Abstract
This study examined trajectories of smoking during pregnancy among low-income smokers and differences on demographics, psychopathology, and smoking outcome expectancies among women with different smoking trajectories. The sample consisted of 215 urban pregnant smokers living in the United States. Results indicated four trajectories of smoking and significant changes over time within each trajectory. Persistent smokers had the highest demographic and mental health risks, reported higher craving compared to light smokers, and were more likely to endorse smoking to reduce negative affect, for state enhancement motives. Implications for intervention are discussed. The study was funded by the National Institute on Drug Abuse.
Keywords: nicotine, pregnant smokers, expectancies, smoking trajectories, depression, stress
Introduction
Tobacco is one of the most commonly used drugs during pregnancy. In 2006, mean smoking prevalence was 13.2%, with rates as high as 33% among women with high school or less education (Martin et al., 2006). Smoking during pregnancy is harmful for mother and child, with increased risk for prenatal complications such as risk for abruption, placenta previa, perinatal mortality, impaired fetal growth, higher neonatal stress and irritability, hearing loss, and respiratory problems (Cornelius & Day, 2000; Cornelius, Leech, & Goldschmidt, 2004; Fried, 1998; McCartney, Fried, & Watkinson, 1994; Stroud et al., 2009). Cigarette exposure also has long-lasting effects on developmental outcomes such as externalizing behavior problems (Buschgens et al., 2009; Porath & Fried, 2005; Silberg et al., 2003; Wakschlag et al., 2011) and higher risk for smoking in later childhood and adolescence (Cornelius, Leech, Goldschmidt, & Day, 2000; O'Callaghan et al., 2009).
Given the long-lasting effects of cigarettes on maternal and child health, more research is needed on patterns of smoking and factors associated with changes in smoking during pregnancy. Such knowledge may have significant implications for timing and content of smoking cessation interventions. To date, only three studies have examined variations in smoking patterns through pregnancy. Unlike the high stability in smoking over time among adult daily smokers (Zhu, Sun, Hawkins, Pierce, & Cummins, 2003), these studies noted large variations in self-reported smoking patterns in pregnancy (Munafo, Heron, & Araya, 2008; Pickett, Rathouz, Kasza, Wakschlag, & Wright, 2005; Pickett, Wakschlag, Dai, & Leventhal, 2003), with repeated quit attempts followed by relapse and within person variations in amount smoked across pregnancy. However, with two exceptions (Munafo et al., 2008; Pickett et al., 2003), there have been few attempts to classify patterns of change in intensity and duration of smoking during pregnancy. This is important because these patterns of change in smoking behavior may be a more accurate predictor of maternal and child health than smoking intensity at a single point in time during pregnancy. Based on the limited literature on trajectories of smoking during pregnancy, we expect that there are light intermittent smokers who may quit upon pregnancy recognition; heavier chronic smokers who may reduce the number of cigarettes smoked upon pregnancy recognition but increase by the third trimester, and more moderate smokers who are more variable in smoking patterns.
Understanding of patterns of smoking during pregnancy and potential changes in these patterns over time is essential not only for examining the impact of these trajectories on maternal and child health, but also because this has the potential to inform timing of smoking cessation intervention. About a third of women smokers who smoked before pregnancy are known to significantly reduce their cigarette consumption upon pregnancy recognition (Centers for Disease Control and Prevention, 2009). However, little is known regarding the stability of these cessation efforts and about factors that differentiate women who successfully reduce their smoking throughout pregnancy compared to women who are more persistent smokers or those who quit later in pregnancy. This may be important information for determining the content of cessation interventions during pregnancy in addition to timing issues.
