Abstract
Response inhibition is considered a core dimension in alcoholism and its co-existing disorders. The major objective of this study is to compare the magnitude and spatial distribution of ERP components during response activation and inhibition in alcoholics (N = 30) and normal controls (N = 30) using a visual Go/No-Go task. The results indicate that alcoholics manifest a decreased P3(00) amplitude during Go as well as No-Go conditions. The difference between Go and No-Go processing was more evident in controls than in alcoholics. The topography of current source density in alcoholics during the P3 response was found to be very different from that of normals, suggesting that alcoholics perhaps activated inappropriate brain circuitry during cognitive processing. The significantly reduced No-Go P3 along with the relatively less anteriorized CSD topography during No-Go condition suggests poor inhibitory control in alcoholics. It is proposed that the No-Go P3, the electrophysiological signature of response inhibition, can be considered as an endophenotypic marker in alcoholism.
Keywords: Event-related potentials, P3, Go/No-Go, Alcoholism, Disinhibitory disorders, Inhibitory control, Endophenotype
1. Introduction
The event-related potentials (ERPs) using Go/No-Go tasks have been widely examined to elucidate the possible neural correlates of response activation and inhibition in normals as well as in clinical groups (Jodo and Inoue, 1990; Falkenstein et al., 1995, 1999; Shibata et al., 1999; Weisbrod et al., 2000; Kaiser et al., 2003). These tasks require the subjects to respond to one type of stimuli (Go condition), but to withhold the response to the other (No-Go condition). In the No-Go condition, two major ERP components have been identified as the markers for response inhibition: first, the N2, a negative deflection with a frontocentral maximum around 200-300 ms, and second, referred to as “No-Go P3”, an augmented positive-going peak usually peaking between 300 and 600 ms (Pfefferbaum et al., 1985; Eimer, 1993; Jodo and Inoue, 1990; Jodo and Kayama, 1992; Kopp et al., 1996). However, the N2 and P3 components during the No-Go condition may represent different processing of response inhibition and hence the dysfunction in either or both of these components in different mental disorders may suggest the deficiency of inhibitory control (Kaiser et al., 2003).
Response inhibition requires the activation of the executive system of the frontal lobes (Barkley, 1997; Weisbrod et al., 2000; Kaiser et al., 2003). On the other hand, the neural basis of this executive system is thought to be a distributed network involving the prefrontal areas and anterior cingulate gyrus (Posner and DiGirolamo, 1998; Smith and Jonides, 1999). However, theories based on the findings of lesion studies stressed the importance of the orbitofrontal cortex in inhibitory control (i.e., Mishkin, 1964; Fuster, 1989). Consistent with the distributed activations that underlie most of the cognitive processes, neuroimaging studies have revealed cerebral activation beyond ventral frontal regions during response inhibition (Brown et al., 1999; Garavan et al., 1999, 2002). The distributed network thought to underlie inhibitory control, as observed with neuroimaging studies, includes the dorsal and ventral prefrontal regions (Kawashima et al., 1996; Tsujimoto et al., 1997; Smith et al., 1998; Konishi et al., 1998; Watanabe et al., 2002), anterior cingulate cortex (Casey et al., 1997; Liddle et al., 2001; Menon et al., 2001; Garavan et al., 2002; Durston et al., 2002), premotor and supplementary motor areas (Ullsperger and von Cramon, 2001; Garavan et al., 2002; Sylvester et al., 2003), and parietal regions (Garavan et al., 1999; Watanabe et al., 2002; Durston et al., 2002).
A robust finding in ERP studies on alcoholism is that alcoholics as well as individuals at high risk to develop alcoholism have been shown to have low P3 amplitude in various task paradigms (Begleiter et al., 1984; Porjesz et al., 1987; Porjesz and Begleiter, 1990, 1991, 1996; Rodriguez Holguin et al., 1999; Hada et al., 2000; Prabhu et al., 2001; Cohen et al., 2002; Suresh et al., 2003). In Go/No-Go tasks, the anteriorly distributed No-Go P3 potential has a markedly reduced amplitude in alcoholic subjects as well as in high-risk individuals, indicating impaired inhibitory control in these individuals (Pfefferbaum et al., 1991; Cohen et al., 1997a, 1997b). However, the deficits in inhibitory control have been reported in a variety of behavioral disorders, which share disinhibitory psychopathology in common, including OCD and Tourette syndrome (Schall et al., 1996; Johannes et al., 2001, 2003), ADHD (Frank et al., 1998; Rubia et al., 1998; Pliszka et al., 2000; Brandeis et al., 2002), ASP and conduct disorder (Bauer and Hesselbrock, 1999a, 1999b; Kiehl et al., 1999, 2000), schizophrenia (Weisbrod et al., 2000; Fallgatter and Muller, 2001), and drug use (Kouri et al., 1996; Bauer, 2001; Kaufman et al., 2003). Based on the patterns of comorbidity, it was suggested that the common psychiatric and substance use syndromes may be divisible into two broad groups of internalizing and externalizing disorder (Kendler et al., 2003). Despite the fact that the addictive disorders inclusive of alcoholism would also involve very specific aspects of disinhibition such as drug incentive salience, drug expectation or craving, and compulsive drug intake, the electrophysiological markers of response inhibition specific to alcoholism, as distinct from other disinhibitory disorders, are poorly understood. Nevertheless, it is important to determine not only the magnitude but also the topographic distribution of averaged brain potentials as well as the estimated surface Laplacian in alcoholism, as this might explain the cortical dynamics and networks during cortical processing.
In the present study, along with ERPs, we have therefore attempted to examine the spatial distribution of Current Source Density (CSD) which may give distinct topographic features specific to alcoholism during response inhibition. The CSD is a method which applies an estimate of surface Laplacian and can provide differential topographic features of cortical surface potentials devoid of volume conduction effects (Nunez, 1995; Srinivasan et al., 1998; Wang and Begleiter, 1999). Although the CSD has successfully differentiated alcoholics and controls in terms of topographic differences (Hada et al., 2000, 2001), this method has not been studied in a Go/No-Go paradigm in alcoholics. The objective of the present study was to examine the ERP as well as CSD correlates of response inhibition in alcoholics and control subjects using a Go/No-Go task. By comparing the magnitude, spatial and temporal characteristics of these measures in alcoholic subjects and healthy controls, it may be possible to elucidate the specific neuro-cognitive abnormalities related to response inhibition in alcoholics. Further, recent advances in understanding the brain mechanisms involved in inhibitory control, impulsivity, motivation, reward, and decision-making might permit a discussion of neural circuitry underlying the pathology of addiction.
2. Methods
2.1. Subjects
The demographic and clinical characteristics of the sample are presented in Table 1. A sample of 30 alcoholics (16 males, 14 females) with an age-range of 19-42 years and 30 gender-matched healthy controls aged between 18 and 35 years were selected. Control subjects were recruited through newspaper advertisements and notices. The alcoholic group comprised diagnosed alcoholic patients from the de-addiction centers of the hospitals in New York, primarily from Kings County Hospital Center at Brooklyn. A team consisting of psychiatrists and psychologists diagnosed the patients according to the DSM-IIIR or DSM-IV criteria for alcohol dependence. The Bard/Porjesz Adult Alcoholism Battery (BAAB), a semi-structured clinical assessment schedule was used to obtain the clinical data related to alcohol dependence and alcohol-related medical problems. The patients who were receiving treatment medication such as antabuse and psychoactive drugs were excluded from the study to avoid the possible interaction of drugs with the EEG profile. Although neuroradiological investigations were not performed, the individuals with severe cognitive deficits based on their score on the Mini Mental State Examination (MMSE; Folstein et al., 1975) were excluded from the study. The control subjects did not have any personal and/or family history of major medical or psychiatric disorders and substance-related addictive illnesses. However, subjects with present history of substance use and/or ASP as co-existing conditions and with a past history of conduct disorder, ADHD, and Oppositional Defiant Disorder (ODD) were also included in the alcoholic sample. Subjects who had positive findings (for their recent drug use) in the urine screen and Breathalyzer test were excluded from the study. Subjects with hearing or visual impairment, liver disease, or head injury were also excluded from the study. Experimental procedures and ethical guidelines were in accordance with approval from the Institutional Review Board (IRB).
Table 1.
Demographic and clinical characteristics of the sample.
| VARIABLES | ALCOHOLICS (N=30) | CONTROLS (N=30) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |
| Age (yrs.) | 34.34 | 4.68 | 19-42 | 24.06 | 5.46 | 18-35 |
| Education (yrs.) | 12.03 | 1.83 | 5-15 | 14.73 | 3.06 | 4-20 |
| Age of onset of drinking (yr) |
15.00 | 3.90 | 12-26 | NA | NA | NA |
| No. of drinking days per month* |
19.97 | 10.41 | 0-30 | 2.07 | 2.65 | 0-10 |
| No. of drinks† per drinking day* |
7.87 | 4.78 | 0-16 | 1.60 | 1.75 | 0-6 |
NA = Not Applicable.
