The nonapeptide oxytocin is considered beneficial to mental health due to its anxiolytic, prosocial, and anti-stress effects. Unexpectedly, evidence for anxiogenic actions of oxytocin in humans has recently emerged. Using region-specific manipulations of the oxytocin receptor gene (Oxtr), we identify the lateral septum as the brain region mediating fear-enhancing effects of Oxtr. These effects emerge after social defeat, and engage Oxtr distinctively coupled to the extracellular signal-regulated protein kinase pathway.
The established view that oxytocin reduces fear and anxiety1–3 has recently been challenged, as humans given oxytocin intranasally show increased recollection of aversive events4 and startle responses to stressful stimuli5,6 Consistent with these data, oxytocin reactivity is associated with increased post-conflict anxiety7.
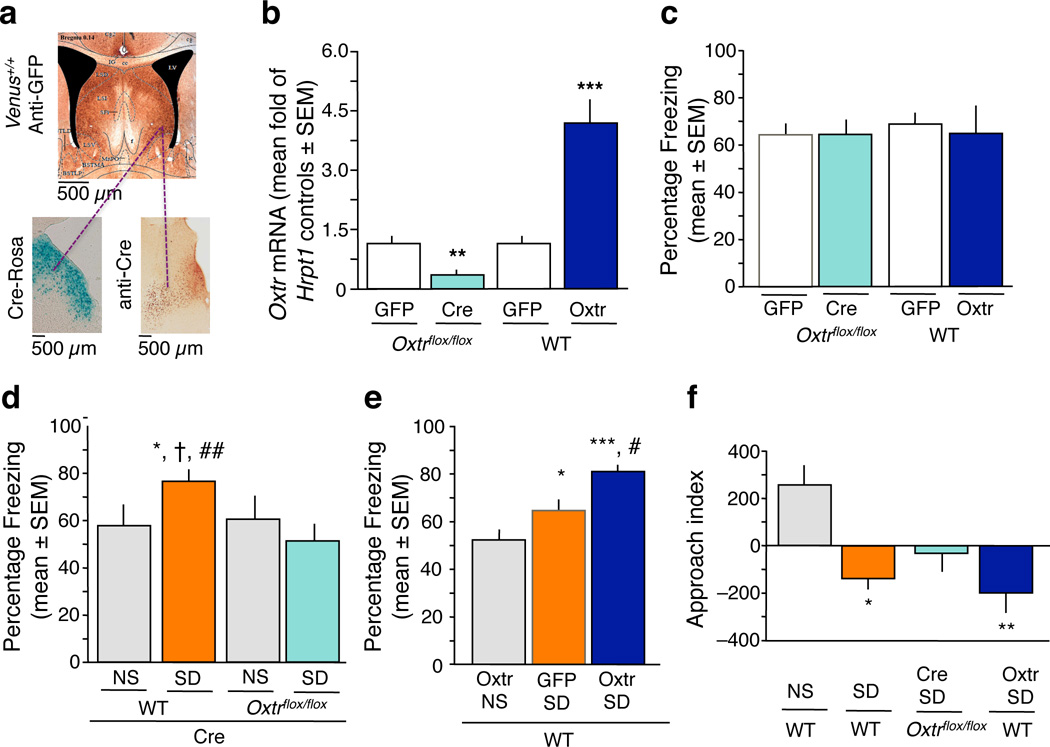
To understand the neurobiological basis of fear regulation by oxytocin, we focused on Oxtr-mediated signaling in the lateral septum, a brain area with high levels of Oxtr8 and a significant involvement in stress and fear9. We manipulated the levels of lateral septal Oxtr with two region-specific genetic approaches: for Oxtr knockdown, we injected a replication-defective adeno-associated virus (rAAV) encoding Cre recombinase (Cre) in Oxtrflox/flox mice (Cre group) ; for overexpression, we infused wild type mice with rAAV vector encoding Oxtr12 (Oxtr group). Corresponding controls received rAAV-green fluorescent protein (GFP) vector (GFP group). The vectors were targeted to the area of the lateral septum containing a dense population of Oxtr-positive neurons (Fig. 1a) and induced down-regulation (Cre Oxtrflox/flox, n = 7 mice/group, vs GFP Oxtrflox/flox, n = 4 mice/group, t9 = 3.49, P < 0.01) or up-regulation [wild type (WT) Oxtr, n = 5 mice/group, vs wild type GFP, n = 7 mice/group, t10 = –5.280, P < 0.001] of Oxtr mRNA levels (Fig. 1b). We next studied the role of Oxtr knockdown or overexpression in context-dependent fear conditioning. One day after pairing context and shock, we assessed freezing behavior to the context as index of fear. Oxtr down-regulation did not affect fear conditioning (t20 = 0.00, P = 1.00), as revealed by similar freezing levels in Oxtrflox/flox mice injected with Cre (n = 11 