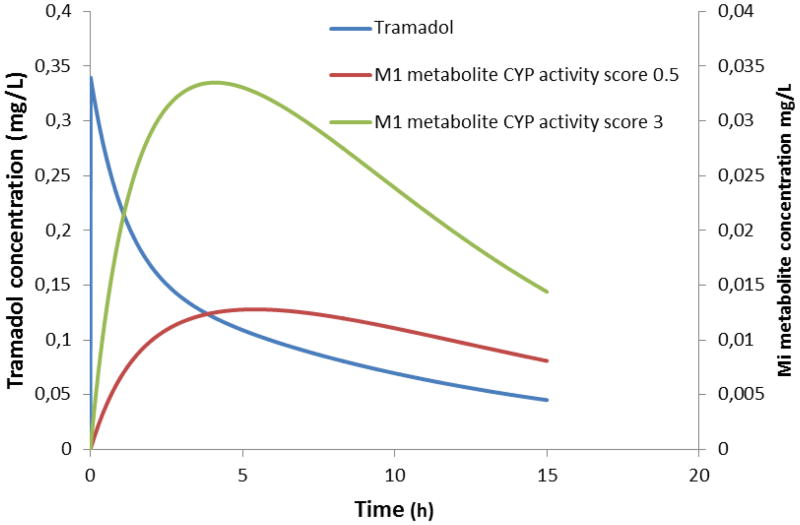
The concept of pharmacogenetics reflects the assumption that a specific effect or risk is not randomly distributed in a (sub)population. This obviously also hold promises for improved predictability and tailored drug therapy in neonates and young infants (43). There are published observations on the impact of pharmacogenetics on in vivo phenotypic CYP2D6 (e.g. tramadol, codeine)(41,42,43), CYP2C19 (e.g. pantoprazole)(43) or N-acetyl transferase (NAT)2 (44) (e.g. isoniazid) activity in early life. However, such iso-enzyme specific observations have been explored based on genotype-phenotype concordances as described in adults and – to a certain extent – still approach the infant as ‘a small adult’ (when does genotype-phenotype concordance appears ?). Figure 2 illustrates the impact of CYP2D6 activity score (0.5 versus 3) on the tramadol and O-demethyl tramadol profiles in a full term neonate (3.2 kg, 1.5 mg.kg−1 intravenous bolus tramadol) [Figure 2] (41,42).
Figure 2.
The impact of pharmacogenetics (the CYP2D6 activity score, either 0,5 or 3) on the O-demethyl tramadol (M1) profile in a full term newborn (3.2 kg) following single iv bolus (1.5 mg.kg−1) administration of tramadol (41,42).
In addition to ‘adult type’ approaches, there are also potential age-specific concordances between genotype and phenotype only present in perinatal life. In this way, pharmacogenetics should be tailored to clinical research questions in perinatal life, and not only mirrors findings in adults. There are however a limited number of maternal-infant pharmacogenetic studies that serve as illustrations of the potential relevance to apply the concepts of pharmacogenetics to perinatal life when tailored to neonates and infants related to codeine and paracetamol respectively (24,33,43).
Sistonen et al. documented that a combination of maternal genetic markers (i.c. CYP2D6 and P-glycoprotein polymorphisms) predicted 87 % of the infant and maternal codeine related CNS depression cases with a sensitivity of 80 % and a specificity of 87 % in a cohort of 111 breastfeeding mother-infant pairs (24). The same approach has been used in the Avon Longitudinal Study and relates to paracetamol. This study explored the impact of polymorphisms [nuclear erythroid 2 p45-related factor 2 (Nrf2) polymorphism and glutathione S-transferase (GST, M1, T1, and P1) polymorphisms] on the strength of the association between paracetamol exposure during pregnancy and the risk to develop atopy during infancy (33). It was observed that the increased risk of atopy was associated with exposure to paracetamol in late gestation in the presence of maternal GSTM1, and was further enhanced when GSTM1 was also present in the infant. Consequently, it seems that maternal antioxidant gene polymorphisms modified the relation between prenatal acetaminophen exposure and childhood asthma, strengthening evidence for a causal, polymorphisms related association (33).
How to handle ever more compounds in search of guidance ?
