Abstract
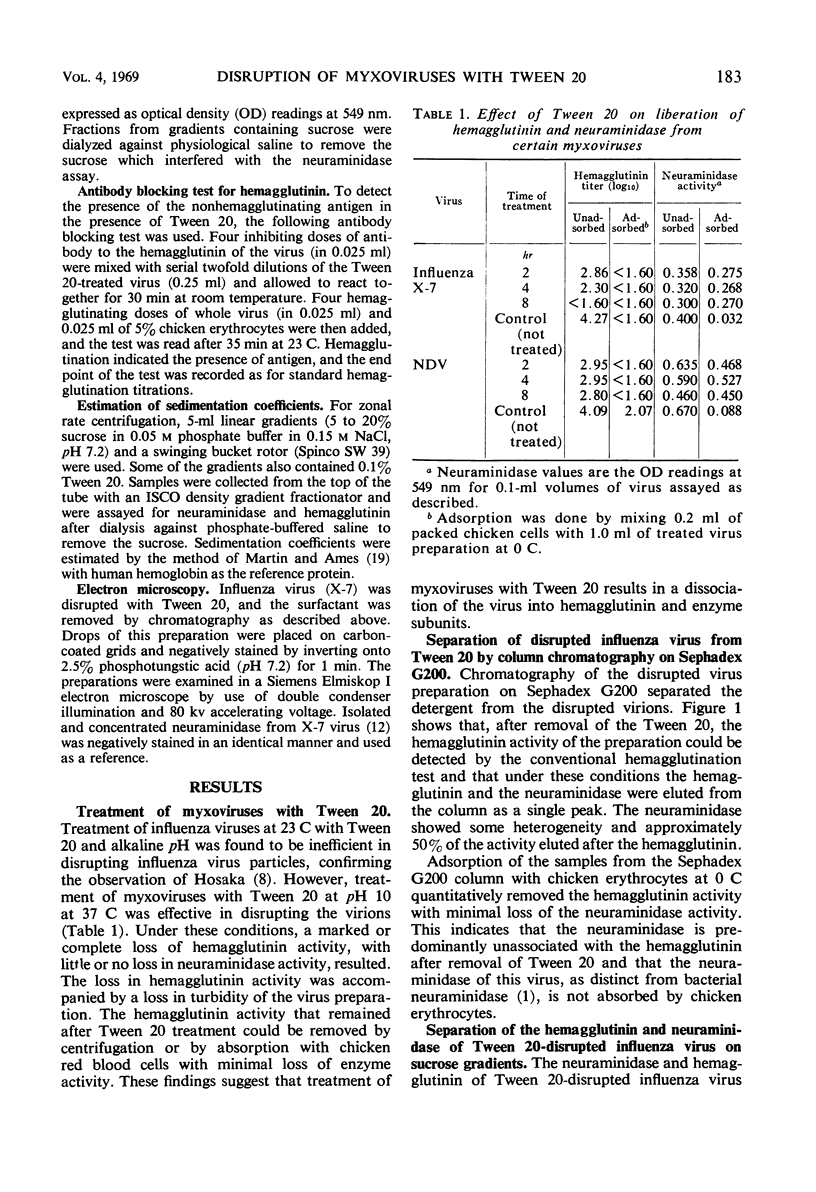
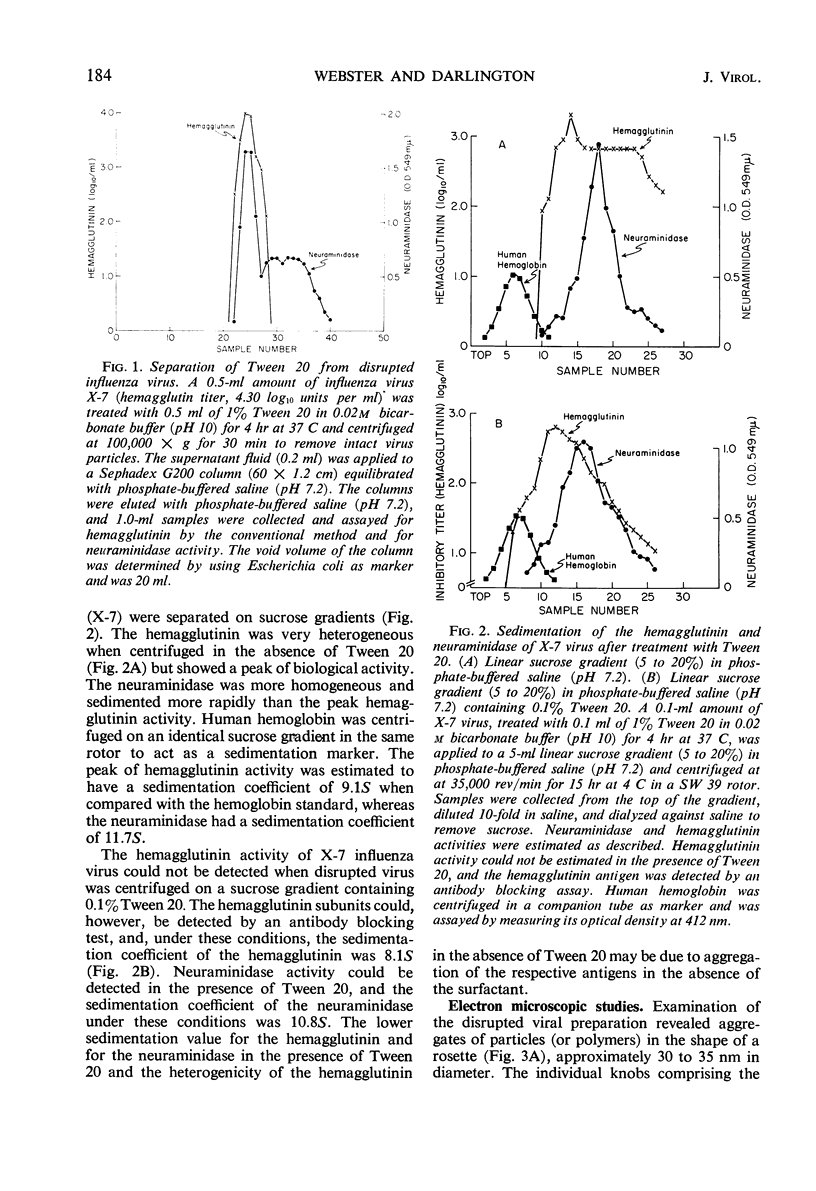
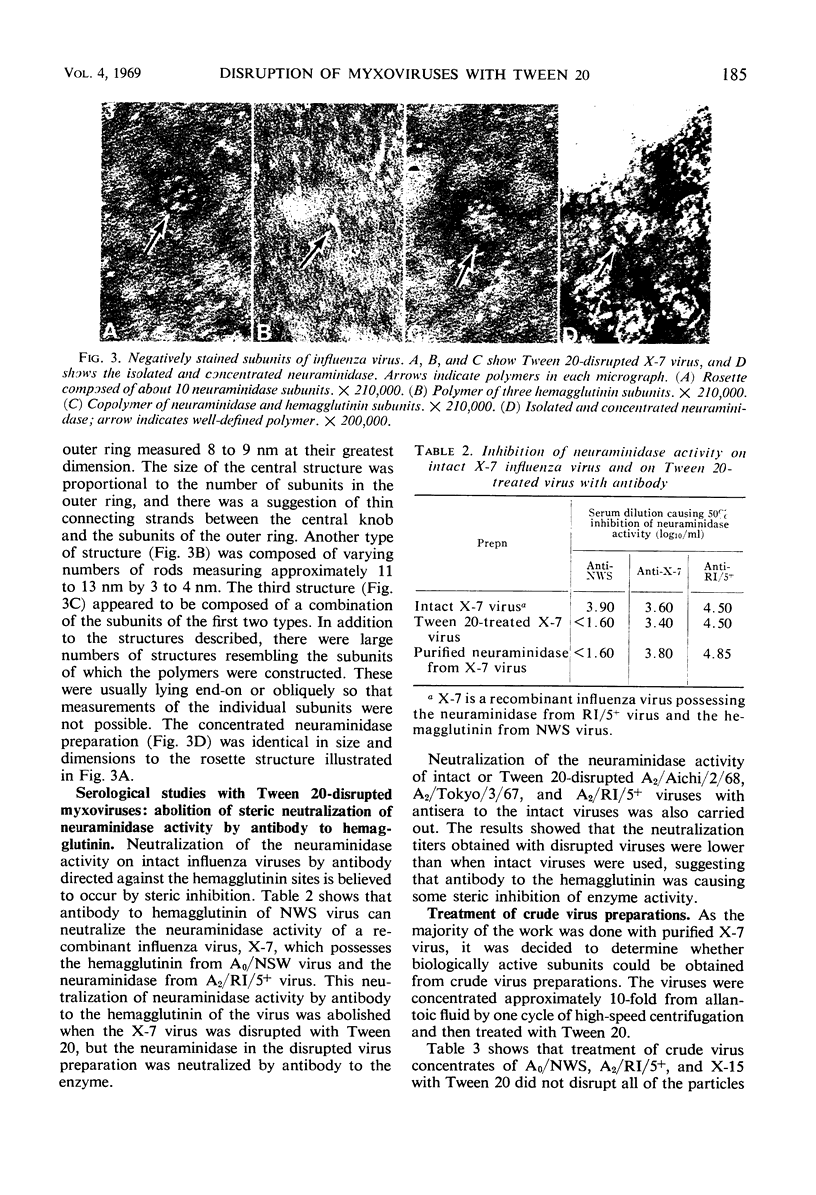
Myxoviruses were disrupted with Tween 20 at high pH, and the major surface antigens were separated in biologically active form. The neuraminidase had a sedimentation coefficient of 10.8S, and the hemagglutinin had a sedimentation coefficient of 8.1S. Electron microscopic examination of negatively stained preparations revealed structures identical in size and morphology to the neuraminidase and hemagglutinin subunits described by others. Inhibition of neuraminidase activity by antibody to the hemagglutinin which occurred with intact viruses (probably for “steric” reasons) did not occur after the viruses were disrupted with Tween 20. Serological assays for neuraminidase were possible in the presence of the mild surfactant, whereas serological assays for hemagglutinin were possible after removal of the reagent. Disruption of myxoviruses with Tween 20 therefore provides a method for the independent study of these antigens during antigenic drift.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADA G. L., FRENCH E. L., LIND P. E. Purification and properties of neuraminidase from Vibrio cholerae. J Gen Microbiol. 1961 Mar;24:409–425. doi: 10.1099/00221287-24-3-409. [DOI] [PubMed] [Google Scholar]
- Coleman M. T., Dowdle W. R., Pereira H. G., Schild G. C., Chang W. K. The Hong Kong-68 influenza A2 variant. Lancet. 1968 Dec 28;2(7583):1384–1386. doi: 10.1016/s0140-6736(68)92683-4. [DOI] [PubMed] [Google Scholar]
