Abstract
Objective
To evaluate agreement between fluorescein angiography (FA) and optical coherence tomography (OCT) for diagnosis of macular edema in patients with uveitis.
Design
Multicenter cross-sectional study
Participants
Four hundred seventy-nine eyes with uveitis of 255 patients
Methods
The macular status of dilated eyes with intermediate, posterior or panuveitis was assessed via Stratus-3 OCT and FA. Kappa statistics evaluated agreement between the diagnostic approaches.
Main Outcome Measures
Macular thickening (center point thickness ≥240 μm per reading center grading of OCT images-“MT”) and macular leakage (central subfield fluorescein leakage ≥0.44 disk areas per reading center grading of FA images-“ML”); agreement amongst these outcomes in diagnosing “macular edema.”
Results
OCT (90.4%) more frequently returned usable information regarding macular edema than FA (77%) and biomicroscopy (76%). Agreement in diagnosis of MT and ML (κ=0.44) was moderate. ML was present in 40% of cases free of MT, whereas MT was present in 34% of cases without ML. Biomicroscopic evaluation for macular edema failed to detect 40% and 45% of cases of MT and ML respectively and diagnosed 17% and 17% of cases with macular edema which did not have MT or ML respectively; these results may underestimate biomicroscopic errors (ophthalmologists were not explicitly masked to OCT and FA results). Among eyes free of ML, phakic eyes without cataract rarely (4%) had MT. No factors were found that effectively ruled out ML when MT was absent.
Conclusion
OCT and FA offered only moderate agreement regarding macular edema status in uveitis cases, probably because what they measure (MT and ML) are related but non-identical macular pathologies. Given its lower cost, greater safety, and greater likelihood of obtaining usable information, OCT may be the best initial test for evaluation of suspected macular edema. However, given that ML cannot be ruled out if MT is absent and vice versa, obtaining the second test after a negative result on the first seems justified when detection of ML or MT would alter management. Given that biomicroscopic evaluation for macular edema frequently erred, ancillary testing for macular edema seems indicated when knowledge of ML or MT status would affect management.
Uveitis is an important cause of blindness and vision loss, often causing visual impairment during the working years. 1–6 Macular edema, a potentially reversible complication of uveitis, is the most common cause of visual impairment among patients with uveitis in tertiary settings. 1,4 When macular edema is detected and treated, vision loss often can be reversed, particularly if treated early. 7 Hence, detection of macular edema is a high priority in the management of patients with uveitis.
Macular edema often is detectable by biomicroscopy, but is diagnosed more easily with fluorescein angiography (FA) or optical coherence tomography (OCT) imaging. In 2004, an expert consensus panel, the Standardization of Uveitis Nomenclature (SUN) Working Group, concluded that reporting of macular edema in clinical studies based on clinical observation of definite macular edema was acceptable, but that use of confirmatory ancillary testing was preferable for prospective studies. 8 The group did not specify whether FA or OCT imaging was preferred. Historically, FA was the method most commonly used for this purpose, imaging fluorescein leakage and accumulation in the macula. Because OCT imaging does not require intravenous administration of fluorescein and directly assesses macular thickness and the presence of intraretinal cysts—key aspects of macular edema—it has begun to supplant FA as the method most commonly used for evaluation of macular edema in clinical trials regarding retinal diseases.
Because the anatomic macular thickening assessed by OCT (MT) and the physiological macular leakage assessed by FA (ML) are different but related entities within the spectrum of “macular edema,” it would be valuable to identify whether there are circumstances wherein the tests are likely to be complementary rather than redundant in the setting of uveitis. In the Multicenter Uveitis Steroid Treatment (MUST) Trial, a large group of patients with intermediate, posterior and panuveitis received protocol-driven fluorescein angiography and Stratus-3 time-domain OCT imaging at the same point in time regardless of whether macular edema was suspected. To evaluate the interrelationship between these alternative approaches for diagnosing macular edema, we used these results to calculate measures of agreement and of the ability of each alternative approache to predict MT (per OCT) or ML (per FA). We also sought to identify risk factors for ML when MT was absent and vice versa.
Patients and Methods
The MUST Trial is a randomized, parallel treatment clinical trial comparing fluocinolone acetonide implant therapy vs. systemic corticosteroids plus immunosuppression when indicated for non-infectious intermediate, posterior, and panuveitis (ClinicalTrials.gov Identifier: NCT00132691, accessed on January 18, 2013). Two hundred fifty-five patients (479 eyes with uveitis) have been enrolled at 23 centers in the United States, the United Kingdom, and Australia. Institutional review board approval was obtained and maintained throughout the study for each participating center.
We have published the study’s methodologic details and primary results previously. 9,10 In brief, patients enrolled had currently or recently active uveitis (within 60 days) of a degree for which systemic corticosteroid therapy was indicated in at least one eye. Inability to image the fundus was not an exclusion criterion. Information from the baseline visit for all eyes with uveitis, regardless of whether that particular eye met eligibility criteria, are included in this analysis.