The current special issue on neonates reflects the increase in knowledge on different aspects of (patho)physiology in this specific population, commonly referred to pediatric anesthesiologists because of surgical interventions or analgosedation to facilitate procedural interventions. This increase in knowledge also applies to pharmacotherapy in neonates. However, off label administration is still common while evidence based pharmacotherapy in neonates is limited. The best way to address this gap is to design and to contribute to properly designed trials on PK/PD of the compounds commonly administered by pediatric anesthesiologists, using appropriate formulations and assessment techniques. The recently published paper on the opioid sparing effects of intravenous paracetamol in non-cardiac neonates is a good illustration of its feasibility 32. Taking the emerging data on neuro-apoptosis into account, the relevant outcome variables of opioid reduction in neonates may differ from the commonly considered (e.g. sedation, bladder retention, constipation), but may relate to neurodevelopmental outcome (11). The good news is that there is indeed an ongoing research activity.
A search on the www.clinicaltrials.gov website (February 2013) revealed 50 ongoing studies for the search terms ‘newborn and ‘anesthesia’, ‘newborn’ and ‘pain’ revealed 84 ongoing studies (45). Similarly, the revised priority list for studies into off-patent paediatric medical products (13 January 2012) includes tetrastrach, clonidine, propofol, thiopental, diclofenac, gabapentin or ibuprofen and provide opportunities to further improve perioperative pharmacotherapy (46). Moreover, for new compounds of potential relevance in paediatric medicine, manufacturers need to perform the appropriate studies as part of their product development plan (pediatric investigation plan). This increase in studies is also associated with an increase in methodologies how to design perform these studies.
Modelling - based on either physiology-based or mechanism PK modelling - improved the knowledge on maturation and subsequent dose estimations to guide study design or clinical decisions. Physiology based PK models combine the available physiological and biochemical information that drive absorption, distribution, metabolism, and excretion (47,48). The same aim, i.e. improved predictability and study design based on the best possible guess, can be attained through mechanism based modeling (6,49). In these models, already available information on the PK of a given drug will be applied to estimate the PK of a drug that has similar chemical characteristics and elimination routes. In general, there has been a renewed interest in modelling to improve drug development efficiency, especially in populations where designing and conducting clinical studies is more challenging, such as infants or newborns. In contrast to the emerging PK modeling, there remains a need for clinically applicable tools to assess PD which can provide response feedback. This has been achieved for neuromuscular monitoring, but not yet fully for depth of anaesthesia, sedation or pain in early infancy.
Irrespective of the availability of trial data to guide pharmacotherapy in neonates, clinicians need to be aware that most trials are primary useful to evaluate efficacy. Studies are not the most appropriate method to collect data on (rare) adverse drug effects, where pharmacovigilance and surveillance following the widespread use is needed (50). Alongside the regulatory agencies, investigators and clinicians should also remain vigilant for such effects and report specific observations. To illustrate this, we refer to recent reports on opioid induced hyperalgesia (51), delirium (52) and propofol infusion syndrome (53) in early infancy.
Acknowledgments
Karel Allegaert is supported by the Fund for Scientific Research, Flanders (Belgium) (F.W.O. Vlaanderen) by a Fundamental Clinical Investigatorship (1800209N). Johannes van den Anker is supported in part by NIH grants (R01HD060543, K24DA027992, R01HD048689, U54HD071601) and FP7 grants TINN (223614), TINN2 (260908), and NEUROSIS (223060). We are grateful for the advice of Brian Anderson and thank him for the figures provided to illustrate the covariates of tramadol disposition.
