Abstract
Objective
The purpose of this study is to develop a nanoemulsion formulation for its use as a transcutaneous vaccine delivery system.
Materials and methods
With bovine albumin-fluorescein isothiocyanate conjugate (FITC-BSA) as a vaccine model, formulations were selected with the construction of pseudo-ternary phase diagrams and a short-term stability study. The size of the emulsion droplets was furthered optimized with high-pressure homogenization. The optimized formulation was evaluated for its skin permeation efficiency. In vitro skin permeation studies were conducted with shaved BALB/c mice skin samples with a Franz diffusion cell system. Different drug concentrations were compared, and the effect of the nanoemulsion excipients on the permeation of the FITC-BSA was also studied.
Results
The optimum homogenization regime was determined to be five passes at 20 000 psi, with no evidence of protein degradation during processing. With these conditions, the particle diameter was 85.2 nm ± 15.5 nm with a polydispersity index of 0.186 ± 0.026 and viscosity of 14.6 cP ± 1.2 cP. The optimized formulation proved stable for 1 year at 4 °C. In vitro skin diffusion studies show that the optimized formulation improves the permeation of FITC-BSA through skin with an enhancement ratio of 4.2 compared to a neat control solution. Finally, a comparison of the skin permeation of the nanoemulsion versus only the surfactant excipients resulted in a steady state flux of 23.44 μg/cm2/h for the nanoemulsion as opposed to 6.10 μg/cm2/h for the emulsifiers.
Conclusion
A novel nanoemulsion with optimized physical characteristics and superior skin permeation compared to control solution was manufactured. The formulation proposed in this study has the flexibility for the incorporation of a variety of active ingredients and warrants further development as a transcutaneous vaccine delivery vehicle.
Keywords: FITC-BSA, nanoemulsion, nanotechnology, skin, squalane, transcutaneous delivery, vaccine delivery
Introduction
Non-invasive transcutaneous delivery of vaccines is an appealing alternative to the traditional immunization routes, such as subcutaneous or intramuscular injections, for many reasons. Transcutaneous immunization avoids gastric degradation or hepatic first-pass metabolism, can initiate an immune response by accessing the antigen-presenting cells in the epidermis and dendritic cells in the dermis and has the potential to be administered painlessly and in a lower dose1. Once the antigen-presenting cells and dendritic cells within the epidermis and dermis are activated, they migrate out of the skin and into lymph nodes, presenting the antigen to B cells and T cells – thereby, transcutaneous immunization has the ability to achieve both humoral and cellular immune responses2. Also, eliminating the need for syringes and other medical devices allows for broader distribution of the vaccine, especially for use in developing nations. However, the most prominent physical hindrance to transcutaneous vaccine delivery is the stratum corneum, which restricts the passage of most high-molecular weight molecules3. Therefore, vigorous research is underway to develop sufficiently small individual particles that can be applied topically and diffuse through the stratum corneum to the epidermis and evoke the desired immune response.
One approach to aid in transcutaneous drug/vaccine delivery is the utilization of nanoemulsions to facilitate and/or enhance the diffusion of the drug or vaccine through the skin barrier4–10. Nanoemulsions are kinetically-stable and typically have a particle size range of 50–200 nm11. One of the most crucial aspects of developing a nanoemulsion formulation is the selection of the formulation constituents. Squalene, which is the un-oxidized form of squalane, has already been shown to be an effective parenteral vaccine adjuvant, proven to lower the necessary antigen dose and to improve both the humoral and cellular immune response of the vaccine12. The oxidized form squalane itself is currently used in cosmetic applications because it effectively moisturizes and absorbs into the skin. Both for its proven non-irritancy and its potential as a vaccine adjuvant, squalane may prove to be an optimal component for a nanoemulsion transcutaneous vaccine delivery system13.
The purpose of this study is the selection and characterization of the most promising squalane nanoemulsion for its use as a non-invasive transcutaneous vaccine vehicle. To aid in the selection of the most promising nanoemulsion formulations, pseudo-ternary phase diagrams were constructed with the surfactants Span 80 and Tween 80, and bovine albumin-fluorescein isothiocyanate conjugate (FITC-BSA) is employed as a vaccine model. The selected formulations were then studied for their short-term stability, primarily characterized by their viscosity and particle size. Additionally, high-pressure homogenization was utilized to further tune the nanoemulsion characteristics, resulting in a promising, novel nanoemulsion formulation. In vitro skin diffusion studies were conducted to assess the potential of our nanoemulsion formulation as a transcutaneous vaccine vehicle.
Materials and methods
Materials
The Span 80 (sorbitan monooleate, HLB 4.3), Tween 80 (polyoxyethylene sorbitan monooleate, HLB 15.0), bovine albumin-fluorescein isothiocyanate conjugate (FITC-BSA, 67 kDa) and squalane were obtained from Sigma-Aldrich (St. Louis, MO). The water used was deionized by a Milli-Q filtration system (Millipore Corporation, Bedford, MA).
Construction of pseudo-ternary phase diagram
Pseudo-ternary phase diagrams were prepared for three different Span 80/Tween 80 volume ratios (Smix), 1:2, 1:1 and 2:1, and following a similar procedure as described by Shafiq-un-Nabi et al14. For the construction of each diagram, squalane and Smix were combined in 11 different volume ratios in 20 mL scintillation vials: 95:5, 9:1, 4:1, 7:3, 3:2, 1:1, 2:3, 3:7, 1:4, 1:9 and 5:95, resulting in an even distribution of data points across the oil–surfactant axis. The aqueous phase was then added to each vial at small aliquots starting with 25 μL. Following the addition of the aliquot of water, the vial was capped and vortexed for 2 min to accelerate equilibration. The samples were allowed to equilibrate for a further 15 min, whereupon the mixture in each vial was visually examined for clarity and photographed (PowerShot A400, Canon, Inc., Lake Success, NY). The amount of water added to the vials was increased with each assessment to ensure even spacing of data points on the finished phase diagram. The following categories were used during visual observation: (1) completely transparent, easily flowable and no phase separation; (2) translucent, easily flowable and no phase separation; (3) opaque, easily flowable and no phase separation; (4) opaque, increased viscosity and possible phase separation and (5) opaque, semi-solid and possible phase separation. Any samples with evidence of phase separation were automatically qualified as either class (4) or (5), depending on the relative viscosity of the sample.
