Abstract
Plexin C1 is a type I transmembrane receptor with intrinsic R-Ras GTPase activity, which regulates cytoskeletal remodeling and adhesion in normal human melanocytes. Melanocytes are pigment-producing cells of the epidermis, precursors for melanoma, and express high levels of Plexin C1, which is lost in melanoma in vitro and in vivo. To determine if Plexin C1 is a tumor suppressor for melanoma, we introduced Plexin C1 into a primary human melanoma cell line, and phenotypes including migration, apoptosis, proliferation and tumor growth in mice were analyzed. Complimentary studies in which Plexin C1 was silenced in human melanocytes were performed. Plexin C1 significantly inhibited migration and proliferation in melanoma, whereas in melanocytes, loss of Plexin C1 increased migration and proliferation. In mouse xenografts, Plexin C1 delayed tumor growth of melanoma at early time points, but tumors eventually escaped the suppressive effects of Plexin C1, due to Plexin C1-dependent activation of the pro-survival protein Akt. R-Ras activation stimulates melanoma migration. Plexin C1 lowered R-Ras activity in melanoma and melanocytes, consistent with inhibitory effects of Plexin C1 on migration of melanocytes and melanoma. To determine if R-Ras is expressed in melanocytic lesions in vivo, staining of tissue microarrays of nevi and melanoma were performed. R-Ras expression was highly limited in melanocytic lesions, being essentially confined to primary melanoma, and almost completely absent in nevi and metastatic melanoma. These data suggest that loss of Plexin C1 in melanoma may promote early steps in melanoma progression through suppression of migration and proliferation, but pro-survival effects of Plexin C1 ultimately abrogate the tumor suppressive effects of Plexin C1. In primary melanoma, loss of Plexin C1 may function in early steps of melanoma progression by releasing inhibition of R-Ras activation, and stimulating migration.
Keywords: melanoma, semaphorin, plexin, R-Ras, melanocyte
INTRODUCTION
Plexin receptors are a family of type I transmembrane proteins (A–D) that bind to semaphorins.1–3 Plexin receptors were identified based on their homology to the extracellular domain of the scatter factor receptors, and their cytoplasmic domains are highly conserved.1,4 While they do not have intrinsic kinase activity, the intracellular domain of Plexins contain an intrinsic Ras-GTPase activating (GAP) domain, resulting in exchange of GTP for GDP on M-Ras and R-Ras.5–8 A recent report shows that Plexin receptors also have Rap1-GAP activity, with particularly high levels of Rap1-GAP activity in Plexin C1.9 Binding sites for Rho-guanine nucleotide exchange factors and Rho-GAPs have also been identified in the cytoplasmic domain of Plexin receptors, which mediate pleiotropic effects on cell migration, and neurite outgrowth through regulation of Rho and Rac activity.10,11 Semaphorins are a large family of transmembrane and secreted proteins that are ligands for Plexin receptors and were originally identified in the nervous system.12 Depending upon the semaphorin, they bind both neuropilin and Plexin receptors, or Plexin receptors alone, to mediate their effects.
Plexin C1, also known as Virus-encoded semaphorin protein receptor, is an ~210 kDa transmembrane receptor expressed by human melanocytes, neurons, neutrophils, dendritic cells and pre-osteoblasts.13–16 Plexin C1 is a receptor for vaccinia virus A39R protein, and stimulates intracellular adhesion molecule-1 expression and cytokine production from human monocytes. Plexin C1 inhibits neutrophil phagocytosis, indicating a potential immune function for Plexin C1.17 Plexin C1 plays a role in the partitioning of paraventricular and supraoptic neurons in the hypothalamus, as indicated by specific defects seen in mice deleted for Plexin C1, and is involved in neural guidance.18,19 The naturally occurring ligand for Plexin C1 is unclear. Semaphorin 7A (Sema7A) shares homology with A39R, and the crystal structure of Sema7A–Plexin C1 complex shows interaction of the ligand-receptor pair through their respective Sema domains, with Plexin C1 dimerization upon ligand binding in vitro.20 Binding of Sema7A to Plexin C1 inhibits integrin activation in monocytes, melanocytes and other cell types; Sema7A also binds to β1-integrins, stimulating adhesion and dendrite outgrowth.15,16,21,22 In melanocytes, Plexin C1 suppresses the activity of cofilin, an actin binding protein necessary for adhesion and migration, and decreases melanocyte adhesion and dendricity, supporting a role for Plexin C1 in regulating cytoskeletal remodeling in normal human melanocytes.16,23
Melanoma is a highly aggressive tumor derived from melanocytes, in which ultraviolet irradiation plays an important inductive role.24 We recently reported that Plexin C1 is lost in melanoma cells lines in part through DNA methylation, a common mechanism for silencing of tumor suppressor proteins, and in a large panel of primary and metastatic melanoma tumors from human subjects.23,25,26 Near total loss of Plexin C1 expression was observed in metastatic melanoma, with graded loss of Plexin C1 in primary melanoma, with expression inversely correlating with tumor depth of invasion. Somatic mis-sense mutations and copy number loss of Plexin C1 have also been identified in subjects with metastatic melanoma.26 Because Plexin C1 regulates cytoskeletal remodeling in human melanocytes, loss of Plexin C1 could promote melanoma progression through effects on cell adhesion and migration. In this report, we determined functional effects of introduction of Plexin C1 into a human melanoma cell line that lacks the receptor, including analysis of Plexin C1 on R-Ras and Rap1 activity in melanocytic cells. Our results support a role for Plexin C1 as a tumor suppressor protein for early steps in melanoma tumor growth, and suggest that suppression of R-Ras activity by Plexin C1 is a potential mechanism by which Plexin C1 blocks early steps in melanoma progression.
RESULTS
Plexin C1 regulates migration in melanoma and melanocytes
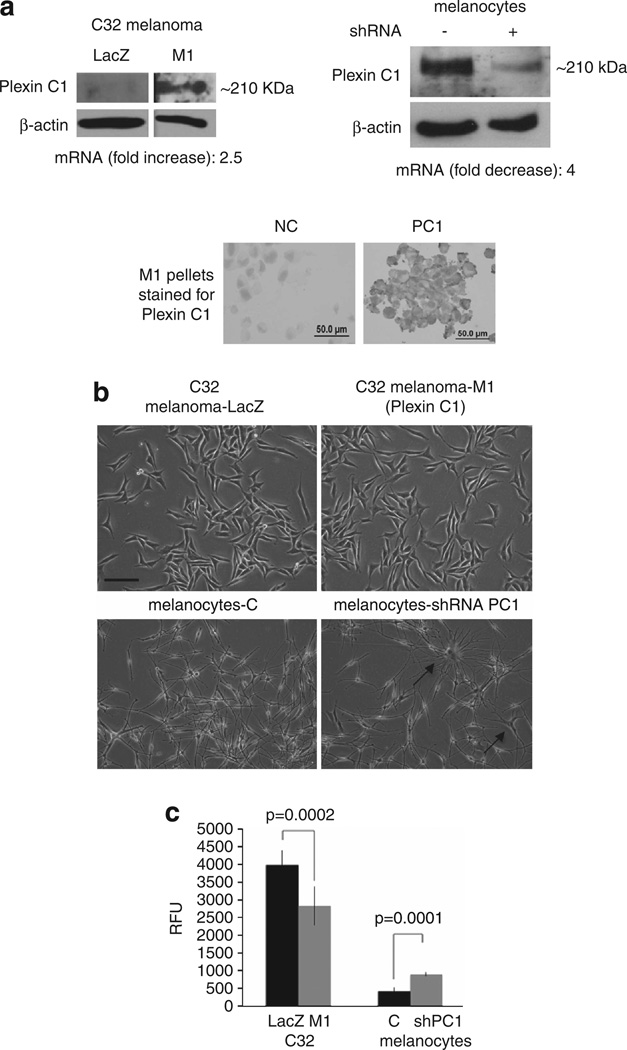
Full-length human Plexin C1 receptor was introduced into C32 primary melanoma cells using a lentiviral vector, and a mixed population of transduced cells (‘M1’) was used at early passage numbers (3–5). Controls consisted of cells expressing empty vector (‘LacZ’). We directly compared melanoma cells expressing Plexin C1 with normal human melanocytes established from neonatal foreskins, in which Plexin C1 was silenced. Plexin C1 expression in M1 cells was lower than melanocytes, but was easily detectable on the cell membrane of C32 cells (Figure 1a). Expression of Plexin C1 had no discernable effects on cellular morphology of melanoma, although increased dendricity of melanocytes was observed in Plexin C1 silenced cells, as previously described (Figure 1b).16 To determine the effects of Plexin C1 on migration, cells were placed in serum-free media overnight (melanoma), or media without growth factors or serum (melanocytes) for 24 h, then plated in the top well of a migration chamber in basal media and allowed to migrate towards media containing 10% fetal bovine serum for 18 h (Figure 1c). As expected, melanoma cells migrated significantly faster than normal melanocytes. Introduction of Plexin C1 reduced migration of melanoma cells by 30% compared with LacZ controls (P = 0.0002), although they still showed greater migration than melanocytes. Silencing of Plexin C1 in melanocytes doubled migration compared with non-target controls (P = 0.0001). Plexin C1 had no effects on invasion of melanoma through Matrigel, or on colony formation in soft agar (data not shown). The addition of Sema7A to the top well of the chamber had no effect on migration of melanoma cells or melanocytes, regardless of Plexin C1 expression, even at doses as high as 200 ng/ml (data not shown).
