Abstract
Effects of prenatal alcohol exposure (PAE) on central nervous system function include an increased prevalence of mental health problems, including substance use disorders (SUD). The hypothalamic-pituitary-adrenal (HPA) and dopamine systems have overlapping neurocircuitries and are both implicated in SUD. PAE alters both HPA and dopaminergic activity and regulation, resulting in increased HPA tone and an overall reduction in tonic dopamine activity. However, effects of PAE on the interaction between HPA and dopamine (DA) systems have not been investigated. The present study examined PAE effects on basal regulation of central stress and dopamine systems in key brain regions where these systems intersect. Adult Sprague-Dawley male and female offspring from prenatal alcohol-exposed (PAE), pairfed (PF), and ad libitum-fed control (C) groups were subjected to chronic variable stress (CVS) or remained as a no stress (non-CVS) control group. Corticotropin releasing hormone (CRH) mRNA, as well as glucocorticoid and DA receptor (DA-R) expression were measured under basal conditions 24 hours following the end of CVS. We show, for the first time, that regulation of basal HPA and DA systems, and likely, HPA-DA interactions, are altered differentially in males and females by PAE and CVS. PAE augmented the typical attenuation in weight gain during CVS in males and caused increased weight loss in females. Increased basal corticosterone levels in control, but not PAE, females suggest that PAE alters the profile of basal hormone secretion throughout CVS. CVS downregulated basal CRH mRNA in the prefrontal cortex and throughout the bed nucleus of the stria terminalis (BNST) in PAE females but only in the posterior BNST of control females. PAE males and females exposed to CVS exhibited more widespread upregulation of basal mineralocorticoid receptor (MR) mRNA throughout the hippocampus, and an attenuated decrease in DA-R expression throughout the nucleus accumbens and striatum compared to CVS-exposed control males and females. Overall, these findings enhance our understanding of PAE effects on the cross-talk between HPA and DA systems, and provide insight into possible mechanisms underlying mental health problems that are related to stress and DA signaling, including SUD, which have a high prevalence among individuals with FASD.
INTRODUCTION
Alcohol consumption during pregnancy can result in fetal alcohol spectrum disorder (FASD) in the children, with a prevalence of 9/1,000 births in North America (Thanh and Jonsson, 2010). The adverse effects of FASD depend on dose, timing and duration of alcohol exposure, and include impairments in neurocognition, self-regulation, and adaptive functioning (Riley et al., 2003, Streissguth et al., 2004). Numerous ‘secondary’ disabilities, including substance use disorders (SUD), have been reported following PAE (O’Connor and Paley, 2009). Consistent with these findings, rodent models of PAE show enhanced preference for alcohol (Barbier et al., 2009) and increased voluntary alcohol consumption in PAE offspring (Chotro et al., 2007), supporting the hypothesis that PAE results in neurobiological vulnerability to SUD. The present study focuses on elucidating the effects of PAE on neurobiological mechanisms underlying vulnerability to SUD.
Dysregulation of hypothalamic-pituitary-adrenal (HPA) function is typically associated with SUD (Lovallo, 2006, Koob and Kreek, 2007). Moreover, data indicate that PAE reprograms the fetal HPA axis such that basal HPA tone is increased, and greater HPA activation and/or delayed or deficient recovery following stress are observed (Haley et al., 2006, Weinberg et al., 2008). Importantly, it appears that maturation of HPA responsivity may have a different developmental trajectory in alcohol-exposed compared to non-exposed infants during the critical first year of life (Ramsay et al., 1996, Jacobson et al., 1999, Haley et al., 2006).
While stress-induced HPA activation is adaptive in the short term, long-term increases in basal HPA tone and/or frequent or sustained HPA activation can lead to HPA dysregulation and alterations in physiological responsiveness and behavior (McEwen, 2008). Thus, the ability to maintain basal hormone levels within the normal physiological range and to turn off a stress response once initiated are important as the ability to respond appropriately to stress initially. The finding that all facets of HPA regulation are altered by PAE has important implications for normal offspring development.
The mesocorticolimbic DA pathway is implicated in functions such as motivation, attention, reinforcement, executive function and emotional regulation. Importantly, PAE produces significant alterations in DA systems by delaying development, reducing the size, structure and electrical activity of DA neurons, and reducing DA synthesis, binding, and metabolism (Rathbun and Druse, 1985, Cooper and Rudeen, 1988, Druse et al., 1990, Shetty et al., 1993, Shen et al., 1995, Shen et al., 1999). These findings support the suggestion that PAE results in reduced overall baseline activity of DA systems. Of relevance to the present study, there is significant overlap between central stress and DA pathways, resulting in a bi-directional interaction between stress and DA systems (Cabib and Puglisi-Allegra, 2012). For example, stress sensitizes healthy individuals to the rewarding effects of substance use, and can induce relapse after abstinence (Sarnyai et al., 2001), suggesting that HPA alterations provide a pathway for increased vulnerability to SUD.
Both HPA and DA systems are important for resilience against, or vulnerability to, addiction (Sinha, 2009); however, effects of PAE on the interaction between HPA and DA systems have not been investigated. In the present study we examined PAE effects on basal regulation of central stress and DA systems in key brain regions where these systems intersect (medial prefrontal cortex [mPFC], bed nucleus stria terminalis [BNST], striatum, nucleus accumbens [NAc], amygdala, hippocampus [HPC]). Basal levels of mRNA for corticotropin releasing hormone (CRH) and glucocorticoid receptors (mineralocorticoid, MR; glucocorticoid, GR) were measured as indicators of dysregulation of the stress system, as, alterations in these measures of stress system function have been implicated in vulnerability to SUD (Koob and Kreek, 2007, Le Moal, 2009, Sinha, 2011). Alterations in basal expression of DA receptors (DA-R) are also implicated in SUD (Everitt et al., 2008, Volkow et al., 2011), and were investigated. All neural measures were examined under basal conditions, either with or without prior exposure to chronic variable stress (CVS). Additionally, we investigated sex differences in outcome as: 1) there are sex differences in HPA function across species, with females typically showing greater HPA responses and resistance to negative feedback compared to males (Young, 1998); 2) PAE differentially alters HPA activity in males and females (Haley et al., 2006, Weinberg et al., 2008); and 3) sex differences are observed in SUD (Becker and Hu, 2008). Given the links between HPA and DA systems, and the effects of PAE on these systems, we hypothesized that PAE will enhance the adverse effects of CVS on basal/tonic regulation of HPA and DA systems in the brain, and that sex differences will be present in the effects of both PAE and CVS, with greater effects of CVS on basal regulation in females compared to males.
MATERIALS AND METHODS
1. Breeding, experimental diets and feeding
Animal procedures were in accordance with the National Institutes of Health guidelines, and were approved by the institutional Animal Care Committee. Adult virgin female (225–260 g; n=31) and male (275–300 g; n=12) Sprague-Dawley rats were obtained from Charles River (St Constant, PQ, Canada) and put onto a 0800–1600 light schedule. Briefly, a male and female were paired, and the presence of vaginal plugs indicated day 1 of gestation (GD 1), which followed previous procedures (Hellemans et al., 2010, Uban et al., 2010). On GD 1, females were moved to a new colony room and randomly assigned to one of three groups: 1) Prenatal alcohol exposure (PAE) - liquid ethanol diet, ad libitum (n = 10); 2) Pair-fed (PF) - liquid control diet; maltose dextrin isocalorically substituted for ethanol, in the amount consumed by a PAE partner (g/kg/body weight/GD), which controls for the reduced food intake typical in alcohol-consuming dams (n = 11), 3) Control (C), standard rat chow consumed ad libitum (n = 11). All dams had ad libitum access to water throughout gestation. Experimental diets provide optimal nutrition during pregnancy (prepared by Dyets Inc. Bethlehem, PA). The current model of PAE utilized moderate levels of chronic alcohol consumption throughout gestation, which is equivalent to the first and second trimesters of human pregnancy. In the present study, alcohol consumption of pregnant dams in the PAE condition averaged ~ 14, 17, and 16 g/kg body wt/day for gestation weeks 1, 2 and 3, respectively. Previous studies that have employed the same breeding and feeding protocols found mean blood alcohol levels in dams of ~140–150 mg/dl during the third week of gestation (Hellemans et al., 2010, Uban et al., 2010). Fresh diet was presented daily at 1700–1800 hr. This schedule permits maintenance of typical corticoid circadian rhythms in PF dams, as the corticoid rhythm in animals receiving a reduced ration re-entrains to the time of feeding (Gallo and Weinberg, 1981). Experimental diets were provided from GD1- 21, at which time they were replaced with standard laboratory chow ad libitum.
On postnatal day 1 (PND 1), pups were weighed and litters culled to 10 (5 females, 5 males when possible (Uban et al., 2010). Dams and pups were weighed and cages were changed on PND 1, 8, 15 and 22, but remained otherwise undisturbed throughout lactation. Pups were weaned on PND 22 and group-housed by litter and sex. On PND 35, same-sex animals were pair-housed with a non-littermate partner from the same prenatal group. To control for potential litter effects, only one male and one female from each litter were assigned to each experimental condition of the study.
