Abstract
Purpose
Although the impact of stigma has been highlighted for epileptic populations, the experiences of people living with other neurological conditions have been less studied. In order to promote research on stigma among people with neurological conditions, we sought to develop and psychometrically validate an 8-item questionnaire measuring internalised and enacted stigma experienced by people with epilepsy, multiple sclerosis (MS), Parkinson’s disease (PD), stroke, and amyotrophic lateral sclerosis (ALS).
Methods
We used Item Response Theory (IRT) methodologies to select items and field-tested our items with 587 participants from 8 academic medical centres across the United States.
Results
We conducted Exploratory and Confirmatory Factor Analysis as well as examined scale the reliability and validity. In addition, we conducted an analysis of variance (ANOVA) test to examine mean total score differences across the 5 neurological conditions. Data from people across conditions revealed that the shortened instrument conformed to an essentially unidimensional model of multi-faceted stigma as a 1-factor questionnaire with correlated residuals on a pair of items that distinctly measured internalised stigma.
Conclusions
Preliminary evidence suggests that the Stigma Scale for Chronic Illness 8-item version (SSCI-8) fits a unidimensional model, which assesses enacted and internalised stigma, and has adequate internal consistency/reliability and validity in relation to psychological distress and patient performance. Our results suggest fairly low stigma for neurological populations. In addition, our results suggest that stigma may be more severe for patients with ALS relative to those with MS and PD. Our results suggest that the scale could be used practically in the clinic setting to examine stigma without the patient burden associated with lengthier scales.
Keywords: neurological disorder, epilepsy, quality of life, stigma, measurement
Neurological disorders often produce permanent disabilities that can cause impairment in almost any aspect of physical, emotional or cognitive functioning [1–2]. Patients diagnosed with neurological disorders similarly experience physical [3–4], emotional [1,4] and cognitive impairments [5–6]. Unique burdens can also be found across the spectrum of neurological disorders. For instance, differences in illness manifestation appear to contribute to differences in quality of life across populations; greater anxiety and lower perceptions of control has been documented for epileptic populations relative to healthy populations and other groups living with chronic illnesses [7]. Certain characteristics of neurological disorders (e.g., seizures, tremors) may be visible to others, resulting in stigmatising social experiences in addition to emotional, cognitive and physical impairments [8].
The stigma associated with neurological conditions and illness manifestation may contribute to poorer quality of life outcomes. Stigma theorists have put forward a set of terms and dimensions that are helpful in describing stigma’s impact [9]. These include multiple dimensions, such as enacted and internalised stigma, which are detailed below.
Enacted stigma and its consequences in neurological populations
‘Enacted stigma’ refers to the negative attitudes expressed by members of the public (e.g. healthcare professionals, clergy, or employers) that are experienced by an individual with devalued characteristics. Literature on stigma experienced by neurological populations has revolved around epileptic populations, who can be susceptible to adverse cultural beliefs concerning disease origin, perceptions of epilepsy as a mental illness, and worries associated with the unpredictable nature of seizures [e.g., 7, 10–15]. Studies in other neurological populations have also indicated significant societal misconceptions [16], but these may vary as much as the visibility of illnesses and result in differences in the frequency of stigmatising situations across neurological disorders. Harden and colleagues [10] found greater discomfort levels concerning working with epileptic co-workers in comparison with workers diagnosed with multiple sclerosis (MS) and depression, because of the unpredictability of seizures. Other neurological conditions, such as stroke, have been reported to be relatively non-stigmatising [17–18].
The importance of enacted stigma has been documented in terms of consequences across a number of neurological diseases. Greater frequency of enacted stigma has been implicated in poorer quality of life and psychological functioning for epileptic patients [13, 19–20]. Patients living with amyotrophic lateral sclerosis (ALS), whose illness results in increasing demands placed upon their caregivers and others [1], have also reported experiences of marginalisation and their impact on psychosocial adjustment [21].
Internalised stigma and its consequences in neurological populations
If public/enacted stigmas are experienced or perceived, they can be ‘internalised’ by the stigmatised individual. Internalised stigma occurs when people with devalued conditions come to believe that negative public attitudes apply to them [22–23] and suffer numerous negative consequences as a result [24]. Internalised stigma is the endorsement of public stereotypes (e.g. “I am dangerous”), prejudice (e.g. “I am afraid of myself”), and resulting self-discrimination (e.g. self-imposed isolation). Once a person internalises negative stereotypes, they may have negative reactions. Self-discrimination, particularly in the form of self-isolation, has many pernicious effects leading to decreased healthcare service use, poor health outcomes, and poor quality of life [25–27]. Low self-esteem and poor self-efficacy are primary examples of these negative reactions [28], and have been associated with not taking advantage of opportunities that promote employment and independent living [29].
Considering the risks of rejection and enacted stigma, individuals with relatively less visible illnesses may not disclose their condition, even in medical settings [8]; Moore and Knowles [16] reported that over 50% of a healthy sample would restrict to whom they disclosed they had Parkinson’s disease (PD). Concealment can be problematic [30] and has been suggested to be more disruptive for certain conditions, such as epilepsy, than enacted stigma [31]. Despite the relative importance often placed on internalised stigma for other patient populations [23–24], there has been a significant gap in the literature for neurological populations. A combined measure assessing internalised and enacted stigma may be particularly helpful for quality of life research and psychosocial interventions.
Stigma measurement
Despite the growing body of evidence suggesting the importance of stigma measurement in clinical populations, there have been two major limitations concerning instrument development and use. First, extant quantitative stigma measures administered to neurological populations have predominantly measured in one aspect: enacted stigma [32–33]. Simultaneous assessment of internalised and enacted stigma is critical, given the growing body of theory and evidence indicating internalised stigma negatively impacts health-related outcomes [23–27]. One potential reason for relatively limited comprehensive instrument development and use is patient and clinical administrative burden; accordingly several studies have used short stigma scales (3–4 items; 13, 32, 34]. Development of short versions of comprehensive stigma scales may enhance instrument utilisation and significantly increase attention given to stigma for neurological populations.
Second, despite high levels of enacted stigma experienced by people across neurological and other disorders, few measures have been developed to measure enacted, or internalised, stigma across conditions. One unexplored reason why such measures have not been developed is the notion that multiple components of stigma might be experienced differently or more severe across conditions. Given this, psychometric assessment of multi-faceted stigma instruments is warranted, given that, if psychometrically sound, brief instruments can measure stigmas across conditions, allowing researchers and clinicians to compare and contrast severity of enacted and internalised stigmas across conditions and appropriately refer clients for emerging therapies that are becoming available to help people cope with stigma [35].