Models of substance use expectancies (Baker, Morse, & Sherman, 1986; Goldman, 1999; Witkiewitz & Marlatt 2004) suggest that expectancies may be the critical “final common pathway” by which individual difference factors such as personality, social contextual factors, and cultural information may influence substance use. Outcome expectancies of substance use may be defined as beliefs about potential positive and negative consequences of use. Smoking outcome expectancies may be significantly different among women with different patterns of smoking during pregnancy. The negative reinforcement model of addiction emphasizes the importance of positive expectancies such as negative affect reduction for substance use, conceptualizing this as the single most important motivational factor for continued substance use (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004). However, few studies examined smoking outcome expectancies among pregnant women, and none examined if different trajectories of smoking during pregnancy were associated with different outcome expectancies. Although models of substance use expectancies have been used extensively to guide development of cessation interventions, these have not been uniformly successful, especially for low-income pregnant smokers (Fiore, Jaen, & Baker, 2008). Increased knowledge about differences in these social cognitive factors for women with different patterns of change in smoking during pregnancy may be important for determining the content of smoking cessation efforts for pregnant women.
In addition to social cognitive factors such as outcome expectancies, low-income pregnant smokers are known to experience a multitude of stressors (Kendzor et al., 2010). These include demographic factors such as financial difficulties, psychological symptoms such as depression and anger, and high levels of perceived stress (Allen, Prince, & Dietz, 2009; Kendzor et al., 2010). To date, only two studies examined correlates of patterns of change in smoking during pregnancy, with one focused exclusively on demographic correlates (Pickett et al., 2003) and the other on depression (Munafo et al., 2008). Results from the first study indicated that women with high school education and above were more likely to be intermittent smokers and more likely to be successful quitters (Pickett et al., 2003). However, smoking intensity and persistence across pregnancy were examined separately in this study, possibly resulting in some confusion since heavier smokers are more likely to have difficulty quitting. Results from the second study indicated that while pre-pregnancy depression was not predictive of trajectories of smoking during pregnancy, reduction in symptoms of depression during pregnancy was associated with reductions in smoking (Munafo et al., 2008). However, this study examined trajectories of smoking at only two time points in pregnancy and several time points in the postnatal period, thus providing limited data about changes during pregnancy. Other studies have noted associations between smoking and hostility in the general population (Houston, 1991; Miller, 1996). Trait hostility consistently predicted higher smoking rates in men and women (Lipkus, 1994; Siegler, Peterson, Barefoot, & Williams, 1992; Whiteman, Fowkes, Deary, & Lee, 1997), and smokers reported higher levels of anger/hostility during pregnancy (Schuetze, Lopez, Granger, & Eiden, 2008). Finally, several studies have noted significant associations between high levels of stress and smoking during pregnancy (Businelle et al., 2010; Ludman et al., 2000; Reitzel et al., 2010). Results from a prospective study (Ludman et al., 2000) indicated that low depression and stress predicted high quit rates in pregnancy with stress decreasing over time among nonsmokers but increasing among smokers (Ludman et al., 2000). Thus, the multiple stressors of financial strain, maternal psychological symptoms of depression and hostility, and perceived stress may differentiate smoking trajectories during pregnancy in addition to social cognitive factors.
We hypothesized that pregnant smokers may be classified into different groups based on their smoking trajectories during pregnancy: light, intermittent smokers; heavier chronic smokers; and moderate smokers with variable patterns of smoking during pregnancy. We expected the majority of change in smoking patterns to occur upon pregnancy recognition with some variability in patterns of reduction and increase thereafter. We expected that heavier, persistent smokers to have more positive smoking outcome expectancies compared to those with lighter, intermittent patterns. We also expected there to be higher sociodemographic risk, higher maternal depression, anger/hostility, and perceived stress among heavier persistent smokers compared to light intermittent smokers or quitters.
Methods
Sample Selection
Pregnant women who presented for prenatal care at a large city hospital were asked to complete a self-report screening form at their first prenatal appointment. Current smokers who met initial eligibility criteria were invited to participate in the study described as one focusing on maternal health and child development. Eligible families were informed of the purpose and nature of the study tasks, duration, and confidentiality procedures. Initial eligibility criteria included the following: <20 weeks gestation, maternal age ≥18, no illicit drug use (other than cannabis), no heavy alcohol (>1 drink/day or 4 drinks on one occasion) or cannabis consumption (>1 joint/day or 4 joints on one occasion) after pregnancy recognition, and no multiple births. Women who agreed to participate were scheduled for a total of four initial interviews; a prenatal interview once in each trimester and a postnatal interview at 2 months of infant age. The first and second prenatal interviews were scheduled within the last 2 weeks of each trimester at a time convenient for the participant. The third prenatal interview was scheduled 6–8 weeks before the expected delivery date. Five women who delivered before their third prenatal appointment completed this interview at their 2-month appointment. Transportation to the University interview site was provided for all participants who did not have their own transportation. Prenatal cigarette and other drug use was ascertained by a combination of self-report, maternal oral fluids collected at each assessment, and meconium collected at delivery.