- DAVENPORT F. M., ROTT R., SCHAEFER W. Physical and biological properties of influenza virus components obtained after ether treatment. J Exp Med. 1960 Nov 1;112:765–782. doi: 10.1084/jem.112.5.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzeniek R., Frank H., Rott R. Electron microscopy of purified influenza virus neuraminidase. Virology. 1968 Dec;36(4):703–707. doi: 10.1016/0042-6822(68)90209-2. [DOI] [PubMed] [Google Scholar]
- HENLE W., LIEF F. S. Studies on the soluble antigen of influenza virus. I. The release of S antigen from elementary bodies by treatment with ether. Virology. 1956 Dec;2(6):753–771. doi: 10.1016/0042-6822(56)90056-3. [DOI] [PubMed] [Google Scholar]
- HOYLE L. Structure of the influenza virus; the relation between biological activity and chemical structure of virus fractions. J Hyg (Lond) 1952 Jun;50(2):229–245. doi: 10.1017/s0022172400019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka Y. Isolation and structure of the nucleocapsid of HVJ. Virology. 1968 Jul;35(3):445–457. doi: 10.1016/0042-6822(68)90223-7. [DOI] [PubMed] [Google Scholar]
- Kilbourne E. D., Laver W. G., Schulman J. L., Webster R. G. Antiviral activity of antiserum specific for an influenza virus neuraminidase. J Virol. 1968 Apr;2(4):281–288. doi: 10.1128/jvi.2.4.281-288.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbourne E. D. Recombination of influenza A viruses of human and animal origin. Science. 1968 Apr 5;160(3823):74–76. doi: 10.1126/science.160.3823.74. [DOI] [PubMed] [Google Scholar]