Recently, a 24-item measure of stigma assessing internalised and enacted components has been initially validated with people living with various neurological conditions and also adapted for people with HIV [36–38]. Preliminary validation of the Stigma Scale for Chronic Illnesses (SSCI) indicated relatively comparable structure and stability of multi-faceted stigma across patients with epilepsy and stroke [37]. Further comparison, however, is warranted concerning assessment of stigma across a broader range of neurological disorders. As well, the relatively high internal consistency of the original SSCI suggests item redundancy (Cronbach’s alpha = .96, [37]) suggesting a shorter version of this instrument may comprehensively measure different aspects of stigma with minimal time and effort for patients and clinicians.
Current Study
The current study is a part of the Patient Reported Outcomes Measurement Information System (PROMIS) and Quality of Outcomes in Neurological Disorders (Neuro-QOL) initiatives. To address the current need for a brief measure assessing multiple dimensions of stigma, we sought in the present study to a) present initial psychometric data on an 8-item short form of the SSCI developed to assess enacted and internalised stigma across neurological conditions and to reduce patient and clinician administrative burden of stigma measurement as well as b) assess the severity of stigma across neurological conditions. We took multiple steps to develop an 8-item stigma scale as part of the Neuro-QOL study in line with the PROMIS, which prioritises patient information to guide survey measure development [39–41]). We first utilised item response theory (IRT) parameters collected from previous research [37, 39–40] on the original 24-item stigma scale to select items which provided the most information (Study 1). Second, the shorter SSCI was administered to a new clinical sample of individuals diagnosed with various neurological disorders (stroke, MS, ALS, PD, epilepsy) within clinic settings (N = 577; Study 2), thus giving us the unique opportunity to investigate how the shorter instrument performs, and to compare the stability and construct of stigma across conditions. We hypothesized this short form would perform similarly to the 24-item instrument as a bi-factor solution which would measure different aspects of stigma (internalised, enacted), but function as a unidimensional model of multifaceted stigma.
Study 1: Item Selection of SSCI-8
During Study 1, the SSCI-8 was developed from Item Response Theory (IRT) psychometric models, which are used to examine characteristics of test items and respondents’ severity levels on a latent construct [42]. We used the 2 parameter IRT model (item difficulty and discrimination) to guide selection from the 24-item original scale, which yields information concerning an item’s ability to differentiate between people across different levels of stigma being measured [43–45]. In terms of a stigma scale, an item’s difficulty (threshold) indicates the severity needed to have a 50% chance of endorsing an item (a given response category or higher). Item quality is closely related to the discrimination (slope) parameter, which may be interpreted as an index of an individual item’s association with the overall construct of stigma and are related to items’ loadings in factor analyses.
The original 24 item SSCI conformed to the bifactor model, measuring two highly correlated facets of stigma (EFA, r = .81): enacted (e.g. experiences of stigma) and internalised, with a strong general factor. We hoped to generate a scale which could be used to assess multi-faceted stigma as well as the two separate assets of stigma (enacted, internalised) and selected items that provided high discrimination for enacted and internalised stigma respectively.
Method
Detailed information concerning the study population and procedures for this study have been reported elsewhere [37]. We selected the ten items with the high discrimination/slope values and a range of threshold parameter values to optimally measure stigma frequency at various severity levels [46]. Items were additionally excluded on the basis of similar wording and content. For example, if two items appeared redundant, one item was not included in the short form of the scale.
The two facets of stigma were considered content ‘bins’ as opposed to substantively distinct constructs, and this method of ‘binning’ and selecting items has been used to develop static short forms tested as part of the PROMIS and Neuro-QOL initiatives [39–41].
Study 1 Results and Discussion
For the SSCI, we examined 10 items that had the largest information expected over different stigma levels on both facets of stigma. We eliminated two items, which loaded on internalised stigma on the original 24-item SSCI, because of shared wording with other short form items which originally loaded on enacted stigma (“Because of my illness, I felt embarrassed in social situations”; “Because of my illness, I felt emotionally distant from other people”). The remaining 8 items are listed with the facet of stigma they measured, according to the standardized factor loadings from an exploratory factor analysis on the original 24-item SSCI instrument [37] and their IRT parameter estimates in Table 1: 2 items were expected to measure internalised stigma 5 were expected to measure enacted stigma, and 1 item, which exhibited split-loading, was noted to potentially measure either or both internalised and enacted stigma.
Table 1.
Stigma Scale for Chronic Illness 8 items, factor loadings and item response parameters from original SSCI measure development [37].
| Item | Bins | Original Study Factor Loadings |
Discrimination | Average Threshold |
|
|---|---|---|---|---|---|
| Enacted (SE) |
Internalised (SE) |
||||
| Because of my illness, some people seemed uncomfortable with me. | Enacted | 0.86 (0.05) | 0.06 (0.06) | 3.44 | 1.17 |
| Because of my illness, some people avoided me. | Enacted | 0.95 (0.01) | −0.01 (0.01) | 4.06 | 1.25 |
| Because of my illness, I felt left out of things | Enacted/Internalised | 0.52 (0.05) | 0.43 (0.05) | 4.00 | 0.71 |
| Because of my illness, people were unkind to me | Enacted | 1.10 (0.05) | −0.21 (0.07) | 3.31 | 1.77 |
| Because of my illness, people avoided looking at me | Enacted | 0.73 (0.06) | 0.19 (0.07) | 3.92 | 1.60 |
| I felt embarrassed about my illness | Internalised | −0.01 (0.03) | 0.94 (0.03) | 3.46 | 0.91 |
| I felt embarrassed because of my physical limitations | Internalised | −0.01 (0.02) | 0.93 (0.02) | 3.39 | 0.72 |
| Some people acted as though it was my fault I have this illness | Enacted | 0.53 (0.06) | 0.33 (0.07) | 2.88 | 1.30 |
Generally-worded items appeared to allow for better measurement of experiences and attitudes than specifically-worded items across a range of different neurological conditions. Selected items described more generalised ostracising social situations (“some people avoided looking at me”) and generalised illness-specific limitations (“I felt embarrassed because of my physical limitations”) relative to excluded items that were more relevant for specific social contexts (“strangers tended to stare at me”) and characteristics specific to certain illnesses (e.g., “I felt embarrassed about my speech”). For example, a person with epilepsy may have frequent seizures that render him/her unable to drive and a person with advanced ALS may have frequent muscle cramps and twitching that render him/her uncomfortable in public social settings. Both types of individuals may feel embarrassed by their different physical limitations. This type of embarrassment can be captured for these and other neurological disorders with the more generally worded item: “I felt embarrassed because of my physical limitations.”