mice/group) or GFP (n = 11 mice/group)(Fig. 1c). Similarly ineffective was Oxtr overexpression, as revealed by indistinguishable freezing in the Oxtr (n = 5 mice/group) and GFP (n = 7 mice/group) groups (t10 = 0.344, P = 0.738) (Fig. 1c). We therefore hypothesized that if oxytocin played a role in fear regulation via the lateral septum, this role would be modulatory rather than direct. To test this possibility, we established a mouse model of stress-enhanced fear10 using acute social defeat, a stressor that significantly increases oxytocin release within the septal area11, and preferentially enhanced context- over tone-dependent fear conditioning (Supplementary Fig. 1). Wild type or Oxtrflox/flox mice were injected with Cre and divided into either non-stressed (NS, separated from the aggressor with a transparent barrier), or stressed groups (SD, exposed to social defeat)(see Supplementary Fig. 2a for experimental design). Six hours after stress, all mice were trained in contextual fear conditioning and tested one day later. We found significant group differences of context fear (F3, 28 = 4.034, P < 0.05), as revealed by higher freezing of stressed Cre mice (SD-Cre, n = 7 mice/group) when compared to their NS-Cre control (P < 0.05, n = 7 mice/group). However, this effect was lacking in Oxtr knockdown mice (Oxtrflox/flox Cre, n = 8 mice/group) that froze similarly to their NS littermates (n = 10; P = 0.759) and significantly less than the wild type SD group (P < 0.01) (Fig 1d). Stress-enhanced fear was also abolished by pharmacological inhibition of Oxtr (Supplementary Fig. 3). In contrast to knockdown, Oxtr overexpression further exacerbated stress-enhanced fear (SD Oxtr, n = 9 mice/group), F2, 25 = 13.67, P < 0.001, when compared to the NS Oxtr (n = 9 mice/group, P < 0.001) and SD GFP (n = 10 mice/group) groups (Fig. 1e). Unlike Oxtr overexpression, however, oxytocin injection did not further increase freezing in response to SD (Supplementary Fig. 3) possibly because endogenous oxytocin saturated available Oxtr and prevented further actions of exogenously added peptide. Taken together, these findings demonstrated that lateral septal Oxtr mediate the enhancement of fear by social defeat stress and that Oxtr are necessary but not sufficient to exert a fear enhancing action.
Figure 1.