Short-term stability assessment
Formulations were chosen from the phase diagrams based on their physical assessment and homogeneity. Emphasis was placed on o/w formulations with a low surfactant content and high oil content. Prior to making any formulations, the surfactant blend was prepared at least 24 h to ensure proper equilibration between the two surfactants. To make the formulation, the Smix was added to a 20 mL scintillation vial. Then the desired volume of squalane was added to the Smix, followed by the addition of the aqueous phase with 0.1 mg/mL of FITC-BSA. Control formulations without FITC-BSA within the aqueous phase were also created. The samples were gently agitated by hand and vortexed for at least 1 min. The samples were emulsified with a high-speed homogenizer PowerGen 700 (Fisher Scientific, Pittsburgh, PA) for an additional 1 min. All formulations were made in triplicate.
The samples were stored at 4 °C for the duration of the 14-day stability study. Every 24 h the formulations were visually assessed for signs of agglomeration and phase separation. The sample was deemed stable if the sample was visually homogeneous with no visible evidence of phase separation. Only those samples categorized as stable underwent viscosity and particle size analysis.
Characterization of nanoemulsions
Viscosity
The viscosity of the nanoemulsion formulations was measured using a Brookfield DV-III Ultra Programmable Rheometer (Brookfield Engineering Laboratories, Middleboro, MA) without dilution of the emulsion prior to measurement. 0.5 mL of the formulation was used for the viscosity measurement. All viscosity measurements were done using a CPE40 spindle (Brookfield Engineering Laboratories, Middleboro, MA) at a room temperature of 21.9 ±1.1 °C. The torque was maintained at 20% for all measurements. All measurements were done in triplicate.
Particle size analysis
The particle size and distribution were measured using a DelsaNano C Particle Analyzer (Beckman Coulter, Inc., Fullerton, CA). This equipment uses photon correlation spectroscopy, which determines the particle size based on the rate of intensity fluctuations of a laser scattered by the particles within the emulsions. Those nanoemulsions used for the 14-day stability study were not diluted prior to analysis. All other readings were diluted prior to size analysis15,16. All measurements were done in triplicate.
Zeta potential
The zeta potential was also measured with the DelsaNano C Particle Analyzer. All samples were diluted 150 times in 1 mM KCl prior to analysis. All measurements were done in triplicate.
High-pressure homogenization optimization
In order to optimize the high-pressure homogenization methodology, the most promising formulation following the short-term stability assessment, formulation B1, was used to evaluate the effect of homogenization pressure and pass number on the particle size, size distribution and viscosity of the nanoemulsion. Samples were prepared as previously mentioned for the short-term assessment, except, rather than using the PowerGen 700, the high-pressure homogenizer EmulsiFlex-C5 (Avestin Inc., Ottawa, Canada) was utilized. Nanoemulsions were prepared at three different pressures, 10, 15 and 20 kpsi, and the number of passes through the homogenizer was also varied, evaluating three, four and five passes. Formulation B1 was further refined by adjusting the surfactant content to reduce the particle size. Following manufacturing at room temperature, all samples were analyzed for their particle size and viscosity. All samples were made in triplicate.
To ensure that the protein was not damaged following homogenization, the structural integrity of BSA during processing of the nanoemulsion was analyzed by PAGE using the method described by Laemmli17. Nanoemulsions containing FITC-BSA were compared with native FITC-BSA, alkaline-hydrolyzed FITC-BSA and molecular weight markers (16–250 kDa). After the protein samples (10 μg) were combined with loading dye, the electrophoresis was performed using 10% Tris-Glycine precast gels (NU Sep, Bogart, GA) at a constant voltage of 90 V in a 10 × Tris-glycine-SDS buffer using a Bio-Rad Mini-Protean II electrophoresis system (Bio-Rad Laboratories, Hercules, CA). After migration of the protein bands, the gel was stained with 0.05% w/v Coomassie Blue G-250 (MP Biomedicals, Solon, OH) in methanol:acetic acid:water (5:1:4) for 3 h, then destained18. The destained gel was scanned in a Versa Doc 4000MP Bio-Rad Molecular Imager system (Bio-Rad Laboratories) using Quantity One software (Bio-Rad Laboratories) for image acquisition.
In vitro skin diffusion study
The final optimized nanoemulsion formulation was evaluated for its skin permeation capability. Nanoemulsions with three different concentrations of FITC-BSA were compared including 0.5, 2.5 and 4.0 mg/mL. Control FITC-BSA solutions, corresponding to the concentration within the nanoemulsions, were prepared with FITC-BSA in DI water. The in vitro skin diffusion studies were performed on a MicroettePlus system (Hanson Research, Chatsworth, CA) with 6 Franz diffusion cells, each with a diffusional area of 1.767 cm2 and a volume of 7 mL. BALB/c mouse skin samples were obtained from Charles Rivers Laboratories International Inc. (Wilmington, MA) and shaved prior to excision from the animal. Samples were frozen until use, not to exceed 6 weeks of storage time prior to use19. The skin samples were sectioned into 3 cm × 3 cm segments while frozen. The skin samples were thawed to room temperature and allowed to equilibrate in 0.01 M PBS (pH = 7.4) prior to loading the skin onto the diffusion cell. The MicroettePlus syringe pumps, diffusion cells and sampling needles were primed with the test media (0.01 M PBS, pH 7.4) prior to loading the skin, and the water bath was maintained at 32 °C for the duration of the study. The skin samples were then mounted on the diffusion cells with the stratum corneum side facing the donor compartment, all air bubbles below the skin surface were removed, the cap assembly was placed on the skin and the skin was allowed to equilibrate with the media and temperature in the diffusion cell for approximately 1 h. After equilibration, 2.0 mL of the sample nanoemulsion or control solution was loaded into the donor compartment of the custom-modified cell cap assembly with a 22G1 hypodermic needle. The collection procedure was initiated immediately after loading the formulations into the donor compartments. The stirrer within each cell was maintained at 400 RPM, and 1 mL samples were withdrawn from the receiver compartment with the MultiFill Collector (Hanson Research, Chatsworth, CA) at the following intervals: 1 min, 30 min, 1, 2, 3, 6, 9, 12, 15, 18, 21, 24, 30, 36, 42 and 48 h. At each collection time, the donor compartment and sampling tubing was rinsed with 1.5 mL to remove any residual sample from the previous collection time. All collected samples were stored in 1 mL amber vials at 4 °C until analysis. Each sample was analyzed with a Cary Eclipse fluorescence spectrophotometer (Varian Inc., Palo Alto, CA). A standard curve with FITC-BSA was made prior to analysis utilizing the same test media and same dilution as the collected samples. While any samples with evidence of skin breakage or leakage of the formulation were removed from the sample set, a minimum samples size of 3 was obtained for all nanoemulsion and control formulations.