Figure 1.
Plexin C1 inhibits directed migration of melanoma and melanocytes. (a) Total cellular lysates of LacZ and M1 cells were resolved on 7.5% SDS–PAGE and blotted for Plexin C1. A band of the expected size (~210 kDa) was detected in M1 cells. Western blot of normal human melanocytes silenced for Plexin C1 show near total silencing of Plexin C1 compared with non-target controls. Plexin C1 was identified at the membrane in cell pellets of M1 cells stained for Plexin C1. ‘NC’ = cells stained with non-immune serum instead of primary antibody. (b) Phase contrast micrograph of melanoma cells expressing Plexin C1, and melanocytes in which Plexin C1 is silenced. Silencing of Plexin C1 increased dendricity in melanocytes, but introduction of Plexin C1 had not obvious effect on melanoma morphology. Bar ~100 µm. (c) Migration of melanoma cells expressing Plexin C1 (M1) was significantly less compared with LacZ controls. In contrast, melanocytes in which Plexin C1 was silenced showed significantly more migration compared with control cells. Results are representative of four separate experiments. Each bar is the average of nine wells ± s.d. RFU, relative fluorescence units.
Plexin C1 regulates R-Ras activation in melanocytes and melanoma
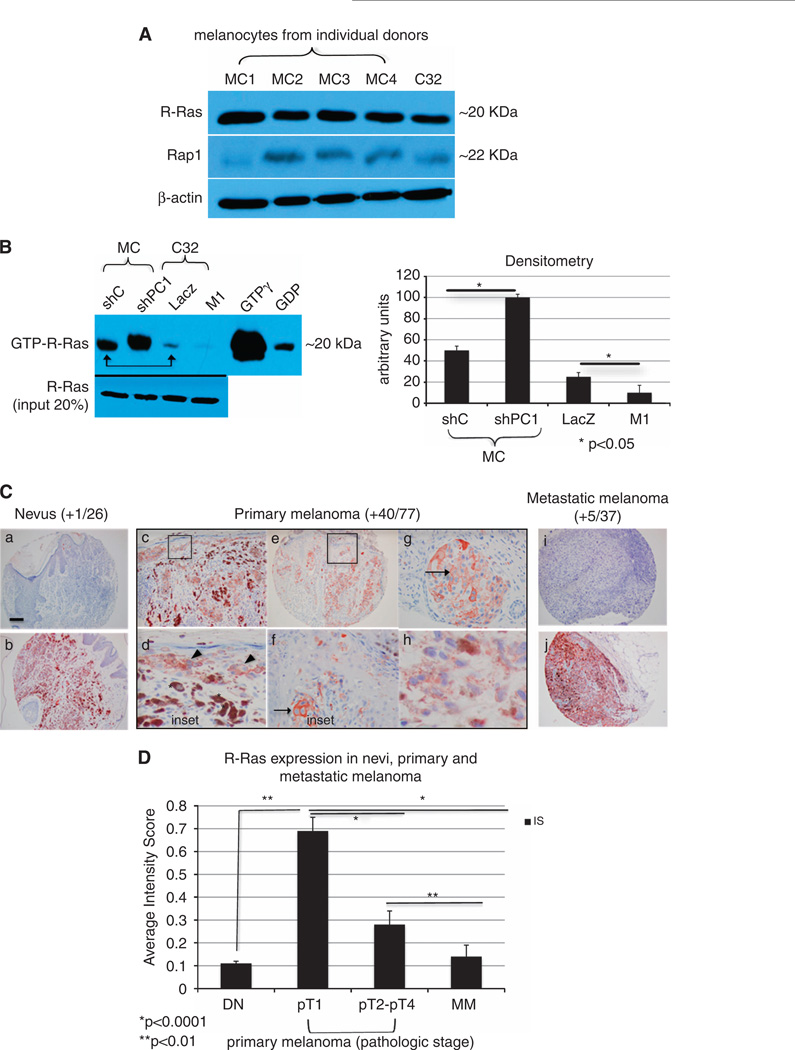
Plexin C1 has intrinsic R-Ras and Rap1 GAP activity.7,9 Because R-Ras and Rap1 regulate melanoma migration,27–30 we determined if Plexin C1 regulates the activation of R-Ras and Rap1 in melanoma. The steady-state levels of R-Ras and Rap1 protein were similar in melanocytes and melanoma, with higher levels of R-Ras compared with Rap1 (Figure 2A). Other melanoma cell lines tested (WW165 and WM115) showed similar results (data not shown). Cells were serum starved (melanoma) or grown in medium without growth factors or serum (melanocytes) for 24 h before affinity capture of GTP-R-Ras. Surprisingly, melanocytes had significantly higher levels of active R-Ras compared with C32 melanoma (Figure 2B). Even when melanoma cells were analyzed under growth conditions containing serum, levels of active R-Ras were higher in melanocytes (data not shown). Plexin C1 expression was inversely correlated with activation of R-Ras in melanocytes and melanoma. Silencing of Plexin C1 doubled levels of active R-Ras in melanocytes, and expression of Plexin C1 in melanoma lowered active R-Ras by almost 40%. In both cell types, changes in R-Ras-GTP were significant (P < 0.05) compared with controls. Active Rap1 was not detected in melanoma, regardless of Plexin C1 expression, or culture conditions (Supplementary Figure 1).
Figure 2.
Plexin C1 negatively regulates R-Ras activity in melanocytic cells. (A) Total cellular lysates of melanocytes (MC) from four individual donors, and C32 melanoma were blotted for R-Ras and Rap1. Shown is a 12% SDS–PAGE. Levels of R-Ras and Rap1 are similar in melanocytes and C32 melanoma, with higher levels of R-Ras compared with Rap1. (B) Affinity pull down of R-Ras performed on lysates of LacZ and M1 cells, and melanocytes silenced for Plexin C1 (~300 µg). A representative blot (n = 3) and densitometry, is shown. Melanocytes have higher levels of GTP-R-Ras under basal conditions compared with melanoma (arrow), which was further increased by silencing Plexin C1. In melanoma, detectable GTP-R-Ras was identified in control cells, which was completely lost in cells expressing Plexin C1. Shown is a 12% SDS–PAGE blotted for R-Ras. (C) R-Ras expression in melanocytic lesions in vivo. Shown are representative photomicrographs of nevi, primary melanoma and metastatic melanoma stained for R-Ras and counterstained with hematoxylin. The majority of nevi and metastatic melanoma did not expression R-Ras (a, i), but those that did showed strong staining (b, j). Expression of R-Ras in primary melanoma was frequently observed (c–h), with particularly strong, membranous staining in the in situ component of early (pT1) melanomas (inset). Asterisks show macrophages with ingested melanin, which also expressed R-Ras. Arrows show R-Ras on the cell membrane. Bar ~1 mm. (D) R-Ras expression, shown as an averaged intensity score (IS) for primary melanoma by pathologic stage, and metastatic melanoma (MM). Differences in R-Ras expression between primary melanomas pT1 and pT2-pT4 are significant, as are differences between primary melanomas (pT1–pT4) and metastatic melanoma. Each bar represents the average IS ± s.e.m.