Data are for the 6 months prior to the treatment in alcoholic group.
One drink = 1 shot glass of hard liquor; 1 glass of wine; 1 bottle of beer.
2.2. Experimental paradigm
There were three visual stimuli in the task: (i) a cross (fixation stimulus), (ii) a circle (Go or No-Go stimulus), and (iii) a dollar sign (reinforcement sign). These stimuli subtended a visual angle of approximately 1°, and were presented on a computer monitor. The Go and No-Go stimuli were always preceded by a fixation stimulus that appeared at the center of the monitor. The circles that appeared at the top right and bottom left corners served as Go stimuli, to which the subjects had to respond by pressing a button as quickly as possible. The No-Go stimuli, to which the subjects were asked to withhold their response, appeared at the top left and bottom right corners. The dollar-sign appeared whenever there was a correct button-press response to indicate a reward. The inter-trial intervals (ITI) for the Go condition with and without the dollar sign (i.e., correct and incorrect Go trials) were 2100 ms and 1550 ms respectively; the ITI for the No-go trails was 1550 ms (as the dollar sign never appeared in the No-go trials). The probabilities of occurrence of Go and No-go stimuli were equal (50/50), and the order of these stimuli was randomized.
The experiment consisted of a practice phase and a recording phase. The practice phase consisted of twenty Go and No-Go trials, respectively. The subjects were instructed to press a button as quickly as possible whenever they saw a circle in either the top right or bottom left corner. The speed of response was stressed, as the subjects had to respond within 400 ms, and responses beyond this window were deemed incorrect. A feedback signal (a beep) was given whenever the subject’s button-press response was wrong; the practice phase did not accrue any reward. The EEG activity was recorded only during the recording phase which consisted of 100 trials (50 Go and 50 No-Go stimuli). The appearance of a dollar sign in this phase indicated a reward of 25 cents for each correct button-press response, while there was no feedback signal provided for the incorrect responses. The total amount gained as reward was not displayed during the stimulus presentation. The subjects received the full amount at the end of the experiment without deductions for errors, although they were not informed of this while performing the experiment. The task characteristics are identical to our earlier study using this paradigm (see: Kamarajan et al., 2004).
2.3. ERP data acquisition and analysis
The subjects were seated in a comfortable, reclining chair located in a dimly-lit sound-attenuated RF-shielded room (IAC, Industrial Acoustics, Bronx, NY) and were instructed regarding the task requirements. EEG activity was recorded on a Neuroscan system (Version 4.1) (Neurosoft, Inc., El Paso, TX) using a 61-channel electrode cap (Electro-cap International, Inc., Eaton, OH), which included 19 channels of the 10-20 International System and 42 additional electrode sites (Electrode Position Nomenclature, American Electroencephalographic Association, 1991) as shown in Fig. 1. The electrodes were referenced to the tip of the nose and the ground electrode was at the forehead (frontal midline). Eye movements were monitored with a supraorbital vertical lead and a horizontal lead placed on the external canthus of the left eye. Electrode impedance was maintained below 5 kΩ. The EEG signals were recorded continuously with a bandpass at 0.02-100 Hz and amplified 10,000 times using a set of amplifiers (Sensorium, Charlotte, VT). The data consisted of sampling rates of either 256 or 512 Hz, and were resampled at 256 Hz during the signal analysis for the sake of uniformity.
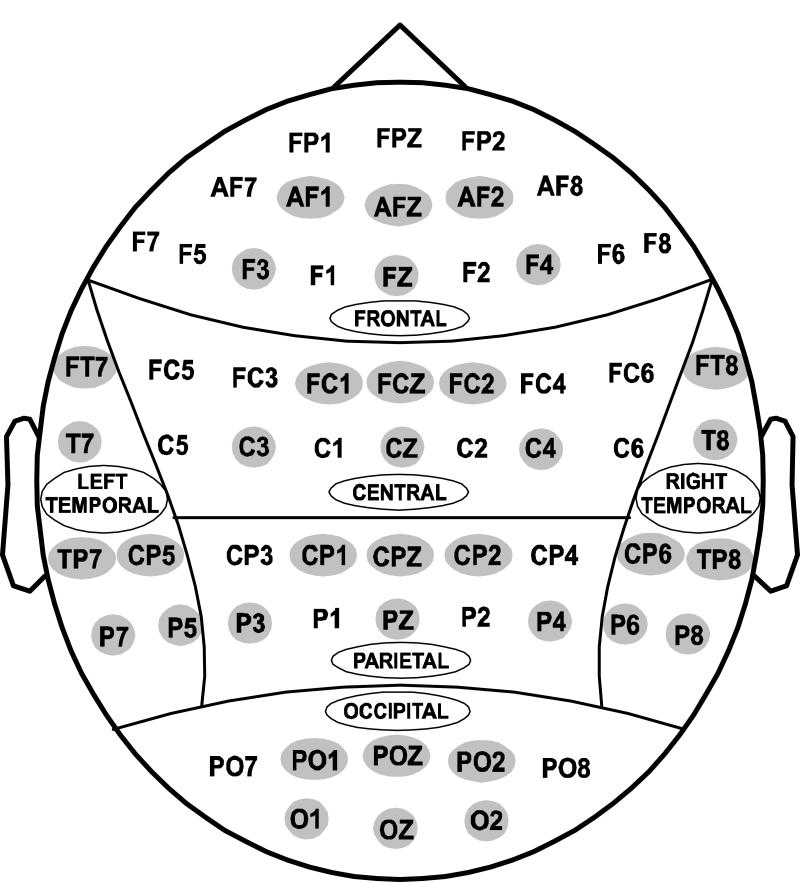
Fig. 1.
Regional grouping of electrodes: (1) Frontal, (2) Central, (3) Parietal, (4) Occipital, (5) Left-temporal, and (6) Right-temporal. The representative electrodes included for statistical analysis are highlighted.
The continuous EEG was digitally low-pass filtered at 32 Hz and then segmented into epochs of 100 ms pre-stimulus to 750 ms post-stimulus. The mean EEG activity for 100 ms prior to stimulus onset served as the pre-stimulus baseline. All segments exceeding ± 75 μV threshold were automatically excluded from further processing. After excluding eye-movement artifacts, the averaged segments for each individual were screened visually for further artifact rejection. The artifact detection was done on all the channels including the electroocculogram (EOG) channels. The trials with reaction times greater than 400 ms (for Go trials) were considered as error responses and therefore were rejected. Only the trials with correct response (button press) for the Go condition and correct inhibition (no button press) for the No-Go condition were averaged. The P3 amplitude was measured as the voltage difference between the pre-stimulus baseline and the largest positive going peak in the latency window 300-600 ms after stimulus onset. For each individual, the amplitude and latency measures were calculated using a semi-automatic peak-picking program, wherein the time window for each component was manually selected in the computer while the peak within the window was automatically detected, measured, and tabulated for each channel. However, operator intervention was possible during the process to ensure that the computer did not make anomalous peak selections. Each subject had a minimum of 20 good trials in each condition with a mean number of about 35 good trials in each group for the purpose of averaging. The grand averages were computed and plotted to determine the components and time windows. The amplitude and latency values of the P3 component obtained separately for Go and No-Go conditions for each subject were used in statistical analyses.
For statistical comparison, the electrodes were grouped into 6 scalp regions, and 6 representative electrodes from each region were taken for analysis as shown in Fig. 1. The demographic and behavioral data (i.e., age, education, MMSE score, reaction time, and error rates) were analyzed using t-test. The comparison of P3 amplitude and latency between the control and alcoholic group were performed using a Multivariate Analysis of Covariance (MANCOVA). Age was used as a covariate in the MANCOVA model for two reasons: the alcoholics were significantly older than the controls in the sample, and age as a factor is known to have an effect on ERP parameters. On the other hand, the comparison between Go and No-Go trial conditions within each group was performed using Repeated Measures Analysis of Variance (RMANOVA). The p-values were adjusted using Bonferroni correction for multiple comparisons during the region-wise statistical analysis of P3 amplitude.
2.4. CSD mapping
The voltage at each electrode is a voltage difference between the recording electrode and the reference electrode. Spatial resolution of these scalp-recorded potentials can be severely limited by (i) reference electrode effects, (ii) “blurring” effects due to the tissue between electrode and sources, and (iii) large electrode spacing (Nunez, 1981). The recorded potential at each electrode thus represents integrated contributions from several sources between the reference and recording electrode and thus provides a blurred picture of the electrical activity that generates the observed field. The CSD, derived using the surface Laplacian method, acts as a spatial filter and provides an estimate of the local radial current density rather than distant/deep (neural) sources (Hjorth, 1975; Nunez, 1981; Nunez and Pilgreen, 1991). The CSD presents a more differentiated picture emphasizing the local components in the electrical state of the scalp surface. These components theoretically represent the primary activity entering the surface from below before it has been integrated into the more smeared potential field (Hjorth, 1991). In the present study, in order to examine the topographic changes in the Laplacian transformed data, the CSD maps were constructed separately for controls and alcoholics using the grand mean values as described by Wang and Begleiter (1999).