Once an optimal FITC-BSA concentration was determined, the optimized nanoemulsion formulation was compared to a solution of the emulsifiers used in the formulation (i.e. Span-80 and Tween-80). A 10% v/v solution of Smix was made in D.I. water, and 2.5 mg/mL FITC-BSA was incorporated. Then, this emulsifier solution, the nanoemulsion (2.5 mg/mL FITC-BSA) and a FITC-BSA control solution (2.5 mg/mL FITC-BSA) underwent in vitro skin diffusion experiments following the same procedure as recorded above.
The cumulative amount of FITC-BSA penetrated through the skin per diffusional area was plotted against time. The steady-state flux (Jss) was calculated as the slope of the linear portion of the plot. The permeability coefficient (Kp) was calculated by dividing the steady-state flux by the initial concentration of the drug in the donor cell. The enhancement ratio (Er) was calculated as the ratio of the flux of the formulation to the flux of the control solution20.
Long-term stability assessment
With the formulation chosen following the short-term stability study and the high-pressure homogenization methodology optimized, samples with and without FITC-BSA were prepared and evaluated for prolonged stability for 1 year. The viscosity of each sample was measured immediately after preparation. The particle size and zeta potential were measured immediately after preparation, at 1 month, 3 months, 6 months, 9 months and 1 year. Both measurements, along with visual inspection, were used to monitor instability phenomena such as coalescence and phase separation. All samples were stored at 4 °C throughout the study duration. 4 °C was chosen as the study temperature because 2–8 °C is the acceptable storage temperature for the majority of commonly recommended vaccines21. Samples were made in triplicate.
Statistical analysis
Statistical comparisons were conducted between samples using ANOVA and the Holm–Sidak method for multiple pairwise comparisons. Differences between two related parameters were considered statistically significant at p<0.05. SigmaPlot (Systat Software, Inc., San Jose, CA) software was used for all statistical analysis.
Results
Pseudo-ternary phase diagrams and formulation stability
The pseudo-ternary phase diagrams all exhibited similar regions, with the central region composed of semi-solid cream formulations and the transparent nanoemulsions located along the oil–surfactant axis (Figure 1). While transparent nanoemulsions are achievable for the current components, the low water content and high surfactant content translates into low drug concentrations, increased chance of skin irritation and a relatively high viscosity. Thus, rather than focusing on the transparent nanoemulsion region along the oil–surfactant axis, the focus of this study was shifted to the region of low viscosity, opaque o/w emulsions along the oil–water axis. Furthermore, formulations from this region require less surfactant and have higher water contents; however, they are usually categorized as simply emulsions, having a larger particle size than the typically-transparent nanoemulsions14. Since the goal of this study is to make emulsions with particle sizes in the nanometer range, the choice of manufacturing procedure becomes paramount. Thus, for the short-term assessment of the different emulsion formulations, a homogenizer was used to reduce the particle size and improve the stability of the nanoemulsions.
Figure 1.
Pseudo-ternary phase diagrams were constructed for three different surfactant ratios: (a) 1:1, (b) 1:2 and (c) 2:1 (Span 80:Tween 80). Within the Smix 1:1 pseudo-ternary phase diagram, each black marker represents the formulations evaluated for stability. The numbered markers are those that proved to be stable for the duration of the study. The exact formulations of these nanoemulsions are contained in Table 1.
Table 1 shows the exact formulation for each of the stable formulations indicated in Figure 1(a), which were stable for the full 2-week duration. Smix 1:1 was chosen for the nanoemulsion formulations because the proportion of approximately 50:50 gives the maximum possible water solubilization for a mixture of Span 80 and Tween 8022. While a 2-week duration is too brief to make definitive conclusions about the overall stability of a formulation, the time frame is acceptable to discern promising formulations. Those formulations in Figure 1(a) which were deemed unstable usually exhibited phase separation within the first 72 h after homogenization. The particle diameters of the nanoemulsion formulations are shown in Figure 2(a). Formulations A1, B1 and C1 consistently had the smallest particle diameter, nearly always below 400 nm, with the exception of formulation A1 on day 1. Formulation D1 closely followed these formulations. While the particle size of some of the formulations fluctuated considerably (e.g. formulation H1), none of the nanoemulsions exhibited a clear decreasing or increasing trend over time. As noted in Table 1, formulations E1, F1 and H1 did not have three stable replicates which may explain the large interday variations observed for these formulations. Stated another way, the visual instability observed among replicates is mirrored in the interday measurement fluctuations. These fluctuations are likely related to instability of the system; the exact form of instability was not identified. Overall, the particle size results for formulations A1, B1, C1 and D1 show potential for future studies because of their small particle size and low viscosity.
Table 1.
Stable nanoemulsion formulations
| Formulation Designation |
Squalane (% v/v) |
Smix 1:1 (% v/v) |
Aqueous Phase* (% v/v) |
|---|---|---|---|
| A1 | 45 | 5 | 50 |
| B1 | 40 | 5 | 55 |
| C1 | 35 | 5 | 60 |
| D1 | 40 | 10 | 50 |
| E1† | 35 | 10 | 55 |
| F1† | 30 | 10 | 60 |
| G1 | 25 | 15 | 60 |
| H1† | 25 | 20 | 55 |
0.1 mg/mL FITC-BSA in DI water.
Stable for two of the three replicates.
Figure 2.
Graphs of the change in the (a) median particle diameter (volume distribution) and (b) viscosity of the stable nanoemulsion formulations for the 2-week duration of the study. The particle size of formulation G1 was omitted due to the over-range particle size readings. Data represent mean ± SD.
To better understand the particle size distribution of the formulations, Table 2 summarizes the results measured after 72 h. The polydispersity index of formulations D1, E1, G1 and H1 are relatively high, reflecting a broader particle size distribution in comparison to the other formulations, which means that these formulations may be prone to instability, especially in light of the fact that E1 and H1 were not stable for all three replicates. Formulations A1, B1, C1 and F1 had the lowest polydispersity indices which were again much higher than would be desirable for an optimized formulation given that a polydispersity index of 0.2 indicates high homogeneity within a formulation. Overall, the distribution of the particle size within each formulation is larger than is desirable and is attributable to the manufacturing techniques employed. Similar to the particle size results, formulations A1, B1 and C1 consistently had the lowest viscosity of all those nanoemulsions analyzed (Figure 2b). Formulations D1, G1 and H1 had considerably higher viscosities over time than the other formulations, indicating that the strongest determinant of the viscosity of the formulations is the amount of surfactant present. The higher viscosity may be attributed to excess surfactant in the aqueous phase, which translated into increased bulk viscosity of the system. Formulations D1 and E1 do display a trend of decreasing viscosity over time, but none of the other formulations showed increasing or decreasing trend for viscosity measurements over the study time period. Thus, formulations A1, B1 and C1 showed the most potential for future investigation.