Oxtr mediate the enhancement of fear by social defeat stress. a, Localization of the Oxtr-positive neurons in the lateral septum of Venus+/+ mice (upper panel); localization of Cre, as determined by Cre immunostaining in wild type mice (lower left panel), and function of Cre, as determined by lacZ staining in Rosa reporter mice (lower right panel). We targeted the viral injections to antero-posterior coordinates corresponding to the highest number of Oxtr-positive neurons (AP +0.14 to +0.38). b, Levels of Oxtr mRNA in the septal area after injection of GFP or Cre in Oxtrflox/flox mice, or after injection of GFP or Oxtr in wild type mice. The septal Oxtr knockdown and overexpression resulted in an approximately 3-fold decrease and increase, respectively, of Oxtr mRNA levels when compared to corresponding GFP controls (**P < 0.01, ***P < 0.001). c, Floxed Oxtr mice injected with Cre froze indistinguishably from GFP controls. Similarly, freezing did not differ between wild type mice injected with Oxtr or GFP. d, Pre-exposure to SD significantly enhanced fear conditioning. This effect was completely abolished in Oxtrflox/flox, but not wild type mice injected with Cre. *P < 0.05 vs NS wild type; †P < 0.05 vs NS Oxtrflox/flox ; ##P < 0.01 vs SD Oxtrflox/flox. e, On the other hand, Oxtr overexpression further enhanced fear (*P < 0.05, ***P < 0.01 vs NS group; #P < 0.05 vs SD GFP group). f, All SD mice except for the Oxtr knockdown group (Oxtrflox/flox Cre) interacted significantly less with the aggressor than NS mice (*P < 0.05, **P < 0.01), indicating that mice lacking Oxtr did not form a persistent memory of the aggressor. Data are expressed as mean ± standard error of the mean (SEM).
Manipulations of Oxtr did not alter the behavioral measures of social defeat or exploratory activity to the context (Supplementary Fig. 4). We therefore hypothesized that, rather than affecting the experience of social defeat, Oxtr exhibited a delayed effect by enhancing aversive social memory13,14. We measured the approach behavior of defeated mice toward the aggressive resident six hours post-stress. NS controls (n = 11 mice/group) approached the resident (approach score 262 ± 62 or 36 % of total time) (Fig. 1f), consistent with a lack of lasting memory of their interaction15. Conversely, SD mice (n = 7 mice/group) and SD mice overexpressing