Demographic and clinical features used in this analysis included: age, race, presence of associated systemic disease, visual acuity, lens status, presence vs. absence of anterior and vitreous cells, vitreous haze grading, 8,11 and whether the uveitis was judged to be active or inactive at baseline. Baseline assessments also included evaluation for macular edema using OCT imaging of macular thickening and fluorescein angiography imaging of macular fluorescein leakage. In addition, clinical evaluation for macular edema using slit-lamp biomicroscopy was performed (the protocol did not explicitly forbid viewing of OCT and FA images prior to grading, although in most cases imaging would have been performed afterward). Stereoscopic macular photographs also were evaluated initially, but were less sensitive than the first three techniques for detection of uveitic macular edema.
Ocular imaging—performed through a dilated pupil—included time domain OCT (Zeiss Stratus 3) and fluorescein angiography. Fast Macular Thickness scans evaluated the center point of macula of each eye with uveitis, and high resolution scans were used for evaluation of intraretinal cystic spaces. Data were acquired from the images by trained Reading Center graders following a detailed protocol for OCT grading. Central macular thickness data were derived directly from scans determined to be of high quality, to serve as a measure of macular edema using this modality. Scans of suboptimal quality (low signal strength, high standard deviation, decentration or misidentification of internal limiting membrane and retinal pigment epithelium boundaries by the automated algorithm) were manually measured at the fovea to avoid artifacts. 12,13 OCT-measured macular thickness at the center point, as an indicator of macular edema status, was categorized for purposes of this analysis as <200 μm (no macular thickening), 200–239 μm (borderline), ≥240 μm (macular thickening) or Missing (when images were either unable to be obtained or unable to be graded). For the primary analysis, an eye was considered to have macular thickening (MT) if the retinal thickness was ≥240 μm. Sensitivity analyses based upon a cut-point of 200 μm also were performed.
Fluorescein angiography included stereo photos of the macula in each eye obtained sequentially over a period of 10 minutes after fluorescein injection. These were graded for the presence and area of macular leakage in the central 0.44 disc areas of the macula (Early Treatment of Diabetic Retinopathy Study (ETDRS) central subfield) by Reading Center graders, as an indicator of macular edema using this modality. Eyes that were not evaluable or with unavailable images were listed as ‘Cannot assess’ or ‘Missing’, respectively. For the purposes of this analysis, eyes with leakage of 0.44 disc areas (i.e., covering the entire central subfield of the macula) were categorized as having macular leakage (“ML”). Sensitivity analyses examining the effect of any leakage produced similar results.
Throughout the study, the Reading Center conducted inter-observer and re-grading exercises in order maintain a high level of quality control on the reproducibility of OCT and FA gradings.
Clinical evaluation of uveitic eyes included slit-lamp biomicroscopy with a condensing lens to evaluate the macula of each eye with uveitis through a dilated pupil. Macular edema was graded clinically as: Present, Absent, Borderline/Equivocal, or Cannot Assess. For the primary analysis, only eyes graded as ‘Present’ were considered to have macular edema. Sensitivity analyses based upon the combination of ‘Present’ and ‘Borderline/Equivocal’ were performed/No specific training in the clinical diagnosis of macular edema was given to the uveitis experts participating in the study, who typically use biomicroscopy to diagnose macular edema (“biomicroscopic ME”). These ophthalmologists were not explicitly forbidden from viewing OCT or FA images prior to grading, although typically photography would be done after grading.
Logistic regression models using robust standard errors were used to assess success in evaluating macular edema status, and risk factors. Estimates of the probability of a certain test result when an alternative test result was positive or negative were computed using generalized estimating equation models with an exchangeable correlation structure to account for correlated within-individual repeated measurements if both eyes had uveitis, and to account for missing data. Kappa statistics and conditional probabilities were used to evaluate the agreement between different methods for diagnosing macular edema; the bootstrap approach was used to estimate the 95% confidence intervals adjusting for between-eye correlation. Statistical analyses were performed using SAS Version 9.1 (SAS Institute, Cary, NC) and R Version 2.11.1 (The R Project for Statistical Computing, Vienna, Austria).
Results
Ability to Asses Macular Edema Status
Among 479 uveitic eyes of 255 patients enrolled in the MUST Trial, 436 (91%, 95% Confidence Interval (CI): 87% to 94%) eyes were successfully imaged and graded for quantitative macular center point thickness (MT) using OCT. In contrast, only 369 (77%, 95% CI: 72% to 82%) eyes were successfully imaged and graded as having or not having macular leakage throughout the central 0.44 disc area (ML) using FA. Clinical examination also succeeded in categorizing only 366 (76%, 95% CI: 72% to 80%) eyes as having or not having biomicroscopic ME, and 49 additional eyes were classified as “borderline/equivocal.” Inability to image and grade eyes in most instances was the result of media opacity in this cohort of eyes with uveitis. Additional characteristics of eyes and patients enrolled in the MUST Trial have been reported previously. 9
Among the 436 eyes successfully assessed for macular thickness by OCT, 54 (12%) could not be assessed by FA and 19 had missing angiograms (4%); by clinical examination, 27 (6%) could not be assessed and for 6 (2%) the grading was missing. In contrast, among cases which were not successfully assessed using OCT, few were successfully categorized using the other approaches: 6 using fluorescein angiography, and 10 using clinical examination (not counting “borderline/equivocal” gradings).