Table 2.
Comparison of formulations on day 3 of stability study
| Formulation | Particle diameter (10%) (nm, mean ± SD) |
Particle diameter (50%) (nm, mean ± SD) |
Particle diameter (90%) (nm, mean ± SD) |
Viscosity (cP, mean ± SD) |
Polydispersity index (mean ± SD) |
|---|---|---|---|---|---|
| A1 | 101.4 ± 53.5 | 143.1 ± 67.0 | 335.5 ± 167.4 | 12.09 ± 6.26 | 0.696 ± 0.358 |
| B1 | 62.3 ± 26.2 | 98.6 ± 42.1 | 228.1 ± 102.0 | 7.25 ± 2.50 | 0.845 ± 0.639 |
| C1 | 54.7 ± 69.0 | 128.1 ± 116.0 | 294.4 ± 266.1 | 4.92 ± 1.26 | 0.478 ± 0.091 |
| D1 | 106.6 ± 47.0 | 176.3 ± 88.0 | 407.3 ± 212.6 | 66.92 ± 56.60 | 1.931 ± 1.232 |
| E1 | 186.1 ± 148.4 | 273.5 ± 192.2 | 645.1 ± 442.8 | 23.00 ± 7.93 | 1.076 ± 0.770 |
| F1 | 144.4 ± 108.3 | 257.3 ± 198.6 | 590.6 ± 465.4 | 10.77 ± 5.82 | 0.582 ± 0.167 |
| G1 | 308.5 ± 243.5 | 489.2 ± 396.0 | 1106.0 ± 877.2 | 94.63 ± 57.47 | 2.528 ± 0.998 |
| H1 | 506.5 ± 417.6 | 855.1 ± 713.6 | 1896.5 ± 1602.7 | 191.60 ± 85.42 | 2.899 ± 0.406 |
High-pressure homogenization optimization
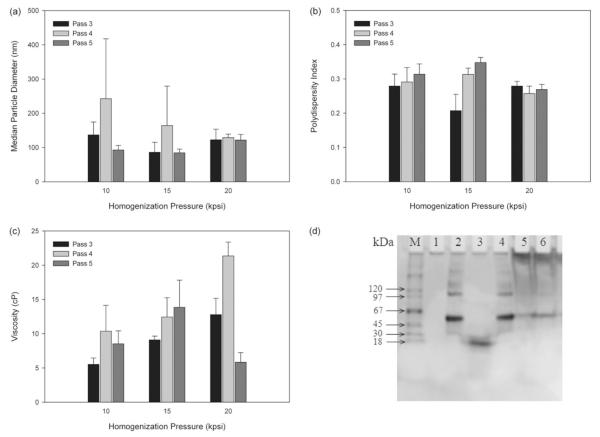
Formulation B1 was chosen to evaluate the potential of high-pressure homogenization to both reduce the particle size and the distribution of particle sizes observed within each sample. Based on the short-term study, formulation B1 was chosen for further investigation based on finding a balance between a low viscosity and a small particle size. Since the short-term study revealed a correlation between viscosity and surfactant amount, a balance must be struck between having sufficient surfactant content to achieve a nano-sized emulsion (overcoming the high surface-to-volume ratio of small particles), but without excess surfactant to increase the viscosity and possibly form secondary structures within the formulation. Figure 3 summarizes the effects of the homogenization pressure and the number of passes through the homogenizer on the nanoemulsion characteristics. Based on the 50% particle diameter, the pressure of 20 000 psi had the most uniform results irrespective of the pass number with no statistical difference among different pass numbers (p = 0.768). Regarding the impact of pass number on the particle size, five passes showed the lowest particle diameters, especially for pressures of 10 000 and 15 000 psi. All of the polydispersity index measurements in Figure 3(b) were well below any reading achieved with the smaller PowerGen 700 homogenizer, with 20 000 psi resulting in the lowest polydispersity index irrespective of the pass number. Finally, three passes at 10 000 psi and five passes at 20 000 psi produced the lowest viscosity samples, but no trend was found between the viscosity and the pass number or the homogenization pressure. Thus, optimizing for the lowest particle size, polydispersity index and viscosity, the most advantageous manufacturing regime appears to be five passes at 20 000 psi. Furthermore, no protein degradation, when compared to a forced degradation FITC-BSA solution, was observed for any of the three triplicates of formulation B1 homogenized at 20 000 psi for five passes relative to the control FITC-BSA solution (Figure 3d).
Figure 3.
Optimization of high pressure homogenization processing by assessing the (a) median particle diameter (volume distribution), (b) polydispersity index, (c) viscosity of formulation B1 and (d) validation of protein integrity by gel electrophoresis following high-pressure homogenization. Lanes: (M) protein marker, (1) blank lane, (2) control FITC-BSA solution, (3) alkaline-hydrolyzed FITC-BSA solution, (4) FITC-BSA solution processed for five passes at 20 000 psi, (5) nanoemulsion formulation B2 and (6) nanoemulsion formulation B1. Data represent mean ± SD.
To further optimize the formulation and manufacturing technique, the surfactant content of the nanoemulsion was increased to achieve an even smaller particle size than previously achieved with formulation B1. The ratio of aqueous phase to oil phase remained constant, while the surfactant content was increased from 5% to 10%. The new variation of formulation B1 was designated as formulation B2. The viscosity, zeta potential and polydispersity index were all slightly increased, while the particle size was decreased when processed for five passes at 20 000 psi (Table 3). 50% of the oil droplets had a diameter below 85 nm regardless whether FITC-BSA was present or not. In general, a high zeta potential (i.e. ± 30 mV) translates into improved stability of the formulation by resisting agglomeration23, indicating that formulation B2 should be as stable, if not more stable, than formulation B1. The combination of reduced particle size and increased zeta potential makes formulation B2 the most promising formulation for further development while still maintaining a relatively low viscosity.
Table 3.
Comparison of formulation B1 and B2
| Formulation | Particle diameter (10%) (nm, mean ± SD) |
Particle diameter (50%) (nm, mean ± SD) |
Particle diameter (90%) (nm, mean ± SD) |
Viscosity (cP, mean ± SD) |
Zeta potential (mV, mean ± SD) |
Polydispersity index (mean ± SD) |
|---|---|---|---|---|---|---|
| B1, blank | 87.7 ± 5.0 | 125.3 ± 5.9 | 210.4 ± 5.7 | 9.34 ± 0.43 | −30.23 ± 5.95 | 0.157 ± 0.028 |
| B2, blank | 58.2 ± 15.1 | 85.2 ± 18.9 | 151.0 ± 25.1 | 11.6 ± 0.7 | −43.13 ± 6.85 | 0.210 ± 0.042 |
| B1, FITC-BSA* | 90.9 ± 6.2 | 128.8 ± 7.6 | 213.9 ± 8.2 | 9.81 ± 0.74 | −24.34 ± 10.14 | 0.165 ± 0.053 |
| B2, FITC-BSA* | 58.2 ± 12.1 | 85.2 ± 15.5 | 148.6 ± 21.0 | 14.6 ± 1.2 | −45.17 ± 4.77 | 0.186 ± 0.026 |
Formulation includes 0.55 mg/mL FITC-BSA.