Study 2: Field Testing SSCI-8
The purpose of the second study was to collect data on the psychometric properties of our new instrument, examine its factor structure, and study the severity of stigma across conditions. We administered a survey which contained the SSCI-8 items, as well as measures of psychological distress and patient performance. Using the structure of the original 24 item SSCI instrument [37] and Study 1 (see Table 1), our short form measure had five items measuring enacted stigma and two items measuring internalised stigma in the SSCI-8. We expected a bifactor model for our short form SSCI-8, similar to the original instrument, wherein two three items measuring internalised stigma would have local dependence, but we hypothesised that the overall scale structure could be analysed as essentially unidimensional. Given extant literature, we anticipated that individuals experiencing more stigma would be more psychologically distressed and have poorer performance capabilities. Finally, we hypothesised that people with some neurological conditions, those that were more visible, would experience more severe stigmas than people with other neurological conditions.
Method
Participants
Participants were among the 581 respondents from 8 academic medical centres who comprised the second wave of a study on the quality of life for people with neurological disorders (NeuroQOL). Interviews were conducted between January and September of 2009.
Recruitment
Site coordinators identified, enrolled, and conducted assessments with eligible participants who met the following criteria: 18 years or older, English-speaking, and diagnosed with selected neurological conditions (stroke, MS, ALS, PD, epilepsy). Participants were excluded if they exhibited cognitive impairment such that informed consent and/or completion of test items with the assistance of an interviewer were not possible.
Eligible patients were approached in a clinic or mailed an invitation to physician-identified patients informing them that someone would contact them about the study at their next clinic appointment. If identified and approached, the site coordinator arranged a meeting to introduce and describe the study, confirm eligibility, and explain participants’ rights. If the eligible participant was interested, informed consent and HIPAA authorisation (Health Insurance Portability and Accountability Act) was obtained. Participants were reimbursed according to local Institutional Review Board (IRB)-approved standards. Surveys were then immediately administered by a research assistant or were scheduled for a later date. Measured included in the survey are described below.
Measures
Stigma Scale for Chronic Illnesses-Short Form (SSCI-8)
The 8-item short form, developed through methods described, was administered with the following response format to assess frequency: 1 = Never, 2 = Rarely, 3 = Sometimes, 4 = Often, and 5 = Always. The raw summed score range for the SSCI-8 accordingly was 8–40. We used raw summed scores in the present analyses, but the SSCI has been calibrated such that scores has been and could be converted into IRT scaled scores in future work [47]. We provide a conversion table to convert raw scores to IRT scores in Table 2 to facilitate use in future research. IRT-based T-score distributions are a result of rescaling raw scores into standardized scores with a mean of 50 and a standard deviation of 10. For example, given a raw score of 15 (T-score = 53.7, SE = 2.0), we can say with 95% confidence that the true score is within twice the standard error of the T-score (53.7 ± 4 = 49.7 to 57.7). Values in Table 2 are only valid when all questions on the SSCI-8 have been completed.
Table 2.
Conversion table for SSCI-8 raw and IRT scores [47]
| Raw Score | T-Score | SE |
|---|---|---|
| 8 | 39.2 | 5.8 |
| 9 | 45.7 | 3.3 |
| 10 | 47.6 | 3.0 |
| 11 | 49.3 | 2.6 |
| 12 | 50.6 | 2.4 |
| 13 | 51.7 | 2.2 |
| 14 | 52.8 | 2.1 |
| 15 | 53.7 | 2.0 |
| 16 | 54.6 | 2.0 |
| 17 | 55.4 | 2.0 |
| 18 | 56.2 | 1.9 |
| 19 | 57.0 | 1.9 |
| 20 | 57.8 | 1.9 |
| 21 | 58.5 | 1.9 |
| 22 | 59.3 | 1.9 |
| 23 | 60.1 | 1.9 |
| 24 | 60.8 | 1.9 |
| 25 | 61.6 | 1.9 |
| 26 | 62.4 | 1.9 |
| 27 | 63.2 | 1.9 |
| 28 | 64.0 | 1.9 |
| 29 | 64.8 | 1.9 |
| 30 | 65.7 | 2.0 |
| 31 | 66.6 | 2.0 |
| 32 | 67.5 | 2.0 |
| 33 | 68.5 | 2.1 |
| 34 | 69.6 | 2.1 |
| 35 | 70.8 | 2.2 |
| 36 | 72.2 | 2.3 |
| 37 | 73.7 | 2.4 |
| 38 | 75.6 | 2.6 |
| 39 | 78.1 | 3.0 |
| 40 | 81.5 | 3.5 |
Psychological distress
We used a one-item self-rating of psychological distress (“Please indicate the statement that best describes your current level of anxiety/depression”) with the following response categories: 1 = Not anxious/depressed, 2 = Moderately anxious/ depressed, and 3 = Extremely anxious/depressed. Given the few number of individuals (n = 10) who reported extreme anxiety/depression, categories were collapsed to examine individuals who were anxious/depressed or were not.
Performance status
We used a one-item self-rating of patient performance status (“Please indicate which statements best describe your own health state today”) with the following response categories: 1 = I have no problems with performing my usual activities, 2 = I have some problems with performing my usual activities, and 3 = I am unable to perform my usual activities. Given the few number of individuals (n = 11) who reported extreme inability to perform usual activities, categories were collapsed to examine individuals who were able to perform their usual activities or were not.
Analysis Plan
We included participants who answered at least one stigma-related survey item. To assess the psychometric properties of our instrument, we randomly split the sample to conduct an exploratory factor analysis (EFA) with data from 288 subjects and a confirmatory factor analysis (CFA) with data from the remaining 289 subjects using Mplus 6.11 [48]. The split-half method has been used to assess constructs whose structure are relatively unknown or unique [49] and for validation of brief forms of survey measures [50]. Both EFA and CFA used polychoric correlation coefficients, which are considered robust with ordinal item responses. Weighted least squares with adjustment for means and variances estimation for categorical variables (WLSMV) with CFA and oblique Geomin rotation was used for EFA.