Diagnosis of Macular Edema
The percentage of eyes diagnosed with macular edema varied according to the method of evaluation. Respectively for OCT and FA, the percentage of eyes with macular edema was 36% (95% CI: 31%–42%) and 39% (95% CI: 33%–42%). By biomicroscopy, 32% (95% CI: 27%–39%) were diagnosed as having macular edema; if cases diagnosed clinically as “borderline/equivocal” were considered to be macular edema, then the percentage of eyes with macular edema identified by clinical examination increased to 44% (95% CI: 38%–51%).
Agreement i Diagnosing Macular Edema
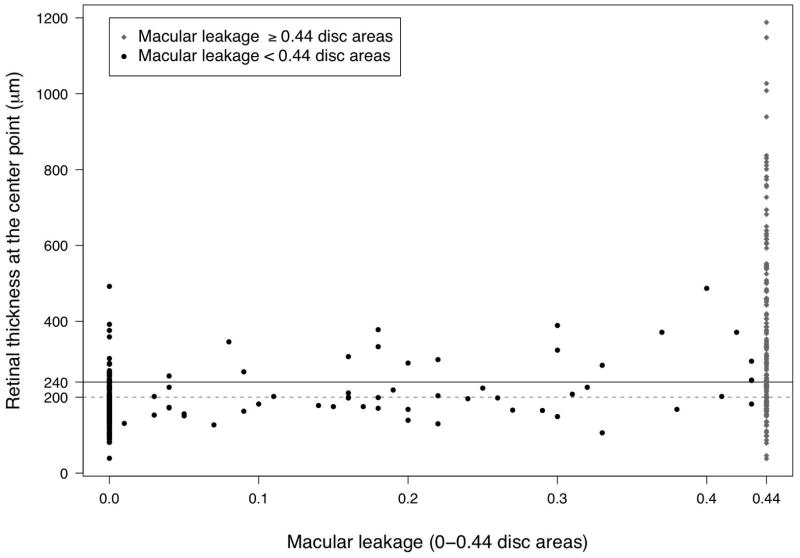
The distribution of macular center point thickness by macular edema status as determined by alternative methods of assessing macular edema is given as Table 1. Macular edema status as determined by fluorescein angiography (macular leakage) and clinical biomicroscopic examination each were strongly associated with increased center point retinal thickness (p<0.0001 for all comparisons; see Table 1), supporting the concept that all approaches are assessing related findings. However, the methods did not agree perfectly (see Figure 1 and Table 2 (available at http://aaojournal.org)). Kappa values among those eyes with each pair of measurements available indicated fair to moderate agreement 14 between OCT-measured center point thickening (≥240 _m) and either fluorescein angiography-measured leakage ≥0.44 disc areas (Kappa=0.44, 95% CI: 0.34 to 0.54) or biomicroscopic findings that macular edema was present (Kappa=0.46, 95% CI: 0.35–0.57). Agreement between fluorescein angiography and clinical examination was similar (Kappa=0.42, 95% CI: 0.30–0.53). Sensitivity analyses considering a “borderline/equivocal” clinical grading to indicate the presence of macular edema yielded similar results.
Table 1.
Relationship between center point macular thickness, measured by OCT, central macular leakage measured by fluorescein angiography, and clinical diagnosis of macular edema via biomicroscopy
| Characteristic | Eyes N (%) | MT, μm Median (25th to 75th percentile) | P-value* | MT Missing N (%) |
|---|---|---|---|---|
| Alternative approach to macular edema diagnosis | ||||
| Fluorescein Angiography | < 0.0001 | |||
| No ML | 167 (46%) | 173 (148 to 200) | 3 (50%) | |
| 0<ML< 0.44 DA | 54 (15%) | 201 (168 to 267) | ||
| ML>0.44 DA | 142 (39%) | 300 (198 to 503) | 3 (50%) | |
| Cannot assess | 54 | 238 (179 to 334) | 26 | |
| Missing | 19 | 186 (144 to 237) | 11 | |
| Clinical Examination | < 0.0001 | |||
| No Biomicroscopic ME | 225 (56%) | 179 (152 to 214) | 7 (58%) | |
| Borderline/Equivocal Biomicroscopic ME | 47 (12%) | 200 (168 to 265) | 2 (17%) | |
| Biomicroscopic ME present | 131 (33%) | 307 (205 to 518) | 3 (25%) | |
| Cannot assess | 27 | 213 (180 to 444) | 13 | |
| Missing | 6 | 369 (173 to 737) | 18 | |
N = number of eyes; MT = retinal thickness at the center point of the macula; ML=leakage of fluorescein throughout the central 0.44 disk areas in the macula; OCT=Stratus-3 Optical Coherence Tomography; ME = macular edema; DA = disc areas.