Oxtr (n = 10 mice/group) approached significantly less, F3, 32 = 3.582, P < 0.05 (Fig. 1f), indicating prolonged social memory of the aggressor16. Such memory was not observed in Oxtr knockdown mice (n = 8 mice/group) whose approach index did not show preference for the aggressor and did not differ from the NS group (P = 0.0828). These findings showed that the potentiation of fear by lateral septal Oxtr might be due, at least in part, to the maintenance of social memory after defeat, rather than general enhancement of fear or anxiety.
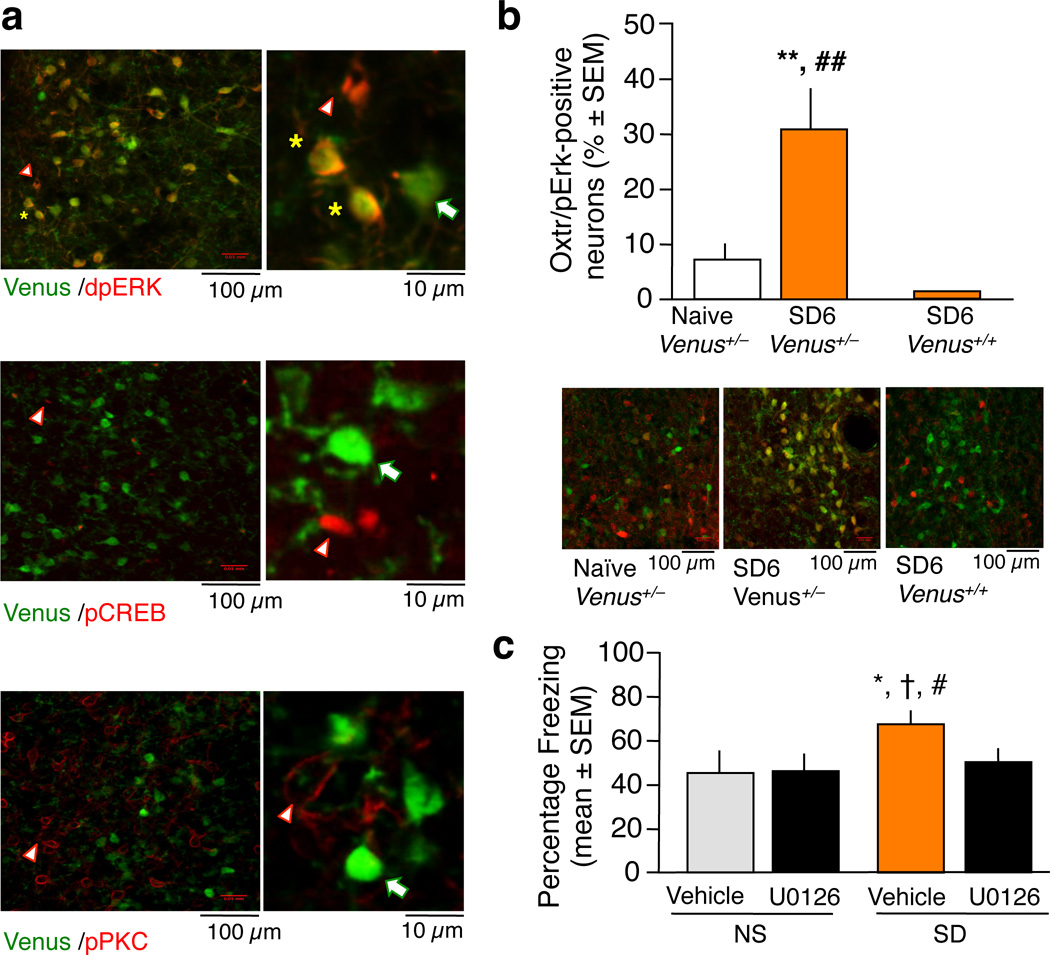
The adverse effects of stress on emotional behavior have been linked to distinct signaling pathways17. To identify the protein kinases activated within Oxtr-bearing neurons, we used reporter mice in which one or both Oxtr gene alleles were replaced with Venus, coding for a variant of GFP. Social defeat did not affect the number of Venus-positive cells (Supplementary Fig. 5), suggesting that the activity of the Oxtr promoter did not change in response to stress. However, the level of phosphorylated extracellular signal-regulated kinase-1/2 (pErk-1/2) was significantly increased in Oxtr-bearing neurons of heterozygous, Venus+/− mice (n = 4 septi/group) 6 hr after SD [(F2, 12 = 13.329, P < 0.01) versus naïve (n = 5 septi/group)] (Fig. 2a,b), an effect that was robustly abolished by Oxtr knockout in homozygous Venus+/+ neurons (n = 6 septi/group). Notably, Venus-positive cells were completely devoid of phosphorylated protein kinase C (pPKC) or phospho-cAMP-response element binding protein (pCREB) activity (Fig 2a, middle and lower panel), demonstrating highly specific coupling of lateral septal Oxtr to the Erk-1/2 signaling pathway. In this way, Oxtr signaling in the lateral septum differs from other tissues, where coupling to PKC has been established18. The role of septal Erk-1/2 signaling as a mediator of Oxtr effects on fear was further supported by the findings that inhibition of Erk-1/2 activity (Supplementary Fig. 6), similar to Oxtr down-regulation, abolished the enhancement of fear in the SD group [F3, 24 = 3.569, P < 0.05; SD vehicle (n = 5 mice/group) vs SD U0126 (n = 7 mice/group), P < 0.05] without affecting fear conditioning of the NS group [(NS U0126 (n = 8 mice/group) vs NS vehicle (n = 8 mice/group), P = 0.8283, Fig. 2c)].
Figure 2.