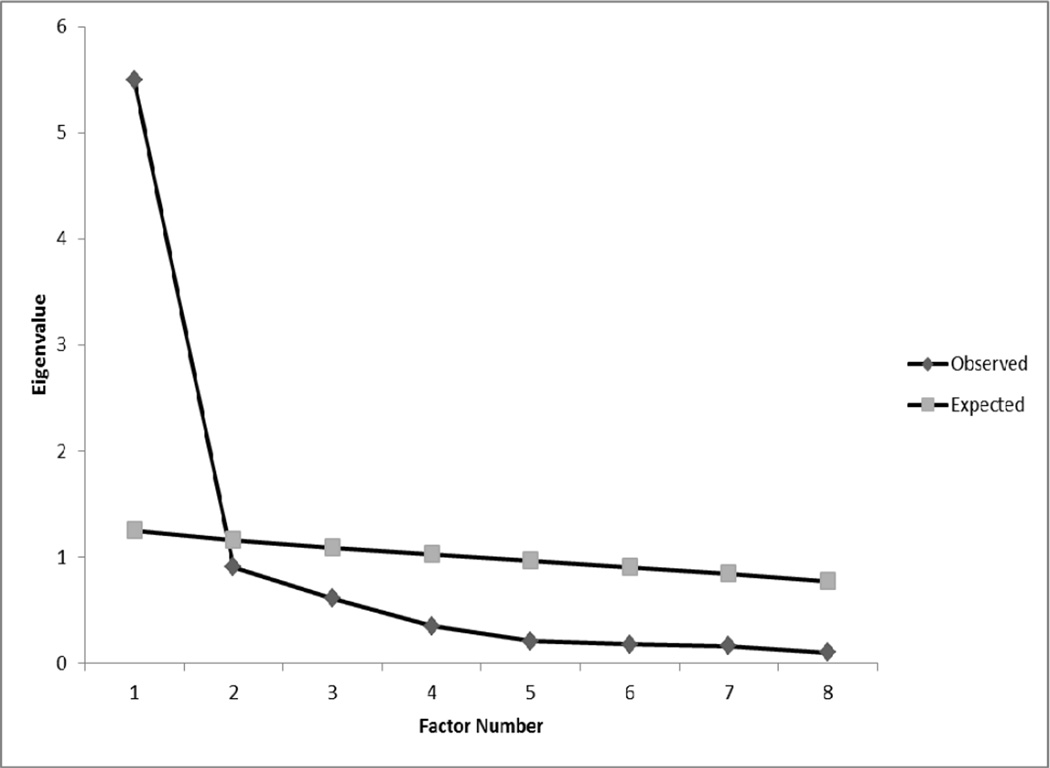
For the EFA sample, we assessed the number of factors through scree plots, initial eigenvalues from EFA, and parallel analysis. Parallel analysis is a recommended technique for identifying the optimal number of factors to extract [51]. It involves generating random normal numbers for expected data sets with the characteristics of the actual or observed dataset to be analysed (e.g., number of subjects and items). EFAs are conducted for each random data set and averages and standard deviations of eigenvalues are calculated across all replications. The number of factors to extract is identified according to the number of observed/actual eigenvalues which exceed the expected eigenvalues conducted from random data sets. For this study, we conducted 1,000 replications. For individual items, we reviewed factor loadings and flagged items with loadings of 0.60 or greater.
For the CFA sample, goodness of model fit was examined using the comparative-fit index (CFI), the Tucker-Lewis Index (TLI) and the root-mean-square error of approximation (RMSEA). For CFA, the criteria for good (or acceptable) model fit were: CFI and TLI values greater than 0.95 (0.90) and RMSEA values less than 0.05 (0.08). We used this sample to assess reliability and validity. For reliability, we examined Cronbach’s alpha and item-total correlations of the bank; the criteria for these were respectively .60 and .40. To test the validity of the SSCI-8, we generated a structural equation model with WLSMV estimation to assess relationships between stigma, psychological distress, and patient performance, using the same criteria for model fit as those described for the CFA. To further understand the scale’s usefulness, we examined severity of stigma across neurological conditions, we conducted an analysis of variance (ANOVA) with condition analysed as the independent variable and the sum of the 8 stigma scale items, the total score, analysed as the dependent variable.
Results
Our data set had an extremely low amount of missing data, wherein four individuals did not answer any stigma items. The percentage of missing data for individual stigma items ranged between 0–.3% per item (n = 1–2). Given the majority of participants completed all items, we kept respondents who answered at least one of the items in the short-form stigma scale (n = 577). Relative to the respondents included in analyses, the four excluded individuals were comparable in gender, (χ2 = 0.03, df = 1, p = .87), race/ethnicity (χ2 = 0.60, df = 4, p = .97), age (F(1, 574) = 2.19, p = .14), education (F(1, 567) = 0.11, p = .74), and income (F(1, 512) = 0.04, p = .84). Three respondents who did not answer any of the items, but did report their neurological condition had ALS; we found no significant differences in year diagnosed, F(1, 420) = 1.01, p = .32.
Socio-demographic and clinical characteristics
The sample comprised 577 participants, 264 men and 313 women. The participants’ ages ranged from 18 to 93 years old (M = 55.14, SD = 14.32). Approximately 50% of the participants had completed college-level courses. On average, participants’ family household annual income ranged between $20,000 and $49,999. Seventy-seven percent of our sample identified as non-Hispanic White, 10% as Hispanic, 10% as African American, 2% as American Indian/Alaska Native and 1% as Asian. Participants were diagnosed with at least one of the following neurological conditions: MS (n = 161, 28.2%), PD (n = 120, 21%), epilepsy (n = 116, 20.3%), stroke (n = 100, 17.5%), and ALS (n = 74, 13%). Over half of our sample had been diagnosed between 2003 and 2009 and the median of year when diagnosed was 2003 or 6 years after 2009, when data were collected.
Factor structure
Table 3 provides information concerning 1- and 2-factor solutions from EFA for the SSCI-8 on 288 subjects. Initial eigenvalues (5.2, 0.9, 0.6, etc.) combined with parallel analysis and scree plots (Figure 1) suggested that a 1-factor solution best fit the data. When examining a 2-factor solution, factor loadings were in line with the items’ proposed classification of enacted and internalised stigma respectively, The item that had exhibited split-loading in the previous study, “Because of my illness, I felt left out of things” (Tables 1, 3), now loaded on enacted stigma. Given these findings, we conducted single factor CFAs with and without correlated residuals for the two items measuring internalised stigma. A single factor CFA would indicate all eight related to the same aspect of stigma, whereas a single CFA with correlated residuals would indicate a unidimensional model with two items sharing variance related to another aspect of stigma (internalised).
Table 3.
Pattern matrix factor loadings from exploratory factor analysis, n = 287
| 1-Factor Solution | 2-Factor Solution | M | SD | ||
|---|---|---|---|---|---|
| Items | Stigma Loadings (SE) |
Internalised Loadings (SE) |
Enacted Loadings (SE) |
||
| Lately…because of my illness, some people seemed uncomfortable with me. | 0.89 (0.02) | 0.15 (0.22) | 0.79 (0.16) | 1.80 | 0.95 |
| Lately…because of my illness, some people avoided me. | 0.90 (0.02) | −0.04 (0.20) | 0.95 (0.13) | 1.69 | 0.95 |
| Lately…because of my illness, I felt left out of things. | 0.80 (0.03) | 0.11 (0.21) | 0.74 (0.15) | 2.03 | 1.09 |
| Lately…because of my illness, people were unkind to me. | 0.54 (0.04) | −0.02 (0.11) | 0.58 (0.09) | 1.30 | 0.63 |
| Lately…because of my illness, people avoided looking at me. | 0.88 (0.02) | 0.07 (0.24) | 0.84 (0.17) | 1.51 | 0.84 |
| Lately…I felt embarrassed about my illness. | 0.82 (0.03) | 0.99 (0.10) | −.001 (0.01) | 1.82 | 1.06 |
| Lately…I felt embarrassed because of my physical limitations. | 0.86 (0.02) | 0.63 (0.24) | 0.31 (0.21) | 2.04 | 1.17 |
| Lately…some people acted as though it was my fault I have this illness. | 0.60 (0.06) | −0.03 (0.12) | 0.65 (0.10) | 1.30 | 0.71 |
Figure 1.