P-values assess the association between log (MT) on ME and cyst variables adjusting for within-person, between-eye correlation. The log transformation is used to adjust for skewness.
Figure 1.
Distribution between retinal thickness at the center point per optical coherence tomography in μm vs. macular leakage in disc areas per fluorescein angiography
The presence of cysts on OCT (p<0.0001) and of cystoid changes on FA (p<0.0001) also were strongly associated with increased macular center point thickness.
Diagnosis of Macular Thickening by Fluorescein Angiography or Clinical Examination
Among the 155 eyes with central macular thickening ≥240 μm (based on Generalized Estimating Equations models accounting for excess correlation between eyes of the same patient and missing data, see above), FA-determined macular leakage was absent or involved less than the entire 0.44 disc area ETDRS central subfield for an estimated 34% (95% CI: 26%–43%) of eyes with macular thickening, whereas macular leakage was present involving at least 0.44 disc area for 25% (95% CI: 19%–32%) of eyes without macular thickening. The cases without fluorescein leakage tended to have less thickening than those with leakage (see Table 1, Figure 1), but a substantial minority of eyes without leakage over the central 0.44 disc areas had macular thickening ≥240 μm.
Among those for whom clinical classification of macular edema was achieved, clinical diagnosis of macular edema was made in 60% (95% CI: 51%–68%) of eyes with retinal thickening whereas macular edema was judged to be absent in 83% (95% CI: 76%–87%) of eyes without macular thickening. If borderline/equivocal clinical diagnoses are considered to indicate macular edema, the positive predictive value of clinical examination for macular thickening would be 72%. Similar results were observed when defining macular thickening as ≥200 μm (results not shown).
Diagnosis of Macular Leakage by Optical Coherence Tomography or Clinical Examination
Among 145 eyes with fluorescein leakage affecting ≥0.44 disc areas (DA) of the central macula, macular thickness ≥240 μm was absent in 40% (95% CI: 32%–49%), whereas the macular thickness was 240 μm or more in 17% (95% CI: 12%–24%) of the eyes with no leakage or leakage less than 0.44 DA. Alternatively defining MT as a central point thickness ≥200 μm instead of ≥240 μm had a higher sensitivity but lower specificity for macular leakage>0.44 DA.
Biomicroscopic macular edema was present for 55% (95% CI: 45%–64%) of eyes for which macular leakage covering the central 0.44 disc areas was detected by FA, whereas biomicroscopic macular edema was graded as absent in 83% (95% CI: 75%–88%) of eyes with absent macular leakage or macular leakage involving less than 0.44 DA. Alternatively, if eyes with borderline/equivocal clinical diagnoses were considered to have biomicroscopic macular edema, the sensitivity of biomicroscopic macular edema for macular leakage>0.44 DA increased to 66%, but the specificity declined.
Factors Associated with Macular Leakage in Eyes Free of Macular Thickening
To provide guidance regarding the usefulness of FA following an OCT demonstrating normal or minimally abnormal macular findings, we evaluated the yield of FA investigation in cases free of macular thickening ≥240 μm. Among these, 23% (95% CI. 18%–29%) were found to have macular leakage ≥0.44 disc areas by FA. Our evaluation of factors associated with a higher or lower risk of macular leakage in eyes free of macular thickening is summarized in Table 3. Factors associated with higher risk of macular leakage included best-corrected visual acuity worse than 20/40 vs 20/40 or better (odds ratio (OR)=4.45, 95% CI: 2.42, 8.17), evidence of macular edema on clinical examination (OR=3.81, 95% CI: 1.73, 8.38), the presence of cataract or prior cataract surgery vs. no cataract (OR=3.01, 95% CI: 1.25, 7.24), and age ≥55 years vs less (OR=2.42, 95% CI: 1.13, 5.17). After adjusting for confounding via multiple logistic regression, only visual acuity and biomicroscopic macular edema were statistically significantly associated with macular leakage among eyes free of macular thickening. However, among these factors, none of the lower risk categories were associated with a risk of macular leakage less than 10%; none of the characteristics effectively ruled out the possibility of macular leakage.
Table 3.