Of the 3,583 women who completed the screening form over a 3-year period, 860 smokers met initial eligibility criteria. Of these, 305 smokers completed the first prenatal interview. Among these 305 smokers, 2 had an abortion, 7 had a miscarriage, 31 did not meet eligibility criteria after the first prenatal interview (used illicit substances or had major mental illness), and 50 could not be located, were not interested, or did not show for follow-up interviews, resulting in a final sample of 215 smokers.
Participants
The final sample consisted of 215 pregnant smokers. Women ranged in age from 18 to 39 (M = 24.18, SD = 4.87). The sample was 47% African-American, 18% Hispanic, 36% Caucasian, 2% American Indian/Alaskan Native, and 7% other or mixed race. Women were allowed to endorse more than one race. Forty-six percent of the women were married or living with their partner, 32% were in a relationship but not living with a partner, and the rest were single. Thirty-two percent of women had less than a high-school education, 29% earned their high-school diploma, 27% completed some college courses, 8% had a vocational or technical training degree, and 4% had a bachelor's degree. Forty-three percent were working at a paid job for 4–45 hours per week. At recruitment, 10% received mental health services in the past 6 months, 14% Temporary Assistance for Needy Families (TANF), 56% food stamps, 68% were on Medicaid, and 66% received assistance from Women Infants and Children program. Thus, the sample consisted mostly of young, low-income minority pregnant women. Mean gestational age at the first prenatal interview was 12 weeks (SD = 2.2; range = 8–19) and mean gestational age at delivery was 38 weeks (SD = 1.95; range= 26–42).
Procedure
All mothers were screened for initial eligibility and informed consent was obtained from those interested and eligible. Mothers were interviewed and maternal oral fluid samples were collected at the end of each trimester and at 2 months' postpartum, and infant meconium samples were collected at delivery. The study was approved by the appropriate institutional review board. Participants were informed that data confidentiality was protected by a US Federal Certificate of Confidentiality issued by the National Institute on Drug Abuse. Participants received a $20.00 check for each prenatal interview and $50 payment in the form of check, gift card, and toys at the 2-month postnatal interview. Maternal interviews were conducted by trained research assistants at the University laboratory. All interview questions were read aloud to the participants in order to address possible literacy issues. A portion of maternal interviews (the depression, stress, and anger/hostility measures) were self-administered audio computer-assisted interviews that was touch screen enabled. Participants wore head phones and heard the questions and response items read to them. To select an answer, participants used the touch screen interface to select a response and move to the next question. Research assistants were present in the room during this portion of the interview to answer any questions that arose. The average length of the first prenatal interview was 1 hour and the average length of all subsequent interviews was about 2 hours.
Maternal Substance Use
Maternal smoking status was determined through a combination of self-report, infant meconium, and maternal oral fluid results. At each prenatal interview, the Timeline Follow-Back Interview (TLFB) (Sobell, Sobell, Klajner, Pavan, & Basian, 1986) was used to assess maternal substance use. The TLFB was used to gather daily tobacco, alcohol, and cannabis use for the previous 3 months. The primary measure of interest in examining patterns of change in smoking during pregnancy was the average number of cigarettes per month during the 3 months before conception and each month of pregnancy. In addition to cigarette use, TLFB also yielded information about the average number of standard drinks per day and average number of joints per day. This method has been established as a reliable and valid method of obtaining longitudinal data on substance-use patterns, has good test-retest reliability, and is highly correlated with other intensive self-report measures (Brown et al., 1998).
Maternal oral fluid was collected at each prenatal interview to provide objective evidence of recent exposure. The oral fluid specimens were analyzed by a commercial laboratory for cotinine, the primary nicotine biomarker, with enzyme-linked immunosorbent assay (ELISA at the first prenatal interview only for the first 32 women recruited into the study at 10ng/mL cutoff) or liquid chromatography-tandem mass spectrometry (LC-MSMS) at 5ng/mL cutoff for cotinine for active smoking.