- LAVER W. G. Purification, N-terminal amino acid analysis, and disruption of an influenza virus. Virology. 1961 Aug;14:499–502. doi: 10.1016/0042-6822(61)90348-8. [DOI] [PubMed] [Google Scholar]
- LAVER W. G. STRUCTURAL STUDIES ON THE PROTEIN SUBUNITS FROM THREE STRAINS OF INFLUENZA VIRUS. J Mol Biol. 1964 Jul;9:109–124. doi: 10.1016/s0022-2836(64)80094-2. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Valentine R. C. Morphology of the isolated hemagglutinin and neuraminidase subunits of influenza virus. Virology. 1969 May;38(1):105–119. doi: 10.1016/0042-6822(69)90132-9. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Paniker C. K. Serological relationships between the neuraminidases in influenza viruses. J Gen Virol. 1968 May;2(3):385–394. doi: 10.1099/0022-1317-2-3-385. [DOI] [PubMed] [Google Scholar]
- ROTT R., SCHAEFER W. Fine structure of subunits isolated from Newcastle disease virus (NDV). Virology. 1961 Jun;14:298–299. doi: 10.1016/0042-6822(61)90210-0. [DOI] [PubMed] [Google Scholar]
- SCHAFER W. Structure of some animal viruses and significance of their components. Bacteriol Rev. 1963 Mar;27:1–17. doi: 10.1128/br.27.1.1-17.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Pereira H. G. A common surface antigen in influenza viruses from human and avian sources. J Gen Virol. 1968 Sep;3(2):201–208. doi: 10.1099/0022-1317-3-2-201. [DOI] [PubMed] [Google Scholar]