The CSD maps were compared using Efron’s bootstrap technique as described by Srebro (1996). Based on this method, two sets of data were initially created by selecting randomly from the control group and alcoholic group respectively. Then, another two sets of data were obtained from the pooled sample consisting of both alcoholic and control subjects. These random assignments to all four sets of data were repeated 200 times, so that each set would have 200 items (or subjects). Pearson correlation (R) was performed for the sets of controls vs. alcoholics and pool-1 vs. pool-2. The Fisher Z transform of Pearson R (Fisher and Yates, 1957) was computed and the two sets of Z estimates were compared using F-tests. If the F-value was significantly large, then the maps would be considered to be different from each other.
3. Results
3.1. Demographic and behavioral data
The behavioral and cognitive performance scores between control and alcoholic subjects are shown in Table 2. The alcoholics were significantly older than the controls (t = 7.832; p = 0.000), and age as a variable has been included in the MANCOVA model for group comparison. Although the control subjects were relatively more educated than the alcoholics (t = 4.147; p = 0.000), education was not included in the MANCOVA model, as this variable has not been consistently shown to affect the electrophysiological profile. Although MMSE scores of alcoholics were significantly lower than that of controls, the difference was very minimal. The MMSE score was also not included in the MANCOVA model, as it was assumed that the lower score in alcoholics could have been the direct result of alcoholism per se. However, there was no significant difference in the reaction time between controls and alcoholics. Although alcoholics committed more errors during the button-press responses of Go and No-Go condition separately, this difference was not statistically significant. On the other hand, the total response error (both Go and No-Go errors put together) was significantly higher in alcoholics than in controls.
Table 2.
Performance scores between control and alcoholic subjects.
| VARIABLE | CONTROL | ALCOHOLIC | t-value | p-value | ||
|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | |||
| MMSE† score | 28.87 | 1.20 | 27.80 | 2.23 | 2.305 | 0.025* |
| Reaction time (in ms) | 291.38 | 22.37 | 293.04 | 23.48 | 0.281 | 0.780 |
| Error1† (Go) | 4.93 | 3.54 | 6.90 | 6.21 | 1.508 | 0.138 |
| Error1† (No-Go) | 1.10 | 1.35 | 3.30 | 7.39 | 1.605 | 0.119 |
| Error† (Total) (Go + No-Go) |
6.03 | 4.00 | 10.20 | 10.42 | 2.044 | 0.048* |
p < 0.05
scores represented in absolute values
3.2. ERP data
The Go/No-Go paradigm used in the present study elicited robust P3 components and also yielded significant statistical differences between groups and between trial conditions as illustrated in Figs. 2 & 3 and Tables 3-6. Other components in the ERP waveforms did not elicit observable differences and hence were not analyzed. There were no significant differences observed in P3 latency and therefore only the findings on P3 amplitude are reported here.
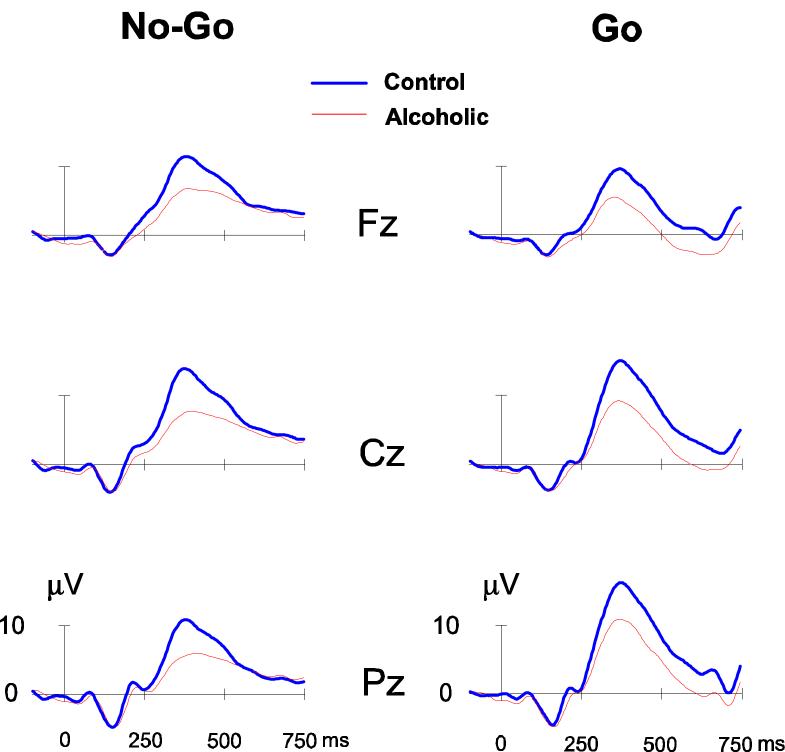
Fig. 2.
ERP waveforms of control versus alcoholic groups during No-Go and Go condition.
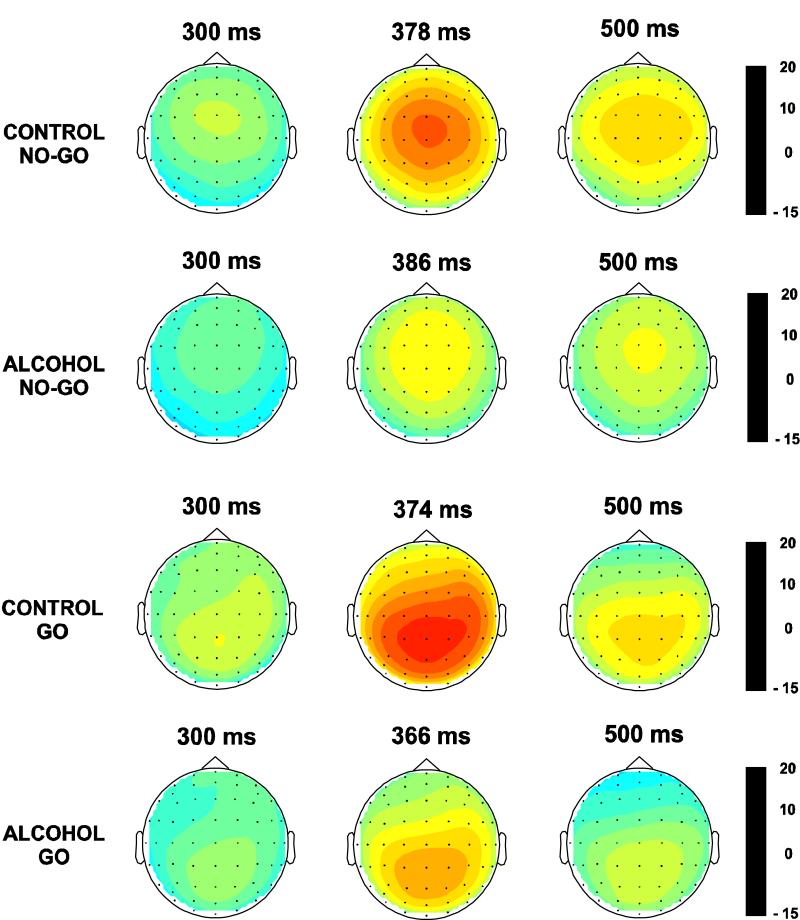
Fig. 3.
The spatial distribution of ERP amplitudes (in μV) at three time intervals of the P3 component in control and alcoholic groups during No-Go and Go condition.
Table 3.
Comparison of P3 amplitude (in μ,V) between control and alcoholic groups during the No-Go condition (using MANCOVA).
| REGION | CONTROL | ALCOHOLIC | F-value (df = 1, 58) |
p-value† | ||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | |||
| Frontal | 12.115 | 0.660 | 9.142 | 0.827 | 3.948 | 0.003** |
| Central | 15.347 | 0.883 | 10.195 | 0.916 | 3.899 | 0.003** |
| Parietal | 12.895 | 0.800 | 8.784 | 0.819 | 2.416 | 0.039* |
| Occipital | 8.097 | 0.643 | 5.692 | 0.669 | 1.241 | 0.301 |
| Left- Temporal |
7.992 | 0.613 | 5.450 | 0.606 | 2.951 | 0.015* |
| Right- Temporal |
9.309 | 0.614 | 5.955 | 0.545 | 3.439 | 0.006** |
SE = Standard Error
Bonferroni corrected
p < 0.05
p < 0.01
Table 6.