Comparison of the risk factors for having leakage among eyes without macular thickening.*
| Characteristic | No Macular Leakage (N = 183) | Macular Leakage (N = 56) | Unadjusted Odds Ratio (95% CI) | P-value | Adjusted Odds Ratio (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Age 55 or greater, N (%) | 2.42 (1.13, 5.17) | 0.022 | ||||
| No | 151 (81%) | 36 (19%) | ||||
| Yes | 32 (62%) | 20 (38%) | ||||
| Race, N (%) | 0.84 (0.42, 1.67) | 0.62 | ||||
| Caucasian | 103 (75%) | 34 (25%) | ||||
| Non-caucasian | 80 (78%) | 22 (22%) | ||||
| Any systemic disease, N (%) | 1.56 (0.76, 3.21) | 0.22 | ||||
| No | 139 (79%) | 37 (21%) | ||||
| Yes | 44 (70%) | 19 (30%) | ||||
| Visual acuity, N (%) | 4.45 (2.42, 8.17) | < 0.0001 | 3.44 (1.80, 6.57) | 0.0002 | ||
| 20/40 or better | 140 (86%) | 22 (14%) | ||||
| Worse than 20/40 | 42 (55%) | 34 (45%) | ||||
| Missing | 1 (100%) | |||||
| Lens status, N (%) | 3.01 (1.25, 7.24) | 0.014 | ||||
| No cataract | 66 (88%) | 9 (12%) | ||||
| Cataract or Aphakic/Pseudophakic | 117 (71%) | 47 (29%) | ||||
| Current activity, N (%) | 1.44 (0.74, 2.78) | 0.28 | ||||
| No | 52 (80%) | 13 (20%) | ||||
| Yes | 131 (75%) | 43 (25%) | ||||
| Anterior chamber cells, N (%) | 0.97 (0.5, 1.86) | 0.93 | ||||
| No cells present | 104 (76%) | 33 (24%) | ||||
| Cells present | 79 (77%) | 23 (23%) | ||||
| Vitreous cells, N (%) | 1.16 (0.46, 2.90) | 0.75 | ||||
| No cells present | 37 (79%) | 10 (21%) | ||||
| Cells present | 146 (77%) | 44 (23%) | ||||
| Missing | 2 (100%) | |||||
| Vitreous haze, N (%) | 1.68 (0.87, 3.26) | 0.12 | ||||
| 0 | 77 (82%) | 17 (18%) | ||||
| ≥ 1+ | 105 (73%) | 38 (27%) | ||||
| Missing | 1 (50%) | 1 (50%) | ||||
| Clinical examination, N (%) | 3.81 (1.73, 8.38) | 0.0009 | 2.99 (1.33, 6.69) | 0.0076 | ||
| No macular edema | 159 (82%) | 34 (18%) | ||||
| Macular edema | 22 (56%) | 17 (44%) | ||||
| Missing | 2 (29%) | 5 (71%) | ||||
| OCT cystoid spaces, N (%) | 1.48 (0.72, 3.02) | 0.29 | ||||
| No | 148 (77%) | 43 (23%) | ||||
| Yes | 35 (73%) | 13 (27%) |
N=number of eyes; OCT=Stratus 3 Optical Coherence Tomography; CI=Confidence Interval
Factors Associated with Macular Thickening in Eyes Free of Macular Leakage
To provide guidance regarding the usefulness of OCT following an FA which demonstrates normal or minimally abnormal macular findings, we also evaluated the yield of OCT investigation in cases free of macular leakage>0.44 DA. Among these, 17% (95% CI: 12%–24%) were found to have macular thickening ≥240 μm by OCT. Our reciprocal evaluation of factors associated with higher odds of macular thickening among those without macular leakage is given in Table 4. Factors associated with higher risk included the presence of cataract or prior cataract surgery vs. no cataract (OR= 6.89, 95% CI: 2.11, 22.5), presence of macular edema on clinical examination (OR=4.87, 95% CI: 2.14, 11.1), cystoid changes on the fluorescein angiogram vs not (OR=4.74, 95% CI: 1.79–12.5), age ≥55 years vs less (OR=3.83, 95% CI: 1.74–8.41), Caucasian vs. non-Caucasian race (OR=2.94, 95% CI: 1.25–7.14), and visual acuity worse than 20/40 vs 20/40 or better (OR=2.81, 95% CI: 1.36, 5.79). The observed proportion with macular thickening in a phakic eye free of cataract was only 4% (3 cases); otherwise, none of the lower risk categories of these factors was associated with a risk of macular thickening less than 10%.
Table 4.