Coupling of lateral septal Oxtr to Erk-1/2 signaling. a, Reporter neurons of heterozygous Venus+/− mice co-localized with pErk-1/2 (upper panel) but not pCREB (middle panel) or pPKC (lower panel). Yellow star indicates co-localization, white arrow Venus-positive neurons, and white triangle protein kinase-positive neurons. b, Social defeat led to a significant increase of the number of pErk-1/2 in Venus+/− neurons 6 hours later (SD6 group). This effect was abolished in Venus+/+ SD6 group lacking Oxtr (**P < 0.01 versus naïve; ##P < 0.01 versus Venus+/− SD6). c, Mice of the SD vehicle group exhibited a significant increase of freezing when compared to vehicle- and U0126- injected NS controls as well as U0126-injected SD mice. Thus, the inhibitor of the mitogen-activated protein kinase kinase (Mek1/2) completely abolished the enhancement of fear by social defeat without affecting fear conditioning of NS mice. *P < 0.05 vs vehicle control; †P < 0.05 vs U0126 NS; #P < 0.05 vs U0126 SD.
Our results demonstrate that social defeat significantly activates the lateral septal Oxtr/Erk pathway and thereby enhances contextual fear conditioning. This mechanism may underlie the unexpected anxiogenic and fear-enhancing effects of oxytocin in humans4–7 and mice19, as opposed to anxiolytic actions mediated by the central amygdala20. We therefore propose that rather than displaying a unidirectional influence on anxiety, the oxytocin system plays a modulatory role, possibly by changing the salience or emotional valence of social and nonsocial contexts. This could improve the cognitive tuning of emotional processes and thus provide superior behavioral adaptation.
Methods
Subjects
Wild type, C57BL/6N mice (Harlan), floxed Oxtr mice14 backcrossed for 9 generations with C57BL/6N mice (Harlan), and Venus reporter driven by the Oxtr promoter8 backcrossed for 9 generations with C57BL/6N mice (Harlan) were used in the experiments. B6.129S4-Gt(ROSA) mice were provided by Warren Tourtellotte. The mice were bred in the CCM facility of Northwestern University. One week prior to experiments, at the age of 8 weeks, the mice were transferred to our satellite facility and individually housed in cabinets (Scanbur) under standard conditions (temperature 22°C, humidity 40–60%, 12/12 light-dark cycle, water and food ad libitum). Experiments were performed with 9 week-old male mice during the light cycle. All studies were approved by the Animal Care and Use Committee at Northwestern University and are in compliance with the National Institute of Health standards.
Surgery
Double-guided cannula (Plastic One) were implanted into the lateral septum of mice as described previously21. Mice were anesthetized with 1.2% Avertin and implanted with bilateral 26-gauge cannulae using a stereotaxic apparatus. Lateral septum was targeted using coordinates: AP+0.2 mm, ML±0.5 mm, DV−3.0 mm22. The mice were allowed to recover for one week before injection of peptides or viruses.
Injections
Oxytocin (Sigma), [1-D(CH2)5,Tyr(ME)2,Thr4,Tyr-NH2(9)]ornithine vasotocin (OTA, Bachem) and MEK inhibitor (U0126, Sigma) were injected over 30 sec, 0.2 µl/per side. The viral vectors rAAV-Cre (driven by a synapsin promoter and obtained from Pavel Osten), rAAV-GFP (driven by a synapsin promoter, University of Pennsylvania Viral Vector Core), rAAV-Oxtr (driven by a CMV promoter12) and and rAAV-GFP (driven by a CMV promoter12), were infused over 2 min (0.1 µl/min) intraseptally in mice under a light isoflurane anesthesia. All viruses had a titer of 1–2 × 1012 particles/ml. Oxytocin (1 ng/0.4 µl/mouse) and OTA (10 ng/0.4 µl/mouse) were dissolved in artificial cerebrospinal fluid (aCSF) and aCSF alone served as vehicle. U0126 (1 µg/0.4 µl/mouse) was dissolved in 50% DMSO and controls for this study were injected with 50% DMSO. After the end of each experiment, all brains were collected for verification of cannula placement following methylene blue injection unless used for immunohistochemical or qPCR validation. Only mice with correct cannula placement (Supplementary Figure 3a) were included in the data analysis.