References
- 1.Anderson BJ, Allegaert K. The pharmacology of anaesthetics in the neonate. Best Pract Res Clin Anaesthesiol. 2010;24:419–431. doi: 10.1016/j.bpa.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Smits A, Kulo A, de Hoon JN, Allegaert K. Pharmacokinetics of drugs in neonates: pattern recognition beyond compound specific observations. Curr Pharm Des. 2012;18:3119–3146. doi: 10.2174/1381612811209023119. [DOI] [PubMed] [Google Scholar]
- 3.Anderson BJ. Pharmacology in the very young: anaesthetic implications. Eur J Anaesthesiol. 2012;29:261–270. doi: 10.1097/EJA.0b013e3283542329. [DOI] [PubMed] [Google Scholar]
- 4.Sumpter A, Anderson BJ. Pediatric pharmacology in the first year of life. Curr Opin Anaesthesiol. 2009;22:469–475. doi: 10.1097/aco.0b013e32832bc7ff. [DOI] [PubMed] [Google Scholar]
- 5.Allegaert K, Verbesselt R, Naulaers G, et al. Developmental pharmacology: neonates are not just small adults. Acta Clin Belg. 2008;63:16–24. doi: 10.1179/acb.2008.003. [DOI] [PubMed] [Google Scholar]
- 6.Anderson BJ, Holford NH. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24:25–36. doi: 10.2133/dmpk.24.25. [DOI] [PubMed] [Google Scholar]
- 7.van den Anker JN. Developmental pharmacology. Dev Disabil Res Rev. 2010;16:233–238. doi: 10.1002/ddrr.122. [DOI] [PubMed] [Google Scholar]
- 8.de Wildt SN. Profound changes in drug metabolism enzymes and possible effects on drug therapy in neonates and children. Expert Opin Drug Metab Toxicol. 2011;7:935–948. doi: 10.1517/17425255.2011.577739. [DOI] [PubMed] [Google Scholar]
- 9.Coté CJ, Ward RM, Lugo RA, Goudsouzian N. Pharmacokinetics and pharmacology of drugs used in children (section II, chapter 6) In: Coté CJ, Lerman J, Todres ID, editors. A practice of anesthesia for infants and children. 4th. Saunders Elsevier; Philadelphia: 2009. [Google Scholar]
- 10.Hines RN. Developmental expression of drug metabolizing enzymes: impact on disposition in neonates and young children. Int J Pharm. doi: 10.1016/j.ijpharm.2012.05.079. [DOI] [PubMed] [Google Scholar]
- 11.Davidson AJ. Anesthesia and neurotoxicity to the developing brain: the clinical relevance. Paediatr Anaesth. 2011;21:716–721. doi: 10.1111/j.1460-9592.2010.03506.x. [DOI] [PubMed] [Google Scholar]
- 12.Shinwell ES, Eventov-Friedman S. Impact of perinatal corticosteroids on neuromotor development and outcome: review of the literature and new meta-analysis. Semin Fetal Neonatal Med. 2009;14:164–170. doi: 10.1016/j.siny.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Zaffanello M, Bassareo PP, Cataldi L, Antonucci R, Biban P, Fanos V. Long-term effects of neonatal drugs on the kidney. J Matern Fetal Neonatal Med. 2010;23(Suppl 3):87–89. doi: 10.3109/14767058.2010.501156. [DOI] [PubMed] [Google Scholar]
- 14.Yilmaz D, Teziç HT, Zorlu P, Firat S, Bilalo(x0011F)lu E, Kutlu AO. Single dose povidone-iodine on thyroid functions and urinary iodine excretion. Indian J Pediatr. 2003;70:675–677. doi: 10.1007/BF02724261. [DOI] [PubMed] [Google Scholar]
- 15.Smits A, Kulo A, Verbesselt R, et al. Cefazolin plasma protein binding and its covariates in neonates. Eur J Clin Microbiol Infect Dis. 2012;31:3359–3365. doi: 10.1007/s10096-012-1703-x. [DOI] [PubMed] [Google Scholar]
- 16.Calder A, Bell GT, Andersson M, Thomson AH, et al. Pharmacokinetic profiles of epidural bupivacaine and ropivacaine following single-shot and continuous epidural use in young infants. Paediatr Anaesth. 2012;22:430–437. doi: 10.1111/j.1460-9592.2011.03771.x. [DOI] [PubMed] [Google Scholar]
- 17.Allegaert K, de Hoon J, Verbesselt R, Naulaers G, Murat I. Maturational pharmacokinetics of single intravenous bolus of propofol. Paediatr Anaesth. 2007;17:1028–1034. doi: 10.1111/j.1460-9592.2007.02285.x. [DOI] [PubMed] [Google Scholar]
- 18.Smits A, Verbesselt R, Kulo A, Naulaers G, de Hoon J, Allegaert K. Urinary metabolites after intravenous propofol bolus in neonates. Eur J Drug Metab Pharmacokinet. doi: 10.1007/s13318-012-0109-6. [DOI] [PubMed] [Google Scholar]