Skin diffusion study
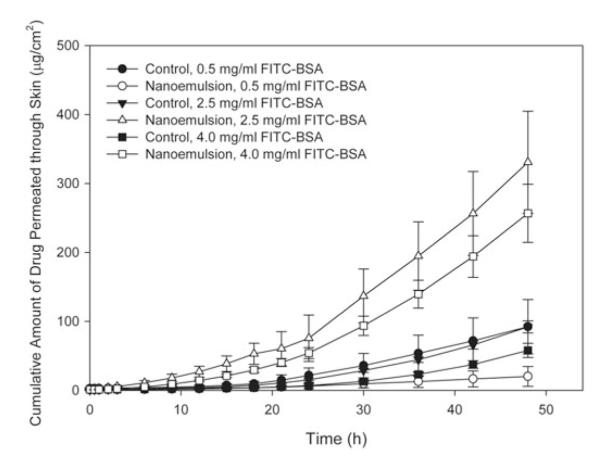
Figure 4 shows the permeability profile of the formulation B2 containing different concentrations of FITC-BSA and their corresponding control solutions, and Table 4 contains the permeability data associated with each formulation. At a concentration of 0.5 mg/mL FITC-BSA, the cumulative amount of drug diffused through the skin for the control solution was statistically different from the nanoemulsion after 36 h (p ≤ 0.006), which may be related to some binding of the surfactant to the skin surface which actually reduces the amount of drug present in the receiver compartment. However, both nanoemulsions at 2.5 and 4.0 mg/mL of FITC-BSA were statistically different from that of their respective control solutions much earlier: after 30 h for 2.5 mg/mL FITC-BSA (p<0.001) and after 21 h for 4.0 mg/mL FITC-BSA (p ≤ 0.020). There was no statistically significant difference among the steady-state flux measurements for the control solutions (p = 0.494). For the nanoemulsion formulations, the steady-state flux for both the nanoemulsion with 2.5 mg/mL FITC-BSA and 4.0 mg/mL FITC-BSA were statistically different from that of the nanoemulsion with 0.5 mg/mL FITC-BSA. Likewise, both the 2.5 and the 4.0 mg/mL FITC-BSA nanoemulsions were statistically different from their control solutions (p<0.001), while the 0.5 mg/mL FITC-BSA nanoemulsion was not significantly different from its corresponding control solution (p = 0.131). Similarly, the enhancement ratios for the two highest concentrations of FITC-BSA were considerably higher than that of the nanoemulsion with 0.5 mg/mL FITC-BSA. Thus, it can be deduced that an initial FITC-BSA concentration of 2.5 mg/mL or higher should be used to evaluate how effectively a nanoemulsion formulation permeates through mouse skin when measured by fluorescence spectrophotometry. While increasing the concentration of the nanoemulsion, thereby increasing the concentration gradient across the skin would be expected to correspond to an increase in FITC-BSA diffused through the skin, no statistical difference was observed between the steady-state flux at 2.5 and 4.0 mg/mL (p = 0.253).
Figure 4.
Permeation profile of FITC-BSA through mouse skin from nanoemulsions (formulation B2) containing different concentrations of FITC-BSA and their corresponding control solutions containing FITC-BSA. Data represent mean ± SEM. Statistically significant differences in steady-state flux (p<0.05): 0.5 mg/mL nanoemulsion versus 2.5 mg/mL nanoemulsion and 4.0 nanoemulsion, 2.5 mg/mL nanoemulsion versus 2.5 mg/mL control, 4.0 mg/mL nanoemulsion versus 4.0 mg/mL control.
Table 4.
Nanoemulsion permeability parameters at different concentrations of FITC-BSA
| Formulation, concentration of FITC-BSA | Jss (μg/cm2/h, mean ± SD) | Kp (×10−3 cm/h, mean ± SD) | E r | r2 (mean ± SD) |
|---|---|---|---|---|
| Control, 0.5 mg/mL | 2.864 ± 2.114 | 5.73 ± 4.23 | – | 0.987 ± 0.011 |
| Control, 2.5 mg/mL | 2.965 ± 0.531 | 1.19 ± 0.21 | – | 0.976 ± 0.008 |
| Control, 4.0 mg/mL | 1.901 ± 0.728 | 0.48 ± 0.18 | – | 0.946 ± 0.027 |
| B2, 0.5 mg/mL | 0.575 ± 0.868 | 1.15 ± 1.74 | 0.20 | 0.995 ± 0.004 |
| B2, 2.5 mg/mL | 10.144 ± 3.022 | 4.06 ± 1.21 | 3.42 | 0.995 ± 0.002 |
| B2, 4.0 mg/mL | 7.993 ± 2.661 | 2.00 ± 0.67 | 4.20 | 0.989 ± 0.004 |
Jss Steady-state flux, Kp permeability coefficient, Er enhancement ratio, r2 determination coefficient of linear portion of plots.
As an initial step to examine the role of the nanoemulsion excipients in FITC-BSA permeation, Figure 5 depicts the FITC-BSA permeation profile comparing formulation B2 versus its surfactant excipients at a concentration of 2.5 mg/mL FITC-BSA. The steady-state flux measurements among the nanoemulsion, emulsifiers and control solution were statistically different (p = 0.036). Additionally, the enhancement ratio for the nanoemulsion was nearly fours times greater than that of its emulsifiers, and three times more FITC-BSA diffused through the skin when incorporated into the nanoemulsion as opposed to the surfactant emulsifiers (Table 5).
Figure 5.
Comparison of FITC-BSA permeation profile through mouse skin for nanoemulsion B2, the emulsifiers contained in formulation B2, and a control solution of FITC-BSA. All contained 2.5 mg/mL FITC-BSA. Data represent mean ± SEM.
Table 5.