Comparison of P3 amplitude (in μ,V) between No-Go and Go conditions in the alcoholic group (using RMANOVA).
| REGION | NO-GO | GO | F-value (df = 1, 29) |
p-value† | ||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | |||
| Frontal | 9.142 | 0.827 | 6.354 | 1.072 | 9.201 | 0.005** |
| Central | 10.195 | 0.916 | 9.348 | 1.345 | 0.696 | 0.411 |
| Parietal | 8.784 | 0.819 | 11.965 | 1.407 | 7.297 | 0.011* |
| Occipital | 5.692 | 0.669 | 9.418 | 1.197 | 10.647 | 0.003** |
| Left- Temporal |
5.450 | 0.606 | 6.848 | 1.037 | 2.237 | 0.146 |
| Right- Temporal |
5.955 | 0.545 | 7.456 | 0.989 | 2.989 | 0.094 |
Bonferroni corrected
p < 0.05
p < 0.01
3.3. P3 amplitude: between-group comparisons
The ERP waveforms and topography of P3 amplitude in control and alcoholic subjects during the No-Go as well as the Go condition are illustrated in Figs. 2 and 3 respectively. In the No-Go condition, the maximum amplitude was observed in the central region, whereas the Go condition showed a parietal maximum. Alcoholics were found to have significantly lower amplitude in both the No-Go and Go conditions (Tables 3 and 4). The frontal and central regions showed a significant difference between groups in the No-Go not in the Go condition. In the Go condition, only the parietal region showed the significant effects. Post-hoc comparisons (adjusted for multiple comparisons) between controls and alcoholics showed that the significance was more robust in the No-Go condition as compared to the Go condition at each of the electrodes.
Table 4.
Comparison of P3 amplitude (in μ,V) between the control and alcoholic groups during the Go condition (using MANCOVA).
| REGION | CONTROL | ALCOHOLIC | F-value (df = 1, 58) |
p-value† | ||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | |||
| Frontal | 10.350 | 0.756 | 6.354 | 1.072 | 1.354 | 0.251 |
| Central | 14.713 | 1.058 | 9.348 | 1.345 | 0.676 | 0.670 |
| Parietal | 16.989 | 1.038 | 11.965 | 1.407 | 2.388 | 0.041* |
| Occipital | 13.412 | 1.004 | 9.418 | 1.197 | 1.089 | 0.381 |
| Left- Temporal |
11.176 | 0.873 | 6.848 | 1.037 | 1.012 | 0.428 |
| Right- Temporal |
11.503 | 0.893 | 7.456 | 0.989 | 1.061 | 0.398 |
Bonferroni corrected
p < 0.05
p < 0.01
p < 0.001
3.4. P3 amplitude: within-group comparisons
In the within-group comparisons of ERP waveforms, while central electrodes showed higher P3 amplitude for the No-Go condition than Go condition in both groups, parietal electrodes displayed higher amplitude for the Go condition. Both groups showed significant condition effects at frontal, parietal, and occipital regions (Table 5 and 6). However, at the temporal regions, only the control group showed significant condition effects. The post-hoc comparison at each of the electrodes showed that the differences between trial conditions were more robust in the control group than in the alcoholic group.
Table 5.
Comparison of P3 amplitude (in μ,V) between the No-Go and Go conditions in the control group (using RMANOVA).
| REGION | NO-GO | GO | F-value (df = 1, 29) |
p-value† | ||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | |||
| Frontal | 12.115 | 0.660 | 10.350 | 0.756 | 4.616 | 0.040* |
| Central | 15.347 | 0.883 | 14.713 | 1.058 | 0.439 | 0.513 |
| Parietal | 12.895 | 0.800 | 16.989 | 1.038 | 21.278 | 0.000*** |
| Occipital | 8.097 | 0.643 | 13.412 | 1.004 | 35.656 | 0.000*** |
| Left- Temporal |
7.992 | 0.613 | 11.176 | 0.873 | 15.798 | 0.000*** |
| Right- Temporal |
9.309 | 0.614 | 11.503 | 0.893 | 9.692 | 0.004** |
Bonferroni corrected
p < 0.05
p < 0.01
p < 0.001
3.5. CSD maps
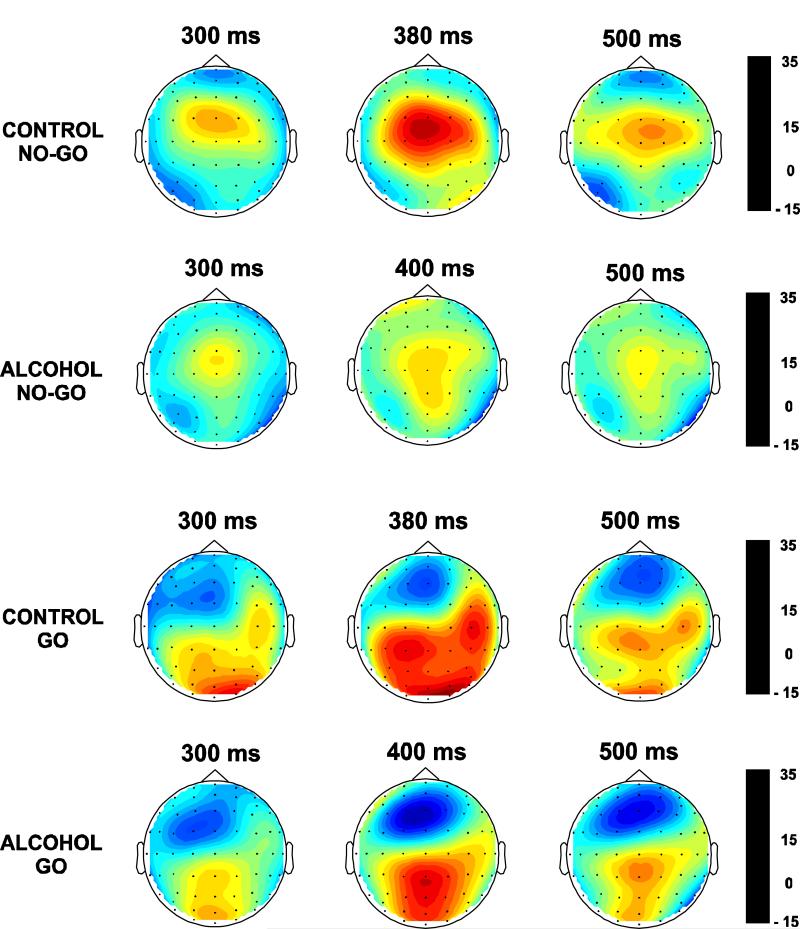
The CSD maps for control and alcoholic groups during No-Go and Go conditions at three time points are shown in Fig. 4. The sources were found to be more anterior in the No-Go condition as compared to the Go condition. The current density of the P3 component spread from 300 to 500 ms and the CSD plots were created for every 10 ms interval. It was found that within the latency window of the P3 (i.e., 300-500 ms) the alcoholic subjects exhibited a 20 ms delay in the overall peak amplitude for both Go and No-Go conditions. The analysis of sources and sinks in the CSD topography clearly differentiates the groups and experimental conditions. In the No-Go condition, the controls have a strong centrally focused source whereas the alcoholics have a diffused and weak source. A smaller prefrontal sink observed in the controls was absent in alcoholics. On the other hand, during the Go condition, the controls manifested three distinct posterior sources at left parietal, right centro-temporal, and occipital regions, while the alcoholics showed a single parieto-occipital source. The negative sink was more pronounced in alcoholics than in controls. Srebro’s bootstrap method revealed that the correlation coefficients of CSD values obtained from control-alcoholic pairs were significantly different from those of the pairs of random pools in each region as shown in Table 7. This result suggests that the CSD maps of controls and alcoholics were significantly different from each other in both Go and No-Go conditions.
Fig. 4.
The spatial distribution of CSD (in μV/r2cm2, where r = head radius) at three time intervals of the P3 component in control and alcoholic groups during No-Go and Go conditions.
Table 7.
Comparison of the Fisher Z transform (of Pearson R) in the No-Go and Go conditions based on bootstrap method.
| REGION | NO-GO CONDITION | GO CONDITION | ||||
|---|---|---|---|---|---|---|
| Control vs. Alcoholic |
Pool-1 vs. Pool-2 |
p-value† | Control vs. Alcoholic |
Pool-1 vs. Pool-2 |
p-value† | |
| Frontal | 0.0026 | 0.5258 | 0.0059** | 1.0735 | 1.1685 | 0.0313* |
| Central | 0.7227 | 1.1119 | 0.0000*** | 0.8501 | 1.3562 | 0.0000*** |
| Parietal | 0.8491 | 1.2457 | 0.0000*** | 0.1895 | 0.8303 | 0.0000*** |
| Occipital | 0.1559 | 0.9807 | 0.0000*** | 1.0930 | 1.5684 | 0.0000*** |
| Left- Temporal |
−0.0552 | 0.4194 | 0.0000*** | 0.8384 | 1.0592 | 0.0003*** |
| Right- Temporal |
0.0798 | 0.4194 | 0.0000*** | 1.0478 | 1.0592 | 0.0024** |
| Overall | 0.0599 | 0.5835 | 0.0000*** | 0.8230 | 1.0147 | 0.0000*** |
Based on F-values (df = 1, 398)
p < 0.05
p < 0.01
p < 0.001
4. Discussion
The amplitude and topographic features of ERPs and CSD were assessed in alcoholic subjects and in healthy controls using a visual Go/No-Go task. The main objective of this study was to examine P3 characteristics between alcoholics and controls during response inhibition (No-Go condition) as well as response activation (Go condition). The results yielded four important findings: 1) alcoholics manifested significantly lower P3 amplitudes during the No-Go as well as Go conditions, implying deficient processing of both response inhibition and response activation, 2) the statistical difference between the No-Go and Go conditions was more robust in controls than in alcoholics, suggesting poor differentiation of task conditions in alcoholics, 3) relatively less anteriorization of CSD polarity in alcoholics during No-Go processing indicates an impaired/decreased frontal lobe contributions, and 4) the topographic patterns (involving sources and sinks) of CSD in alcoholics are significantly different from controls, implying that the network systems associated with cognitive processing are perhaps different between alcoholics and control subjects.