Because the expression of R-Ras in tissues in vivo is quite restricted, the significance of Plexin C1-dependent regulation of R-Ras in melanoma was further explored through analysis of R-Ras expression in melanocytic lesions from patient samples. Immunohistochemical stained tissue microarrays of nevi, primary melanoma and metastatic melanoma showed that R-Ras expression is highly restricted in the skin, being absent in the epidermis, appendages, smooth muscle, lymphocytes and endothelial cells, and was only detectable in resident skin macrophages. In fact, R-Ras expression was essentially restricted to the melanocytic cells within the lesions (Figure 2C). R-Ras was predominantly expressed in primary melanoma, in which 40/77 (52%) of tumors expressed R-Ras. R-Ras was identified on the plasma membrane of melanoma, with particularly strong expression in the in situ (intra-epidermal) compartment. When intensity scores (IS) of R-Ras expression in primary melanoma were broken down by pathologic stage, 73% of pT1 melanomas expressed R-Ras with an average IS of 0.69 (± 0.44), compared with melanomas pT2 and higher, in which only 11/38 (29%) expressed R-Ras, with an average intensity score of 0.28 ± 0.04 (P < 0.001; Figure 2D). Only 1/26 (4%) nevi (IS 0.11 ± 0.01) and 5/37 (14%) metastatic melanomas expressed R-Ras. Differences in R-Ras expression between primary melanoma, and metastatic melanoma and nevi were highly significant (P < 0.0001).
Plexin C1 negatively regulates proliferation but protects melanoma from apoptosis
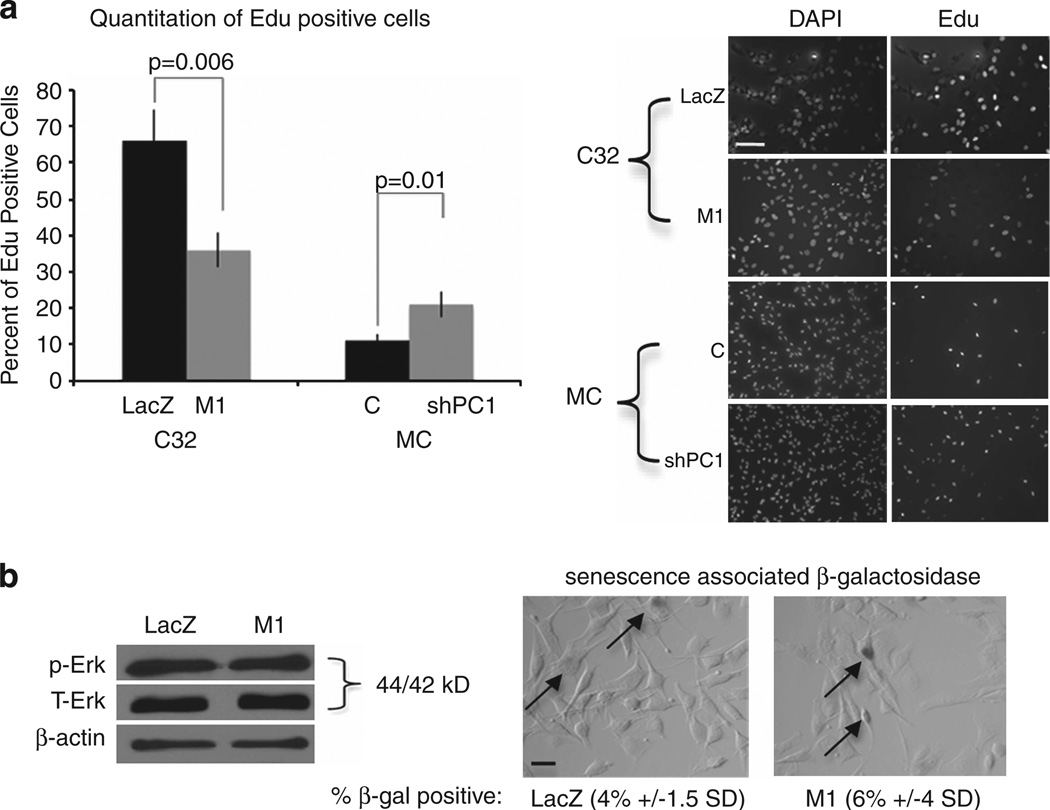
To determine if Plexin C1 regulates cell proliferation, cells were plated onto Purcol-coated coverslips in complete media and proliferation was assessed by Click-IT analysis 18 h later. Figure 3a shows the percentage of Edu-positive nuclei in C32 melanoma cells expressing Plexin C1, and in melanocytes silenced for Plexin C1. Melanoma cells expressing Plexin C1 showed a 45% decrease in proliferation compared with LacZ controls (P = 0.006). Melanocytes in which Plexin C1 was silenced showed a 45% increase in proliferation compared with non-target controls (P = 0.01). Levels of active Erk1/Erk2 were unchanged in melanoma cells expressing Plexin C1 compared with LacZ controls, and Plexin C1 did not induce senescence of melanoma, as evaluated by senescence associated β-galactosidase expression (Figure 3b).
Figure 3.
Plexin C1 negatively regulates proliferation of melanoma and melanocytes. (a) Proliferation of melanoma cells expressing Plexin C1 (M1) was reduced by almost 50% compared with LacZ controls. In contrast, melanocytes (‘MC’) in which Plexin C1 was silenced show significantly increased proliferation. The right panel shows representative images from Click-IT studies. Results are representative of three separate experiments. Bars represent the average percentage of Edu-positive cells ± s.d. (b) Cells were placed in basal media for 24 h, then lysed and total cellular protein were resolved on 10% SDS–PAGE and blotted for phosphorylated Erk1/Erk2. No differences in levels of active Erk1/Erk2 were detected. Senescence associated β-galactosidase (pH 6.0) expression was quantified in LacZ and M1 cells. Shown are representative bright field images of cells stained for β-galactosidase. No significant differences in percentage of β-galactosidase-positive cells were detected (P = 0.12). Results are representative of two experiments. Arrows indicate β-galactosidase positive cells; Bar ~30 µm.