2. Chronic variable stress (CVS) paradigm
In adulthood (70±5 d), pairs of rats were randomly assigned to either non-CVS (no stress) or CVS conditions. Animals in the non-CVS and CVS conditions were housed in separate colony rooms. A CVS paradigm was utilized because of its utility in mimicking the often variable, unstable, and unpredictable challenges typically experienced by individuals with a FASD. CVS treatment consisted of ten consecutive days of exposure to variable stressors presented in random order, with 2 stressors per day, separated by at least 2 hours, with one occurring ante meridiem (0900–1200hr) and the other post meridiem (1300–1900 hr). The CVS paradigm consisted of the following stressors: 1) tail nick; 2) social isolation; 3) restraint; 4) novel cage; 5) cage tilt; 6) soiled cage; 7) platform; and 8) white noise as previously described (Hellemans et al., 2010). The following two modifications were made: 1) a single stressor (blood collection via tail nick, weigh and cage change) was performed on days 1, 5 and 10; and 2) overnight social isolation occurred individually in small cages with corncob bedding. Blood samples were collected via tail nick, under basal conditions, on days 1, 5, and 10 of CVS, for analysis of plasma corticosterone (CORT) levels.
3. Brain perfusion
24 hr following the last day of CVS, all rats in both the non-CVS and CVS conditions were perfused (0900–1100 hr). Cage partners were anesthetized with isoflurane (Baxter Corporation, Mississagua, Ontario). Blood was collected from the heart for measurement of CORT, testosterone (T), estradiol (E2) and progesterone (P4) within 1–2 min after removal of the cage from the colony room. Rats were then perfused transcardially and extracted brains were prepped for in situ hybridization (ISH) and immunohistochemistry (IHC) as previously described (Williamson et al., 2010). Coronal sections were obtained throughout the entire brain (30 µm; Bregma: 4.20mm to −7.32mm (Paxinos and Watson, 2005).
4. Determination of stage of estrous cycle
At the time of perfusion, stage of estrous cycle was determined as described (Uban et al., 2012). Vaginal cytology was assessed under a light microscope to identify stages of the estrus cycle.
5. Radioimmunoassays (RIA)
Blood samples were collected in tubes containing 100 µl EDTA, centrifuged at 3200 rpm (15 min at 4 °C), and plasma stored at 80 °C until assayed. Tail poke samples were used to measure CORT (collected between 0900–1000), and perfusion blood samples were used to measure T, P4, E2 (collected between 0900–1100).
CORT
Total CORT levels (bound plus free) were determined using the ImmuChemTM Corticosterone I125 RIA Kit (MP Biomedicals). The specificity of the antibody to CORT is 100% (minimum detectable CORT concentration was 7.7 ng/mL), and the intra-assay coefficient of variation was 7.1%.
Testosterone
ImmuChemTM Testosterone I125 RIA Kit (MP Biomedicals) was used. The antibody is 100% specific for testosterone and has less than 0.01% cross-reactivity with estradiol-17β, CORT and progesterone (minimum detectable testosterone concentration was 0.1 ng/mL), and the intra-assay coefficient of variation was 6.0%.
Estradiol
Siemens/DPC Estradiol Double Antibody RIA kit was used. The antiserum cross-reacts 100% for estradiol without detectable cross-reactivity with CORT or aldosterone (minimum detectable concentration was 8 pg/mL), and the intra-assay coefficient of variation was 7.0%.
Progesterone
ImmuChemTM Progesterone RIA Kit (MP Biomedicals) was used. The antibody is 100% specific to progesterone with no detectable cross-reaction with CORT or aldosterone (minimum detectable progesterone concentration was 0.10 ng/mL), and the intra-assay coefficient of variation was 6.4%.
6. Preparation of brain tissue for in situ hybridization (ISH) and immunohistochemistry (IHC)
Incubations were performed at room temperature and on a shaker unless stated otherwise. In cold PBS (0.1 M phosphate buffer in 0.9% saline; pH 7.4), brain sections were transferred to a petri dish over ice, rinsed (3× 10 min), mounted onto slides, placed into an RNAse-free desiccator, and stored under vacuum overnight. Every 5th section throughout the region of interest was used per probe (n=5–8 rats per experimental group) for both ISH and IHC.
7. In situ hybridization
Probes and Labeling
Antisense oligonucleotide probes were used to detect mRNA levels for CRH and eCB receptor CB1 with a standard protocol (Lan et al., 2006). Riboprobes were used to detect mRNA levels for mineralocoid (MR) and glucocorticoid (GR) receptors (Glavas et al., 2007). Briefly, slides were removed from the desiccator, fixed in 10% Formalin (30 min), subjected to a series of washes (Lan et al., 2006), air-dried, and stored in a desiccator overnight. 50% oligo hybridization buffer (Lan et al., 2006) or 75% riboprobe hybridization buffer (Glavas et al., 2007) mixed with the probe (at a hybridization activity of 600,000 cpm/slide for CRH and CB1; 2,000,000 cpm/slide for GR and MR) were applied to slides, and slides covered with hybrislips (Sigma-Aldrich Canada Ltd., ON, Canada). Sections were incubated overnight (38°C) in 50% formamide humidified containers for oligonucleotide probes, or at 55°C in 75% formamide humidified containers for riboprobes. The following morning, the hybrislips were removed and post-hybridization washes comprised a series of decreasing salt concentrations (Lan et al., 2006, Glavas et al., 2007). Sections were dehydrated in 70% EtOH (5 min) and airdried overnight. Sections for CB1, MR and GR were placed in light tight cassettes under Kodak BioMax MR film (Eastman Kodak Co., NY, USA) and were exposed for 16 days (CB1 and GR) or 9 days (MR). Film was developed with Kodak GBX developer and GBX fixer, rinsed in water and hung to dry overnight. X-ray autoradiographs were then digitized for measuring. Slides for CRH were dipped in Kodak NTB2 autoradiography emulsion (Eastman Kodak Co.) and exposed for 132 days (CeA), 28 days (PVN), 104 days (mPFC,NAc) or 99 days (BNST) sealed in desiccated, light tight boxes (4°C). Slides were developed with Kodak D-19 developer (14°C) and fixed with Kodak fixer (14°C), then counterstained with Toluidine Blue, and coverslipped with Permount (Fisher Scientific Ltd., ON, Canada).
8. Immunohistochemistry
Fluorescent double-staining for DA receptors (D1 and D2) was performed on every 5th section from bregma 3.72 – 0.84 (Paxinos and Watson, 2005) using a standard IHC protocol (Malone et al., 2008, Uban et al., 2010) with adaptations suitable for the peptides being measured and using the following antibodies: 1) primaries: mouse monoclonal Anti-Dopamine D1 Receptor (1:450, Novas Biologicals, Littleton, CO, USA and rabbit polyclonal Anti-Dopamine D2 Receptor (1:300, Millipore, Temecula, CA, USA); and 2) secondaries: goat anti-mouse Alexa 594 for D1 (1:400; Invitrogen, Eugene, OR, USA) with goat anti-rabbit Alexa 488 for D2 (1:400; Invitrogen, Eugene, OR, USA) . Briefly, a circle was traced around the tissue on the slide using a Super HT Hydrophonic Pen (Research Products International Corp.). Sections were rinsed, blocked for 2 hr, and incubated in primaries for 22 hr in a Nunc box lined with moistened Benchkote, with TBS at 4°C. Slides were then rinsed, and incubated in secondaries for 1 hr, then rinsed, followed by a brief dip in dH20. Slides were left to dry for 3 hours prior to being cover-slipped. These D1 and D2 antibodies have been previously assessed for specificity (Rajput et al., 2009, Oda et al., 2010). In the present study, controls included a series of slides involving omission of the primary and/or secondary antibodies to assess for specificity of binding using optical density measurements.
9. Quantification of data
Densometric Analysis
Experimenters were blind to experimental conditions. ISH data from the X-ray autoradiographs were scanned and measured (Scion Image 4.0.2 software, NIH, USA). Grey level measurements were taken in the following subregions of the HPC: 1) GR: DG, CA1; 2) MR: DG, CA1–3 (Figure 4C); and CB1: DG, CA1/2, CA3. Images from CRH nuclear-emulsion dipped slides were captured under dark field on a Zeiss Axioskop2 under 5× magnification (except PVN at 10×) using Northern Eclipse Software, and measured with ImageJ. Briefly, a customized circle covering the region of interest was used for measurements (8 sections bilaterally per rat) as follows: 1) mPFC: prelimbic (PL), infralimbic (IL); 2) NAc: core, shell; 3) CeA; 4) BNST: anterior (dorsal plus ventral), posterior (fusiform plus dorsal medial); 5) PVN (Lan et al., 2006).