After birth, meconium specimens were collected from soiled diapers twice daily until the appearance of milk stool, transferred to storage containers, and frozen until transport to the National Institute on Drug Abuse for analysis. Meconium specimens were assayed with a validated LS-MSMS method for nicotine, cotinine, and 3′-trans-hydroxycotinine (Gray et al., 2010a; Gray et al., 2010b).
Maternal Psychosocial Variables
The association between patterns of smoking and three psychosocial variables assessed in the second trimester interview were examined in this study: symptoms of depression, stress, and anger/hostility. Symptoms of depression were assessed using the Beck Depression Inventory (BDI) (Beck & Steer, 1984; Sharp & Lipsky, 2002). The BDI has high reliability in a range of populations, high internal consistency, and well-established construct validity (Beck & Steer, 1984; Sharp and Lipsky, 2002). The internal consistency of the BDI was quite high for this sample, Cronbach's alpha = 0.87. Maternal stress during pregnancy was assessed with the Perceived Stress Scale (PSS) (Cohen, Kamarck, & Mermelstein, 1983) which has good reliability and validity (Cohen et al., 1983). The internal consistency of the PSS for this sample was α = 0.80. Symptoms of anger, hostility, and aggression were measured during pregnancy with the Buss-Perry Aggression Questionnaire (Buss & Perry, 1992). This measure yields four subscales: anger, hostility, physical, and verbal aggression as well as a total score. The total anger/hostility score was used in this study with internal consistency of α = 0.92 for this sample.
Smoking Outcome Expectancies
The Smoking Consequences Questionnaire—Adult (Copeland, Brandon, & Quinn, 1995) was used to examine smoking outcome expectancies in the second trimester interview. The scale comprises 10 factors: Negative Affect Reduction, Stimulation/State Enhancement, Health Risk, Taste/Sensorimotor Manipulation, Social Facilitation, Weight Control, Craving/Addiction, Negative Physical Feelings, Boredom Reduction, and Negative Social Impression. The internal consistency for these subscales ranged from 0.70 (for negative social impression) to 0.95.
Analytic Approach
To estimate trajectories of smoking during pregnancy, mixture models were estimated using the SAS procedure TRAJ (Jones, Nagin, & Roeder, 2001). This semi-parametric procedure uses longitudinal data to estimate group membership. Missing data are accommodated through the use of a maximum likelihood estimation procedure (Jones et al., 2001). Two, three, four, and five group models were fit. To select the most appropriate model, a number of criteria were assessed. First, Bayesian Information Criteria (BIC) were compared for each set of models with the goal of identifying the group that had the maximum BIC (i.e., least negative) (Nagin, 1999). However, for some applications, the addition of new groups often leads to an improvement in BIC that does not result in a better model (Nagin, 1999). Therefore, it is also important to consider other factors. For example, the addition of one group may result in splitting one trajectory class into two smaller groups with parallel trajectories (Nagin, 1999).
Consistent with previous work (e.g., Chung, Maisto, Cornelius, Martin, & Jackson, 2005; Clark, Jones, Wood, & Cornelius, 2006; Edwards, Homish, Eiden, Grohman, & Leonard, 2008), groups that resulted in a small number of individuals were also not considered for the final models. Finally, standard errors were examined because the presence of large standard errors can be a sign that a model was overparameterized (Nagin, 1999). In addition to selecting the correct number of groups, a determination of the appropriate polynomial to describe the curve must be made. A significant zero-order polynomial would indicate a group that did not experience a change over time, whereas a first-order and second-order polynomial would describe linear and quadratic changes in the trajectory group over time, respectively (Nagin, 1999). After selecting an appropriate model to describe changes in smoking during pregnancy, MANOVAs were used to examine differences between groups for theoretically associated constructs.