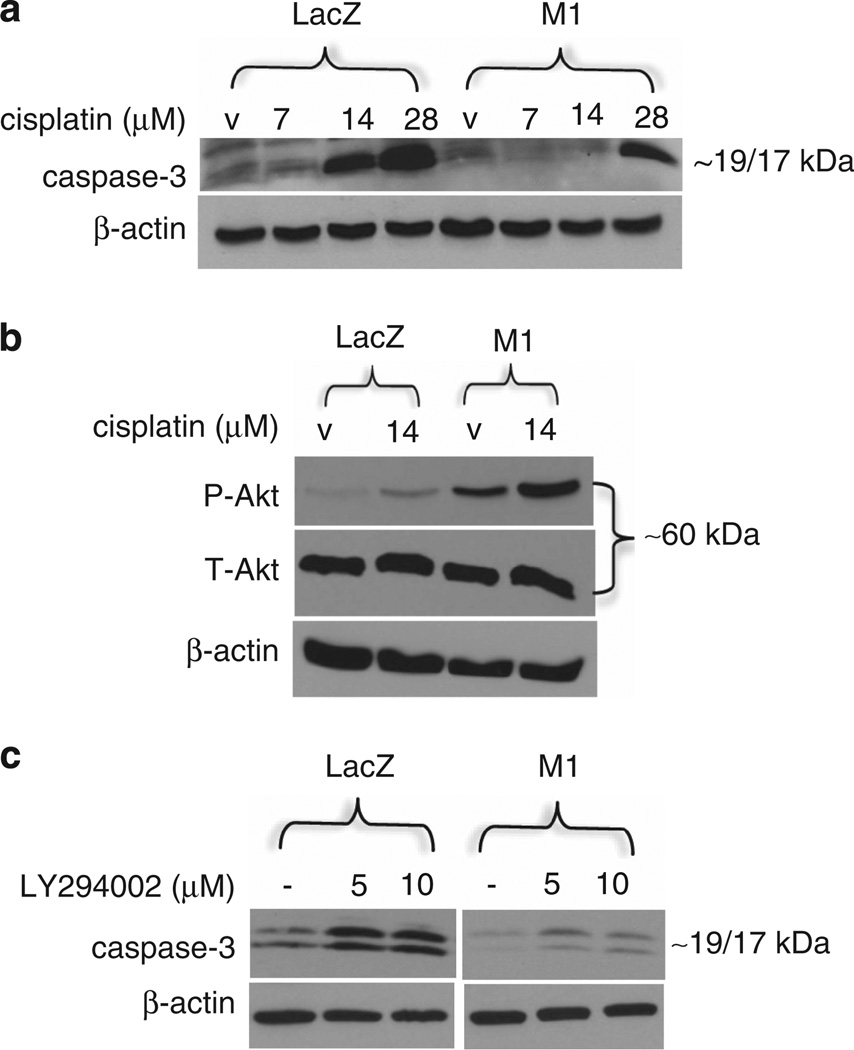
Plexin B1, a receptor that shares 30% homology with Plexin C1, activates the phosphotidyl-inositol-3 (PI3)-kinase-Akt pathway and protects melanoma from cisplatin-induced apoptosis.30 To determine if Plexin C1 renders melanoma resistant to apoptosis, C32 melanoma expressing Plexin C1 were treated with cisplatin for 48 h. Floating cells were collected and combined with adherent cells, and the presence of cleaved caspase-3 were determined. Plexin C1 rendered melanoma cells relatively resistant to cisplatin-induced apoptosis, as shown by reduced levels of active caspase-3 in treated cells (Figure 4a), which was also observed in cells treated for 24 h (Supplementary Figure 2). Basal levels of active Akt were higher in melanoma cells expressing Plexin C1, and were further induced by treatment with cisplatin (Figure 4b), suggesting that sustained activation of Akt by Plexin C1 rendered cells relatively resistant to cisplatin. Akt is phosphorylated and activated by the PI3-kinase pathway. To determine if Plexin C1 activates PI3-kinase, cells were treated with cisplatin (14 µm) for 24 h in the presence of the PI3-kinase inhibitor LY294002, and caspase-3 levels were determined (Figure 4c). As expected, cisplatin induced more apoptosis in LacZ cells compared with M1 cells, as shown by greater expression of activated caspase-3. Inhibition of PI3-kinase further increased apoptosis in LacZ cells, but had only a minor effect on apoptosis in M1 cells, consistent with higher levels of PI3-kinase activity in melanoma cells expressing Plexin C1.
Figure 4.
Plexin C1 protects melanoma from apoptosis. (a) Following treatment with cisplatin, total cellular lysates of adherent and floating cells were resolved on 12% SDS–PAGE and blotted for cleaved (active) caspase-3. LacZ cells are more apoptotic than M1 cells when treated with cisplatin, as shown by increased expression of active caspase-3. Results shown are representative of four separate experiments. (b) Cells were treated with cisplatin (14 µm) for 24 h and total cellular lysates of adherent and floating cells were resolved on 10% SDS–PAGE and blotted for phosphorylated Akt (P-Akt). Basal levels of P-Akt are higher in M1 cells compared with LacZ controls, and are further increased by cisplatin. Shown are representative results from three separate experiments. (c) Cells were treated with cisplatin in the presence of the PI3-kinase inhibitor LY294002. As expected, cells treated with cisplatin (28 µm) showed more apoptosis in LacZ compared with M1 cells (first lane). Treatment with cisplatin and LY294002 induced much higher levels of apoptosis in LacZ compared with M1 cells, indicating that resistance to apoptosis by Plexin C1 is due to higher levels of PI3-kinase activity. Shown are lysates reolved on 12% SDS–PAGE and blotted for caspase-3. Results are representative of two separate experiments.
Plexin C1 delays tumor formation in mice
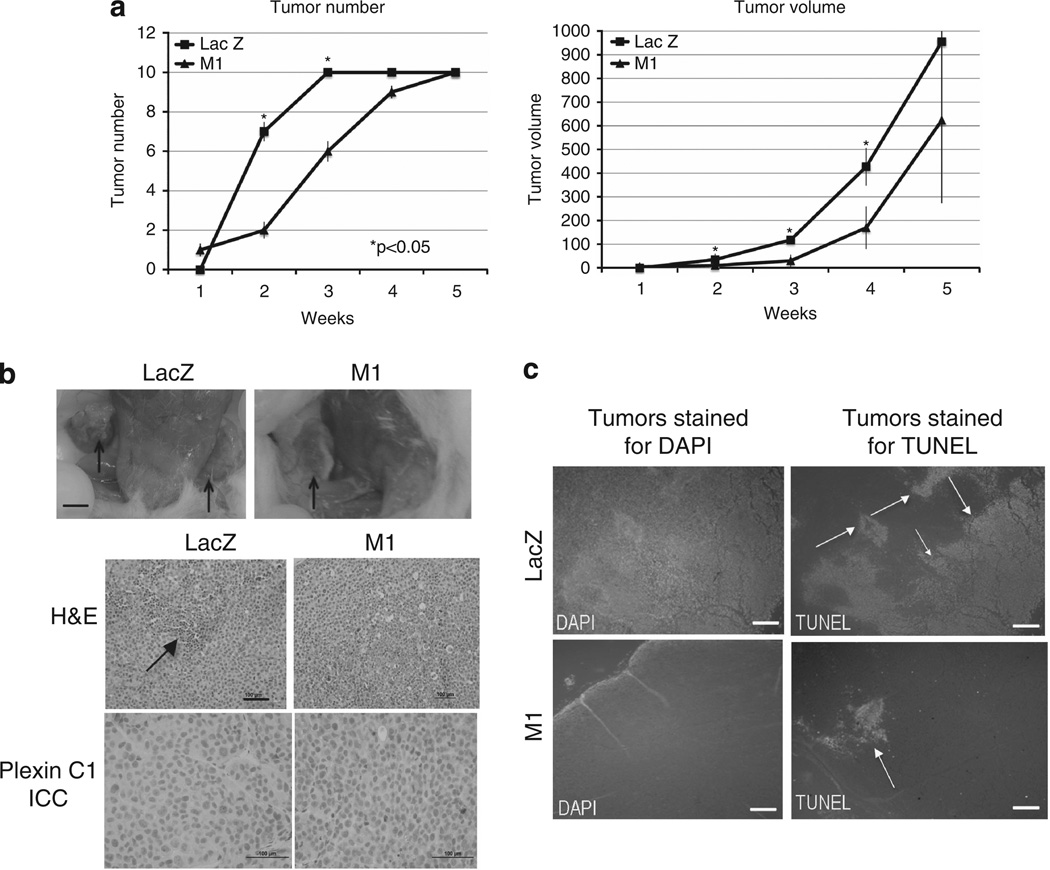
Our studies show that expression of Plexin C1 in a human melanoma cell line inhibits two key steps of melanoma progression, migration and proliferation, but renders them more resistant to apoptosis through PI3-kinase-dependent Akt activation. To determine if Plexin C1 suppresses tumor progression in vivo, we measured tumor formation in NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice. Cells were injected bilaterally into the flanks of mice (n = 5) and development of palpable tumors and tumor volume were documented weekly. Plexin C1 significantly delayed development of tumors at weeks 2 and 3 compared with tumors from LacZ-expressing control cells (P < 0.05), and tumor volume was also significantly reduced at weeks 2–4 (Figure 5a). At 5 weeks, however, all mice had developed tumors on both flanks, and tumor weights and volumes were not significantly different from control mice. To determine if loss of Plexin C1 in M1 xenografts accounted for loss of tumor suppressive effects, tumors were stained for Plexin C1 (Figure 5b). Tumors derived from M1 cells retained Plexin C1 expression at the time of sacrifice. Hematoxylin and eosin-stained sections of tumors showed geographic areas of necrosis in LacZ controls, which was more extensive than that seen in M1 xenografts. TUNEL staining of tumors showed less apoptosis in M1 xenografts compared with LacZ controls, consistent with in vitro data showing that Plexin C1 suppresses apoptosis (Figure 5c).