Using experiments of both equiprobable and non-equiprobable stimuli, Eimer (1993) suggested that the frontocentrally distributed No-Go P3 was related to response inhibition while the parietally distributed Go P3 was related to response activation. Our finding that alcoholics displayed significantly lower P3 amplitude during Go as well as No-Go conditions, therefore, suggests that both response activation and response inhibition are dysfunctional in alcoholic individuals. However, in our study, we have not found any group effects in No-Go N2, which is considered to be an important index of response inhibition. We argue that the absence of N2 effect does not exclude the presence of response inhibition deficits in alcoholics. In the literature on Go/No-Go tasks, it has been widely reported that both No-Go N2 and No-Go P3 have been considered to be the electrophysiological signatures of response inhibition. In our study, we do get a trend that there is a greater N2 on No-Go relative to go trials, although this was not statistically significant. Further, in our study, there is also a trend that alcoholics have reduced N2 in No-Go condition at FZ and CZ electrodes, but this reduction was not statistically significant, as against the robust reduction in the P3 component. Further, in an equiprobable Go/No-Go task, P3 has been found to reflect response inhibition in alcoholics and in individuals at risk for alcoholism (Cohen et al., 1997a, 1997b). Therefore, as suggested by many researchers, both No-Go N2 and No-Go P3 may index response inhibition (Cohen et al., 1997a, 1997b; Weisbrod et al., 2000; Kaiser et al., 2003), though they may represent different stages of cognitive processing and may underlie different neural generators. Further, the paradigm used in our study was unique and required speeded reaction time less than 400 ms; in this paradigm, the N2 was perhaps camouflaged by the early P3.
It is also possible that the ERP index that underlies response inhibition might be different in different clinical groups. For example, schizophrenic patients showed normal activation in No-Go N2, but a lack of lateralization in the No-Go P3 (Weisbrod et al., 2000), while depressive patients showed significantly lower activation in the No-Go N2 but normal activation in No-Go P3 as compared to controls (Kaiser et al., 2003). Our finding is identical to the study by Cohen et al. (1997a) who reported that alcoholics showed lower P3 amplitudes in Go as well as No-Go conditions, with the absence of group effects in No-Go N2. The differences in findings could also be due to the fact that some studies reported larger amplitudes in the Go condition (Pfefferbaum et al., 1980, 1984, 1985; Pfefferbaum and Ford, 1988), while other studies observed larger P3 amplitudes in the No-Go condition (Karlin et al., 1970; Simson et al., 1977; Roberts et al., 1994). The diverse task characteristics in Go/No-Go paradigms including stimulus probability, induction of prepotency of response, and speed-accuracy trade off could have also contributed for the differential findings. It is to be noted that the paradigm used in our study involves the emphasis of speed in response and therefore the response time was shorter compared to other Go/No-Go tasks.
At the behavioral level, the dysfunction in response inhibition can also be evidenced by the finding that alcoholics committed more commission errors than controls during No-Go conditions. Response inhibition is considered to be a behavioral measure (encompassing sensory, cognitive and motor components) subserved by cortical inhibition and frontal executive processes which could be elicited by a neurophysiological index such as No-Go P3. Although the P3 component as elicited from different task paradigms has been shown to be associated with cortical inhibition, the No-Go P3 as an electrophysiological index of response inhibition, is considered to involve more than generalized cortical inhibition; it reflects activity at the cortical network subserving executive control involving prefrontal areas, anterior cingulate, and left premotor cortex (Kiefer et al., 1998). Our results showed that alcoholics had decreased amplitude in the parietally distributed Go-P3 which was related to response activation, as well as in the fronto-centrally distributed No-Go P3 that was associated with response inhibition, implying that alcoholics have dysfunctions in both cortical inhibition and frontal inhibitory mechanisms. This view can be supported by the evidence that alcoholics manifest abnormality in inhibition-based rhythms and in inhibition-related receptor mechanism like GABA at the physiological level (Volkow et al., 1993; Behar et al., 1999; Lingford-Hughes et al., 1998; 2000) and impairment in frontal executive functions at the cognitive level (Giancola and Moss, 1998; Kamarajan et al., 2004).
The topography of ERP and CSD potentials clearly delineated the controls from alcoholics. The No-Go anteriorization in the surface potential, as reported by other studies (e.g., Nativ et al., 1992; Fallgatter et al., 1998), has been observed in controls as well as in alcoholics. However, in the CSD topography, alcoholics, as compared to controls, showed less anteriorization effect in the No-Go condition and thus a reduced frontal lobe contribution during response inhibition. Further, in consensus with earlier P3 findings on alcoholism, our finding that lower P3 amplitude in the Go condition in alcoholics suggests a processing deficit during response activation as well. The processing deficits attributed to lower P3 amplitude mostly include attentional, executive, and working memory systems (e.g., Coull, 1998; Noldy et al., 1990; Perchet et al., 2001). Prefrontal cortex is recruited when there is a need to select between competing responses (Bunge et al., 2002). The executive control system regulates response selection in situations where routine mechanisms are unavailable or inadequate for task performance (Norman and Shallice, 1986; Posner and Dehaene, 1994). In an fMRI study, Pfefferbaum et al. (2001) demonstrated that alcoholics showed diminished activation of frontal cortical systems compared with controls during a task involving executive control. Further, Rangaswamy et al. (2004) reported that a dysfunctional fronto-parietal circuit involving the rehearsal component of the working memory system may underlie the low P300 responses seen in subjects who were at high risk to develop alcoholism. Therefore, our finding that alcoholics have lower P3 during response activation and inhibition perhaps indicates impaired executive control in alcoholic subjects and further suggests an insufficient input from the prefrontal cortex or circuitry.
As noted earlier, inhibitory control is considered to be an important executive function (Barkley, 2001; Collette and Van der Linden, 2002), and thought to be subserved by prefrontal circuits (Casey et al., 2002). The cognitive control model provided by Casey and colleagues suggests the involvement of fronto-striatal circuits during behavioral inhibition (Casey et al., 2001, 2002). Specific to addiction behavior, various neuro-cognitive models have been proposed to explain the vulnerability, genesis, and maintenance of alcohol/drug dependence. Giancola and Moss (1998) theorized that executive cognitive functioning (ECF) was an important determinant in the etiology of alcoholism and comorbid disinhibitory pathology, and proposed a cognitive-neurobehavioral model of alcoholism by implicating the fronto-striatal system. According to Begleiter and Porjesz (1999), the genetic predisposition to develop alcoholism primarily involves a state of CNS disinhibition/hyperexcitability which is reflected by the electrophysiological and cognitive anomalies in alcoholics as well as in the offspring of alcoholics. Goldstein and Volkow (2002) conceptualized alcohol/drug addiction as a syndrome of impaired response inhibition and salience attribution, and summarized the involvement of the fronto-subcortical circuits in addiction disorders. Chambers et al. (2003) proposed that the primary motivation circuitry involving cortical-striatal-thalamic-cortical loops were putatively involved in impulsivity, decision making and alcohol/drug addiction. Recently, we proposed the prefrontal network systems (PNS) model of alcoholism based on available evidence on compromised frontal function in alcoholism and on our finding that the brain oscillatory responses during inhibitory processes (No-Go condition) were attenuated in alcoholics (Kamarajan et al., 2004)
It is often discussed that the electrophysiological deficits observed in alcoholics could have been a trait variable rather than drinking-related state variable. By documenting the evidence from the Collaborative Study on the Genetics of Alcoholism (COGA), Porjesz et al. (1996, 1998) demonstrated that P3 amplitude meets all the criteria to be considered as a phenotypic marker for alcoholism. Further, the P3 amplitude has been consistently proposed to be a trait marker in alcoholism as the decreased P3 amplitude has also been frequently identified in children of alcoholics compared to normal controls (e.g., Begleiter et al., 1984; Porjesz and Begleiter, 1990, 1991; Polich et al., 1994; Porjesz et al., 1998). Moreover, many researchers have proposed that ERP features and EEG oscillations are highly heritable (Begleiter et al., 1998; van Beijsterveldt and Boomsma, 1994; van Beijsterveldt et al., 1996; van Beijsterveldt et al., 1998; van Beijsterveldt et al., 2001; van Beijsterveldt and van Baal, 2002; Almasy et al., 1999; Anokhin et al., 2001; Hesselbrock et al., 2001; Porjesz et al., 2002a, 2002b; Winterer and Goldman, 2003). In the face of these observations, our finding that alcoholics display anomalies during response activation and inhibition may also suggest an impaired genetic mechanism in alcoholics, which could have perhaps led to deficient cognitive processing.