Observed and expected eigenvalues for Exploratory Factor Analysis
Notes. Expected eigenvalues were derived from 1,000 replications assuming 8 variables and 287 participants.
Single factor CFAs with and without correlated residuals on 2 internalised stigma items were conducted on the other 289 subjects. The single factor solution without correlated residuals on two items (χ2 = 237.03 df = 20, p < 0.0001) appeared to fit the data much worse than the single factor solution with correlated residuals (χ2 = 51.34, df = 19, p = 0.0001). In both cases, χ2 is significant, but this statistic is highly sensitive to sample size. For single factor CFA, without residual correlation, RMSEA was 0.19, TLI was 0.91 and CFI was 0.94. A single factor solution with correlated residuals on two items fit adequately based on our criteria (CFI: 0.99; TLI: 0.99, RMSEA: 0.08). The two items with correlated residuals appeared to both measure internalised stigma on the original scale, whereas the other 6 items measured enacted stigma.
Internal Consistency of Items
We examined internal consistency of items on data from the sample of participants we used to conduct CFA (n = 289). The 8-item version of the SSCI had a Cronbach’s alpha of 0.89 and item-total correlations equal to .45 or higher (n = 287). Enacted and internalised stigma portions of the scale exhibited adequate Cronbach’s alphas (0.85, 0.87, respectively). Item-total correlations were equal to .49 or higher.
Convergent Validity
To assess convergent validity, we computed point-biserial correlations between stigma, psychological distress, and performance on data from the sample of participants we used to conduct CFA (n = 289). As predicted, individuals with greater stigma summed scores were more likely to exhibit psychological distress (r = .31, df = 286, p < .0001) and more likely to report problems conducting their usual activities (r = .23, df = 287, p < .0001).
Socio-demographic characteristics
To determine if stigma varied by socio-demographic and clinical variables, we conducted ANOVAs and Pearson’s correlations. Men and women did not vary in stigma, ANOVA, F(1, 287) = 0.42, p = .52. Given the small samples of racial/ethnic minorities, we collapsed groups to assess differences between racial/ethnic minority and non-Hispanic White participants. Racial/ethnic minority participants (M = 15.12, SD = 7.23) reported greater amounts of stigma than non-Hispanic White counterparts, M = 13.13, SD = 5.25, F91, 287) = 5.62, p = .02. Stigma was not correlated with year when diagnosed (r = −.04, df = 275, p = .57) nor education (r = −.10, df = 285, p = .08), but was negatively correlated with age (r = −.12, df = 286, p = .04) and household income (r = −.20, df = 259, p < .001). Younger individuals and individuals coming from lower income households appeared to report greater amounts of stigma.
Multi-faceted Stigma across Neurological Conditions
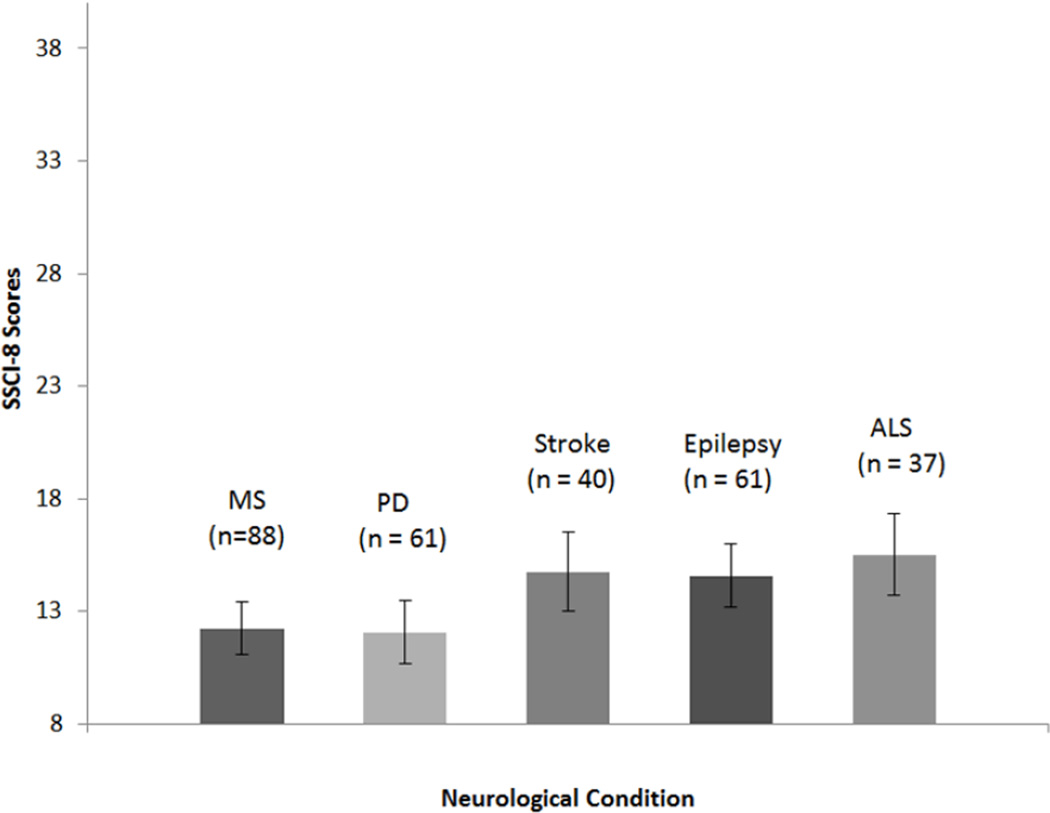
Given limited sample sizes for and lack of representation for all response categories for the 8 items across conditions, we were unable to examine the structure and stability of the SSCI-8 across neurological conditions. We examined severity of stigma across neurological conditions, conducting ANOVA. Significant differences were found in total scores on the 8 item stigma scale across conditions (F (4, 286) = 4.41, p < 0.01). People with ALS reported the most stigma (M = 15.51, SD = 5.47), followed by people with stroke (M = 14.75, SD = 7.16), and epilepsy (M = 14.56, SD= 7.03), with people with MS (M = 12.23, SD = 4.34), and PD (M = 12.07, SD = 4.28), reporting the least amount of stigma. Post-hoc comparisons revealed that significant differences were driven by patients with ALS, who experienced more stigma than patients with MS and PD (both p = .02). Figure 2 depicts the estimated marginal mean SSCI-8 scores for the 5 conditions studied.