Results
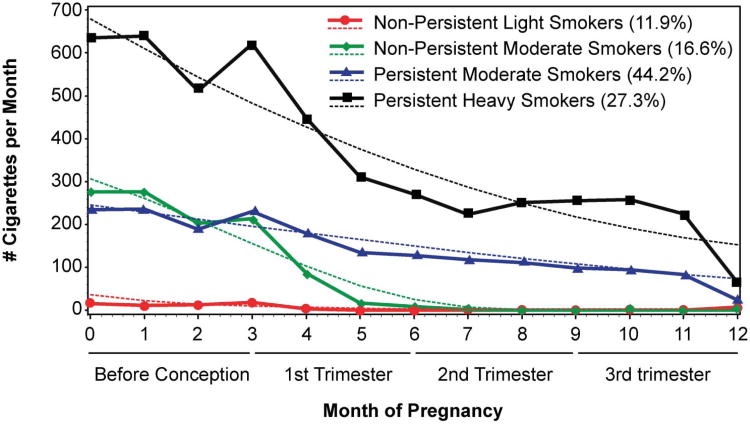
The trajectory analyses identified a four and five group model that fit the data. The five group model, however, resulted in low prevalence rates for one trajectory class (prevalence was 6.0%; N = 10). Therefore, the four class model was selected. The four group model yielded a group of non/light smokers (N = 26; 12.1%), a nonpersistent moderate smoking group exhibiting significant declines in smoking between months 3 and 5 corresponding approximately to the time of pregnancy recognition (N = 36; 16.7%), a persistent moderate smoking group (N = 94; 43.7%), and a persistent heavy smoking group (N = 59; 27.4%) (Figure 1). Months 1–3 in the figure reflect the 3 months before conception, and the months thereafter, reflect the months of pregnancy.
Figure 1.
Although changes in smoking in the non/light smoker group were minimal, there was evidence of a statistically significant quadratic effect with rates of smoking slightly higher at baseline and at 12 months compared to the middle months (quadratic effect, coefficient = 2.36, p < .05). The nonpersistent moderate smoking group exhibited a significant linear decline in rates of smoking over time (linear effect, coefficient = −43.34, p < .001). The persistent moderate smoker group also had a linear decline in smoking over time; however, this decline was gradual (linear effect, coefficient = −17.7, p > .001). The fourth group, the heavy smoking group, demonstrated a quadratic effect with rates of smoking decreasing initially followed by a period of increased smoking (quadratic effect, coefficient −2.28, p < .001).
Group Differences in Substance Use
MANOVA with the trajectory groups as the independent variable and maternal cigarette, alcohol, and marijuana use before conception as dependent variables yielded a significant multivariate effect of group, F(9, 508.80) = 45.09, p < .001. Univariate analyses followed by Bonferroni post-hoc comparisons indicated that, as would be expected, the persistent heavy smokers smoked the highest number of cigarettes per day, followed by persistent and then the non-persistent moderate smokers, with light smokers reporting the lowest number of cigarettes per day (see Table 1). There were also significant group differences in average number of standard drinks per day, with persistent heavy smokers reporting higher number of drinks per day compared to light smokers (see Table 1). The pattern of group differences with regard to cigarette smoking was similar across all three trimesters, with heavy smokers reporting the highest number of cigarettes per day across pregnancy compared to the other group, as indicated in Table 1, as would be expected.
Table 1. Differences on smoking and other drug use among trajectory groups.