In conclusion, the results of the present study indicate that alcoholics manifest deficient cognitive processing mechanisms as evidenced by suppressed P3 amplitude during response activation and inhibition. The topographic pattern of alcoholics was found to be different from that of normals, suggesting the possibility that alcoholics activate different/inappropriate brain circuitry during cognitive processing. The relatively weaker No-Go P3 in surface potential and less anteriorization in CSD topography are suggestive of poor inhibitory control in alcoholics. Evidence suggests that the spectrum of disinhibitory or externalizing disorders, that includes alcoholism, share common inherent causality and external manifestations of symptoms. It is suggested that the No-Go potential can be considered as an endophenotypic marker in alcoholism and related disinhibitory disorders. However, future studies should attempt to elucidate the relative as well as interactive contributions of genetic and environmental factors.
Acknowledgments
The authors are grateful to the valuable assistance of Aquanette Sass, Aleksey Dumer, Lakshmi Krishnamurthy, Glenn Murawski, Tracy Crippen, Carlene Haynes, and Joyce Alonzia. This study was supported by the NIH grant # 5 RO1 AA002686 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA).
References
- Almasy L, Porjesz B, Blangero J, Chorlian DB, O’Connor SJ, Kuperman S, Rohrbaugh J, Bauer LO, Reich T, Polich J, Begleiter H. Heritability of event-related brain potentials in families with a history of alcoholism. American Journal of Medical Genetics. 1999a;88:383–390. [PubMed] [Google Scholar]
- Anokhin AP, van Baal GC, van Beijsterveldt CE, de Geus EJ, Grant J, Boomsma DI. Genetic correlation between the P300 event-related brain potential and the EEG power spectrum. Behavior Genetics. 2001;31:545–554. doi: 10.1023/a:1013341310865. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barkley RA. The executive functions and self-regulation: an evolutionary neuropsychological perspective. Neuropsychology Review. 2001;11:1–29. doi: 10.1023/a:1009085417776. [DOI] [PubMed] [Google Scholar]
- Bauer LO. CNS recovery from cocaine, cocaine and alcohol, or opioid dependence: a P300 study. Clinical Neurophysiology. 2001;112:1508–1515. doi: 10.1016/s1388-2457(01)00583-1. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. P300 decrements in teenagers with conduct problems: implications for substance abuse risk and brain development. Biological Psychiatry. 1999a;46:263–272. doi: 10.1016/s0006-3223(98)00335-7. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. Subtypes of family history and conduct disorder: effects on P300 during the stroop test. Neuropsychopharmacology. 1999b;21:51–62. doi: 10.1016/S0893-133X(98)00139-0. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcoholism: Clinical and Experimental Research. 1999;23:1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Reich T, Edenberg HJ, Goate A, Blangero J, Almasy L, Foroud T, Van Eerdewegh P, Polich J, Rohrbaugh J, Kuperman S, Bauer LO, O’Connor SJ, Chorlian DB, Li TK, Conneally PM, Hesselbrock V, Rice JP, Schuckit MA, Cloninger R, Nurnberger J, Jr., Crowe R, Bloom FE. Quantitative trait loci analysis of human event-related brain potentials: P3 voltage. Electroencephalography and Clinical Neurophysiology. 1998;108:244–250. doi: 10.1016/s0168-5597(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Behar KL, Rothman DL, Petersen KF, Hooten M, Delaney R, Petroff OA, Shulman GI, Navarro V, Petrakis IL, Charney DS, Krystal JH. Preliminary evidence of low cortical GABA levels in localized 1H-MR spectra of alcohol-dependent and hepatic encephalopathy patients. American Journal of Psychiatry. 1999;156:952–954. doi: 10.1176/ajp.156.6.952. [DOI] [PubMed] [Google Scholar]
- Brandeis D, Banaschewski T, Baving L, Georgiewa P, Blanz B, Warnke A, Steinhausen HC, Rothenberger A, Scheuerpflug P. Multicenter P300 brain mapping of impaired attention to cues in hyperkinetic children. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:990–998. doi: 10.1097/00004583-200208000-00018. [DOI] [PubMed] [Google Scholar]
- Brown GG, Kindermann SS, Siegle GJ, Granholm E, Wong EC, Buxton RB. Brain activation and pupil response during covert performance of the Stroop Color Word task. Journal of the International Neuropsychological Society. 1999;5:308–319. doi: 10.1017/s1355617799544020. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JD. Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage. 2002;17:1562–1571. doi: 10.1006/nimg.2002.1252. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Durston S, Fossella JA. Evidence for a mechanistic model of cognitive control. Clinical Neuroscience Research. 2001;1:267–282. [Google Scholar]
- Casey BJ, Tottenham N, Fossella J. Clinical, imaging, lesion, and genetic approaches toward a model of cognitive control. Developmental Psychobiology. 2002;40:237–254. doi: 10.1002/dev.10030. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor R, Orendi J, Schubert A, Nystrom LE, Giedd JN, Castellanos FX, Haxby JV, Noll DC, Cohen JD, Forman SD, Dahl RE, Rapoport JL. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. The American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HL, Ji J, Chorlian DB, Begleiter H, Porjesz B. Alcohol-related ERP changes recorded from different modalities: a topographic analysis. Alcoholism: Clinical and Experimental Research. 2002;26:303–317. [PubMed] [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H, Wang W. Neuroelectric correlates of response production and inhibition in individuals at risk to develop alcoholism. Biological Psychiatry. 1997a;42:57–67. doi: 10.1016/S0006-3223(96)00221-1. [DOI] [PubMed] [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H, Wang W. Neurophysiological correlates of response production and inhibition in alcoholics. Alcoholism: Clinical and Experimental Research. 1997b;21:1398–1406. [PubMed] [Google Scholar]
- Collette F, Van der Linden M. Brain imaging of the central executive component of working memory. Neuroscience and Biobehavioral Reviews. 2002;26:105–125. doi: 10.1016/s0149-7634(01)00063-x. [DOI] [PubMed] [Google Scholar]
- Coull JT. Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Progress in Neurobiology. 1998;55:343–361. doi: 10.1016/s0301-0082(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Worden MS, Yang Y, Casey BJ. The effect of preceding context on inhibition: an event-related fMRI study. Neuroimage. 2002;16:449–453. doi: 10.1006/nimg.2002.1074. [DOI] [PubMed] [Google Scholar]
- Eimer M. Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biological Psychology. 1993;35:123–138. doi: 10.1016/0301-0511(93)90009-w. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychologica. 1999;101:267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Koshlykova NA, Kiroj VN, Hoormann J, Hohnsbein J. Late ERP components in visual and auditory Go/Nogo tasks. Electroencephalography and Clinical Neurophysiology. 1995;96:36–43. doi: 10.1016/0013-4694(94)00182-k. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Muller TJ. Electrophysiological signs of reduced prefrontal response control in schizophrenic patients. Psychiatry Research. 2001;107:19–28. doi: 10.1016/s0925-4927(01)00092-0. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Wiesbeck GA, Weijers HG, Boening J, Strik WK. Event-related correlates of response suppression as indicators of novelty seeking in alcoholics. Alcohol and Alcoholism. 1998;33:475–481. doi: 10.1093/alcalc/33.5.475. [DOI] [PubMed] [Google Scholar]
- Fisher RA, Yates F. Statistical Tables for Biological, Agricultural and Medical Research. Hafner; New York: 1957. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frank Y, Seiden JA, Napolitano B. Electrophysiological changes in children with learning and attentional abnormalities as a function of age: event-related potentials to an “oddball” paradigm. Clinical Electroencephalography. 1998;29:188–193. doi: 10.1177/155005949802900412. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe. 2nd ed. Raven Press; New York: 1989. [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Moss HB. Executive cognitive functioning in alcohol use disorders. Recent Developments in Alcoholism. 1998;14:227–251. doi: 10.1007/0-306-47148-5_10. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. The American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hada M, Porjesz B, Begleiter H, Polich J. Auditory P3a assessment of male alcoholics. Biological Psychiatry. 2000;48:276–286. doi: 10.1016/s0006-3223(00)00236-5. [DOI] [PubMed] [Google Scholar]
- Hada M, Porjesz B, Chorlian DB, Begleiter H, Polich J. Auditory P3a deficits in male subjects at high risk for alcoholism. Biological Psychiatry. 2001;49:726–738. doi: 10.1016/s0006-3223(00)01049-0. [DOI] [PubMed] [Google Scholar]