Comparison of permeability parameters for formulation B2 and its surfactants
| Formulation, concentration of FITC-BSA |
Jss (μg/cm2/h, mean ± SD) |
Kp (×10−3 cm/h, mean ± SD) |
E r |
r2 (mean ± SD) |
Percentage of drug recovered at 48 h* (mean ± SD) |
|---|---|---|---|---|---|
| B2, 2.5 mg/mL | 23.439 ± 17.230 | 9.38 ± 6.89 | 7.44 | 0.995 ± 0.004 | 30.21 ± 18.05 |
| Emulsifiers, 2.5 mg/mL† | 6.102 ± 0.977 | 2.44 ± 0.39 | 1.94 | 0.995 ± 0.002 | 10.20 ± 1.70 |
| Control, 2.5 mg/mL | 3.152 ± 0.897 | 1.26 ± 0.36 | – | 0.987 ± 0.002 | 3.00 ± 0.67 |
Jss Steady-state flux, Kp permeability coefficient, Er enhancement ratio, r2 determination coefficient of linear portion of plots.
Relative to amount of drug loaded in donor compartment.
10% Smix aqueous solution.
Long-term study
Formulation B2 proved stable for the entire duration of the 1 year study independent of the presence of FITC-BSA (Figure 6). After allowing for the effects of differences in time, the particle size measurements for the formulation with and without FITC-BSA were not significantly different (p = 0.739). The formulation without FITC-BSA showed no significant temporal change for the particle size or polydispersity index measurements (p ≥ 0.07), except for a significant result when comparing the particle size measurements from day 1 to month 12 (p = 0.040). Likewise, the formulation with FITC-BSA did not have any significant fluctuations in particle size either (p ≥ 0.118), but the polydispersity index measurement at month 1 was significantly different from subsequent measurements (p ≤ 0.021).
Figure 6.
Long-term stability assessment of formulation B2 with and without FITC-BSA, (a) median particle diameter, (b) polydispersity index and (c) zeta potential measurement. Data represent mean ± SD. Statistically significant differences (p<0.05) between nanoemulsion with and without FITC-BSA for viscosity measurements on month 6, month 9 and month 12.
However, both formulations ±showed an increasing zeta potential over the first 6 months, with a statistically significant increase from month 1 to month 3 (p<0.001) (Figure 6c). The zeta potential of the formulation without FITC-BSA changed by 17.48 mV over the first 6 months, from −43.13 to −25.65 mV, and with FITC-BSA the zeta potential of the nanoemulsion differed by 14.69 mV, from −45.17 to −30.48 mV. Overall, there was no statistically significant difference for the zeta potential measurements for the nanoemulsion with FITC-BSA when comparing measurements on day 1 with those at 12 months (p = 0.438), but the formulation with FITC-BSA differed from the formulation without FITC-BSA for the measurements at month 6, month 9, and 1 year (p ≤ 0.002).
Discussion
A blend of two different non-ionic surfactants with different HLB values were used to minimize the amount of surfactant required for a stable formulation. Minimizing the concentration of surfactant reduces the occurrence of skin irritation associated with large surfactant volumes24. Additionally, our results show that minimizing the amount of surfactant results in a lower overall viscosity of the system. A low-viscosity transcutaneous vaccine delivery vehicle is desirable because the formulation could easily cover a large area of skin and could potentially reduce the inhibition of the diffusion of the vaccine antigen through the nanoemulsion. Formulation B2 has only 10% surfactant volume which is considerably lower than other reported surfactant volumes for nanoemulsion formulations and which is due to the use of a high-pressure homogenizer rather than relying on spontaneous emulsification methods20,23,25. Additionally, since using a small high-performance homogenizer did not guarantee uniform mixing and shear over the entire batch, high-pressure homogenization was utilized to produce a more uniform size distribution. Careful control over the size of the formulation and the selection of excipients in the nanoemulsion is vital to the final success of a vaccine delivery vehicle because the two most important parameters to consider for transcutaneous particle-based vaccines are the size of the particles and their physical and chemical properties that facilitate their internalization by the antigen-presenting cells and other immune system cells within the skin3.
While the optimum homogenization procedure was determined, the processing procedure may need to be adjusted when incorporating other vaccine antigens into the nanoemulsion. The model protein FITC-BSA may have resisted degradation in the high-shear conditions of homogenization, but this result will not be applicable to all proteins. The homogenization parameters must be reassessed when integrating a different protein to mitigate the effects of homogenization on protein damage or denaturation. Furthermore, the aqueous phase of the formulation must be adjusted to suit the vaccine antigen to optimize its stability within the formulation and/or to suit the desired physical characteristics of the delivery method. One precedence for this adjustment of the nanoemulsion parameters after antigen selection is GlaxoSmithKline’s Adjuvant Systems (GlaxoSmithKline Biologicals S.A., Rixensart, Belgium). Both their AS04 and AS03 nanoemulsion formulations start with the same precursor emulsion called SB6226–29. To transform the bulk nanoemulsion SB62 into the final adjuvant AS03 or AS04, SB62 is diluted with PBS, stirred for 15–20 min and sterilized by filtration using a 0.22 μm filter. The vaccine antigen is packaged separately and combined with the nanoemulsion immediately prior to intramuscular injection26. Additionally, the manufacturing regime in this study is not too disparate from those reported in clinical and preclinical development currently. The preclinical work from the Infectious Disease Research Institute (IDRI, Seattle, WA) involves the use of their nanoemulsion formulation for incorporation of antigens against a variety of diseases, including polio, influenza, tuberculosis and Leishmania30–33. Their processing procedure is similar to this study in that their formulation is processed at 30 000 psi for 12 passes to produce the final formulation34.
The skin permeation results clearly demonstrate the improvement of transport across the skin of FITC-BSA by incorporation of FITC-BSA in the nanoemulsion formulation compared to a neat solution. However, the results of the permeation studies with different concentrations of FITC-BSA are somewhat unexpected. These initial results indicate that the permeation of the FITC-BSA is not only driven by concentration gradient and that solubility and partitioning play a meaningful role in the permeation of the formulation. In concentrated solutions and complex vehicles, such as formulation B2, the interaction among the drug molecules and the formulation excipients can be significant, and the diffusion of the drug will be highly dependent on the thermodynamic activity of the protein35. Squalane, Span-80 and Tween-80 have all been shown to have permeation enhancement properties, and the interaction of these excipients with each other, with the FITC-BSA, and with the skin create a complex diffusion environment – further work must be done to untangle these interactions and understand the mechanism of action of the nanoemulsion formulation. In an initial step to understand the impact of each excipient’s role in the transcutaneous transport of the FITC-BSA, the permeation enhancement of the surfactants was analyzed. The results demonstrated the importance of the nanoemulsion as a transcutaneous vehicle rather than its independent components. The fact that the nanoemulsion more effectively facilitated the diffusion of the drug than the emulsifiers may be attributed to the presence of squalane within the nanoemulsion and/or a synergistic effect between squalane and the emulsifiers. More research must be done to determine the exact role squalane plays in the transcutaneous diffusion of the FITC-BSA. The permeability coefficient, encompassing both diffusion effects and skin-vehicle partition effects, reflects the same results as the steady-state flux since all of the samples had the same FITC-BSA concentration in the donor chamber.