- 19.Welzing L, Kribs A, Eifinger F, Huenseler C, Oberthuer A, Roth B. Propofol as an induction agent for endotracheal intubation can cause significant arterial hypotension in preterm neonates. Paediatr Anaesth. 2010;20:605–611. doi: 10.1111/j.1460-9592.2010.03330.x. [DOI] [PubMed] [Google Scholar]
- 20.Allegaert K, Palmer GM, Anderson BJ. The pharmacokinetics of intravenous paracetamol in neonates: size matters most. Arch Dis Child. 2011;96:575–580. doi: 10.1136/adc.2010.204552. [DOI] [PubMed] [Google Scholar]
- 21.Anderson BJ, Allegaert K. Intravenous neonatal paracetamol dosing: the magic of 10 days. Paediatr Anaesth. 2009;19:289–295. doi: 10.1111/j.1460-9592.2008.02680.x. [DOI] [PubMed] [Google Scholar]
- 22.Anderson BJ, Larsson P. A maturation model for midazolam clearance. Paediatr Anaesth. 2011;21:302–308. doi: 10.1111/j.1460-9592.2010.03364.x. [DOI] [PubMed] [Google Scholar]
- 23.Larson A, Stidman T, Banerji S, Kaufman J. Seizures and methemoglobinemia in an infant after excessive EMLA application. Pediatr Emerg Care. 2013;29:377–379. doi: 10.1097/PEC.0b013e3182854790. [DOI] [PubMed] [Google Scholar]
- 24.Sistonen J, Madadi P, Ross CJ, et al. Prediction of codeine toxicity in infants and their mothers using a novel combination of maternal genetic markers. Clin Pharmacol Ther. 2012;91:692–699. doi: 10.1038/clpt.2011.280. [DOI] [PubMed] [Google Scholar]
- 25.Holford NH, Ma SC, Anderson BJ. Prediction of morphine dose in humans. Paediatr Anaesth. 2012;22:209–222. doi: 10.1111/j.1460-9592.2011.03782.x. [DOI] [PubMed] [Google Scholar]
- 26.Anderson BJ, van Lingen RA, Hansen TG, Lin YC, Holford NH. Acetaminophen developmental pharmacokinetics in premature neonates and infants. Anesthesiology. 2002;96:1336–1345. doi: 10.1097/00000542-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Anderson BJ, Pons G, Autret-Leca E, Allegaert K, Boccard E. Pediatric intravenous paracetamol (propacetamol) pharmacokinetics: a population analysis. Paediatr Anaesth. 2005;15:282–292. doi: 10.1111/j.1460-9592.2005.01455.x. [DOI] [PubMed] [Google Scholar]
- 28.Allegaert K, Rayyan M, De Rijdt T, Van Beek F, Naulaers G. Hepatic tolerance of repeated intravenous paracetamol administration in neonates. Paediatr Anaesth. 2008;18:388–392. doi: 10.1111/j.1460-9592.2008.02535.x. [DOI] [PubMed] [Google Scholar]
- 29.Anderson BJ. Paracetamol (acetaminophen): mechanisms of action. Paediatr Anaesth. 2008;18:915–921. doi: 10.1111/j.1460-9592.2008.02764.x. [DOI] [PubMed] [Google Scholar]
- 30.Allegaert K, Naulaers G, Vanhaesebrouck S, Anderson BJ. The paracetamol concentration-effect relation in neonates. Paediatr Anaesth. 2013;23:45–50. doi: 10.1111/pan.12076. [DOI] [PubMed] [Google Scholar]
- 31.Anderson BJ, Woollard GA, Holford NH. Acetaminophen analgesia in children: placebo effect and pain resolution after tonsillectomy. Eur J Clin Pharmacol. 2001;57:559–569. doi: 10.1007/s002280100367. [DOI] [PubMed] [Google Scholar]
- 32.Ceelie I, de Wildt SN, van Dijk M, et al. Effect of intravenous paracetamol on postoperative morphine requirements in neonates and infants undergoing major noncardiac surgery: a randomized controlled trial. JAMA. 2013;309:149–154. doi: 10.1001/jama.2012.148050. [DOI] [PubMed] [Google Scholar]
- 33.Shaheen SO, Newson RB, Ring SM, et al. Prenatal and infant acetaminophen exposure, antioxidant gene polymorphisms, and childhood asthma. J Allergy Clin Immunol. 2010;126:1141–8.e7. doi: 10.1016/j.jaci.2010.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowe AJ, Carlin JB, Bennett CM, et al. Paracetamol use in early life and asthma: prospective birth cohort study. BMJ. 2010;341:c4616. doi: 10.1136/bmj.c4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oncel MY, Yurttutan S, Degirmencioglu H, et al. Intravenous paracetamol treatment in the management of patent ductus arteriosus in Extremely Low Birth Weight Infants. Neonatology. 2012;103:165–168. doi: 10.1159/000345337. [DOI] [PubMed] [Google Scholar]