- Hesselbrock V, Begleiter H, Porjesz B, O’Connor S, Bauer L. P300 event-related potential amplitude as an endophenotype of alcoholism--evidence from the collaborative study on the genetics of alcoholism. Journal of Biomedical Science. 2001;8:77–82. doi: 10.1007/BF02255974. [DOI] [PubMed] [Google Scholar]
- Hjorth B. An on-line transformation of EEG scalp potentials into orthogonal source derivations. Electroencephalography and Clinical Neurophysiology. 1975;39:526–530. doi: 10.1016/0013-4694(75)90056-5. [DOI] [PubMed] [Google Scholar]
- Hjorth B. Principles for transformation of scalp EEG from potential field into source distribution. Journal of Clinical Neurophysiology. 1991;8:391–396. doi: 10.1097/00004691-199110000-00004. [DOI] [PubMed] [Google Scholar]
- Jodo E, Inoue K. Effects of practice on the P300 in a Go/NoGo task. Electroencephalography and Clinical Neurophysiology. 1990;76:249–257. doi: 10.1016/0013-4694(90)90019-g. [DOI] [PubMed] [Google Scholar]
- Jodo E, Kayama Y. Relation of a negative ERP component to response inhibition in a Go/No-go task. Electroencephalography and Clinical Neurophysiology. 1992;82:477–482. doi: 10.1016/0013-4694(92)90054-l. [DOI] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Mantey M, Nager W, Rada D, Muller-Vahl KR, Emrich HM, Dengler R, Munte TF, Dietrich D. Altered inhibition of motor responses in Tourette Syndrome and Obsessive-Compulsive Disorder. Acta Neurologica Scandinavica. 2001;104:36–43. doi: 10.1034/j.1600-0404.2001.00308.x. [DOI] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Nager W, Rada D, Muller-Vahl KR, Emrich HM, Dengler R, Munte TF, Dietrich D. Tourette syndrome and obsessive-compulsive disorder: event-related brain potentials show similar mechanisms [correction of mechanisms] of frontal inhibition but dissimilar target evaluation processes. Behavioural Neurology. 2003;14:9–17. doi: 10.1155/2003/326468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser S, Unger J, Kiefer M, Markela J, Mundt C, Weisbrod M. Executive control deficit in depression: event-related potentials in a Go/Nogo task. Psychiatry Research. 2003;122:169–184. doi: 10.1016/s0925-4927(03)00004-0. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. The role of brain oscillations as functional correlates of cognitive systems: a study of frontal inhibitory control in alcoholism. International Journal of Psychophysiology. 2004;51:155–180. doi: 10.1016/j.ijpsycho.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin L, Martz MJ, Mordkoff AM. Motor performance and sensory-evoked potentials. Electroencephalography and Clinical Neurophysiology. 1970;28:307–313. doi: 10.1016/0013-4694(70)90167-7. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. Journal of Neuroscience. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima R, Satoh K, Itoh H, Ono S, Furumoto S, Gotoh R, Koyama M, Yoshioka S, Takahashi T, Takahashi K, Yanagisawa T, Fukuda H. Functional anatomy of GO/NO-GO discrimination and response selection--a PET study in man. Brain Research. 1996;728:79–89. [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kiefer M, Marzinzik F, Weisbrod M, Scherg M, Spitzer M. The time course of brain activations during response inhibition: evidence from event-related potentials in a go/no go task. Neuroreport. 1998;9:765–770. doi: 10.1097/00001756-199803090-00037. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Hare RD, Liddle PF, McDonald JJ. Reduced P300 responses in criminal psychopaths during a visual oddball task. Biolical Psychiatry. 1999;45:1498–1507. doi: 10.1016/s0006-3223(98)00193-0. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Liddle PF. An event-related potential investigation of response inhibition in schizophrenia and psychopathy. Biolical Psychiatry. 2000;48:210–221. doi: 10.1016/s0006-3223(00)00834-9. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Sekihara K, Miyashita Y. No-go dominant brain activity in human inferior prefrontal cortex revealed by functional magnetic resonance imaging. European Journal of Neuroscience. 1998;10:1209–1213. doi: 10.1046/j.1460-9568.1998.00167.x. [DOI] [PubMed] [Google Scholar]
- Kopp B, Mattler U, Goertz R, Rist F. N2, P3 and the lateralized readiness potential in a nogo task involving selective response priming. Electroencephalography and Clinical Neurophysiology. 1996;99:19–27. doi: 10.1016/0921-884x(96)95617-9. [DOI] [PubMed] [Google Scholar]
- Kouri EM, Lukas SE, Mendelson JH. P300 assessment of opiate and cocaine users: effects of detoxification and buprenorphine treatment. Biological Psychiatry. 1996;40:617–628. doi: 10.1016/0006-3223(95)00468-8. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Human Brain Mapping. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingford-Hughes AR, Acton PD, Gacinovic S, Boddington SJ, Costa DC, Pilowsky LS, Ell PJ, Marshall EJ, Kerwin RW. Levels of gamma-aminobutyric acid-benzodiazepine receptors in abstinent, alcohol-dependent women: preliminary findings from an 123I-iomazenil single photon emission tomography study. Alcoholism: Clinical and Experimental Research. 2000;24:1449–1455. [PubMed] [Google Scholar]
- Lingford-Hughes AR, Acton PD, Gacinovic S, Suckling J, Busatto GF, Boddington SJ, Bullmore E, Woodruff PW, Costa DC, Pilowsky LS, Ell PJ, Marshall EJ, Kerwin RW. Reduced levels of GABA-benzodiazepine receptor in alcohol dependency in the absence of grey matter atrophy. British Journal of Psychiatry. 1998;173:116–122. doi: 10.1192/bjp.173.2.116. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Human Brain Mapping. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M. Perseration of central sets after frontal lesions in man. In: Warren JM, Akert K, editors. The Frontal Granular Cortex and Behavior. McGraw-Hill; New York: 1964. pp. 219–294. [Google Scholar]
- Nativ A, Lazarus JA, Nativ J, Joseph J. Potentials associated with the initiation and inhibition of visually triggered finger movement in humans: the “no-go potential” in the go/no-go paradigm. International Journal of Neuroscience. 1992;66:107–118. doi: 10.3109/00207459208999795. [DOI] [PubMed] [Google Scholar]
- Noldy NE, Stelmack RM, Campbell KB. Event-related potentials and recognition memory for pictures and words: the effects of intentional and incidental learning. Psychophysiology. 1990;27:417–428. doi: 10.1111/j.1469-8986.1990.tb02337.x. [DOI] [PubMed] [Google Scholar]
- Norman DA, Shallice T. Attention to action: willed and automatic control of behavior. In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness and Self Regulation: Advances in Research and Theory. Plenum Press; New York: 1986. pp. 1–18. [Google Scholar]
- Nunez PL. Electric Fields of the Brain: the Neurophysics of EEG. Oxford University Press; New York: 1981. [Google Scholar]
- Nunez PL. Neocortical Dynamics and Human EEG Rhythms. Oxford University Press; New York: 1995. [Google Scholar]
- Nunez PL, Pilgreen KL. The spline-Laplacian in clinical neurophysiology: a method to improve EEG spatial resolution. Journal of Clinical Neurophysiology. 1991;8:397–413. [PubMed] [Google Scholar]
- Perchet C, Revol O, Fourneret P, Mauguiere F, Garcia-Larrea L. Attention shifts and anticipatory mechanisms in hyperactive children: an ERP study using the Posner paradigm. Biological Psychiatry. 2001;50:44–57. doi: 10.1016/s0006-3223(00)01119-7. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM. ERPs to stimuli requiring response production and inhibition: effects of age, probability and visual noise. Electroencephalography and Clinical Neurophysiology. 1988;71:55–63. doi: 10.1016/0168-5597(88)90019-6. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Roth WT, Kopell BS. Age-related changes in auditory event-related potentials. Electroencephalography and Clinical Neurophysiology. 1980;49:266–276. doi: 10.1016/0013-4694(80)90221-7. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Weller BJ, Kopell BS. ERPs to response production and inhibition. Electroencephalography and Clinical Neurophysiology. 1985;60:423–434. doi: 10.1016/0013-4694(85)91017-x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Wenegrat BG, Roth WT, Kopell BS. Clinical application of the P3 component of event-related potentials. I. Normal aging. Electroencephalography and Clinical Neurophysiology. 1984;59:85–103. doi: 10.1016/0168-5597(84)90026-1. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, White PM, Mathalon D. Event-related potentials in alcoholic men: P3 amplitude reflects family history but not alcohol consumption. Alcoholism: Clinical and Experimental Research. 1991;15:839–850. doi: 10.1111/j.1530-0277.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Pliszka SR, Liotti M, Woldorff MG. Inhibitory control in children with attention-deficit/hyperactivity disorder: event-related potentials identify the processing component and timing of an impaired right-frontal response-inhibition mechanism. Biological Psychiatry. 2000;48:238–246. doi: 10.1016/s0006-3223(00)00890-8. [DOI] [PubMed] [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychological Bulletin. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Event-related potentials in individuals at risk for alcoholism. Alcohol. 1990;7:465–469. doi: 10.1016/0741-8329(90)90033-9. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Neurophysiological factors in individuals at risk for alcoholism. Recent Developments in Alcoholism. 1991;9:53–67. [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Effects of alcohol on electrophysiological activity of the brain. In: Begleiter H, Kissin B, editors. Alcohol and Alcoholism: The Pharmacology of Alcohol and Alcohol Dependence. Vol. 2. Oxford University Press; New York: 1996. pp. 207–247. [Google Scholar]