Social defeat
Mice of each virus condition were randomly assigned to the NS or SD groups. Mice were placed in a cage of an aggressive resident for 10 minutes. Social defeat was monitored and confirmed based on the number of attacks by the aggressor, and defensive and submissive postures of the defeated mouse23. NS controls were placed in the cage of an aggressive resident for 10 minutes, with a perforated Plexiglas barrier separating the resident and the intruder. Defensive behavior (flurries, submissive, and defensive postures) during social defeat were scored from the video recordings as described23.
Fear conditioning
Fear conditioning took place in a context, a 35 × 20 × 20 cm Plexiglas chamber with a stainless steel rod floor (4 mm diameter, 0.9 cm center to center) located within a sound-attenuating cabinet with black inner walls (TSE Systems, Inc). The box was cleaned after each mouse with 70% ethanol. Mice were placed in the chamber and 3 min later presented with a footshock (2 s, 0.7 mA, constant current). Mice were tested for fear to the conditioning context by returning them to this chamber for 3 min tests. Freezing was scored every 5 s by a trained observer blind to the experimental conditions (the cage cards were replaced by coded cards), and expressed as the percentage of total number of observations.
Social recognition
Six hour after social defeat, mice were first habituated for 3 min to an open field arena, followed by a 5-min re-exposure to the aggressor or novel mouse confined to metal cages inside the same arena. The activity and time spent interacting with the aggressor or novel mouse was recorded automatically by a Videomot II software (TSE Systems, Inc.). The approach index was calculated as a difference between activity at the side of the aggressor and the side of the novel mouse adjusted for the side activity during habituation (test aggressor-novel/habituation aggressor/novel)15.
Immunohistochemistry and immunofluorescence
Immunohistochemistry for Cre (1:2,000; Covance, Cat # MMS-106P) or β-galactosidase (βgal; ICN Pharmaceuticals) were performed on frozen tissue. For β-galactosidase staining, sections were postfixed in 2% paraformaldehyde (PFA), 0.2% glutaraldehyde, 5 mM EGTA, 0.01% NP-40 in PBS-Mg at 4°C and reacted for 6–12 hours at 37°C in reaction buffer (1 mg/ml X-gal, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide). After reaction, the tissues were postfixed/dehydrated in methanol, and cleared in 2:1 benzyl benzoate:benzyl alcohol as described24. For all other stainings, brains were fixed with 4% PFA, dehydrated in 30% sucrose and stained using the Vectastain system (Vector, Cat # BA-9200 for mouse and BA-1000 for rabbit primary antibodies), as described previously10. Immunostaining with anti-Cre antibodies was visualized with d-amino benzidine. Immunostaining with mouse anti-GFP (1:6,000; Abcam, Cat # AB1218) were visualized with fluoresceinisothiocyanate, whereas rabbit anti-pErk-1/2 (1:1,600; Cell Signaling, Cat # 4370), anti-pCREB (1:8,000; Cell Signaling, Cat # 9198), or anti-pPKC (1:400; Cell Signaling, Cat #9371) were visualized with rhodamine. The antibodies were previously validated using peptide preadsorption or knockout approaches26,27.
Quantification of immunostaining signals was performed as described previously25. Slices were mounted in Vectashield (Vector) and observed with a confocal laser scanning microscope (Olympus Fluoview FV10i) for double-labeling at 40 ×. Septal sections were counted for double-labeling at the coordinates22 AP +0.14 to + 0.38 and the number of kinase-positive neurons expressed as percent of total Venus-positive neurons/septum. One septum (with the highest number of Venus-positive neurons)/mouse/group was used to calculate group means.