Figure 2.
Estimated marginal mean total raw scores and 95% CI intervals on the Stigma Scale for Chronic Illness 8 item version (SSCI-8) for 5 neurological conditions
General Discussion
Given the increasing importance placed on stigma among clinical populations, we sought to a) develop a shorter questionnaire measure which assessed multiple aspects of stigma with minimal burden to patients and clinicians and b) to compare severity of multi-faceted stigma across neurological conditions. Our study provides significant contributions in terms of a comprehensive shorter stigma instrument and information concerning the universality of stigma across ALS, epileptic, MS, PD, and stroke populations.
SSCI-8 development and psychometric properties
We took multiple steps to complete our first aim and develop the SSCI-8. During Study 1, we identified the most informative items which had been related to internalised (3 items) and enacted stigma (5 items) through the use of IRT parameter estimates. Similar to the original SSCI [37], our shorter version exhibited essential unidimensionality while measuring two components of stigma (enacted and internalised). In addition, our scale demonstrated adequate consistency and items generally loaded onto factors as theorised, altogether suggesting retention of the major strengths and components of the 24-item SSCI. Importantly, one item exhibited different loadings across studies (“Because of my illness, I felt left out of things.”). Whereas the item exhibited split-loading with the original study sample [Study 1, 37], it loaded on enacted stigma in Study 2. Future research should address this item, which may potentially assess experienced social distancing (enacted stigma) and/or internalised attitudes/expectations concerning social distancing (internalised stigma).
Our findings are in line with literature linking both enacted [52–53] and internalised stigma to psychological distress and healthcare outcomes [e.g., internalised stigma, 23]. A stronger relationship between multi-faceted stigma and severity of impairments/patient performance are in accordance with previous findings highlighting increased enacted stigma experiences for individuals with greater impairment/visibility concerning their neurological condition [13, 15]. Regardless, our study revealed relatively low amounts of stigma for neurological populations. Given our findings, future research should address the amount of stigma among these groups to address the need for stigma reduction interventions in these populations. Preliminary analyses revealed socio-demographic differences in stigma experiences, wherein race/ethnicity, age, and income were associated with stigma scores, which warrants further study. Future research may especially want to utilise multi-ethnic samples, given the small proportion of racial/ethnic minorities in this study’s sample. Further research, especially in terms of internalised stigma, is needed to confirm patterns between multiple aspects of stigma and impairment to aid treatment development in terms of targeting reduction of public/enacted versus internalised stigma.
Stigma across neurological conditions
Enacted stigma experienced by patients with epilepsy [10, 13, 19–20, 29] and PD [16, 34] have been more thoroughly studied than other neurological populations, such as patients with ALS [21]. Neurological populations may differ in terms of exposure to stigmatising events. For example, one person can experience varying levels of functioning and visibility of stroke over periods of time based on rehabilitation, and similarly, people with MS can have relapses and remittances, which can affect the visibility of the condition over time. Rao and colleagues [37] found a few differences in multi-faceted stigma measurement across epileptic and stroke populations, but, given the relatively small magnitude as well as directions of these differences, concluded relative measurement equivalence across these patients.
In the current study, we limited ourselves to examining mean differences across conditions because of small sample sizes within conditions. Our results suggested that the severity of multi-faceted stigma is different across neurological disorders: patients with ALS appear to experience significantly greater amounts of stigma than patients with MS and PD. Our instrument may allow for more thorough comparisons across these conditions and allow for greater understanding concerning the impact of stigma on health outcomes for populations with a variety of neurological disorders. For example, future studies could investigate whether there are differences in the factor structure for people with stroke and epilepsy.
Limitations and strengths
Our study had several major limitations concerning modeling multi-faceted stigma across neurological conditions, including a) small sample sizes for certain conditions (e.g., ALS) and b) incomplete representation of all response categories for all SSCI-8 items for each neurological disorder. In addition to these limitations, we worked with a non-random sample and model fit was not ideal. Future studies with larger sample sizes and a random sample may provide more evidence to support our results here. As well, we appeared to have only two items which measured internalised stigma; future studies wishing to assess internalised stigma more comprehensively should consider inclusion of additional relevant SSCI questionnaire items. Similar to other studies of discrimination and mental health, the findings of this current study may be hampered by the shared nature of measures assessing stigma and psychological distress. Future studies should additionally test the causal nature of this relationship, as those who are more psychologically distressed may also be more likely to attribute others’ intents and behaviours as stigmatising. Future studies should additionally incorporate other constructs, as this current study only incorporated the relationship of stigma to two, psychological distress and performance. Finally, as discussed above, our sample exhibited relatively low amounts of stigma. This finding may be due to the measure and/or the low existing levels of stigma in these populations. The resulting floor effect has implications for the suitability of the SSCI-8 as a measure to address the success of stigma reduction interventions. Interventions wishing to utilise this instrument may need to administer the SSCI-8 along with other stigma-related instruments to assess concurrent validity and assess intervention-related changes in stigma. Future research may rely on the strengths of the SSCI, as a generic instrument, to assess differences in stigma of neurological populations relative to other clinic populations (e.g., people living with HIV/AIDS, schizophrenia).
Despite these limitations, our study had several strengths. First, we used novel psychometric methods (i.e., IRT) to develop the scale. In addition, our participants came from diverse clinical sites across the United States, and the shorter SSCI-8 demonstrated similar strong psychometric properties as the original 24 item version. The shortened measure demonstrated its ease of use to measure the dual facets of enacted and internalised stigma among people with various neurological conditions in a manner that is brief and psychometrically sound. In addition, our results suggested that severities of stigma, and perhaps the experiences of stigma as well, are different for people with epilepsy, PD, MS, stroke, and ALS. These differences are perhaps related to the visibility and changes in symptomatology associated with certain conditions over time. Future studies can lend more evidence to these findings.
Implications for use by clinicians and researchers
Consequently, researchers and clinicians will be able to use the SSCI-8 in the future either as one scale of stigma or utilize specific items to measure internalised or enacted stigma. For instance, clinicians may be able to utilize this scale to assess the role of multi-faceted or specific aspects of stigma in relation to psychosocial outcomes of their patients and recommend therapies designed to reduce the impact of enacted stigma. Researchers may be able to utilize this instrument to determine which interventions are most needed for neurological populations and which aspect of stigma to target. If enacted/public stigma appears to be the most related to psychosocial distress, for example, researchers may develop stigma reduction interventions for general populations. If internalised stigma appears to be particularly relevant, researchers may focus their efforts on designing interventions to reduce stress and promote coping strategies for clinical populations.