Evaluation of risk factors for macular thickening in eyes with uveitis and no macular leakage.*
| Characteristic | No Macular Thickening (N = 183) | Macular Thickening (N = 38) | Unadjusted Odds Ratio (95% CI) | P-value | Adjusted Odds Ratio (95% CI) | P-value |
|---|---|---|---|---|---|---|
| Age 55 or greater, N (%) | 3.83 (1.74, 8.41) | 0.0008 | 2.35 (1.03, 5.36) | 0.042 | ||
| No | 151 (88%) | 21 (12%) | ||||
| Yes | 32 (65%) | 17 (35%) | ||||
| Race, N (%) | 2.94 (1.25, 7.14) | 0.012 | ||||
| Non-caucasian | 80 (91%) | 8 (9%) | ||||
| Caucasian | 103 (77%) | 30 (23%) | ||||
| Any systemic disease, N (%) | 0.94 (0.39, 2.25) | 0.89 | ||||
| No | 139 (83%) | 29 (17%) | ||||
| Yes | 44 (83%) | 9 (17%) | ||||
| Visual acuity, N (%) | 2.81 (1.36, 5.79) | 0.0052 | 2.02 (0.93, 4.34) | 0.072 | ||
| 20/40 or better | 140 (88%) | 20 (13%) | ||||
| Worse than 20/40 | 42 (70%) | 18 (30%) | ||||
| Missing | 1 (100%) | |||||
| Lens status, N (%) | 6.89 (2.11, 22.5) | 0.0013 | 4.12 (1.19, 14.2) | 0.025 | ||
| No cataract | 66 (96%) | 3 (4%) | ||||
| Cataract or Aphakic/Pseudophak | 117 (77%) | 35 (23%) | ||||
| Current activity, N (%) | 1.01 (0.45, 2.23) | 0.98 | ||||
| No | 52 (83%) | 11 (17%) | ||||
| Yes | 131 (83%) | 27 (17%) | ||||
| Anterior chamber cells, N(%) | 0.92 (0.43, 1.94) | 0.82 | ||||
| No cells present | 104 (82%) | 23 (18%) | ||||
| Cells present | 79 (84%) | 15 (16%) | ||||
| Vitreous cells, N (%) | 0.93 (0.40, 2.15) | 0.87 | ||||
| No cells present | 37 (82%) | 8 (18%) | ||||
| Cells present | 146 (83%) | 30 (17%) | ||||
| Vitreous haze, N (%) | 0.87 (0.41, 1.83) | 0.72 | ||||
| 0 | 77 (81%) | 18 (19%) | ||||
| ≥ 1+ | 105 (84%) | 20 (16%) | ||||
| Missing | 1 (100%) | |||||
| Clinical examination, N (%) | 4.87 (2.14, 11.1) | 0.0002 | ||||
| No macular edema | 159 (87%) | 23 (13%) | ||||
| Macular edema | 22 (59%) | 15 (41%) | ||||
| Missing | 2 (100%) | |||||
| FA cystoid changes, N (%) | 4.74 (1.79, 12.5) | 0.0017 | 3.63 (1.43, 9.18) | 0.0065 | ||
| No | 172 (86%) | 29 (14%) | ||||
| Yes | 11 (55%) | 9 (45%) |
N=number of eyes; FA=Fluorescein Angiography; CI=Confidence Interval
Discussion
Although OCT-measured thickening and FA-measured macular leakage both are used widely for diagnosis of macular edema in the setting of uveitis, they do not image the same thing. Fluorescein leakage demonstrates a pathophysiological process of leakage from blood vessels, which is often but not always associated with macular thickening. Possible instances where leakage might be present in the absence of thickening include a severely damaged macula that is atrophic and thin but has ongoing inflammatory leakage, a macula where leakage has recently begun and thickening has not yet occurred, a macula distorted by epiretinal membrane but not thickened, or a macula where leakage is balanced by physiologic outflow of fluid from the macula providing a steady state without thickening. Likewise, macular thickening may occur for reasons other than current vascular leakage (e.g., failure to clear osmotically active molecules from the retina or dysfunction of the retinal pigment epithelial pump 15), and might take some time to clear after leakage is no longer present under otherwise favorable circumstances.
Thus, it is not surprising that our results indicate that these findings are related but not interchangeable. Findings of macular thickening by OCT and macular leakage by FA demonstrated only moderate agreement, presumably because one measures anatomy and the other physiology. The degree of agreement was similar to that observed in the few previous smaller studies of birdshot retinochoroiditis cases, 16 and uveitis cases. 17 An earlier study using an OCT 2000 scanner (Humphrey Instruments, San Leandro, CA) focused on the presence of cysts on OCT and any degree of fluid on FA;18 this study found a better degree of agreement between these outcomes than we observed in the different outcomes that we studied, focusing on macular thickening, which we have demonstrated is more predictive of reduction in visual acuity than macular cysts.6 The presence of cysts on OCT or cystoid changes on FA were strongly associated with macular thickening by OCT and macular leakage on FA respectively in our study.
OCT has logistical advantages over FA, in that it is less expensive, less invasive, faster, and safer/better tolerated. A further advantage is that newer spectral domain OCT imaging—where available—has been reported as having prognostic value regarding the potential for recovery of macular edema with treatment. 19 A new application of OCT, 3-dimensional OCT, has been reported to have higher sensitivity than FA for diagnosis of cystoid macular edema in the context of retinal diseases. 20 The research community is widely adopting the use of OCT imaging in clinical studies, due to high reproducibility and the potential for quantitative outcome variables which is statistically advantageous in some instances. Our observation that OCT is more often successful than FA in imaging the macula in eyes with uveitis (91% vs 77% respectively) is a further advantage of this approach for research studies and clinical care. These considerations would seem to support the use of OCT as an initial test when an eye is suspected of having macular edema, unless fluorescein angiography was specifically indicated to evaluate uveitis (e.g., for suspected vasculitis, vascular closure, neovascularization or optic nerve leakage, or for evaluation of a white dot syndrome).