Finally, the long-term stability study showed the formulation B2 is stable for 1-year duration at 4 °C. While the polydispersity index for formulation B2 with FITC-BSA did fluctuate, the measurements overall still reflect a very homogenous nanoemulsion with only slight fluctuations in size. Since nanoemulsions are not a rigid nanoparticle system, slight fluctuations in size and distribution can occur, but the important result of this long-term study is that no overall increase in nanoemulsion size was observed over the study time period. The increase in zeta potential over time may indicate a reduction in the electrochemical stability of the formulation, but no corresponding increase in particle diameter was observed during the year-long duration, which would be indicative of nanoemulsion destabilization as seen with Ostwald ripening. The stability of our formulation is comparable to those of the nanoemulsion drug delivery vehicles for vaccines currently on the market. For instance, MF59 (squalene emulsion component of the influenze vaccine Fluad®, Novartis, Basel, Switzerland) can be stored at 2–8 °C for 1 year as well without the development of large particles36–38. Given that the formulation with FITC-BSA showed less variation in the zeta potential measurements over the 1-year duration, the negatively-charged FITC-BSA may have played a role in stabilizing the zeta potential measurements over time by protein absorption at the oil–water interface of the formulation. Since BSA can be used to stabilize o/w emulsions and appears to have stabilized the zeta potential measurements of our formulation during long-term storage, BSA could be incorporated as an excipient of formulation to maintain the surface charge of the nanoemulsion39. Maintaining a negative zeta potential may also translate into enhanced permeation since negatively-charged liposomes have resulted in improved permeation through the epidermis40.
Conclusions
The most promising formulations from this study were formulations A1, B1 and C1. The optimized high-pressure homogenization regime was found to be five passes at 20 000 psi, resulting in no evidence of protein degradation. BSA played a significant role in stabilizing the formulation over time since the formulation with FITC-BSA resulted in a more stable zeta potential over the long-term storage period. Formulation B2 was chosen for further study and demonstrated an enhanced ability to transport FITC-BSA through the mouse skin samples compared to control solutions. This study provided a basis for further investigation into the applicability of the proposed nanoemulsion as a transcutaneous vaccine vehicle. The nanoemulsion formulations described and categorized within this study are both flexible in their capacity for active ingredient incorporation and robust in their applications.
This study provided a basis for further investigation into the applicability of the proposed nanoemulsion as a transcutaneous vaccine vehicle. However, the ultimate success of this nanoemulsion as a carrier for transcutaneous vaccine delivery will depend on its ability to influence the biological responses in an appropriate model. Thus, in vivo studies in mice are currently underway, comparing formulation B2 to other emulsion formulations for their permeation efficiency in delivering a candidate vaccine protein. Future work will include the replacement of the model protein FITC-BSA with a known vaccine antigen. Additional work will also include standardized stability studies to assess the robustness of the formulation, further in vitro studies to elucidate the role each excipient plays in enhancing the permeation profile of the drug and the impact of particle size on diffusion, and further formulation optimization to increase the percentage of recovery of the drug. Although hairless mouse skin is widely used for in vitro skin diffusion studies, the final formulation should also be evaluated using human skin sample. Hence, our future work will also include evaluating the permeation efficiency through human skin samples.
Acknowledgments
This work was funded in part by the following grants: Louisiana Board of Regents RC/EEP (2007-11), LEQSF(2007-12)-ENH-PKSFI-PRS-02, DoD W81XWH-07-1-0136 and NIH 1G12RR026260-01.
Footnotes
Declaration of interest The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- 1.Kogan A, Garti N. Microemulsions as transdermal drug delivery vehicles. Adv Colloid Interface Sci. 2006;123–126:369–85. doi: 10.1016/j.cis.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Glenn GM, Kenney RT. Mass vaccination: solutions in the skin. Curr Topics Microbio Immunol. 2006;403:247–68. doi: 10.1007/3-540-36583-4_14. [DOI] [PubMed] [Google Scholar]
- 3.Combadiere B, Mahe B. Particle-based vaccines for transcutaneous vaccination. Comp Immunol Microbiol Infect Dis. 2008;31:293–315. doi: 10.1016/j.cimid.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Baboota S, Shakeel F, Ahuja A, Ali J, Shafiq S. Design, development and evaluation of novel nanoemulsion formulations for transdermal potential of celecoxib. Acta Pharm. 2007;57:315–32. doi: 10.2478/v10007-007-0025-5. [DOI] [PubMed] [Google Scholar]
- 5.Cui Z, Sloat BR. Topical immunization onto mouse skin using a microemulsion incorporated with an anthrax protective antigen protein-encoding plasmid. Int J Pharm. 2006;317:187–91. doi: 10.1016/j.ijpharm.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Foldvari M, Babiuk S, Badea I. DNA delivery for vaccination and therapeutics through the skin. Curr Drug Deliv. 2006;3:17–28. doi: 10.2174/156720106775197493. [DOI] [PubMed] [Google Scholar]
- 7.Guglielmini G. Nanostructured novel carrier for topical application. Clin Dermatol. 2008;26:341–6. doi: 10.1016/j.clindermatol.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Hoeller S, Sperger A, Valenta C. Lecithin based nanoemulsions: a comparative study of the influence of non-ionic surfactants and the cationic phytosphingosine on physicochemical behaviour and skin permeation. Int J Pharm. 2009;370:181–6. doi: 10.1016/j.ijpharm.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Kotyla T, Kuo F, Moolchandani V, et al. Increased bioavailability of a transdermal application of a nano-sized emulsion preparation. Int J Pharm. 2008;347:144–8. doi: 10.1016/j.ijpharm.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 10.Shakeel F, Baboota S, Ahuja A, et al. Nanoemulsions as vehicles for transdermal delivery of aceclofenac. AAPS PharmSciTech. 2007;8:191–9. doi: 10.1208/pt0802042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tadros T, Izquierdo P, Esquena J, Solans C. Formation and stability of nano-emulsions. Adv Colloid Interface Sci. 2004;108:303–18. doi: 10.1016/j.cis.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 12.Tagliabue A, Rappuoli R. Vaccine adjuvants: the dream becomes real. Hum Vaccin. 2008;4:347–9. doi: 10.4161/hv.4.5.6438. [DOI] [PubMed] [Google Scholar]