Acknowledgements
The authors would like to thank two anonymous reviewers for their critical review of this study and Betsy J. Feldman, Ph.D. for her technical assistance with the statistical analysis for this project. This study was support by a contract from the National Institute for Neurological Disorders and Stroke (NINDS) number HHSN265200423601C Cella (PI) and K23 MH 084551 PI: Rao.
References
- 1.Jenkinson C, Fitzpatrick R, Swash M, Peto V. ALS-HPS Steering Group. The ALS Health Profile Study: Quality of life of amyotrophic lateral sclerosis patients and carers in Europe. J. Neurol. 2000;415:835–840. doi: 10.1007/s004150070069. [DOI] [PubMed] [Google Scholar]
- 2.Perez L, Huang J, Jansky L, Nowindki C, Victorson D, Peterman A, Cella D. Using focus groups to inform the Neuro-QOL measurement tool: Exploring patient-centered health-related quality of life concepts across neurological conditions. J Neurosci Nurs. 2007;39:342–353. doi: 10.1097/01376517-200712000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Dallmeijer AJ, de Groot V, Roorda LD, Schepers VPM, Lindeman E, van den Berg LH, Beelen A, Dekker J. Fupro Study Group. Cross-diagnostic validity of the SF-36 physical functioning scale in patients with stroke, multiple sclerosis and amyotrophic lateral sclerosis: A study using Rasch analysis. J Rehab Med. 2007;39:163–169. doi: 10.2340/16501977-0024. [DOI] [PubMed] [Google Scholar]
- 4.Pugh MJV, Copeland LA, Zeber JE, Cramer JA, Cavazos JE, Kazis LE. The impact of epilepsy on health status among younger and older adults. Epilepsia. 2005;46:1820–1827. doi: 10.1111/j.1528-1167.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 5.Heilman KM, Valenstein E, editors. Clinical neuropsychology. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 6.Rasquin SMC, Lodder J, Ponds RWHM, Winkens I, Jolles J, Verhey FRJ. Cognitive functioning after stroke: A one-year follow up study. Dementia Ger Cog Disor. 2004;18:138–144. doi: 10.1159/000079193. [DOI] [PubMed] [Google Scholar]
- 7.Antonak RF, Livneh H. A review of research on psychosocial adjustment to impairment among persons with epilepsy. J Epilepsy. 1992;5:194–205. [Google Scholar]
- 8.Joachim G, Acorn S. Stigma of visible and invisible chronic conditions. J Adv Nurs. 200;32:243–248. doi: 10.1046/j.1365-2648.2000.01466.x. [DOI] [PubMed] [Google Scholar]
- 9.Goffman E. Stigma: Notes on the management of spoiled identity. New York: Simon and Schuster Inc.; 1963. [Google Scholar]
- 10.Harden CL, Kossoy A, Vera S, Nikolov B. Reaction to epilepsy in the workplace. Epilepsia. 2004;45:1134–1140. doi: 10.1111/j.0013-9580.2004.67003.x. [DOI] [PubMed] [Google Scholar]
- 11.Hermann B, Jacoby A. The psychosocial impact of epilepsy in adults. Epilepsy Behav. 2004;15:S11–S16. doi: 10.1016/j.yebeh.2009.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill M. Overcoming the stigma of epilepsy. Neurol Asia. 2010;15:S21–S24. [Google Scholar]
- 13.McLaughlin DP, Pachana NA, Mcfarland KStigma. seizure frequency and quality of life: The impact of epilepsy in late adulthood. Seizure. 2007;17:281–287. doi: 10.1016/j.seizure.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Jacoby A, Snape D, Baker GA. Epilepsy and social identity: The stigma of a chronic neurological disorder. Lancet Neurol. 2005;4:171–178. doi: 10.1016/S1474-4422(05)01014-8. [DOI] [PubMed] [Google Scholar]
- 15.Taylor J, Baker G, Jacoby A. Levels of epilepsy stigma in an incident population and associated factors. Epilepsy Behav. 2011;21:255–260. doi: 10.1016/j.yebeh.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Moore S, Knowles S. Beliefs and knowledge about Parkinson's disease. E-J App Psychol Clin Soc Issues. 2006;2:15–21. [Google Scholar]
- 17.Manzo JF. On the sociology and social orgnaization of stigma: Some ethnomethodological insights. Human Studies. 2004;27:401–416. [Google Scholar]
- 18.McLaughlin ME, Bell MP, Stringer DY. Stigma and acceptance of persons with disabilites: Understudied aspects of work diversity. Group Org Manage. 2004;29:302–333. [Google Scholar]
- 19.Kumari P, Ram D, Nizamie SH, Goyal N. Stigma and quality of life in individuals with epilepsy: A preliminary report. Epilepsy Behav. 2009;15:358–361. doi: 10.1016/j.yebeh.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Suurmeijer TPBM, Reuvekamp MF, Aldenkamp BP. Social functioning, psychological functioning, and quality of life in epilepsy. Epilepsia. 2001;42:1160–1168. doi: 10.1046/j.1528-1157.2001.37000.x. [DOI] [PubMed] [Google Scholar]
- 21.Ye SY. The lived expeirence of struggling against illness for patients with amyotrophic lateral sclerosis. J Kor Acad Nurs. 2008;38:802–812. doi: 10.4040/jkan.2008.38.6.802. [DOI] [PubMed] [Google Scholar]
- 22.Corrigan P, Penn DL. Lessons from social psychology on discrediting psychiatric stigma. Am Psychol. 1999;54:756–776. doi: 10.1037//0003-066x.54.9.765. [DOI] [PubMed] [Google Scholar]
- 23.Corrigan P, Watson A. The paradox of self-stigma and mental illness. Clin Psychol Sci Pract. 2002;9:35–53. [Google Scholar]
- 24.Corrigan P, Watson A, Barr L. The self-stigma of mental illness: Implications for self-esteem and self-efficacy. J Soc Clin Psychol. 2006;25:875–884. [Google Scholar]
- 25.Fung KM, Tsang HW, Corrigan PW. Self-stigma of people with schizophrenia as a predictor of their adherence to psychosocial treatment. Psych. Rehab. J. 32:95–104. doi: 10.2975/32.2.2008.95.104. [DOI] [PubMed] [Google Scholar]
- 26.Rusch N, Corrigan PW, Wassel A, Micahels P, Larson JE, Olschewski M, Wilkniss S, Batia K. Self-stigma group identification perceived legitimacy of discrimination and mental health service use. Brit. J Psych. 2009;195:551–552. doi: 10.1192/bjp.bp.109.067157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rusch N, Holzer A, Hermann C, Schramm E, Jacob GA, Bohus M, Lieb K, Corrigan PW. Self stigma in women with borderline personality disorder and women with social phobia. J. Nerv. Ment Dis. 2006;194:766–773. doi: 10.1097/01.nmd.0000239898.48701.dc. [DOI] [PubMed] [Google Scholar]