RNA extraction and quantitative PCR (qPCR)
Mice were injected with methylene blue to visualize the viral injection site and then killed by cervical dislocation. Brains were dissected and the lateral septum was collected using a brain matrix. Tissue was homogenized in lysis buffer with β-mercaptoethanol and frozen in liquid nitrogen. RNA was extracted using miRCURY total RNA isolation kit (Exiqon), reversely transcribed and subjected to real-time PCR using SYBR Green master mix (Applied Biosystems) and primers for Oxtr or mouse hypoxanthine phosphoribosyl transferase 1 (mHprt1) as an internal control. For Oxtr the forward primer was 5’-GGA GCG TCT GGG ACG TCA AT-3” and the reverse primer was 5’-AGG AAG CGC TGC ACG AGT T-3’. For mHprt1 the forward primer was 5’-GGG CTT ACC TCA CTG CTT TC -3’ and reverse 5’-TCT CCA CCA ATA ACT TTT ATG TCC-3”. The level of Oxtr expression in Oxtrflox/flox mice injected with rAAV-Cre or wild type mice injected with rAAV-Oxtr was normalized to mHprt1 and shown relative to the rAAV-GFP control.
Protein extraction and immunoblot
Individual septi were collected around the tip of the cannula from nonstressed mice injected with vehicle, or mice injected with vehicle or U0126 five hours after stress. Septi were lysed in a modified radioimmunoprecipitation (RIPA) buffer, incubated 15 min on ice and centrifuged for 15 min, 15000 × g, 4°C. The RIPA buffer contained 50 mM Tris-HCl pH 7.4, 1% NP-40, 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, protease inhibitor cocktail (Boehringer Manheim), 1 mM Na3VO4 and 1 mM NaF. After determining the protein concentration for each lysate (Bio-Rad protein assay), input samples (10 µg protein/µl) were reduced in loading buffer with DTT and boiled for 5 minutes, then subjected to SDS polyacrylamide gel electrophoresis (25 µg/well) and blotted to PVDF membranes (Millipore). Using the SnapID system (Millipore), membranes were saturated with I-block (Tropix), incubated with mouse anti-pErk-1/2 (Sigma, 1:16,000, Cat # M8159) or rabbit Erk-1/2 (Santa Cruz, 1:5,000, Cat #K-23) and corresponding secondary antibodies (Goat Anti-Rabbit 1:10,000, Applied Biosystems, Cat # T2191). For detection, we used alkaline phosphatase chemiluminescence.
Statistical analyses
We used about 5–8 mice per experimental group for behavioral and 4–8 biological samples/group for immunohistochemical, qPCR, and immunoblot assays. The numbers for each biochemical assay are biological replicates. The behavioral and molecular studies were replicated at 2–4 times (on average 3 times). These sample sizes gave us statistical power of 80% to detect stress or drug effects with the behavioral and biochemical data. Group sizes were balanced to ensure equal variance between the tested groups, and this was verified with Levene's weighted F test computed by the SPSS package for all comparisons. All data showed normal distribution and were analyzed by two-tailed Student’s t-test for two groups or one-way ANOVA for three or more groups with Treatment as a main factor. Post-hoc comparisons were performed using Fisher’s LSD test. Each key study was replicated at least twice.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the National Institutes of Health grants R01 MH078064 (J.R.) and MH092065 (Y.F.G.). Part of this study carried out by N.K. and K.S. was the result of "Integrated research on neuropsychiatric disorder" in the Strategic Research Program for Brain Sciences by the Ministry of Education, Culture, Sports, Science and Technology of Japan. We thank Dr. Pavel Osten (Cold Spring Harbor) for providing the rAAV-Cre vector, and Lin Li and Warren G. Tourtellotte (Department of Pathology, Northwestern University) for providing B6.129S4-Gt (ROSA) mice and their help with the β-galactosidase staining.