| Nonpersistent light smokers, N = 26 | Nonpersistent moderate smokers, N = 36 | Persistent moderate smokers, N = 94 | Persistent heavy smokers, N = 59 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Variables | M | SD | M | SD | M | SD | M | SD | ||||
| Before conception: | ||||||||||||
| # cigarettes/day | 0.50a | 0.75 | 9.15b | 4.53 | 7.78c | 3.33 | 21.21d | 5.21 | ||||
| # drinks/day | 0.25a | 0.58 | 1.04 | 1.98 | 0.39 | 0.84 | 1.27b | 3.22 | ||||
| # joints/day | 0.50 | 1.59 | 0.85 | 1.34 | 1.33 | 2.54 | 1.23 | 2.32 | ||||
| First trimester | ||||||||||||
| # cigarettes/day | 0.25a | 0.51 | 3.62b | 2.45 | 5.97c | 2.77 | 15.17d | 5.15 | ||||
| # drinks/day | 0.11 | 0.32 | 0.31 | 0.83 | 0.15 | 0.28 | 0.37 | 0.73 | ||||
| # joints/day | 0.28 | 0.78 | 0.38 | 0.77 | 0.94 | 1.96 | 0.79 | 1.53 | ||||
| Second trimester | ||||||||||||
| # cigarettes/day | .00a | .00 | .13a | .35 | 3.91b | 2.79 | 8.18c | 5.54 | ||||
| # drinks/day | .00 | .00 | .00 | .00 | .01 | .02 | .01 | .02 | ||||
| # joints/day | .07 | .35 | .04 | .13 | .17 | .51 | .19 | .57 | ||||
| Third trimester | ||||||||||||
| # cigarettes/day | .04a | .16 | .00a | .01 | 3.17b | 2.35 | 8.50c | 6.96 | ||||
| # drinks/day | .00 | .02 | .00 | .02 | .00 | .01 | .01 | .03 | ||||
| # joints/day | .02 | .10 | .02 | .11 | .07 | .29 | .11 | .47 | ||||
| Positive saliva cotinine | 35% | 36% | 96% | 98% | ||||||||
| Positive meconium | 41% | 27% | 86% | 93% | ||||||||
Note. Means with different superscripts were significantly different from each other.
The trajectory groups were based only on maternal self-report. Thus, we examined if there were differences among groups on the salivary cotinine measures taken once during each trimester, and the infant meconium assays. As indicated in Table 1, the majority of persistent moderate and heavy smokers had an oral fluid sample positive for cotinine at some point during pregnancy, and infant meconium samples that were positive for nicotine or metabolites of nicotine. However, contrary to expectations, a substantial proportion of the light smokers had positive oral fluid cotinine or infant meconium results (see Table 1), raising some questions about the veracity of self-reports among a subset of these women.
Group Differences in Demographics and Social Network Variables
ANOVA with the trajectory groups as the independent variable and maternal education (number of years of education) as the dependent variable indicated a significant group difference in maternal education, F(3, 211) = 4.49, p < .01. As indicated in Table 2, light smokers had more years of education compared to moderate or heavy persistent smokers. ANOVA with number of close relatives who smoked as the dependent measure yielded a significant effect of group, F(3, 211) = 5.13, p < .01, with light smokers having fewer close relatives who smoked compared to persistent heavy smokers (see Table 2). There was a significant association between race (white vs. non-white) and trajectory group, χ2 (3,N = 215) = 9.84, p < .05, and between work status and trajectory group, χ2 (3, N= 215) = 9.84, p < .05. Persistent heavy smokers were more likely to be White compared to the other groups, and persistent moderate smokers were more likely to be working compared to the other groups (see Table 2).
Table 2. Demographic differences among trajectory groups.
| Nonpersistent light smokers N=26 | Nonpersistent moderate smokers N = 36 | Persistent moderate smokers N=94 | Persistent heavy smokers N=59 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Variables | M | SD | M | SD | M | SD | M | SD | ||||
| Maternal age | 23.3 | 3.2 | 23.8 | 6.6 | 24.0 | 4.7 | 24.7 | 5.5 | ||||
| Years of education* | 12.6a | 1.7 | 12.6 | 1.6 | 11.7b | 1.5 | 11.6b | 1.8 | ||||
| Age when first smoked | 16.6 | 1.4 | 15.3 | 3.2 | 15.6 | 3.1 | 14.7 | 3.1 | ||||
| Close relatives smoke* | 1.3a | .87 | 1.2 | .88 | 1.5 | .86 | 1.8b | .89 | ||||
| Friends smoke | 1.4 | 1.1 | 1.6 | 1.0 | 1.8 | 0.97 | 1.8 | 1.0 | ||||
| % white* | 23% | 39% | 29% | 51% | ||||||||
| % on TANF | 4% | 8% | 17% | 19% | ||||||||
| % on food stamps | 54% | 39% | 60% | 61% | ||||||||
| % on medicaid | 65% | 64% | 66% | 76% | ||||||||
| % working for pay* | 42% | 47% | 69% | 49% | ||||||||
| % married/cohabiting | 50% | 56% | 41% | 46% | ||||||||
| % with smoking partner | 54% | 75% | 71% | 78% | ||||||||
| % other household smokers | 15% | 11% | 27% | 32% | ||||||||
Note. Means with different superscripts were significantly different from each other.