- 36.Hammerman C, Bin-Nun A, Kaplan M. Managing the patent ductus arteriosus in the premature neonate: a new look at what we thought we knew. Semin Perinatol. 2012;36:130–138. doi: 10.1053/j.semperi.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 37.Anderson BJ. My child is unique; the pharmacokinetics are universal. Paediatr Anaesth. 2012;22:530–538. doi: 10.1111/j.1460-9592.2011.03788.x. [DOI] [PubMed] [Google Scholar]
- 38.Sneyd JR, Simons PJ, Wright B. Use of proton nmr spectroscopy to measure propofol metabolites in the urine of the female Caucasian patient. Xenobiotica. 1994;24:1021–1028. doi: 10.3109/00498259409043299. [DOI] [PubMed] [Google Scholar]
- 39.Allegaert K, Peeters MY, Verbesselt R, et al. Interindividual variability in propofol pharmacokinetics in preterm and term neonates. Br J Anaesth. 2007;99:864–870. doi: 10.1093/bja/aem294. [DOI] [PubMed] [Google Scholar]
- 40.Hannam JA, Anderson BJ. Contribution of morphine and morphine-6-glucuronide to respiratory depression in a child. Anaesth Intensive Care. 2012;40:867–870. doi: 10.1177/0310057X1204000516. [DOI] [PubMed] [Google Scholar]
- 41.Allegaert K, van den Anker JN, de Hoon JN, et al. Covariates of tramadol disposition in the first months of life. Br J Anaesth. 2008;100:525–532. doi: 10.1093/bja/aen019. [DOI] [PubMed] [Google Scholar]
- 42.Allegaert K, Rochette A, Veyckemans F. Developmental pharmacology of tramadol during infancy: ontogeny, pharmacogenetics and elimination clearance. Paediatr Anaesth. 2011;21:266–273. doi: 10.1111/j.1460-9592.2010.03389.x. [DOI] [PubMed] [Google Scholar]
- 43.Leeder JS, Kearns GL. Intepreting pharmacogenetic data in the developing neonate: the challenge of hitting a moving target. Clin Pharmacol Ther. 2012;92:434–436. doi: 10.1038/clpt.2012.130. [DOI] [PubMed] [Google Scholar]
- 44.Zhu R, Kiser JJ, Seifart HI, et al. The pharmacogenetics of NAT2 enzyme maturation in perinatally HIV exposed infants receiving isoniazid. J Clin Pharmacol. 2012;52:511–519. doi: 10.1177/0091270011402826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. [accessed 09 february 2013]; www.clinicaltrials.gov website.
- 46. [accessed 09 February 2013]; www.ema.europa.eu/docs/en_GB/document_library/others/2009/10/WC500004017.pdf.
- 47.Strougo A, Eissing T, Yassen A, Willmann S, Danhof M, Freijer J. First dose in children: physiological insights into pharmacokinetic scaling approaches and their implications in paediatric drug development. J Pharmacokinet Pharmacodyn. 2012;39:195–203. doi: 10.1007/s10928-012-9241-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson TN, Rostami-Hodjegan A. Resurgence in the use of physiologically based pharmacokinetic models in pediatric clinical pharmacology: parallel shift in incorporating the knowledge of biological elements and increased applicability to drug development and clinical practice. Paediatr Anaesth. 2011;21:291–301. doi: 10.1111/j.1460-9592.2010.03323.x. [DOI] [PubMed] [Google Scholar]
- 49.De Cock RF, Piana C, Krekels EH, et al. The role of population PK-PD modelling in paediatric clinical research. Eur J Clin Pharmacol. 2011;67:5–16. doi: 10.1007/s00228-009-0782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choonara I. Educational Paper: Aspects of clinical pharmacology in children-pharmacovigilance and safety. Eur J Pediatr. doi: 10.1007/s00431-012-1871-9. [DOI] [PubMed] [Google Scholar]
- 51.Hallett BR, Chalkiadis GA. Suspected opioid-induced hyperalgesia in an infant. Br J Anaesth. 2012;108:116–118. doi: 10.1093/bja/aer332. [DOI] [PubMed] [Google Scholar]
- 52.Madden K, Turkel S, Jacobson J, Epstein D, Moromisato DY. Recurrent delirium after surgery for congenital heart disease in an infant. Pediatr Crit Care Med. 2011;12:e413–e415. doi: 10.1097/PCC.0b013e31820ac2bf. [DOI] [PubMed] [Google Scholar]
- 53.Sammartino M, Garra R, Sbaraglia F, Papacci P. Propofol overdose in a preterm baby: may propofol infusion syndrome arise in two hours? Paediatr Anaesth. 2010;20:973–974. doi: 10.1111/j.1460-9592.2010.03395.x. [DOI] [PubMed] [Google Scholar]