However, in this large group of eyes with uveitis, OCT missed a substantial number of cases with macular leakage. Among the cases where OCT did not demonstrate thickening, macular leakage was associated with several-fold greater odds of diminished visual acuity, indicating that such macular leakage often may be of clinical importance. Conversely, a substantial number of cases of macular thickening also were missed by fluorescein angiography, and such thickening also was associated with reduced visual acuity. Thus, both FA and OCT yielded clinically important information in a large minority of cases when the other test was negative. In situations where a positive result on the second test would affect treatment decisions, both tests may be warranted. However, if the first test is positive and provides sufficient grounds to initiate treatment, the second test likely is unnecessary. Longitudinal study would be valuable to clarify the relationship of these pathologies in relation to visual outcomes, to better clarify their importance and interrelationships.
We evaluated whether the presence of some other associated factor would render a second test unnecessary, with a notably small yield. While several factors were predictive of either increased risk of fluorescein leakage in cases without macular thickening per OCT, or of macular thickening in cases without fluorescein leakage per FA, the absence of these factors was not associated with sufficiently low probability of macular leakage to effectively rule out the presence of macular leakage. The only possible exception was that a very small number of cases of macular thickening were present in phakic, noncataractous eyes free of macular leakage, which may represent a group in which OCT would be redundant following FA. Further experience would be valuable to determine whether this finding in fact mitigates the need for an OCT following a negative FA (assuming detection of macular thickening would alter treatment).
Regarding clinical diagnosis of macular edema, an expert consensus group previously concluded that clinically definite macular edema by biomicroscopy is an adequately reliable indicator of the presence of macular edema, if diagnosis based on ancillary testing is not available. 8 However, our results, based on biomicroscopic evaluation by leading uveitis experts, found that clinicians made both positive and negative errors in a substantial proportion of cases. Furthermore, the MUST Trial protocol did not forbid clinicians from viewing OCT and FA results before recording the results of biomicroscopy, and some may have done so, which could have biased results of clinical macular edema grading to be more favorable than they would have been otherwise. An evaluation of detection of foveal macular edema in diabetics by biomicroscopy vs OCT also has demonstrated poor sensitivity for detection of macular thickening between 201–300 μm, although agreement was favorable for greater levels of thickening.20 Based on these considerations, evaluation with an ancillary test would seem justified in settings where the presence of macular edema is suspected, regardless of biomicroscopic findings, although biomicroscopic findings suggesting macular edema make it more likely that positive findings will be identified. Ancillary testing may be especially useful to identify non-cystic thickening of the macula (because cystic changes are more likely to be clinically identifiable), when the ocular media are poor (particularly with OCT), and when it is important to monitor changes in macular status over time. Prior publications which have relied upon clinical diagnosis of macular edema are not necessarily invalidated by these observations, but should be interpreted with the recognition that macular edema outcomes may have been misclassified in both directions to some extent, which tends to reduce the strength of observed associations rather than producing spurious associations.
Limitations of this study include its cross-sectional nature, and the use of time-domain OCT rather than spectral domain OCT, which was introduced into practice after the MUST Trial was initiated. However, cross-sectional observations correspond well to the situation patients and clinicians face in clinical practice, where a decision regarding treatment must be made before follow-up imaging is available. Time-domain OCT, while providing lower resolution images than spectral domain OCT and therefore sometimes missing smaller cysts, nevertheless provides adequate resolution for measuring central macular thickening, the major finding of interest here. Our previous work has demonstrated that thickening is much more predictive of visual loss than cysts,6 and small cysts are unlikely to have as much impact as large ones detectable by time-domain OCT. The protocol explicitly controlled artifacts known to potentially bias measurement of macular thickness, such as eccentric centering, reducing the likelihood that our results have a meaningful degree of measurement bias. Results of the study would be applicable to non-infectious uveitis, given that infectious uveitis was not studied. Strengths of the study include standardized, simultaneous imaging by OCT and FA of a large cohort of eyes with a high prevalence of macular edema, providing favorable statistical power for addressing questions of clinical importance. OCT and FA studies were graded objectively by masked graders in a Reading Center environment subjected to ongoing quality control.
In summary, agreement between central macular thickness per OCT imaging and macular leakage by FA is imperfect (kappa=0.44), likely because the two tests measure different but related aspects of macular pathology, and hence may give complementary information. Given the observed superiority of OCT in obtaining gradable images, along with its lower cost and non-invasive nature, in most cases OCT seems the most appropriate first test to evaluate the macula, unless some other specific indication for FA exists. However, when OCT is negative and findings of macular leakage would be clinically important, FA is likely to provide valuable additional information if the ocular media permit it. Likewise, when FA is performed initially and is negative, it seems reasonable to obtain a follow-up OCT in situations when detection of macular thickening would be clinical relevant. Biomicroscopy misdiagnosed the presence or absence of macular edema sufficiently often that ancillary testing with OCT and/or FA seems to be indicated whenever a reasonable probability of potentially treatable macular edema exists.
Supplementary Material
Acknowledgments
Financial support: Supported by cooperative agreements from the National Eye Institute to Mount Sinai School of Medicine (U10 EY 014655), The Johns Hopkins University Bloomberg School of Public Health (U10 EY 014660), and the University of Wisconsin, Madison, School of Medicine (U10 EY 014656).