Figure 5.
Plexin C1 delays tumor growth in mice. (a) Shown are numbers of tumors, and tumor volumes (mm2) for weeks 1–5 after injection. Tumor numbers were significantly fewer at weeks 2 and 3 in mice injected with M1 cells; tumor volumes were significantly smaller at weeks 2–4. Results are represented as average tumor number/volume ± s.d. (b) Photographs of mice bearing tumors from LacZ controls and cells expressing Plexin C1 (M1). Arrows show subcutaneous tumors present in the flanks at the time of sacrifice. Bar ~5 mm. Hematoxylin and eosin (H&E) stained sections of tumors are shown. Arrows indicate areas of necrosis/apoptosis, which was more extensive in tumors from control mice. Immunocytochemical (ICC) stains for Plexin C1 show retention of Plexin C1 expression in tumors from M1 cells (bottom right panel), and no Plexin C1 expression in tumors from LacZ controls. Bar ~100 µm. (c) TUNEL stain of tumors show more TUNEL-positive nuclei in LacZ tumors compared with tumors from M1 cells. Shown are representative images from fluorescence micrographs. Sections were stained with DAPI to highlight nuclei. Bar = 1 mm.
DISCUSSION
We are interested in Plexin C1 because it is expressed at high levels in normal human melanocytes in vitro and in vivo, regulates melanocyte adhesion and is lost in melanoma in subjects in vivo. Plexin C1 expression is down-regulated by low doses of ultraviolet-B irradiation (personal observations), suggesting that UV-dependent regulation of Plexin C1 may promote melanoma initiation or progression. The purpose of this work was to test the hypothesis that Plexin C1 suppresses melanoma progression. In this report, we show that Plexin C1 delays tumor progression in mouse xenografts, and through in vitro studies have identified a role for Plexin C1 in the regulation of melanoma proliferation and migration. Perhaps because Plexin C1 is a large receptor, we obtained only modest expression in C32 cells, and despite several attempts, we were unable to achieve expression in other melanoma cell lines. However, the receptor was correctly localized to the plasma membrane of transductants, and expression was retained in xenograft tumors. Analysis of effects of Plexin C1 silencing in melanocytes were performed as complimentary studies to over-expression of the receptor in melanoma.
Plexin C1 negatively regulated migration in melanoma and normal melanocytes. In combination with prior studies demonstrating that Plexin C1 regulates melanocyte adhesion and dendricity,16,23 the data suggest that a primary effect of Plexin C1 in melanocytic cells is regulation of cytoskeletal organization. Plexin C1 negatively regulated proliferation in melanoma, without changes in activation of Erk1/Erk2. This is consistent with our previous report in melanocytes, in which knockdown of Plexin C1 had no effect on Erk1/Erk2 activation.23 C32 cells have a V600E mutation in BRAF, a driver mutation that chronically activates the MAP kinase pathway.31 These data suggest that Plexin C1 suppresses cell proliferation through regulation of signaling pathways independent of MAP kinase. Our studies are the first to demonstrate that Plexin C1 regulates the activity of R-Ras in melanoma, and the first to demonstrate effects of a Plexin receptor on R-Ras activity in a normal human primary cell (melanocytes). Although Plexin C1 has Rap1-GAP activity,9 we were unable to detect Rap1-GAP activity in melanoma cells, regardless of Plexin C1 expression. R-Ras increases integrin receptor affinity32,33 and the activity of Rho and Rac, which are critically important in cell adhesion and directed migration.34,35 Because R-Ras promotes melanoma progression through binding to filamin A,28 Plexin C1 may negatively regulate melanoma and melanocyte migration through R-Ras-dependent changes in multiple downstream targets. There are little data on the expression of R-Ras in human tissues in vivo. R-Ras was not detected in the epidermis, or in endothelial cells, lymphocytes, melanocytes or smooth muscle in the skin, but was expressed in dermal macrophages. Because R-Ras is expressed almost solely in primary melanomas in vivo, coordinated loss of Plexin C1 in primary melanoma,23,25 and de novo expression and activation of R-Ras, may function to promote attachment and migration to extracellular matrix or basement membranes, promoting progression.
Somewhat surprisingly, normal melanocytes had higher basal levels of R-Ras activity, compared with melanoma. Similar observations were made when melanocytes were compared with the melanoma cell line WW165 (unpublished observations). Melanocytes are cultured in growth-factor rich medium, whose effects may persist even 24 h after removal, which could account for high levels of resting GTP-bound R-Ras. The function of R-Ras in melanocytes remains to be explored. R-Ras, though expressed by melanocytes in culture, was not detected in melanocytes in skin in vivo, but was detected in transformed melanocytic cells of primary melanoma. Therefore, expression of R-Ras may occur in response to stress response pathways initiated by ultraviolet irradiation, or secondary to oncogenic transformation. Melanocytes are highly dendritic cells, and R-Ras activity is critically important for axon outgrowth and neurite formation.36,37 We speculate that in melanocytes, R-Ras controls dendricity either through regulation of Rac and Rho or through modulation of β1-integirn affinity, key regulatory molecules in melanocyte dendrite formation and maintenance.38–40 Plexin C1 may therefore control melanocyte dendricity in part through regulation of R-Ras activity.
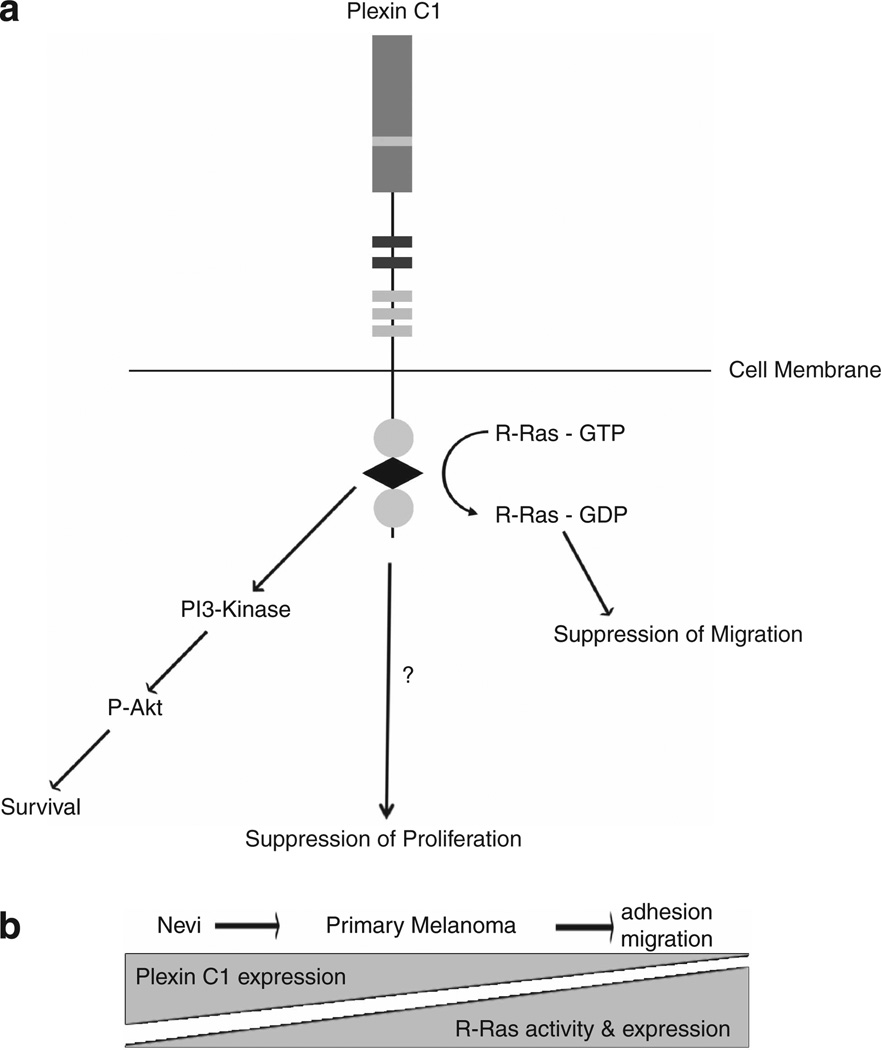
In mouse xenograft studies, Plexin C1 delayed, but did not eliminate, tumor formation. This is in contrast with Plexin B1, which completely blocks melanoma formation in the same mouse strain.41 Similar to Plexin B1, Plexin C1 activates the PI3-kinase pathway, rendering cells more resistant to apoptosis,30 consistent with lower levels of apoptosis in xenograft tumors from Plexin C1 expressing cells. Akt activation (Figure 4b) by cisplatin has been previously described in several tumor cell lines,42 and may be a mechanism for tumor resistance to the drug. Consistent with this, cisplatin induced Akt phosphorylation in C32 melanoma, which was greater in cells expressing Plexin C1 compared with controls, consistent with increased resistance of M1 cells to cisplatin-induced apoptosis (Figure 4a). Despite Akt activation, cisplatin induced apoptosis in C32 melanoma, suggesting that cisplatin-dependent apoptosis occurs through PI3-kinase-dependent and -independent pathways. In aggregate, our data suggest that Plexin C1 has tumor suppressor effects in early stages of melanoma progression, due to effects on proliferation and migration (Figure 6a). As tumors enlarge, and come under survival pressure due to lack of tumor blood supply, tumors expressing Plexin C1 survive and grow due to activation of the PI3-kinase Akt pathway, which renders them relatively resistant to apoptosis. Coordinated loss of Plexin C1, and expression of R-Ras in early primary melanoma, is expected to activate R-Ras, potentially promoting early steps in melanoma progression through enhanced migration and adhesion (Figure 6b).