Optical densities for D1 and D2 receptors were acquired with an Olympus FV1000 confocal microscope (20×) and analyzed with ImageJ. Optimal images were produced by customizing imaging parameters to the brightest immunofluorescence slides for each region to avoid saturation. The middle 1.14 µm of each section was imaged, and background measurements were obtained from the glass slide adjacent to the brain section to control for differential background on slides, as D1 and D2 are widely distributed throughout the brain. Corrected optical density values were averaged across right and left hemispheres and across sections (i.e. 6 measures per subject per subregion).
10. Statistical analyses
All statistical analyses were performed with Statistica 9.0 software (StatSoft, Inc). Developmental data were analyzed using repeated-measures analyses of variance (RM-ANOVA) with prenatal group (C, PF, PAE) as the between-subjects factor; within-subjects factors were day of gestation or lactation for the dams, or postnatal day for the offspring. A separate ANOVA assessed pup body weights on the day of birth (postnatal day (PND) 1). For each probe and subregion, a RM-ANOVA was run, with prenatal group and stress (non-CVS, CVS) as between-subject factors and subregion as the within-subject factor. Data for males and females were analyzed together to evaluate sex differences for data collected prior to perfusion, but separately after perfusion to evaluate effects of estrous stage in females. Stage of estrus was used as a covariate for females and stages were grouped (proestrous/estrous, diestrus I/II) due to the relatively small number of females in any one stage of estrus. All post-hoc analyses utilized Newman-Keuls comparisons. All tests of a priori hypotheses utilized a Bonferroni correction. We hypothesized that 1) stress and prenatal treatment would individually and/or interactively enhance HPA sensitivity and reduce dopaminergic function, as assessed by changes in hormone levels, levels of CRH, MR and GR mRNA, and DA-R densities and; 2) subregions of key brain areas (i.e. mPFC, BNST, HPC, NAc) would be differentially affected by prenatal treatment and stress, as these subregions often interact with brain stress systems in opposing ways.
RESULTS
Developmental Data
Reduced body weight in PAE and PF compared to control dams during gestation but not lactation
Analysis of maternal weight throughout pregnancy revealed a significant prenatal group × gestation day interaction (F6, 72=8.62, p<0.001; Table 1). PAE weighed less than C dams on GD 7 and 21 (p’s<0.02), and PF weighed less than C dams throughout pregnancy (GD 7–21) (p’s<0.03), and less than PAE dams on GD 21 (p<0.03). There were no effects of prenatal group on maternal body weights during lactation (p’s>0.26).
Table 1.
Dam and Offspring Body Weights (g, Mean ± SEM)
| Dam Body Weight |
Prenatal Treatment |
||
|---|---|---|---|
| Control | Pair-fed | Alcohol Exposed |
|
| Pregnant Dams (N) | 11 | 12 | 12 |
| Maternal | 0 | 0 | 0 |
| Death/Illness (N) | |||
| Perinatal Death | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.2 |
| Gestation Length (d) | 22.9 ± 0.3 | 22.7 ± 0.2 | 23.1 ± 0.1 |
| Litter Size | 16.0 ± 0.5 | 15.3 ± 1.1 | 15.6 ± 0.8 |
| Dam Weight (g) | --- | --- | --- |
| GD1 | 252.7 ± 4.6 | 256.0 ± 2.4 | 251.6 ± 3.1 |
| GD21 | 419.9 ± 11.3 | 356.0 ± 5.2a | 375.7 ± 6.6a |
| LD1 | 306.7 ± 6.3 | 297.6 ± 3.4 | 300.2 ± 5.5 |
| LD22 | 323.8 ± 6.1 | 328.1 ± 6.1 | 321.2 ± 5.6 |
| Offspring Body Weight | Control | Pair-fed | PAE |
| Males | |||
| PND1 | 6.7 ± 0.2 | 6.0 ± 0.1b | 6.3 ± 0.2 b |
| PND22 | 57.6 ± 1.0 | 55.4 ± 1.0 | 55.3 ± 1.7 |
| Females | |||
| PND1 | 6.4 ± 0.2 | 5.8 ± 0.2 b | 5.9 ± 0.2 b |
| PND22 | 54.2 ± 0.9 | 54.7 ± 1.2 | 52.3 ± 1.8 |
Dam and offspring body weights
PF<PAE<C.
PAE=PF<C.
Abbreviations: N, sample size; g, gram; d, day; GD, gestation day; LD, lactation day; PND, postnatal day.
Reduced birth weight in PAE and PF compared to control pups, but no differences during the pre-weaning period
At birth, C weighed more than PAE and PF pups (p’s<0.05), and males weighed more than females (p<0.001; main effects of prenatal group (F2,28=4.22, p=0.02) and sex (F1,28=24.10, p<0.001)) (Table 1). During the pre-weaning period there were no significant differences in body weight among prenatal groups for male (F2,84=1.35, p=0.27) or female (F2,84=1.09, p=0.35) pups, and both male (F3,84=4,488.54, p<0.001) and female (F3,84=3,411.47, p<0.001) pups from all prenatal groups gained weight throughout the preweaning period (PND 1–21).
Outcome Measures in Adulthood
Attenuated weight gain in PAE males and weight loss in PAE females during CVS
A prenatal group × sex × stress × day RM-ANOVA on percent change in weight from D1–5 and D1–10 of CVS exposure, revealed two-way interactions of prenatal group × stress (F2,82=7.63, p<0.001), prenatal group × day (F2,82=8.81, p<0.001), stress × day (F1,82=79.87, p<0.001), and sex × day (F1,82=9.65, p<0.01), and main effects of stress (F1,82=301.93, p<0.001), sex (F1,82=49.31, p<0.001), and day (F1,82=107.30, p<0.001; Table 2). Post-hoc analyses revealed lower body weights during CVS in both males and females from all prenatal groups, compared to that in their respective non-CVS counterparts (p’s<0.001). Overall, males exposed to CVS showed attenuated weight gain, while females exhibited weight loss (p’s<0.001) on d 5 and 10 of CVS. Importantly, CVS had a significantly greater effect on PAE than PF and C animals (p’s<0.01), as revealed by a highly significant difference among groups (p’s<0.001) by d 10 of CVS.
Table 2.
Physiological effects of chronic variable stress:
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| Throughout CVS treatment: | ||||||||
| Treatment | Day | Control | Pair-fed | PAE | Control | Pair-fed | PAE | |
|
% change in Body Weight (g) |
Non-CVS |
1–5 5–10 |
3.9±0.4 a 9.8±0.4 a |
4.7±0.5 a 11.4±0.8 a |
6.0±0.8 a 12.3±1.4 a |
1.6±0.5a 7.9±0.7a |
2.8±0.5a 7.6±0.9a |
5.1±1.7a 7.0±0.6a |
| CVS |
1–5 5–10 |
0.4±0.5 2.5±0.8 |
0.7±0.5 2.4±0.8 |
0.0±0.5 0.4±1.6 b |
−1.4±0.6 −0.5±1.3 |
−2.8±0.6 −1.9±1.0 |
−2.4±0.5 −5.2±1.3 b |
|
|
Basal CORT (ng/mL) |
CVS |
1 5 10 |
0.9±0.3 1.3±0.4 1.3±0.6 |
0.8±0.2 0.7±0.0 0.9±0.2 |
0.7±0.1 1.4±0.4 0.8±0.1 |
0.75±0.1 12.4±6.2 2.9±1.6 |
1.8±0.4 8.1±3.4 3.3±1.9 |
2.7±1.6 c 4.3±2.4c 5.7±2.1 c |
| 24 hours post-CVS treatment: | ||||||||
|
Basal CORT (ng/mL) |
Non-CVS | - | 1.1±0.3 | 2.1±1.1 | 1.3±0.4 | 6.1±1.4 | 2.8±0.9 | 3.7±0/9 |
| CVS | - | 2.0±0.5 | 2.2±0.7 | 2.1±0.4 | 3.2±1.0 | 5.0±1.9 | 3.7±1.4 | |
|
Basal T (ng/mL) |
Non-CVS | - | 1.9±0.4 | 2.9±0.6 | 1.2±0.2d | - | - | - |
| CVS | - | 2.4±0.6 | 2.0±0.2 | 1.5±0.2d | - | - | - | |
|
Basal E2 (pg/mL) |
Non-CVS | - | - | - | - | 102±39 | 144±73 | 59±16 |
| CVS | - | - | - | - | 28±13 | 121±42 | 57±21 | |
|
Basal P4 (ng/mL) |
Non-CVS CVS |
- | - | - | - | 85±26 | 51±16 | 84±18 |
| - | - | - | - | 38±12e | 45±13e | 44±6e | ||
Summary of physiological effects of CVS: 1) Throughout CVS on days 1, 5 and 10; and 2) 24 hrs following the last day of CVS (or non-CVS). Mean ±SEM.
Non-CVS > CVS overall, p<0.001;
PAE<C, p<0.001);
C>PAE overall, p<0.05;
C=PF>PAE overall, p’s<0.05;
Non-CVS>CVS overall, p<0.05.
Abbreviations: CVS, chronic variable stress; g, grams; CORT, corticosterone; T, testosterone; E2, estradiol; P4, progesterone.