- 13.Fox CB. Squalene emulsions for parenteral vaccine and drug delivery. Molecules. 2009;14:3286–312. doi: 10.3390/molecules14093286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shafiq-un-Nabi S, Shakeel F, Talegaonkar S, et al. Formulation development and optimization using nanoemulsion technique: a technical note. AAPS PharmSciTech. 2007;8:E12–17. doi: 10.1208/pt0802028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anton N, Vandamme TF. Nano-emulsions and micro-emulsions: clarifications of the critical differences. Pharm Res. 2011;28:978–85. doi: 10.1007/s11095-010-0309-1. [DOI] [PubMed] [Google Scholar]
- 16.Wooster TJ, Golding M, Sanguansri P. Impact of oil type on nanoemulsion formation and Ostwald ripening stability. Langmuir. 2008;24:12758–65. doi: 10.1021/la801685v. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Cardamone JM. Investigating the microstructure of keratin extracted from wool: peptide sequence (MALDI-TOF/TOF) and protein conformation (FTIR) J Mol Struct. 2010;969:97–105. [Google Scholar]
- 19.Davies DJ, Ward RJ, Heylings JR. Multi-species assessment of electrical resistance as a skin integrity marker for in vitro percutaneous absorption studies. Toxicol In Vitro. 2004;18:351–8. doi: 10.1016/j.tiv.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Elshafeey AH, Kamel AO, Fathallah MM. Utility of nanosized microemulsion for transdermal delivery of tolterodine tartrate: exvivo permeation and in-vivo pharmacokinetic studies. Pharm Res. 2009;26:2446–53. doi: 10.1007/s11095-009-9956-5. [DOI] [PubMed] [Google Scholar]
- 21.Centers of Disease control and Prevention (CDC) Guidelines for maintaining and managing the vaccine cold chain. MMWR Morb Mortal Wkly Rep. 2003;52:1023–5. [PubMed] [Google Scholar]
- 22.Porras M, Solans C, Gonzalez C, Gutierrez JM. Properties of water-in-oil (W/O) nano-emulsions prepared by a low-energy emulsification method. Colloids Surf A: Physicochem Eng Asp. 2008;324:181–8. [Google Scholar]
- 23.Bali V, Ali M, Ali J. Study of surfactant combinations and development of a novel nanoemulsion for minimizing variations in bioavailability of ezetimibe. Colloids Surf B: Biointerfaces. 2010;76:410–20. doi: 10.1016/j.colsurfb.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Azeem A, Rizwan M, Ahmad FJ, et al. Nanoemulsion components screening and selection: a technical note. AAPS PharmSciTech. 2009;10:69–76. doi: 10.1208/s12249-008-9178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar M, Pathak K, Misra A. Formulation and characterization of nanoemulsion-based drug delivery system of risperidone. Drug Dev Ind Pharm. 2009;35:387–95. doi: 10.1080/03639040802363704. [DOI] [PubMed] [Google Scholar]
- 26.D’Hondt E, Hehme N, Hanon EJ, Stephenne J. Influenza vaccine. United States of America patent US 2007/0141078 A1. 2007 Jun 21; [Google Scholar]
- 27.Morel S, Didierlaurent A, Bourguignon P, et al. Adjuvant system AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29:2461–73. doi: 10.1016/j.vaccine.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Ferguson M, Risi G, Davis M, et al. Safety and long-term humoral immune response in adults after vaccination with an H1N1 2009 pandemic influenza vaccine with or without AS03 adjuvant. J Infect Dis. 2012;205:733–44. doi: 10.1093/infdis/jir641. [DOI] [PubMed] [Google Scholar]
- 29.Alonso PL, Sacarlal J, Aponte JJ, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364:1411–20. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 30.Baldwin SL, Fox CB, Pallansch MA, et al. Increased potency of an inactivated trivalent polio vaccine with oil-in-water emulsions. Vaccine. 2011;29:644–9. doi: 10.1016/j.vaccine.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 31.Bertholet S, Goto Y, Carter L, et al. Optimized subunit vaccine protects against experimental leishmaniasis. Vaccine. 2009;27:7036–45. doi: 10.1016/j.vaccine.2009.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baldwin SL, Shaverdian N, Goto Y, et al. Enhanced humoral and type 1 cellular immune responses with Fluzone adjuvanted with a synthetic TLR4 agonist formulated in an emulsion. Vaccine. 2009;27:5956–63. doi: 10.1016/j.vaccine.2009.07.081. [DOI] [PubMed] [Google Scholar]
- 33.Baldwin SL, Bertholet S, Kahn M, et al. Intradermal immunization improves protective efficacy of a novel TB vaccine candidate. Vaccine. 2009;27:3063–71. doi: 10.1016/j.vaccine.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fox CB, Anderson RC, Dutill TS, et al. Monitoring the effects of component structure and source on formulation stability and adjuvant activity of oil-in-water emulsions. Colloids Surf B: Biointerfaces. 2008;65:98–105. doi: 10.1016/j.colsurfb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Otto A, DuPlessis J, Wiechers JW. Formulation effects of topical emulsions on transdermal and dermal delivery. Int J Cosmet Sci. 2009;31:1–19. doi: 10.1111/j.1468-2494.2008.00467.x. [DOI] [PubMed] [Google Scholar]
- 36.Ott G, Radhakrishnan R, Fang JH, Hora M. The adjuvant MF59: a 10-year perspective. In: O’Hagan DT, editor. Vaccine adjuvants: preparation methods and research protocols. Humana Press Inc.; Totowa (NJ): 2000. pp. 211–28. [Google Scholar]
- 37.Podda A. The adjuvanted influenza vaccines with novel adjuvants: experience with the MF59-adjuvanted vaccine. Vaccine. 2001;19:2673–80. doi: 10.1016/s0264-410x(00)00499-0. [DOI] [PubMed] [Google Scholar]
- 38.Ott G, Barchfeld GL, VanNest G. Enhancement of humoral response against human influenza vaccine with the simple submicron oil/water emulsion adjuvant MF59. Vaccine. 1995;13:1557–62. doi: 10.1016/0264-410x(95)00089-j. [DOI] [PubMed] [Google Scholar]
- 39.Rangsansarid J, Fukada K. Factors affecting the stability of O/W emulsion in BSA solution: stabilization by electrically neutral protein at high ionic strength. J Colloid Interface Sci. 2007;316:779–86. doi: 10.1016/j.jcis.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 40.Gillet A, Compere P, Lecomte F, et al. Liposome surface charge influence on skin penetration behaviour. Int J Pharm. 2011;411:223–31. doi: 10.1016/j.ijpharm.2011.03.049. [DOI] [PubMed] [Google Scholar]