Figure 6.
Model of Plexin C1 in early stages of melanoma progression. (a) Proposed model of Plexin C1 signaling in melanoma. Plexin C1 suppresses R-Ras activity, which is expected to lower adhesion and migration of melanoma. Proliferation is also lowered, through signaling pathways that are Erk1/Erk2 independent. Activation of the PI3-kinase pathway, however, abrogates the tumor suppressive effects of Plexin C1, promoting cell survival. (b) R-Ras and Plexin C1 show a coordinated increase (R-Ras), and decrease (Plexin C1) in expression in nevi and primary melanoma in vivo. Because Plexin C1 suppresses R-Ras activity in melanoma and melanocytes, the final effect is predicted to be increased R-Ras-dependent downstream phenotypes, including enhanced adhesion and migration.
MATERIALS AND METHODS
Reagents
PureCol was from Inamed Biomaterials (Freemont, CA, USA); the BCA Protein Assay kit was from Pierce Chemical (Rockford, IL, USA). Rabbit polyclonal antibodies to β-actin were purchased from Abcam (Cambridge, MA, USA); goat polyclonal antibodies againt the N-terminal region of Plexin C1 were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA, USA). Rabbit polyclonal antibodies to Sema7A were purchased from Abcam. Rabbit polyclonal antibodies to phospho-AKT (Ser473) and total AKT, rabbit polyclonal antibodies to phospho-p44/42 MAPK (Erk1/Erk2) (Thr202/Tyr204), mouse monoclonal antibodies to total Erk1/Erk2 and rabbit polyclonal antibodies to cleaved caspase-3 (Asp 175) were purchased from Cell Signaling Technology (Danvers, MA, USA). Rabbit polyclonal antibodies to R-Ras used for western blotting were a generous gift of Dr Andrew Chan (Medical College of Wisconsin), and are described in Argast et al.41 R-Ras antibodies used for immunohistochemistry were purchased from Santa Cruz Biotechnologies (sc-523). Horseradish peroxidase-conjugated goat anti-rabbit and goat anti-mouse antibodies were purchased from Sigma Co. (St Louis, MO, USA). The Ras activation assay kit was purchased from Cytoskelton Inc. (Denver, CO, USA); the Rap1 activity assay was purchased from EMD Millipore Corp. (Billerica, MA, USA). The PI3-kinase inhibitor LY294002 was purchased from Cell Signaling Technology. Full-range rainbow molecular weight marker was purchased from Amersham Life Sciences (Arlington Heights, IL, USA). Polybrene was purchased from Santa Cruz Biotechnologies; cisplatin was purchased from Enzo Life Sciences (Farmingdale, NY, USA).
Cells and cell culture
Neonatal foreskins were obtained according to the University of Rochester Research Subjects Review Board guidelines and were the source of cultured human melanocytes. Human melanocytes were cultured in Opti-MEM (Gibco-BRL Grand Island, NY) containing: 5% FBS (fetal bovine serum; Atlanta Biologicals; Lawrenceville, GA, USA), 10−4M iso-butyl-methylxanthine (IBMX), Anti-Anti (Gibco-BRL), 2.5 nm Cholera Toxin, 0.1mm dbcAMP, 25 ng/ml phorbol ester. C32 primary human melanoma cells were obtained Q3 from ATCC (Manassas, VA, USA) and were cultured in EMEM (BioWhittaker Walkersville, MD) and l-Glutamine (Lonza, Walkersville, MD, USA) with 10% FBS, Anti-Anti, and 1 mM sodium pyruvate (Invitrogen Carlsbad, CA). C32 cells harbor the V600E mutation in the BRAF gene31
Establishment of melanoma cells expressing Plexin C1
The open reading frame of human Plexin C1 in Myc-DDK (RC211396; Origene Technologies, Rockville, MD, USA) was subcloned into the biscistronic lentiviral vector pLVX-IRES-NEO (Clontech, Grand Island, NY, USA). High titer replication-incompetent VSV-G-pseudotyped lentivirus was made using Lenti-X HT Packaging System in 293 FT cells (Clontech). Lentivirus-associated p24 ELISA kit (Cell Biolabs, San Diego, CA, USA) was used to quantitate virus. Cells were transduced with lentivirus at a multiplicity of infection of 2.5. Controls consisted of cells transduced with empty vector. Three days after transduction, selection with neomycin (Sigma Co.) was performed.
Silencing of Plexin C1
Human melanocytes were plated at 105 cells in a six-well plate. Cells were infected at 2.5 multiplicity of infection with MISSION Lentivirus particles (Sigma Co.) expressing shRNA in pLKO.1-CMV-neo, targeting human Plexin C1 (Clone TRCN0000060645). Cells infected with non-target shRNA (shRNA-NT) in Lentivirus were used as controls. Silenced cells were selected with neomycin.
Western blotting
Cells were lysed in RIPA buffer (150 mm NaCl, 1% NP-40, 0.5% DOC, 0.1% sodium dodecyl sulfate (SDS), 50 mm Tris–HCl) with protease inhibitors (Boehringer Mannheim, Gmbt, Germany) and protein was quantitated using bovine serum albumen as a standard (Bio-Rad Laboratories, Hercules, CA, USA). Protein was resolved on SDS–PAGE (polyacrylamide gel electrophoresis) gels and blotted using standard procedures. Visualization of the immunoreactive proteins was accomplished with an enhanced chemiluminescence reaction (Pierce Chemical, Rockford, IL, USA).
Construction and analysis of tissue microarrays
The project received Institutional Review Board exemption from the Human Subjects Review Board at the University of Rochester: Category 4 (45 CRF 46.101): secondary use of pre-existing data. Twenty-six cases of benign nevi, 77 cases of melanoma primary to the skin and 37 cases of metastatic melanoma were chosen from formalin-fixed, paraffin-embedded archival material from Strong Memorial Department of Pathology. Three representative areas from each slide were chosen and 1 mm cores corresponding to these areas were sampled from each block. All nevi were dermal nevi; primary melanomas were pathologic state pT1 (n = 36), pT2 (n = 20), pT3 (n = 8) and pT4 (n = 13). Metastatic melanomas were from lymph nodes, (20/37), skin (9/37) and solid organs including lung, liver, bone and gut (8/37). Each stained core was examined under a light microscope by one of the authors (GS). Staining was quantified as absent (0), or present (1 +), and scores from the three cores were averaged to determine an intensity score (IS). Cores with <50% of tissue present were not scored.