Hormones
CVS increased basal CORT in control, but not PAE, females, while there were no differences among groups in males
There were no significant differences in basal CORT levels among prenatal groups prior to CVS (F2,42=0.84, p=0.43). Data were then analyzed with a RM-ANOVA as percent change from D1–5 and D1–10.
For males, there were no significant differences among groups in basal CORT levels over the course of CVS exposure (p’s>0.41; Table 2).
For females, a significant effect of prenatal group (F2,13=3.56, p=0.05; Table 2), indicated greater basal CORT levels in C compared to PAE (p=0.04), and a strong trend for greater levels in C compared to PF (p=0.056) females. Although the interaction between prenatal group and day was not significant, inspection of Table 2 suggests different patterns of CORT in control and PAE animals across days. There are no differences among groups on day 1 (i.e. the start of CVS) or on day 10 (i.e. the last day of CVS), but C females show a directional CORT increase on day 5 that did not occur in PAE females. PF females show a similar, but attenuated, pattern of response as C females, whereas PAE females showed a blunted response to CVS, with no change in basal CORT levels over the 10 days. Together, these findings suggest that profiles of basal CORT across the 10 days of CVS differ in PAE compared to C females, and that the main effect of prenatal group is driven primarily by enhanced levels of CORT in C, and to a lesser extent PF, females on day 5 of CVS.
Importantly, basal CORT at the time of perfusion (i.e. 24 hours following the cessation of CVS) did not differ among prenatal groups or between stress conditions for either males (p’s>0.22) or females (p’s>0.24; Table 2).
Reduced basal levels of T in PAE males
There was a significant effect of prenatal group on basal levels of plasma T at the time of perfusion (F2,41=4.36, p=0.02), but no effect of CVS (p=0.94 ;Table 2). Post-hoc analysis revealed lower levels of T in PAE (x̄ = 1.2 ng/mL) compared to PF (x̄ = 2.4 ng/mL) and C (x̄ = 2.1 ng/mL) males (p’s<0.05), while PF and C males did not differ from each other (p=0.46).
CVS reduced basal P4, but not E2 levels in females
As expected, P4 levels varied as a function of estrous stage (F1,38=5.39, p=0.05); therefore estrous stage was utilized as a covariate. There was a significant effect of stress (F1,38=5.39, p=0.025), with reduced levels of P4 overall following CVS (x̄ = 44.1 ng/mL) compared to those in the non-CVS condition (x̄ = 75.2 ng/mL) (Table 2). There was no significant effect of prenatal group (p=0.53) on P4 levels, and no significant differences among prenatal groups or between stress conditions for E2 levels (p’s>0.10).
CRH mRNA signaling
Within the mPFC, CVS decreased CRH mRNA levels in the PL subregion in PAE females, but increased levels in the IL subregion in PF females
In males, there was a significant main effect of subregion (F1,29=12.56, p<0.001), with CRH mRNA levels higher in the IL compared to the PL (Figure 1). There were no other significant main or interaction effects (p’s>0.23).
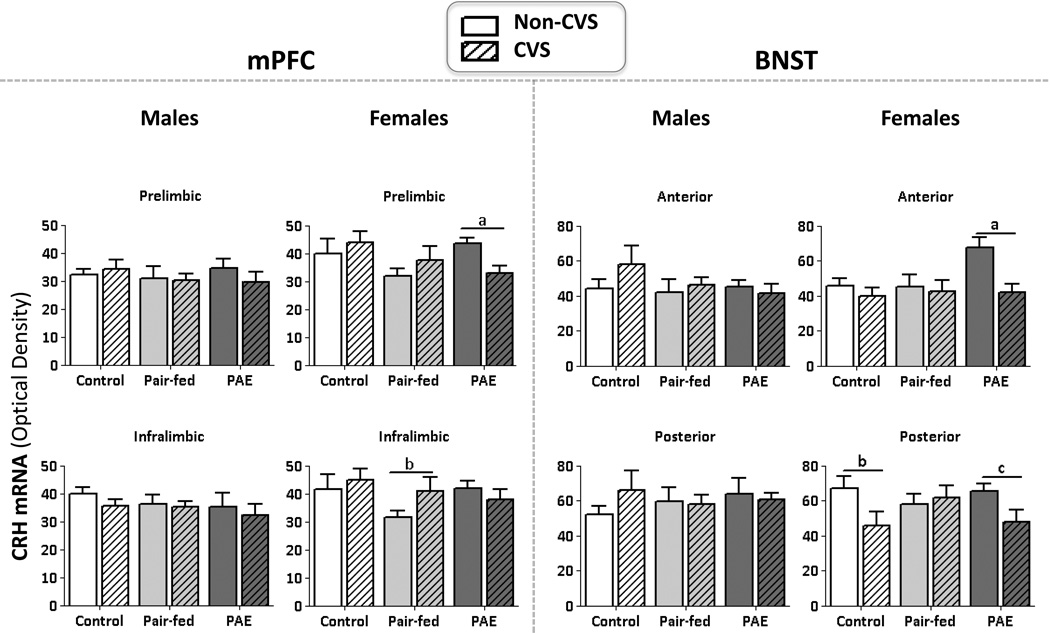
Figure 1. CRH mRNA levels.
Mean ±SEM. Measured 24 hrs following the last day of CVS in the following subregions: 1) the prelimbic and infralimbic (mPFC); and 2) the anterior and posterior (BNST). mPFC: aPAE: non-CVS>CVS; bPF: CVS>non-CVS. BNST: aPAE: non-CVS>CVS; bC: CVS>non-CVS; cPAE: CVS>non-CVS.
In females, the overall ANOVA revealed no significant effects of prenatal group (p’s>0.18), stress or subregion (p’s>0.07). A priori analyses to test our hypothesis that CVS would differentially alter CRH mRNA levels in PAE compared to PF and C rats revealed that CVS decreased CRH mRNA levels in the PL of PAE females, but increased CRH mRNA levels in the IL of PF females, compared to their control counterparts (p’s<0.0008) (Figure 1).
CVS decreased CRH mRNA levels throughout the BNST of PAE females, but only in the pBNST of control females
In males, there was a significant main effect of subregion (F1,30=24.46, p<0.001), with CRH mRNA levels higher overall in the posterior compared to the anterior BNST (Figure 1). There were no other significant main or interaction effects (p’s>0.23).
In females, there was a trend for a prenatal group × subregion interaction (F2,38=2.89, p=0.067; Figure 1) and a significant effect of stress (F1,38=7.10, p=0.01). CVS decreased CRH mRNA levels in the BNST overall. In addition, a priori analyses revealed that CVS reduced CRH mRNA levels in both the anterior and posterior BNST in PAE females, but only in the pBNST of C females (p’s<0.0008).
No significant differences in CRH mRNA levels in the NAc shell, CeA, or PVN
There were no significant differences in CRH mRNA expression in the NAc, CeA and PVN among prenatal groups or following stress in either males (p’s>0.25), or females (p’s>0.16; data not shown).
MR and GR mRNA levels the hippocampus
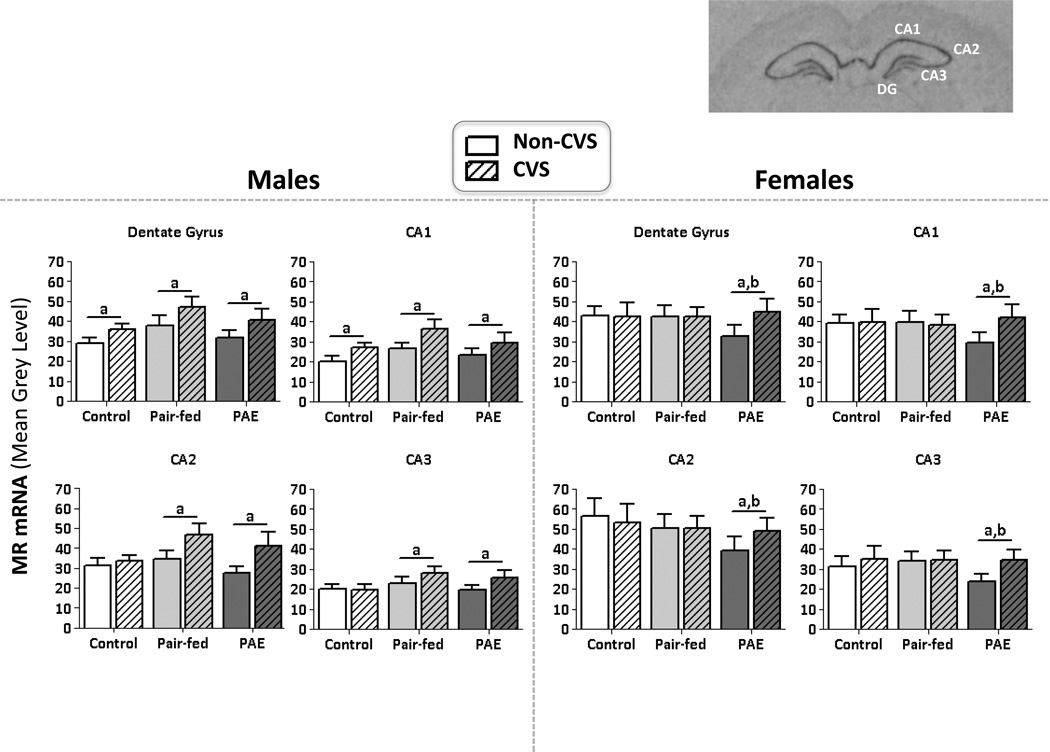
CVS increased MR mRNA levels throughout the HPC in PAE males and females
In males, there was a significant stress × subregion interaction (F3,123=4.86, p=0.003), and a statistical trend for a prenatal group × stress × subregion interaction (F6,123=2.08, p=0.06) (Figure 2), as well as significant main effects of stress (F1,123=5.44, p=0.02) and subregion (F3, 123=138.36, p<0.001). A priori analyses revealed that CVS increased basal MR mRNA levels throughout the HPC in PAE and PF males, but only in the DG and CA1 of C males (p’s<0.0125).