- 28.Watson AC, Corrigan PW, Larson JE, Sells M. Self-stigma in people with mental illness. Schizophr Bull. 2007;33:1312–1318. doi: 10.1093/schbul/sbl076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Link B. Understanding labeling effects in the area of mental disorders: An assessment of the effects of expectations of rejection. Am Sociol Rev. 1987;52:96–112. [Google Scholar]
- 30.Pachankis JE. The psychological implications of concealing a stigma: A cognitive-affective-behavioral model. Psych Bull. 2007;133:328–345. doi: 10.1037/0033-2909.133.2.328. [DOI] [PubMed] [Google Scholar]
- 31.Scambler G. Health-related stigma. Sociol Health Ill. 2009;31:441–455. doi: 10.1111/j.1467-9566.2009.01161.x. [DOI] [PubMed] [Google Scholar]
- 32.Jacoby A. Felt versus enacted stigma: A concept revisited. Soc Sci Med. 1994;38:269–274. doi: 10.1016/0277-9536(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 33.Looper KJ, Kirmayer LJ. Perceived stigma in functional somatic syndromes and comparable medical conditions. J Psychosom Res. 2004;57:373–378. doi: 10.1016/j.jpsychores.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Suzkukamo Y, Ohbu S, Kondo T, Kohmoto J, Fukuhara SS. Psychological adjustment has a greater effect on health-related quality of life than on severity of disease in Parkinson's disease. Move Disord. 2006;21:761–766. doi: 10.1002/mds.20817. [DOI] [PubMed] [Google Scholar]
- 35.Brown L, Macintyre K, Trujillo L. Interventions to reduce HIV/AIDS stigma: What have we learned? AIDS Ed Prev. 2003;151:49–69. doi: 10.1521/aeap.15.1.49.23844. [DOI] [PubMed] [Google Scholar]
- 36.Rao D, Andrasik M, Acharya X, Simoni J. Liamputtong P, editor. Internalized Stigma among African Americans living with HIV: Preliminary scale development based on qualitative data. Stigma, discrimination, and living with HIV/AIDS: A cross-cultural perspective. Springer; In press. [Google Scholar]
- 37.Rao D, Choi S, Victorson D, Bode R, Heinemann A, Peterman A, Cella D. Measuring stigma across neurological conditions: The development of the Stigma Scale for Chronic Illness (SSCI) Qual Life Res. 2009;18:585–595. doi: 10.1007/s11136-009-9475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao D, Feldman B, Fredericksen R, Crane P, Simoni J, Kitahata M, Crane H. A structural equation model of HIV-related stigma, depressive symptoms, and medication adherence. AIDS Behav. 2011 doi: 10.1007/s10461-011-9915-0. DOI: 10.1007/s10461-011-9915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cella D, Gershon R, Lai J, Choi S. The future of outcomes measurement: item banking, tailored short-forms, and computerized adaptive assessment. Qual Life Res. 2007;16(Supplement 1):133–141. doi: 10.1007/s11136-007-9204-6. [DOI] [PubMed] [Google Scholar]
- 40.Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B. The Patient Reported Outcomes Measurement Informational System PROMIS; overview, developmental work 2004–2006. Med Care. 2007;455:S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeWalt DA, Rothrock N, Yount S, Stone AA. Evaluation of item candidates: the PROMIS qualitative item review. Med Care. 2007;455(Supplement 1):S12–S21. doi: 10.1097/01.mlr.0000254567.79743.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hambleton RR, Swaminathan H, Rogers HJ. Fundamentals of Item Response Theory. Newbury Park, CA: Sage Publications, Incl; 1991. [Google Scholar]
- 43.Cella D, Chang C-H. Response to Hays et al. and McHorney and Cohen: A discussion of Item Response Theory and its applications in health status assessment. Med Caer. 2000;38:S66–S72. doi: 10.1097/00005650-200009002-00010. [DOI] [PubMed] [Google Scholar]
- 44.Hays RD, Morales LS, Reise SP. Item Response Theory and health outcomes measurement in the 21st century. Med Care. 2000;38:S28–S42. doi: 10.1097/00005650-200009002-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McHorney CA, Cohen AS. Equating healthponse theory modeling for evaluat status measures with Item Response Theory: Illustrations with functional status items. Med care. 2000;38:S43–S59. doi: 10.1097/00005650-200009002-00008. [DOI] [PubMed] [Google Scholar]
- 46.Reeve B, Fayers P. Applying item response theory modeling for evaluating questionnaire item and scale properties. In: Fayers P, Hays R, editors. Assessing quality of life in clinical trials: Methods of practice. 2nd edition. USA: Oxford University Press; 2005. pp. 55–73. [Google Scholar]
- 47.National Institute of Neurological Disorders and Stroke (NINDS) User manual for the quality of life in neurological disordres (Neuro-QOL) measures, version 1.0. 2010 Sep [Google Scholar]
- 48.Mplus version 6.0. [Computer software] Los Angeles: Muthén Muthén; [Google Scholar]
- 49.Gershon RC, Lai JS, Bode R, Choi S, Moy C, Bleck T, et al. NeuroQOL: Quality of life item banks for adults with neurological disorders: Item development and calibrations based upon clinical and general population testing. Qual Life Res. 2011 doi: 10.1007/s11136-011-9958-8. DOI 10.1007/s11136-011-9958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skevington SM, Lotfy M, O’Connell KA. The World Health Organization’s WHOQUOL-BRIEF quality of life assessment: Psychometric properties and results of the international field trial A report from the WHOQOL Group. Qual Life Res. 2003;13:299–310. doi: 10.1023/B:QURE.0000018486.91360.00. [DOI] [PubMed] [Google Scholar]
- 51.Thompson B, Daniel LG. Factor analytic evidence for construct validity of scores: A historical overview and some guidelines. Ed Psychol Measure. 1996;56:197–208. [Google Scholar]
- 52.Sirey JA, Bruce ML, Alexopoulos GS, Perlick DA, Friedman SJ, Meyers BS. Stigma as a barrier to recovery: Perceived stigma and patient-rated severity of illness as predictors of antidepressant drug adherence. Psych Serv. 2001;52:1615–1620. doi: 10.1176/appi.ps.52.12.1615. [DOI] [PubMed] [Google Scholar]
- 53.Sirey JA, Bruce ML, Alexopoulos GS, Perlick DA, Raue P, Friedman SJ, Meyers BS. Perceived stigma as a predictor of treatment discontinuation in young and older outpatients with depression. Am J Psych. 2001;158:479–481. doi: 10.1176/appi.ajp.158.3.479. [DOI] [PubMed] [Google Scholar]