- Porjesz B, Almasy L, Edenberg HJ, Wang K, Chorlian DB, Foroud T, Goate A, Rice JP, O’Connor SJ, Rohrbaugh J, Kuperman S, Bauer LO, Crowe RR, Schuckit MA, Hesselbrock V, Conneally PM, Tischfield JA, Li TK, Reich T, Begleiter H. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proceedings of the National Academy of Sciences of the United States of America. 2002a;99:3729–3733. doi: 10.1073/pnas.052716399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Bihari B, Kissin B. Event-related brain potentials to high incentive stimuli in abstinent alcoholics. Alcohol. 1987;4:283–287. doi: 10.1016/0741-8329(87)90024-3. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Litke A, Bauer LO, Kuperman S, O’Connor SJ, Rohrbaugh J. Visual P3 as a potential phenotypic marker for alcoholism: Evidence from the COGA national project. In: Ogura C, Koga Y, Shimokochi M, editors. Recent Advances in Event-Related Brain Potential Research. Elsevier Science; Holland: 1996. pp. 539–549. [Google Scholar]
- Porjesz B, Begleiter H, Reich T, Van Eerdewegh P, Edenberg HJ, Foroud T, Goate A, Litke A, Chorlian DB, Stimus A, Rice J, Blangero J, Almasy L, Sorbell J, Bauer LO, Kuperman S, O’Connor SJ, Rohrbaugh J. Amplitude of visual P3 event-related potential as a phenotypic marker for a predisposition to alcoholism: preliminary results from the COGA Project. Collaborative Study on the Genetics of Alcoholism. Alcoholism: Clinical and Experimental Research. 1998;22:1317–1323. [PubMed] [Google Scholar]
- Porjesz B, Begleiter H, Wang K, Almasy L, Chorlian DB, Stimus AT, Kuperman S, O’Connor SJ, Rohrbaugh J, Bauer LO, Edenberg HJ, Goate A, Rice JP, Reich T. Linkage and linkage disequilibrium mapping of ERP and EEG phenotypes. Biological Psychology. 2002b;61:229–248. doi: 10.1016/s0301-0511(02)00060-1. [DOI] [PubMed] [Google Scholar]
- Posner MI, Dehaene S. Attentional networks. Trends in Neuroscience. 1994;17:75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Posner MI, DiGirolamo GJ. Executive attention: conflict, target detection and cognitive control. In: Parasuraman R, editor. The Attentive Brain. MIT Press; Cambridge: 1998. pp. 401–423. [Google Scholar]
- Prabhu VR, Porjesz B, Chorlian DB, Wang K, Stimus A, Begleiter H. Visual p3 in female alcoholics. Alcoholism: Clinical and Experimental Research. 2001;25:531–539. [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Ardekani BA, Choi SJ, Tanabe JL, Lim KO, Begleiter H. A functional MRI study of visual oddball: evidence for frontoparietal dysfunction in subjects at risk for alcoholism. Neuroimage. 2004;21:329–339. doi: 10.1016/j.neuroimage.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Roberts LE, Rau H, Lutzenberger W, Birbaumer N. Mapping P300 waves onto inhibition: Go/No-Go discrimination. Electroencephalography and Clinical Neurophysiology. 1994;92:44–55. doi: 10.1016/0168-5597(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Rodriguez Holguin S, Porjesz B, Chorlian DB, Polich J, Begleiter H. Visual P3a in male alcoholics and controls. Alcoholism: Clinical and Experimental Research. 1999;23:582–591. [PubMed] [Google Scholar]
- Rubia K, Oosterlaan J, Sergeant JA, Brandeis D, v Leeuwen T. Inhibitory dysfunction in hyperactive boys. Behavioural Brain Research. 1998;94:25–32. doi: 10.1016/s0166-4328(97)00166-6. [DOI] [PubMed] [Google Scholar]
- Schall U, Schon A, Zerbin D, Eggers C, Oades RD. Event-related potentials during an auditory discrimination with prepulse inhibition in patients with schizophrenia, obsessive-compulsive disorder and healthy subjects. International Journal of Neuroscience. 1996;84:15–33. doi: 10.3109/00207459608987247. [DOI] [PubMed] [Google Scholar]
- Shibata T, Shimoyama I, Ito T, Abla D, Iwasa H, Koseki K, Yamanouchi N, Sato T, Nakajima Y. Event-related dynamics of the gamma-band oscillation in the human brain: information processing during a GO/NOGO hand movement task. Neuroscience Research. 1999;33:215–222. doi: 10.1016/s0168-0102(99)00003-6. [DOI] [PubMed] [Google Scholar]
- Simson R, Vaughan HG, Jr., Ritter W. The scalp topography of potentials in auditory and visual Go/NoGo tasks. Electroencephalography and Clinical Neurophysiology. 1977;43:864–875. doi: 10.1016/0013-4694(77)90009-8. [DOI] [PubMed] [Google Scholar]
- Smith AM, Kiehl KA, Mendrek A, Forster BB, Hare RD, Liddle PF. Whole brain fMRI of a Go/No Go task. Neuroimage. 1998;7:S971. [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Srebro R. A bootstrap method to compare the shapes of two scalp fields. Electroencephalography and Clinical Neurophysiology. 1996;100:25–32. doi: 10.1016/0168-5597(95)00205-7. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Nunez PL, Silberstein RB. Spatial filtering and neocortical dynamics: estimates of EEG coherence. IEEE Transactions on Bio-medical Engineering. 1998;45:814–826. doi: 10.1109/10.686789. [DOI] [PubMed] [Google Scholar]
- Suresh S, Porjesz B, Chorlian DB, Choi K, Jones KA, Wang K, Stimus A, Begleiter A. Auditory P3 in Female Alcoholics. Alcoholism: Clinical and Experimental Research. 2003;27:1064–1074. doi: 10.1097/01.ALC.0000075549.49800.A0. [DOI] [PubMed] [Google Scholar]
- Sylvester CY, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, Jonides J. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia. 2003;41:357–370. doi: 10.1016/s0028-3932(02)00167-7. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T, Ogawa M, Nishikawa S, Tsukada H, Kakiuchi T, Sasaki K. Activation of the prefrontal, occipital and parietal cortices during go/no-go discrimination tasks in the monkey as revealed by positron emission tomography. Neuroscience Letters. 1997;224:111–114. doi: 10.1016/s0304-3940(97)13480-2. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Boomsma DI. Genetics of the human electroencephalogram (EEG) and event-related brain potentials (ERPs): a review. Human Genetics. 1994;94:319–330. doi: 10.1007/BF00201587. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, van Baal GC. Twin and family studies of the human electroencephalogram: a review and a meta-analysis. Biological Psychology. 2002;61:111–138. doi: 10.1016/s0301-0511(02)00055-8. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Heritability of human brain functioning as assessed by electroencephalography. American Journal of Human Genetics. 1996;58:562–573. [PMC free article] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Individual differences in P300 amplitude: a genetic study in adolescent twins. Biological Psychology. 1998;47:97–120. doi: 10.1016/s0301-0511(97)00025-2. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, van Baal GC, Molenaar PC, Boomsma DI, de Geus EJ. Stability of genetic and environmental influences on P300 amplitude: a longitudinal study in adolescent twins. Behavior Genetics. 2001;31:533–543. doi: 10.1023/a:1013389226795. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Hitzemann R, Fowler JS, Wolf AP, Pappas N, Biegon A, Dewey SL. Decreased cerebral response to inhibitory neurotransmission in alcoholics. The American Journal of Psychiatry. 1993;150:417–422. doi: 10.1176/ajp.150.3.417. [DOI] [PubMed] [Google Scholar]
- Wang K, Begleiter H. Local polynomial estimate of surface Laplacian. Brain Topography. 1999;12:19–29. doi: 10.1023/a:1022225522447. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Sugiura M, Sato K, Sato Y, Maeda Y, Matsue Y, Fukuda H, Kawashima R. The human prefrontal and parietal association cortices are involved in NO-GO performances: an event-related fMRI study. Neuroimage. 2002;17:1207–1216. doi: 10.1006/nimg.2002.1198. [DOI] [PubMed] [Google Scholar]
- Weisbrod M, Kiefer M, Marzinzik F, Spitzer M. Executive control is disturbed in schizophrenia: evidence from event-related potentials in a Go/NoGo task. Biological Psychiatry. 2000;47:51–60. doi: 10.1016/s0006-3223(99)00218-8. [DOI] [PubMed] [Google Scholar]
- Winterer G, Goldman D. Genetics of human prefrontal function. Brain Research: Brain Research Reviews. 2003;43:134–163. doi: 10.1016/s0165-0173(03)00205-4. [DOI] [PubMed] [Google Scholar]