Figure 2. mRNA levels in the HPC.
Mean ±SEM. Measured 24 hrs following last day of CVS. Optical density measures of MR mRNA in males and females. aCVS>non-CVS; bPAE<C=PF. Top right: Image of MR mRNA expression on film with the dentate gyrus (DG), CA1, CA2 and CA3 subregions.
In females, stage of estrus was a significant covariate (F1,114=4.74, p=0.03), such that MR mRNA levels were increased throughout the HPC during proestrus/estrus compared to diestrus. There were no significant main or interaction effects for basal MR mRNA levels (p’s>0.21). However, a priori analyses revealed that under non-CVS conditions, MR mRNA levels were lower in PAE than in PF and C females, and CVS then selectively increased MR mRNA levels in PAE females to the levels seen in PF and C females (p’s<0.0125) (Figure 2).
CVS increased GR mRNA levels in the HPC of males, but not females, overall
In males, there was a statistical trend for an effect of CVS on GR mRNA levels (F1,38=3.72, p=0.06), with higher GR mRNA levels overall following CVS (non-CVS: x̄ = 12.48; CVS: x̄ = 15.81). There were no other significant main or interaction effects (p’s>0.10).
In females there was a statistical trend for stage of estrus cycle as a covariate (F1,35=3.41, p=0.07), with increased GR mRNA during proestrus/estrus, and estrus stage was then utilized as a covariate in this analysis. However, there were no significant main or interaction effects for GR mRNA levels among females (p’s>0.12) (non-CVS: x̄ = 19.91; CVS: x̄ = 23.76; C: x̄ = 22.15; PF: x̄ = 20.30; PAE: x̄ = 23.16).
DA receptor expression
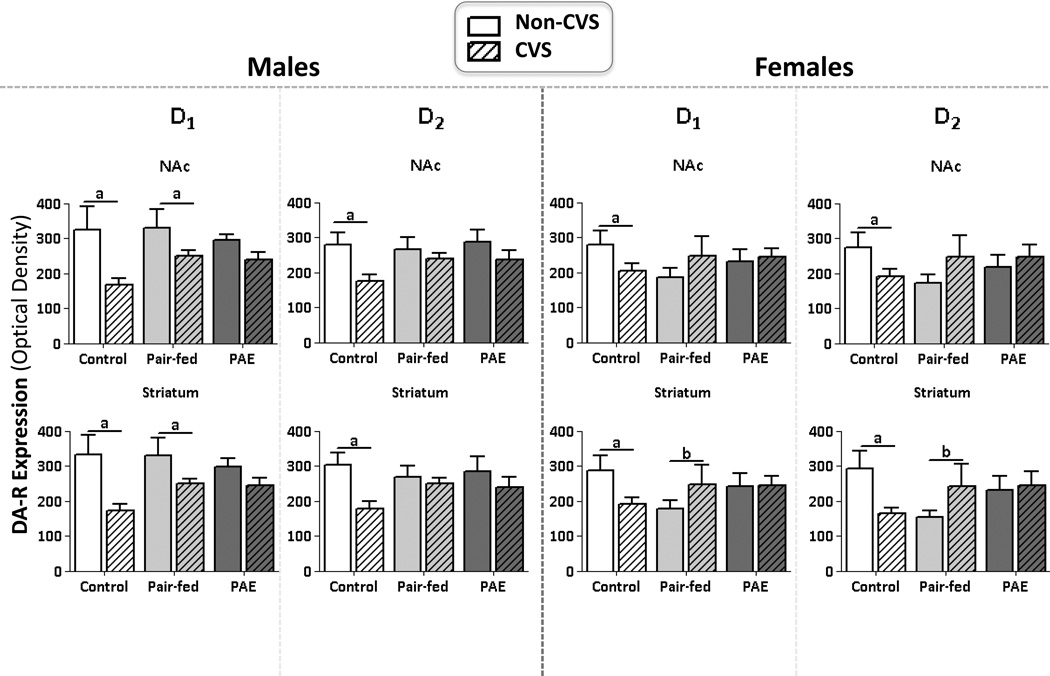
CVS reduced DA-R expression in the NAc in control but not PAE rats
In males, there was a significant DA-R subtype × subregion interaction (F1,41=2.52, p=0.002) and significant main effects of stress (F1,41=10.95, p=0.001), DA-R subtype (F1,41=4.78, p=0.03), and NAc subregion (F1,41=25.83, p=0.001). Post-hoc analyses revealed that despite the interaction, there was greater D1 compared to D2 expression (p’s<0.001), and greater DA-R expression in the core compared to the shell (p’s<0.001). As well, a priori analyses revealed that CVS reduced DA-R expression in the NAc core and shell in C males (p’s<0.0006), reduced D1, but not D2, expression overall in PF males (p’s<0.0006), and had no effect on DA-R expression in PAE males (p>0.26) (Figure 3).
Figure 3. DA-R expression.
Mean ±SEM. Optical densities of D1 & D2 expression was measured 24 hrs following last day of CVS in males and females. The core subregion of the nucleus accumbens core (parallel effects in the shell subregion not shown): anon-CVS>CVS in C and PF, but not PAE males and females. The striatum: anon-CVS>CVS in C but not PAE males and females; bCVS>non-CVS in PF females.
In females, there was a significant effect of subregion (F1,41=14.25, p<0.001), with greater expression of both D1 and D2 overall in the core compared to the shell (Figure 3).There were no other significant main or interaction effects (p’s >0.11). A priori analyses revealed that CVS reduced DA-R expression in both the core and shell in C females (p’s<0.0006), but had no effect in PF and PAE females (p’s>0.50).
CVS reduced DA-R expression in the striatum in control but not in PAE rats
In males, there was a significant effect of stress (F1,41=10.85, p=0.002), where CVS reduced DA-R expression in the striatum overall (Figure 3). Moreover, a priori analyses revealed differential effects of CVS depending on prenatal treatment: similar to what was seen in the NAc, CVS reduced DA-R expression in C males, reduced D1 expression in PF males (p’s<0.016), and had no effect on DA-R expression in PAE males (p’s>0.25). There were no other significant main or interaction effects (p’s>0.10).
In females, there was a statistical trend for an interaction between prenatal group and stress (F2,41=2.74, p=0.07) (Figure 3). Furthermore, a priori analyses revealed that CVS reduced DA-R expression in C females and increased DA-R expression in PF females (p’s<0.016), but had no significant effect in PAE females (p’s>0.43). There were no other significant main or interaction effects (p’s>0.10).
Females had greater expression of D1 compared to D2 receptors throughout the mPFC, while males showed this only in the PL subregion
In males, there was an interaction between DA-R subtype (i.e. D1 or D2) and subregion (F(1,41)=5.32, p=0.02), such that there was similar expression of D1 in the PL and IL (p=0.86), but greater expression of D2 in the PL compared to the IL (p<0.01) (data not shown). There were no other significant main or interaction effects (p’s>0.09).
In females, there was a significant effect of DA-R subtype (F1,41=10.13, p<0.003), with greater expression of D1 than D2 overall (p’s<0.001). There were no other significant main or interaction effects (p’s>0.16) (data not shown).
DISCUSSION
Overall, prior CVS exposure unmasked alterations in basal HPA and DA circuitry in PAE subjects, revealing more widespread changes in basal regulation of central stress circuitry, but a loss of the typical changes in basal DA-R expression in PAE compared to control animals. Specifically, PAE increased sensitivity to stress, and altered interactions between HPA and DA systems in a sexually dimorphic manner, as shown by differential effects of CVS on: 1) body weights and patterns of basal CORT over the course of CVS; 2) basal CRH mRNA levels in the mPFC and BNST; 3) basal MR mRNA levels throughout the hippocampus; and 4) basal DA-R expression. Overall, these results extend our understanding of the sexually dimorphic effects of PAE on basal HPA regulation, and show, for the first time, that regulation of basal HPA and DA systems, and likely, HPA-DA interactions, are altered differentially in males and females by PAE and CVS.