Immunohistochemical staining for Plexin C1 and R-Ras
Sections were de-paraffinized and endogenous peroxidase was quenched by incubation in 3% H2O2. Non-specific staining was blocked by incubation in non-protein blocking solution (Dako, Carpenteria, CA, USA). Antigen retrieval was performed by incubating the slides in Target retrieval Buffer (Dako), pH 6.0, at 98 °C for 15 min. Slides were incubated with goat polyclonal antibodies against Plexin C1 (1/100) or rabbit polyclonal antibodies against R-Ras (1/100) in antibody diluent for 1 h at room temperature. Sections were then incubated with biotin-conjugated species appropriate secondary antibodies for 30 min at room temperature followed by streptavidin horseradish-peroxidase (Vector Laboratories, Burlingame, CA, USA).
β-Galactosidase staining
Cells were plated onto PureCol-coated coverslips and allowed to attach for 24 h. Cells were placed in basal media for 24 h and stained for senescence-associated β-galatosidase as previously described.43 A minimum of 200 cells were evaluated for β-galatosidase expression by bright field microscopy and percent β-galatosidase expressing cells was determined.
Click-IT assays
Staining was performed on cells cultured on PureCol-coated coverslips by Click-IT assay (Invitrogen). Click-IT-positive nuclei were identified using a filter with excitation wavelength of 495 nm. Positive nuclei were counted in a minimum of 200 cells, and percent positive nuclei determined by dividing by total nuclei, identified by DAPI counter-staining, viewed with a filter with excitation wavelength of 341 nm.
Migration assays
In all, 96-well fluorometric migration assays were purchased from Millipore (Billerica, MA, USA). In all, 104 cells were plated in the upper well of a 96-well chamber and allowed to migrate across an 8-µm membrane towards media with 10% FBS. Migration assays were performed in triplicate wells.
TUNEL staining of tumor sections
TUNEL uptake on formalin-fixed paraffin-embedded tumor sections was detected using the DeadEnd Flurometric TUNEL System (Promega, Madison, WI, USA). TUNEL-positive nuclei were visualized using a filter with excitation wavelength of 495 nm. Sections were counter-stained with DAPI, viewed with a filter with excitation wavelength of 341 nm.
Mouse xenograft studies
NSG mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were housed in the animal facility at the University of Rochester Medical Center, in accordance to the animal care guidelines from the Division of Laboratory Animal Medicine at University of Rochester Medical Center. A total of 2 × 106 melanoma cells were injected subcutaneously into both flanks of each mouse (n = 5). Development of tumors was monitored weekly by palpation, and tumor length and width (in mm) were measured with calipers. Tumor volume was calculated as 4π (length × width). Mice were killed 5 weeks after injection and tumors were harvested, measured and weighted.
Reverse transcription polymerase chain reaction and comparative real-time PCR
Total RNA was isolated using the RNeasy Mini Kit (QIAgen, Valencia, CA, USA). Primers for amplification of Plexin C1 were: fwd: 5′-AACCATTGCACTGCAACC-3′ ; rvs: 5′-GATTCCATCTTCAAGAATCACG-3′. The conditions were: 95 °C, 3 min (1 cycle); 95 °C 15 s, 54.5 °C, 30 s, 72 °C, 40 s (40 cycles). Primers used for amplification of β-actin were: fwd: 5′-CACGCACGATTTCCCGCTCGG-3′; rvs: 5′-CAGGCTGTGCTATCCTGTAC-3′. The conditions were 95 °C, 3 min (1 cycle); 95 °C 15 s, 54.5 °C, 30 s, 72 °C, 40 s (40 cycles). PCR cycle number was normalized to β-actin to arrive at an adjusted relative cycle number for each sample.
Purification of Fc-tagged Sema7A
Fc-tagged Sema7A was isolated from culture supernatant of transfectants of 293FT cells (Invitrogen) as previously described.16 The activity of the recombinant protein was analyzed by Erk1/Erk2 activation in normal melanocytes (Supplementary Figure 3).
Statistical analysis
Differences between means were analyzed by two-tailed Student’s t-test. A P-value <0.05 was considered significant.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by R01CA136499 (GS), by NIH training Grant 5T32AR007472 (JS), and by the Department of Biogenetics, University of Rochester (LX We also thank Lori Moll (Oncogene Inc.,) for assistance with subcloning of Plexin C1.).
ABBREVIATIONS
- SD
standard deviation
- Sema7A
Semaphorin 7A
- SEM
standard error of the mean
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
REFERENCES
- 1.Artigiani S, Comoglio PM, Tamagnone L. Plexins semaphorins, and scatter factor receptors: a common root for cell guidance signals? IUBMB Life. 1999;48:477–482. doi: 10.1080/713803563. [DOI] [PubMed] [Google Scholar]
- 2.Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7:211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perala N, Sariola H, Immonen T. More than nervous: the emerging roles of plexins. Differentiation. 2012;83:77–91. doi: 10.1016/j.diff.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Barton WA, Himanen JP, Antipenko A, Nikolov DB. Structures of axon guidance molecules and their neuronal receptors. Adv Protein Chem. 2004;68:65–106. doi: 10.1016/S0065-3233(04)68003-X. [DOI] [PubMed] [Google Scholar]
- 5.Uesugi K, Oinuma I, Katoh H, Negishi M. Different requirement for Rnd GTPases of R-Ras GAP activity of Plexin-C1 and Plexin-D1. J. Biol Chem. 2009;284:6743–6751. doi: 10.1074/jbc.M805213200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puschel AW. GTPases in semaphorin signaling. Adv Exp Med Biol. 2007;600:12–23. doi: 10.1007/978-0-387-70956-7_2. [DOI] [PubMed] [Google Scholar]
- 7.Negishi M, Oinuma I, Katoh H. R-ras as a key player for signaling pathway of plexins. Mol Neurobiol. 2005;32:217–222. doi: 10.1385/MN:32:3:217. [DOI] [PubMed] [Google Scholar]
- 8.Oinuma I, Katoh H, Negishi M. Semaphorin 4D/Plexin-B1-mediated R-Ras GAP activity inhibits cell migration by regulating beta(1) integrin activity. J Cell Biol. 2006;173:601–613. doi: 10.1083/jcb.200508204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, He H, Srivastava N, Vikarunnessa S, Chen Y-b, Jiang J, et al. Plexins are GTPase-activating proteins for Rap and are activated by induced dimerization. Sci Signal. 2012;5:ra6. doi: 10.1126/scisignal.2002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aurandt J, Vikis HG, Gutkind JS, Ahn N, Guan KL. The semaphorin receptor plexin-B1 signals through a direct interaction with the Rho-specific nucleotide exchange factor, LARG. Proc Natl Acad Sci USA. 2002;99:12085–12090. doi: 10.1073/pnas.142433199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barberis D, Casazza A, Sordella R, Corso S, Artigiani S, Settleman J, et al. p190 Rho-GTPase activating protein associates with plexins and it is required for semaphorin signalling. J Cell Sci. 2005;118(Part 20):4689–4700. doi: 10.1242/jcs.02590. [DOI] [PubMed] [Google Scholar]
- 12.Giger RJ, Hollis ER, 2nd, Tuszynski MH. Guidance molecules in axon regeneration. Cold Spring Harbor Perspect Biol. 2010;2:a001867. doi: 10.1101/cshperspect.a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walzer T, Galibert L, De Smedt T. Dendritic cell function in mice lacking Plexin C1. Int Immunol. 2005;17:943–950. doi: 10.1093/intimm/dxh274. [DOI] [PubMed] [Google Scholar]