Footnotes
Potential Conflict of Interests: JHK: consultant (Alcon, Allergan, LuxBio, Sanofi Pasteur, Xoma); JET: advisory board (Allergan, Xoma), consultant (Servier).
Off label use of drugs: Not applicable
Supplemental Material: This article contains online-only material. The following should appear online-only: Table 2 and the Credit Roster.
ClinicalTrials.gov Identifier: NCT00132691 (accessed on January 18, 2013)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Durrani OM, Meads CA, Murray PI. Uveitis: a potentially blinding disease. Ophthalmologica. 2004;218:223–36. doi: 10.1159/000078612. [DOI] [PubMed] [Google Scholar]
- 2.National Advisory Eye Council. Vision Research. A National Plan, 1983–1987. Chapter 1, Introduction. Bethesda, MD: National Institutes of Health, Public Health Service, US Department of Health and Human Services; 1983. p. 13. Support for Vision Research. vol. 1. NIH Publication 83–2469. [Google Scholar]
- 3.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303–8. doi: 10.1007/BF00163549. [DOI] [PubMed] [Google Scholar]
- 4.Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80:332–6. doi: 10.1136/bjo.80.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ten Doesschate J. Causes of blindness in The Netherlands. Doc Ophthalmol. 1982;52:279–85. doi: 10.1007/BF01675857. [DOI] [PubMed] [Google Scholar]
- 6.Taylor SR, Lightman SL, Sugar EA, et al. The impact of macular edema on visual function in intermediate, posterior, and panuveitis. Ocul Immunol Inflamm. 2012;20:171–81. doi: 10.3109/09273948.2012.658467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothova A. Medical treatment of cystoid macular edema. Ocul Immunol Inflamm. 2002;10:239–46. doi: 10.1076/ocii.10.4.239.15589. [DOI] [PubMed] [Google Scholar]
- 8.Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Multicenter Uveitis Steroid Treatment Trial Research Group. The Multicenter Uveitis Steroid Treatment Trial: rationale, design, and baseline characteristics. Am J Ophthalmol. 2010;149:550–61. doi: 10.1016/j.ajo.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kempen JH, Altaweel MM, Holbrook JT, et al. Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the Multicenter Uveitis Steroid Treatment Trial. Ophthalmology. 2011;118:1916–26. doi: 10.1016/j.ophtha.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92:467–71. doi: 10.1016/s0161-6420(85)34001-0. [DOI] [PubMed] [Google Scholar]
- 12.Giani A, Cigada M, Esmaili DD, et al. Artifacts in automatic retinal segmentation using different optical coherence tomography instruments. Retina. 2010;30:607–16. doi: 10.1097/IAE.0b013e3181c2e09d. [DOI] [PubMed] [Google Scholar]
- 13.Leung CK, Chan WM, Chong KK, et al. Alignment artifacts in optical coherence tomography analyzed images. Ophthalmology. 2007;114:263–70. doi: 10.1016/j.ophtha.2006.06.059. [DOI] [PubMed] [Google Scholar]
- 14.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 15.Marmor MF. Mechanisms of fluid accumulation in retinal edema. Doc Ophthalmol. 1999;97:239–49. doi: 10.1023/a:1002192829817. [DOI] [PubMed] [Google Scholar]
- 16.Monnet D, Levinson RD, Holland GN, et al. Longitudinal cohort study of patients with birdshot chorioretinopathy. III. Macular imaging at baseline. Am J Ophthalmol. 2007;144:818–28. doi: 10.1016/j.ajo.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Ossewaarde-Van Norel J, Camfferman LP, Rothova A. Discrepancies between fluorescein angiography and optical coherence tomography in macular edema in uveitis. Am J Ophthalmol. 2012;152:233–9. doi: 10.1016/j.ajo.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Antcliff RJ, Stanford MR, Chauhan DS, et al. Comparison between optical coherence tomography and fundus fluorescein angiography for the detection of cystoid macular edema in patients with uveitis. Ophthalmology. 2000;107:593–9. doi: 10.1016/s0161-6420(99)00087-1. [DOI] [PubMed] [Google Scholar]
- 19.Pelosini L, Hull CC, Boyce JF, et al. Optical coherence tomography may be used to predict visual acuity in patients with macular edema. Invest Ophthalmol Vis Sci. 2011;52:2741–8. doi: 10.1167/iovs.09-4493. [DOI] [PubMed] [Google Scholar]
- 20.Ouyang Y, Keane PA, Sadda SR, Walsh AC. Detection of cystoid macular edema with three-dimensional optical coherence tomography versus fluorescein angiography. Invest Ophthalmol Vis Sci. 2010;51:5213–8. doi: 10.1167/iovs.09-4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown JC, Solomon SD, Bressler SB, et al. Detection of diabetic foveal edema: contact lens biomicroscopy compared with optical coherence tomography. Arch Ophthalmol. 2004;122:330–5. doi: 10.1001/archopht.122.3.330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.