Body weight is influenced by PAE and CVS
As expected, both PAE and PF pups exhibited reduced birth weight but, catch up growth was observed throughout preweaning. These results are consistent with past studies (Hellemans et al., 2010, Uban et al., 2010), showing that our model of PAE produces mild-moderate effects on fetal growth, and is a model of alcohol related neurodevelopmental disorder (ARND) rather than FAS. The finding that CVS in adulthood attenuated weight gain in PAE males and resulted in weight loss in PAE females supports the hypothesis that PAE produces enhanced sensitivity to stress and that PAE females may be more vulnerable to the effects of later life stress than PAE males.
Endocrine regulation is altered by PAE
Prenatal treatment altered the pattern of basal CORT activity during CVS in females. Over the course of CVS, basal CORT levels were higher overall in control compared to PAE females. As there were no significant differences among groups in basal CORT levels on day 1 or day 10 of CVS, this was likely driven by the elevation of basal CORT levels observed in controls midway through CVS treatment. This profile of basal CORT was attenuated in PF females and absent in PAE females, suggesting that PAE may blunt the normal basal CORT response to CVS.
While basal CORT levels were unaltered over the course of CVS in males, basal testosterone levels measured at termination, 24 hr after the last stress exposure, were reduced in PAE compared to control and PF males. As testosterone typically plays an inhibitory role in HPA activity (Viau, 2002), low testosterone levels in PAE males, could diminish the capacity to regulate HPA responsiveness to stressors. Indeed, our previous studies demonstrated that PAE reduces sensitivity of the HPA axis to the regulatory effects of testosterone (Lan et al., 2009). Interestingly, in females, there were no effects of PAE on basal levels of estradiol or progesterone. However, basal progesterone levels were reduced in females exposed to CVS, regardless of prenatal treatment. As progesterone functions as a glucocorticoid antagonist (reviewed in (Kudielka and Kirschbaum, 2005), decreased basal progesterone levels could result in reduced antagonism of stress-induced HPA activity, providing one possible mechanism through which CVS may increase overall vulnerability to subsequent stressors in females.
PAE alters basal HPA signaling in females, but not males, in a region specific manner
There were no differences in baseline CRH mRNA expression between PAE and control males and females. However, CVS revealed selective alterations in basal CRH mRNA levels in the mPFC and BNST of PAE and PF, compared to control, females suggesting enhanced sensitivity to CVS. Within the mPFC, the PL subregion inhibits, while the IL subregion potentiates, activation of brain stress systems (Sullivan and Gratton, 2002). Similarly, subregions within the BNST have opposing effects: the aBNST increases, while the pBNST decreases, CRH mRNA expression in the PVN of the hypothalamus (Herman et al., 1994). Here, we found a decrease in CRH mRNA levels following CVS in the PL of PAE females, but an increase following CVS in the IL subregion of PF females. These data suggest that CVS increases HPA drive within the mPFC in both PAE and PF females, but via different mechanisms, such that inhibitory drive is decreased in PAE females, and excitatory drive is enhanced in PF females. Within the BNST, on the other hand, CRH mRNA levels were reduced in both anterior and posterior subregions in PAE females, but only in the pBNST of control females. Thus, CVS may decrease inhibitory regulation in control females, which may account for the increased basal CORT levels on day 5 of CVS. In contrast, in PAE females, the balance between inhibitory and excitatory drive may have been altered, reflecting broader effects of CVS in PAE than controls, and possibly contributing to the overall blunting of the basal CORT response to CVS.
Interestingly, the effects of CVS and PAE in the mPFC and BNST were sexually-dimorphic, as there were no effects of CVS on CRH mRNA among males. Thus, it appears that prenatal treatments, such as PAE or pair-feeding, have a greater impact on sensitivity to stress and basal CRH activity or regulation in females than in males. Consistent with this, prenatal stress causes HPA hyperactivity selectively in adult females, suggesting sex differences in the sensitivity of the developing brain to stress hormones (Weinstock et al., 1992). By analogy, we propose that prenatal exposure to alcohol, which has both direct and indirect (via maternal HPA activation) effects on the developing fetal HPA axis, may selectively alter CRH regulation in adult female offspring. Importantly, whereas prenatal stress alters subsequent HPA responsiveness to stressors (Weinstock et al., 1992), our study demonstrates alterations in basal HPA regulation in PAE females following prior exposure to stress.
In contrast to the sexually dimorphic effects of CVS and PAE in the mPFC and BNST, we found that CVS produced more widespread increases in basal MR mRNA levels throughout the hippocampus of PAE compared to control males and females, but upregulated basal GR mRNA levels in males, but not females, across all prenatal groups. GRs play a role in suppression of stress-induced HPA activity, whereas MRs play a permissive or tonic role in regulating basal HPA activity (De Kloet et al., 1998, Sapolsky et al., 2000). Shifts in the balance between MR and GR alter stress system activity and regulation, and decrease the ability to maintain homeostasis. The finding of more widespread MR mRNA upregulation under basal conditions in PAE offspring following CVS suggests an increase in basal HPA tone. Furthermore the overall upregulation in GR mRNA levels in males, but not females, suggests that males are more sensitive than females to the effects of CVS on the hippocampus.
A primary goal of the present study was to determine whether prior stress exposure will alter basal HPA regulation, and to compare central and peripheral changes that might occur. Thus animals were terminated under basal conditions, 24 hr after the last exposure to CVS. We identified specific neural measures and brain regions that were more affected by CVS in PAE compared to control males and females, despite similar basal CORT levels among prenatal groups. These data support and extend previous work from our laboratory (Glavas et al., 2007) demonstrating that manipulation of the HPA axis through removal of the CORT feedback signal by adrenalectomy unmasked central HPA dysregulation in PAE animals under basal conditions, with CORT clamped at similar levels among groups. It is noteworthy that measures of central regulation of stress systems are not always correlated with peripheral hormone levels, and a disconnect between central and peripheral measures may in part underlie vulnerability to mental health problems. As de Kloet and colleagues (de Kloet et al., 2005) note, the extrahypothalamic regions of the CRH system are more remote from the hypothalamus, and therefore alterations in basal MR/GR or CRH mRNA expression may contribute to vulnerability to mental health problems in the absence of basal hypothalamic alterations. Alterations within the extrahypothalamic regions may be an antecedent for alterations in cognition, behavior and affect associated with mental health problems.
Stress reduced basal DA-R expression in control but not PAE animals
Exposure to CVS reduced basal DA-R expression in the striatum and NAc in both male and female controls, but not in PAE subjects. While acute stress enhances DA neuron activity, chronic stress may attenuate this effect in controls (Valenti et al., 2012), possibly by initially increasing and then decreasing, tonic DA activity in the NAc (Cabib and Puglisi-Allegra, 2012). In the present study, it is possible that the typical stress-enhanced DA release became increasingly blunted over time by CVS, resulting in reduced tonic DA activity in controls. As chronic stress decreases tonic DA levels in the long-term, DA-Rs may ultimately downregulate to match the reduced tonic DA levels. The downregulation of DA-Rs following CVS observed in controls under basal conditions may result, in part, from an overall downregulation in activity of DA systems. In contrast, it appears that CVS was ineffective at enhancing DA neuron activity and release in PAE subjects. PAE rats show increased HPA responsivity to alcohol and morphine challenges compared to control animals (Taylor et al., 1986), and enhanced stress-induced alcohol consumption (Nelson et al., 1983). Interestingly, attenuated DA release within the NAc and striatum is observed in PAE male and female rats following exposure to a low dose of ethanol (0.5g/kg) in adulthood (Blanchard et al., 1993), and DA neuron activity is decreased overall within the ventral tegmental area in adult PAE rats (Shen et al., 1995, Shen et al., 1999). The present data support and extend these previous findings, indicating that PAE attenuates the effects of CVS on DA-R expression; however, it remains to be known whether this attenuation is due to under- or over- sensitivity of DA-Rs following CVS. Conversely, differing results pertaining to DA function may be due to differing PAE paradigms. Clearly, the effects of PAE on stress and dopamine systems are complex. PAE may result in reduced meta-plasticity of DA systems, where dopaminergic responses are not as finely calibrated to a range of stimuli, including an array of pharmacological agents, and in the present study, stress. As a result, PAE subjects may show exaggerated or blunted behavioral and pharmacological effects in DA systems compared to control subjects.
Additionally, our results suggest that DA regulation is more malleable by CVS in the NAc and striatum compared to the mPFC. The NAc and striatum are regions of importance for DA function in relation to reinforcement and motivational neurocircuitries. The mPFC directly influences tonic DA in the NAc as it evaluates a stressor (Cabib and Puglisi-Allegra, 2012). Therefore alterations in DA-R expression may be revealed in the NAc prior to alterations occurring in the mPFC. Our results are the first to demonstrate that CVS dowregulates basal DA-R expression in both males and females and that PAE attenuates the effects of CVS on DA systems in brain regions highly relevant to the etiology of SUD.