- 14.Lallier TE. Semaphorin profiling of periodontal fibroblasts and osteoblasts. J Dent Res. 2004;83:677–682. doi: 10.1177/154405910408300904. [DOI] [PubMed] [Google Scholar]
- 15.Messina A, Ferraris N, Wray S, Cagnoni G, Donohue DE, Casoni F, et al. Dysregulation of Semaphorin7A/beta1-integrin signaling leads to defective GnRH-1 cell migration, abnormal gonadal development and altered fertility. Hum Mol Genet. 2011;20:4759–4774. doi: 10.1093/hmg/ddr403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott GA, McClelland LA, Fricke AF. Semaphorin 7a promotes spreading and dendricity in human melanocytes through beta1-integrins. J Invest Dermatol. 2008;128:151–161. doi: 10.1038/sj.jid.5700974. [DOI] [PubMed] [Google Scholar]
- 17.Comeau MR, Johnson R, DuBose RF, Petersen M, Gearing P, VandenBos T, et al. A poxvirus-encoded semaphorin induces cytokine production from monocytes and binds to a novel cellular semaphorin receptor. VESPR Immunity. 1998;8:473–482. doi: 10.1016/s1074-7613(00)80552-x. [DOI] [PubMed] [Google Scholar]
- 18.Xu C, Fan CM. Allocation of paraventricular and supraoptic neurons requires Sim1 function: a role for a Sim1 downstream gene PlexinC1. Mol Endocrinol. 2007;21:1234–1245. doi: 10.1210/me.2007-0034. [DOI] [PubMed] [Google Scholar]
- 19.Pasterkamp RJ, Kolk SM, Hellemons AJ, Kolodkin AL. Expression patterns of semaphorin7A and plexinC1 during rat neural development suggest roles in axon guidance and neuronal migration BMC. Devl Biol. 2007;7:98. doi: 10.1186/1471-213X-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H, Juo ZS, Shim AH, Focia PJ, Chen X, Garcia KC, et al. Structural basis of semaphorin-plexin recognition and viral mimicry from Sema7A and A39R complexes with PlexinC1. Cell. 2010;142:749–761. doi: 10.1016/j.cell.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424:398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki K, Okuno T, Yamamoto M, Pasterkamp RJ, Takegahara N, Takamatsu H, et al. Semaphorin 7A initiates T-cell-mediated inflammatory responses through alpha1beta1 integrin. Nature. 2007;446:680–684. doi: 10.1038/nature05652. [DOI] [PubMed] [Google Scholar]
- 23.Scott GA, McClelland LA, Fricke AF, Fender A. Plexin C1, a receptor for semaphorin 7a, inactivates cofilin and is a potential tumor suppressor for melanoma progression. J Invest Dermatol. 2009;129:954–963. doi: 10.1038/jid.2008.329. [DOI] [PubMed] [Google Scholar]
- 24.Maddodi N, Setaluri V. Role of UV in cutaneous melanoma. Photochem Photobiol. 2008;84:528–536. doi: 10.1111/j.1751-1097.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 25.Lazova R, Gould Rothberg BE, Rimm D, Scott G. The semaphorin 7A receptor Plexin C1 is lost during melanoma metastasis. Am J Dermatopathol. 2009;31:177–181. doi: 10.1097/DAD.0b013e318196672d. [DOI] [PubMed] [Google Scholar]
- 26.Balakrishnan A, Penachioni JY, Lamba S, Bleeker FE, Zanon C, Rodolfo M, et al. Molecular profiling of the "plexinome" in melanoma and pancreatic cancer. Hum Mutat. 2009;30:1167–1174. doi: 10.1002/humu.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao L, Feng Y, Bowers R, Becker-Hapak M, Gardner J, Council L, et al. Ras-associated protein-1 regulates extracellular signal-regulated kinase activation and migration in melanoma cells: two processes important to melanoma tumorigenesis and metastasis. Cancer Res. 2006;66:7880–7888. doi: 10.1158/0008-5472.CAN-06-0254. [DOI] [PubMed] [Google Scholar]
- 28.Gawecka JE, Griffiths GS, Ek-Rylander B, Ramos JW, Matter ML. R-Ras regulates migration through an interaction with filamin A in melanoma cells. PLoS One. 2010;5:e11269. doi: 10.1371/journal.pone.0011269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang D, Tao J, Li L, Kedei N, Tóth ZE, Czap A, et al. RasGRP3, a Ras activator, contributes to signaling and the tumorigenic phenotype in human melanoma. Oncogene. 2011;30:4590–4600. doi: 10.1038/onc.2011.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens L, McClelland L, Fricke A, Williamson M, Kuo I, Scott G. Plexin B1 suppresses c-Met in melanoma: a role for Plexin B1 as a tumor-suppressor protein through regulation of c-Met. J Invest Dermatol. 2010;130:1636–1645. doi: 10.1038/jid.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abi-Habib RJ, Urieto JO, Liu A, Leppla SH, Duesbery N, Frankel A. BRAF status and mitogen-activated protein/extracellular signal-regulated kinase kinase 1/2 activity indicate sensitivity of melanoma cells to anthrax lethal toxin. Mol Can Ther. 2005;4:1303–1310. doi: 10.1158/1535-7163.MCT-05-0145. [DOI] [PubMed] [Google Scholar]
- 32.Keely PJ, Rusyn EV, Cox AD, Parise LV. R-Ras signals through specific integrin alpha cytoplasmic domains to promote migration and invasion of breast epithelial cells. J Cell Biol. 1999;145:1077–1088. doi: 10.1083/jcb.145.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z, Vuori K, Wang H, Reed JC, Ruoslahti E. Integrin activation by R-ras. Cell. 1996;85:61–69. doi: 10.1016/s0092-8674(00)81082-x. [DOI] [PubMed] [Google Scholar]
- 34.Holly SP, Larson MK, Parise LV. The unique N-terminus of R-ras is required for Rac activation and precise regulation of cell migration. Mol Biol Cell. 2005;16:2458–2469. doi: 10.1091/mbc.E03-12-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wozniak MA, Kwong L, Chodniewicz D, Klemke RL, Keely PJ. R-Ras controls membrane protrusion and cell migration through the spatial regulation of Rac and Rho. Mol Biol Cell. 2005;16:84–96. doi: 10.1091/mbc.E04-04-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwashita S, Kobayashi M, Kubo Y, Hinohara Y, Sezaki M, Nakamura K, et al. Versatile roles of R-Ras GAP in neurite formation of PC12 cells and embryonic vascular development. J Biol Chem. 2007;282:3413–3417. doi: 10.1074/jbc.C600293200. [DOI] [PubMed] [Google Scholar]
- 37.Iwasawa N, Negishi M, Oinuma I. R-Ras controls axon branching through Afadin in cortical neurons. Mol Biol Cell. 2012;23:2793–2804. doi: 10.1091/mbc.E12-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hara M, Yaar M, Tang A, Eller MS, Reenstra W, Gilchrest BA. Role of integrins in melanocyte attachment and dendricity. J Cell Sci. 1994;107(Part 10):2739–2748. doi: 10.1242/jcs.107.10.2739. [DOI] [PubMed] [Google Scholar]
- 39.Scott G, Leopardi S. The cAMP-PKA signaling pathway has opposing effects on Rac and Rho in B16F10 cells: implications for dendrite formation in melanocytic cells. Pigment Cell Res. 2003;16:139–148. doi: 10.1034/j.1600-0749.2003.00022.x. [DOI] [PubMed] [Google Scholar]
- 40.Scott G. Rac and Rho: the story of melanocyte dendrite formation (Review) Pigm Cell Res. 2002;5:322–330. doi: 10.1034/j.1600-0749.2002.02056.x. [DOI] [PubMed] [Google Scholar]
- 41.Argast GM, Croy CH, Couts KL, Zhang Z, Litman E, Chan DC, et al. Plexin B1 is repressed by oncogenic B-Raf signaling and functions as a tumor suppressor in melanoma cells. Oncogene. 2009;28:2697–2709. doi: 10.1038/onc.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winograd-Katz SE, Levitzk A. Cisplatin induces PKB/Akt activation and p38MAPK phosphorylation of the EGF receptor. Oncogene. 2006;25:7381–7390. doi: 10.1038/sj.onc.1209737. [DOI] [PubMed] [Google Scholar]
- 43.Itahana K, Campisi J, Dimri GP. Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods Mol Biol. 2007;371:21–31. doi: 10.1007/978-1-59745-361-5_3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.