Footnotes
AUTHOR CONTRIBUTIONS
Y.F.G. performed all of the experiments and data analyses, Y.F.G. and J.R. designed the studies and wrote the paper, K.S., H.M., and K.N. developed the rAAV-Oxtr and rAAV-GFP viral vectors, K.N. provided the Oxtrflox/flox and Venus reporter mice, N.C.T. developed the model of social defeat, V.J. helped with the qPCR analyses, A.G. bred and genotyped the mice.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
REFERENCES
- 1.Carter CS. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 2.Ayers LW, Missig G, Schulkin J, Rosen JB. Neuropsychopharmacology. 2011;36:2488–2497. doi: 10.1038/npp.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishak WW, Kahloon M, Fakhry H. J. Affect Disord. 2011;130:1–9. doi: 10.1016/j.jad.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Bartz JA, et al. Proc. Natl. Acad. Sci. USA. 2010;107:21371–21375. doi: 10.1073/pnas.1012669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grillon C, et al. Mol. Psychiatry. 2012 [Google Scholar]
- 6.Striepens N, et al. Proc. Natl. Acad. Sci. U S A. 2012;109:18144–18149. doi: 10.1073/pnas.1208852109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabak BA, McCullough ME, Szeto A, Mendez AJ, McCabe PM. Psychoneuroendocrinology. 2011;36:115–122. doi: 10.1016/j.psyneuen.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida M, et al. J. Neurosci. 2009;29:2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheehan TP, Chambers RA, Russell DS. Brain Res. Rev. 2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Tronson NC, et al. Biol. Psychiatry. 2010;68:1007–1015. doi: 10.1016/j.biopsych.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebner K, Wotjak CT, Landgraf R, Engelmann M. Brain Res. 2000;872:87–92. doi: 10.1016/s0006-8993(00)02464-1. [DOI] [PubMed] [Google Scholar]
- 12.Sato K, et al. Biosc.i Biotechnol. Biochem. 2009;73:2145–2148. doi: 10.1271/bbb.90287. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson JN, Young LJ, Insel TR. Fron.t Neuroendocrinol. 2002;23:200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- 14.Takayanagi Y, et al. Proc. Nat.l Acad. Sci. USA. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thor DH, Holloway WR. J. Comp. Physiol. Psych. 1982;96:1000–1006. [Google Scholar]
- 16.Cao JL, et al. J. Neurosci. 2010;30:16453–16458. doi: 10.1523/JNEUROSCI.3177-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Revest JM, et al. Nat. Neurosci. 2005;8:664–672. doi: 10.1038/nn1441. [DOI] [PubMed] [Google Scholar]
- 18.Devost D, Wrzal P, Zingg HH. Prog. Brain Res. 2008;170:167–176. doi: 10.1016/S0079-6123(08)00415-9. [DOI] [PubMed] [Google Scholar]
- 19.Pagani JH, Lee HJ, Young WS., 3rd Genes Brain. Behav. 2011;10:710–719. doi: 10.1111/j.1601-183X.2011.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knobloch HS, et al. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
REFERENCES FOR SUPPLEMENTARY METHODS
- 21.Radulovic J, Ruhmann A, Liepold T, Spiess J. J. Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paxinos G, Franklin KB. The mouse brain in stereotaxic coordinates. San Diego Academic Press; 2001. [Google Scholar]
- 23.Roche KE, Leshner AI. Science. 1979;204:1343–1344. doi: 10.1126/science.221973. [DOI] [PubMed] [Google Scholar]
- 24.Eldredge LC, et al. Development. 2008;135:2949–2957. doi: 10.1242/dev.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mutso AA, et al. J. Neurosci. 2012;32:5747–5756. doi: 10.1523/JNEUROSCI.0587-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tronson NC, et al. Neuropharmacology. 2008;33:1570–1583. [Google Scholar]
- 27.Stanciu M, et al. Mol. Brain Res. 2001;94:15–24. doi: 10.1016/s0169-328x(01)00174-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.