Of note, the present data demonstrate that sexually dimorphic effects of CVS on basal measures implicated in central stress systems, but not in measures of DA-R expression. This finding of more widespread effects of CVS on stress than on DA systems is consistent with human literature, which suggests that the etiology of SUD is more connected with stress in women than in men. For example, initial motivation to use is sexually dimorphic, with women using more to alleviate stress, and men using more in connection with enhanced risk-taking behavior. Thus, women tend to cycle through the stages of SUD more rapidly than men (Becker et al., 2012). Findings from basic animal models similarly demonstrate sex differences in response to drugs. Specifically, female rats typically show enhanced: 1) DA release to stress or substances with abuse potential (Becker et al., 2012); 2) medium spiny neuron density in the NAc (Wissman et al., 2012); and 3) and sensitization to stimulants (Zhao and Becker, 2010). Together, these findings support enhanced neurobiological vulnerability to SUD in females compared to males, with females entering the cycle of addiction at a more advanced stage because of the connection between their substance use and stress reduction. Our novel finding that PAE females exhibited more widespread alterations in basal stress circuitry compared to control females is consistent with these results, suggesting a possible mechanism that may underlie enhanced vulnerability specifically in PAE females.
Pair-feeding is an experimental treatment in itself
A number of effects of pair-feeding were observed in the present study. It is important to note that although pair-feeding controls for the reduced food intake of PAE dams, it is actually a treatment in itself. Although alcohol-consuming dams eat ad libitum, they typically reduce their intake below what would occur if given the same diet without alcohol. Because the amount of diet presented to PF dams is yoked to that of PAE dams, PF dams are effectively on a “meal feeding” schedule. They are given less food than they would eat ad libitum, and consume their entire ration within a few hours, remaining deprived until the next feeding. It is likely that PF dams experience some level of stress as a result of the hunger that accompanies reduced food intake, and in turn, their offspring may experience some level of prenatal stress. Thus, it is not entirely surprising that some effects of pair-feeding on central HPA and DA systems are observed in the present study and are different from effects observed in PAE subjects.
Conclusions
The present data demonstrate long-lasting alterations in both HPA and DA systems, as well as in HPA-DA interactions, in PAE males and females. Specifically, PAE animals show widespread changes in basal regulation of central stress circuitry, but a loss of the typical changes in basal DA-R expression compared to controls. It is possible that these changes in central regulation of basal HPA activity may underlie the increased stress responsiveness that is consistently observed in PAE animals and in children with FASD. Further, effects of PAE on the cross-talk between HPA and DA systems provide insight into possible mechanisms underlying mental health problems that are related to stress and DA signaling, including SUD, which have a high incidence among individuals with FASD (O’Connor and Paley, 2009). Moreover, optimal dopaminergic function is required for executive function, cognition, and emotional regulation, (Goto et al., 2007), all of which are altered by PAE (Mattson et al., 2011, Schneider et al., 2011). An understanding of how PAE alters the neurobiological mechanisms implicated in mental health problems is vitally important for the development of targeted prevention, intervention and treatment for this population.
Table 3.
Summary of neurbiological effects of chronic variable stress:
| Males | Females | ||||||
|---|---|---|---|---|---|---|---|
| Control | Pair-fed | PAE | Control | Pair-fed | PAE | ||
| mPFC | PL | -- | -- | -- | -- | -- | ↑ CRH mRNA a |
| IL | -- | -- | -- | -- | ↓ CRH mRNA a |
-- | |
| BNST | aBNST | -- | -- | -- | -- | -- | ↓ CRH mRNA a |
| pBNST | -- | -- | -- | ↓ CRH mRNA a |
-- | ↓ CRH mRNA a |
|
| HPC | DG | ↑MR mRNA b |
↑MR mRNA b |
↑MR mRNA b |
-- | -- | ↑MR mRNA b |
| CA1 | ↑MR mRNA b |
↑MR mRNA b |
↑MR mRNA b |
-- | -- | ↑MR mRNA b |
|
| CA2 | -- | ↑MR mRNA b |
↑MR mRNA b |
-- | -- | ↑MR mRNA b |
|
| CA3 | -- | ↑MR mRNA b |
↑MR mRNA b |
-- | -- | ↑MR mRNA b |
|
| NAc | core | ↓D1 & D2a | ↓D1a | -- | ↓D1 & D2a | -- | -- |
| shell | ↓D1 & D2a | ↓D1a | -- | ↓D1 & D2a | -- | -- | |
| Striatum | ↓D1 & D2a | ↓ D1b | -- | ↓D1 & D2b | ↓D1 & D2b | -- | |
Summary of changes in basal expression of HPA and DA systems 24 hrs following the last day of CVS (or non-CVS) in the: 1) prelimbic (PL) and infralimbic (IL) subregions of the mPFC; 2) anterior (aBNST) and posterior (pBNST) subregions of the BNST; 3) in the dentate gyrus (DG), CA1, CA2 and CA3 subregions of the HPC; 4) the core and shell of the NAc; and 5) in the striatum. Abbreviations: corticotropin releasing hormone (CRH); mineralcorticoid receptor (MR); glucocorticoid receptor (GR); dopamine receptor type I (D1) and type II (D2).
p<0.001;
p<0.0125.
ACKNOWLEDGEMENTS
We would like to thank Dr. Douglas Allan and Luba Veverytsa at the Facility for Synaptic Imaging at the University of British Columbia for assistance with and use of the confocal microscope for imaging of dopamine receptors. We would like to thank Stephanie Lieblich for her expert assistance with tissue processing, Wayne Yu for his expert assistance with RIAs, as well as Caitlin Bauermeister, Welan Dionela, and Jimmy Yan for their valuable contributions to this study. This research was funded by grants from the Canadian Foundation for Fetal Alcohol Research (CFFAR) to JW and LAMG, and NIH/NIAAA R37 AA007789 to JW. KAU was funded by IMPART (CIHR STIHR program).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
No financial support from any individual or corporate body has been received for compensation of work over the past three years; therefore the authors declare no potential conflict of interest.
REFERENCES
- Barbier E, Houchia H, Warnaulta V, Pierrefichea O, Daousta M, Naassila M. Effects of prenatal and postnatal maternal ethanol on offspring response to alcohol and psychostimulants in long evans rats. Neuroscience. 2009;161:427–440. doi: 10.1016/j.neuroscience.2009.03.076. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Frontiers in neuroendocrinology. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Perry AN, Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biology of sex differences. 2012;3:14. doi: 10.1186/2042-6410-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard BA, Steindorf S, Wang S, LeFevre R, Mankes RF, Glick SD. Prenatal ethanol exposure alters ethanol-induced dopamine release in nucleus accumbens and striatum in male and female rats. Alcoholism, clinical and experimental research. 1993;17:974–981. doi: 10.1111/j.1530-0277.1993.tb05651.x. [DOI] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S. The mesoaccumbens dopamine in coping with stress. Neuroscience and biobehavioral reviews. 2012;36:79–89. doi: 10.1016/j.neubiorev.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Chotro MG, Arias C, Laviola G. Increased ethanol intake after prenatal ethanol exposure: studies with animals. Neuroscience and biobehavioral reviews. 2007;31:181–191. doi: 10.1016/j.neubiorev.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Cooper J, Rudeen P. Alterations in regional catecholamine content and turnover in the male rat brain in response to in utero ethanol exposure. Alcoholism, clinical and experimental research. 1988;12:282–285. doi: 10.1111/j.1530-0277.1988.tb00195.x. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nature reviews Neuroscience. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocrine reviews. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Druse MJ, Tajuddin N, Kuo A, Connerty M. Effects of in utero ethanol exposure on the developing dopaminergic system in rats. Journal of neuroscience research. 1990;27:233–240. doi: 10.1002/jnr.490270214. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo PV, Weinberg J. Corticosterone rhythmicity in the rat: interactive effects of dietary restriction and schedule of feeding. J Nutrition. 1981;111:208–218. doi: 10.1093/jn/111.2.208. [DOI] [PubMed] [Google Scholar]
- Glavas MM, Ellis L, Yu WK, Weinberg J. Effects of prenatal ethanol exposure on basal limbic-hypothalamic-pituitary-adrenal regulation: role of corticosterone. Alcoholism, clinical and experimental research. 2007;31:1598–1610. doi: 10.1111/j.1530-0277.2007.00460.x. [DOI] [PubMed] [Google Scholar]
- Goto Y, Otani S, Grace AA. The Yin and Yang of dopamine release: a new perspective. Neuropharmacology. 2007;53:583–587. doi: 10.1016/j.neuropharm.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley DW, Handmaker NS, Lowe J. Infant stress reactivity and prenatal alcohol exposure. Alcoholism, clinical and experimental research. 2006;30:2055–2064. doi: 10.1111/j.1530-0277.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Verma P, Yoon E, Yu WK, Young AH, Weinberg J. Prenatal alcohol exposure and chronic mild stress differentially alter depressive- and anxiety-like behaviors in male and female offspring. Alcoholism, clinical and experimental research. 2010;34:633–645. doi: 10.1111/j.1530-0277.2009.01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Watson SJ. Involvement of the bed nucleus of the stria terminalis in tonic regulation of paraventricular hypothalamic CRH and AVP mRNA expression. Journal of neuroendocrinology. 1994;6:433–442. doi: 10.1111/j.1365-2826.1994.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Bihun JT, Chiodo LM. Effects of prenatal alcohol and cocaine exposure on infant cortisol levels. Development and psychopathology. 1999;11:195–208. doi: 10.1017/s0954579499002011. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. The American journal of psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biological psychology. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Lan N, Hellemans KG, Ellis L, Viau V, Weinberg J. Role of testosterone in mediating prenatal ethanol effects on hypothalamic-pituitary-adrenal activity in male rats. Psychoneuroendocrinology. 2009;34:1314–1328. doi: 10.1016/j.psyneuen.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan N, Yamashita F, Halpert AG, Ellis L, Yu WK, Viau V, Weinberg J. Prenatal ethanol exposure alters the effects of gonadectomy on hypothalamic-pituitary-adrenal activity in male rats. Journal of neuroendocrinology. 2006;18:672–684. doi: 10.1111/j.1365-2826.2006.01462.x. [DOI] [PubMed] [Google Scholar]
- Le Moal M. Drug abuse: vulnerability and transition to addiction. Pharmacopsychiatry. 2009;42(Suppl 1):S42–S55. doi: 10.1055/s-0029-1216355. [DOI] [PubMed] [Google Scholar]
- Lovallo WR. Cortisol secretion patterns in addiction and addiction risk. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2006;59:195–202. doi: 10.1016/j.ijpsycho.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone DT, Kearn CS, Chongue L, Mackie K, Taylor DA. Effect of social isolation on CB1 and D2 receptor and fatty acid amide hydrolase expression in rats. Neuroscience. 2008;152:265–272. doi: 10.1016/j.neuroscience.2007.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychology review. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European journal of pharmacology. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LR, Lewis JW, Liebeskind JC, Branch BJ, Taylor AN. Stress induced changes in ethanol consumption in adult rats exposed to ethanol in utero. Proceedings of Western Pharmacological Society. 1983;26:205–209. [PubMed] [Google Scholar]
- O’Connor MJ, Paley B. Psychiatric Conditions Associated with Prenatal Alcohol Exposure. Developmental Disabilities Research Reviews. 2009;15:225–234. doi: 10.1002/ddrr.74. [DOI] [PubMed] [Google Scholar]
- Oda S, Funato H, Adachi-Akahane S, Ito M, Okada A, Igarashi H, Yokofujita J, Kuroda M. Dopamine D5 receptor immunoreactivity is differentially distributed in GABAergic interneurons and pyramidal cells in the rat medial prefrontal cortex. Brain research. 2010;1329:89–102. doi: 10.1016/j.brainres.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Park JH, Yoo KY, Lee CH, Kim IH, Shin BN, Choi JH, Hwang IK, Won MH. Comparison of glucocorticoid receptor and ionized calcium-binding adapter molecule 1 immunoreactivity in the adult and aged gerbil hippocampus following repeated restraint stress. Neurochemical research. 2011;36:1037–1045. doi: 10.1007/s11064-011-0444-z. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; 2005. [DOI] [PubMed] [Google Scholar]
- Rajput PS, Kharmate G, Somvanshi RK, Kumar U. Colocalization of dopamine receptor subtypes with dopamine and cAMP-regulated phosphoprotein (DARPP-32) in rat brain. Neuroscience research. 2009;65:53–63. doi: 10.1016/j.neures.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Ramsay DS, Bendersky MI, Lewis M. Effect of prenatal alcohol and cigarette exposure on two- and six-month-old infants' adrenocortical reactivity to stress. Journal of pediatric psychology. 1996;21:833–840. doi: 10.1093/jpepsy/21.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbun W, Druse M. Dopamine, serotonin, and acid metabolites in brain regions from the developing offspring of ethanol-treated rats. Journal of neurochemistry. 1985;44:57–62. doi: 10.1111/j.1471-4159.1985.tb07112.x. [DOI] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Li TK, Jacobson SW, Coles CD, Kodituwakku PW, Adnams CM, Korkman MI. Neurobehavioral consequences of prenatal alcohol exposure: an international perspective. Alcoholism, clinical and experimental research. 2003;27:362–373. doi: 10.1097/01.ALC.0000052703.38558.B2. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacological reviews. 2001;53:209–243. [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Adkins MM. The effects of prenatal alcohol exposure on behavior: rodent and primate studies. Neuropsychology review. 2011;21:186–203. doi: 10.1007/s11065-011-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen RY, Hannigan JH, Chiodo LA. The effects of chronic amphetamine treatment on prenatal ethanol-induced changes in dopamine receptor function: electrophysiological findings. The Journal of pharmacology and experimental therapeutics. 1995;274:1054–1060. [PubMed] [Google Scholar]
- Shen RY, Hannigan JH, Kapatos G. Prenatal ethanol reduces the activity of adult midbrain dopamine neurons. Alcoholism, clinical and experimental research. 1999;23:1801–1807. [PubMed] [Google Scholar]
- Shetty A, Burrows R, Phillips D. Alterations in neuronal development in the substantia nigra pars compacta following in utero ethanol exposure: immunohistochemical and Golgi studies. Neuroscience. 1993;52:311–322. doi: 10.1016/0306-4522(93)90159-d. [DOI] [PubMed] [Google Scholar]
- Sinha R. Stress and addiction: a dynamic interplay of genes, environment, and drug intake. Biological psychiatry. 2009;66:100–101. doi: 10.1016/j.biopsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. New findings on biological factors predicting addiction relapse vulnerability. Current psychiatry reports. 2011;13:398–405. doi: 10.1007/s11920-011-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O'Malley K, Young JK. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. Journal of developmental and behavioral pediatrics : JDBP. 2004;25:228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002;27:99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Branch BJ, Nelson LR, Lane LA, Poland RE. Prenatal ethanol and ontogeny of pituitary-adrenal responses to ethanol and morphine. Alcohol. 1986;3:255–259. doi: 10.1016/0741-8329(86)90034-0. [DOI] [PubMed] [Google Scholar]
- Thanh NX, Jonsson E. Drinking alcohol during pregnancy: evidence from Canadian Community Health Survey 2007/2008. Journal of population therapeutics and clinical pharmacology = Journal de la therapeutique des populations et de la pharamcologie clinique. 2010;17:e302–e307. [PubMed] [Google Scholar]
- Uban KA, Rummel J, Floresco SB, Galea LAM. Estradiol modulates effort-based decision making in female rats. Neuropsychopharmacology. 2012;37:390–401. doi: 10.1038/npp.2011.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uban KA, Sliwowska JH, Lieblich S, Ellis LA, Yu WK, Weinberg J, Galea LAM. Prenatal alcohol exposure reduces the proportion of newly produced neurons and glia in the dentate gyrus of the hippocampus in female rats. Horm Behav. 2010;58:835–843. doi: 10.1016/j.yhbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti O, Gill KM, Grace AA. Different stressors produce excitation or inhibition of mesolimbic dopamine neuron activity: response alteration by stress pre-exposure. The European journal of neuroscience. 2012;35:1312–1321. doi: 10.1111/j.1460-9568.2012.08038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. Journal of neuroendocrinology. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20:470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M, Matlina E, Maor GI, Rosen H, McEwen BS. Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary adrenal system in the female rat. Brain research. 1992;595:195–200. doi: 10.1016/0006-8993(92)91049-k. [DOI] [PubMed] [Google Scholar]
- Williamson M, Bingham B, Gray M, Innala L, Viau V. The medial preoptic nucleus integrates the central influences of testosterone on the paraventricular nucleus of the hypothalamus and its extended circuitries. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:11762–11770. doi: 10.1523/JNEUROSCI.2852-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissman AM, May RM, Woolley CS. Ultrastructural analysis of sex differences in nucleus accumbens synaptic connectivity. Brain structure & function. 2012;217:181–190. doi: 10.1007/s00429-011-0353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RL, Lightner EN, Harman JS, Meijer OC, Conrad CD. Attenuating corticosterone levels on the day of memory assessment prevents chronic stress-induced impairments in spatial memory. The European journal of neuroscience. 2006;24:595–605. doi: 10.1111/j.1460-9568.2006.04948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA. Sex differences and the HPA axis: implications for psychiatric disease. The journal of gender-specific medicine : JGSM : the official journal of the Partnership for Women's Health at Columbia. 1998;1:21–27. [PubMed] [Google Scholar]
- Zhao W, Becker JB. Sensitization enhances acquisition of cocaine self-administration in female rats: estradiol further enhances cocaine intake after acquisition. Hormones and behavior. 2010;58:8–12. doi: 10.1016/j.yhbeh.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Li L, Tang S, Cao X, Li Z, Li W, Li C, Zhang X. Effects of serotonin depletion on the hippocampal GR/MR and BDNF expression during the stress adaptation. Behavioural brain research. 2008;195:129–138. doi: 10.1016/j.bbr.2008.06.009. [DOI] [PubMed] [Google Scholar]