Variables were significantly different across the groups at p < .05.
Group Differences in Psychological Symptoms
Measures of prenatal stress and depression were highly correlated (r = 0.63) and were, thus, analyzed using MANOVA. MANOVA with depression and stress as the dependent measures yielded a significant group difference, F(6, 388) = 2.20, p < .05. Univariate analyses followed by simple contrasts indicated that heavy and nonpersistent moderate smokers reported higher levels of prenatal depression compared to light smokers. Moderate and heavy smokers also reported higher prenatal stress compared to light smokers (see Table 3). ANOVA with the total BPA score indicated a significant effect of group, F(3, 185) = 2.60, p < .05. As indicated in Table 3, persistent smokers reported higher levels of anger, hostility, and aggression compared to light smokers.
Table 3. Differences on psychosocial variables and smoking expectancies among trajectory groups.
| Nonpersistent light smokers N = 26 | Nonpersistent moderate smokers N = 36 | Persistent moderate smokers N = 94 | Persistent heavysmokers N = 59 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Variables | M | SD | M | SD | M | SD | M | SD | ||||
| Prenatal depression* | 13.2a | 5.8 | 17.7b | 11.4 | 15.2 | 6.8 | 18.7b | 10.6 | ||||
| Prenatal stress* | 23.0a | 6.1 | 27.8b | 9.1 | 26.4b | 6.9 | 26.8b | 8.3 | ||||
| Prenatal Anger/hostility* | 70.4a | 17.6 | 78.3 | 23.4 | 79.9b | 18.9 | 80.3b | 19.2 | ||||
| Smoking expectancies | ||||||||||||
| NA reduction* | 4.4a | 3.8 | 5.4 | 3.0 | 5.7 | 2.3 | 7.1b | 1.6 | ||||
| Stimulation/SE* | 0.73a | 1.6 | 1.3 | 1.7 | 1.4 | 1.6 | 2.2b | 2.2 | ||||
| Craving/addiction* | 3.0a | 3.4 | 4.6 | 2.7 | 5.3b | 2.1 | 6.0b | 2.0 | ||||
| Neg. physical feelings* | 1.9 | 2.3 | 2.2 | 2.4 | 1.5 | 1.9 | 2.6 | 2.5 | ||||
Note. Means with different superscripts were significantly different from each other.
Variables were significantly different across the groups at p < .05.
Group Differences in Smoking Expectancies
MANOVA with the smoking expectancies subscales yielded a significant multivariate effect of group status on smoking expectancies, F(30, 476.18) = 1.78, p < .01. There were significant group differences on four subscales, negative affect reduction, stimulation/state enhancement, craving/addiction, and negative physical feelings. As indicated in Table 3, heavy smokers had higher scores on the negative affect reduction scale and the stimulation/state enhancement scales compared to light smokers, and persistent moderate and heavy smokers had higher scores on the craving/addiction scale compared to light smokers. Although the overall univariate effect was significant for the negative physical feelings scale, none of the simple contrasts with the light smokers as the comparison group were significant.
Discussion
One goal of this study was to examine trajectories of smoking during pregnancy. Results indicated four distinct trajectories of smoking during pregnancy reflecting both persistency and amount of smoking. The persistent moderate and heavy smokers both continued to smoke through pregnancy, but had different patterns of change. Moderate smokers exhibited a gradual decline in number of cigarettes smoked during pregnancy while the heavy smokers exhibited a decrease around the time of pregnancy recognition followed by an increase in number of cigarettes smoked in the second and third trimesters. The nonpersistent moderate smokers smoked similar number of cigarettes as persistent moderate smokers before pregnancy, but exhibited a sharp decline in the number of cigarettes upon pregnancy recognition. With regard to potential fetal exposure effects, both groups of persistent smokers may be the most problematic with continued smoking through second and third trimesters.
Persistent smokers were more likely to have less than high school education compared to the other two groups. This result is similar to that reported by Pickett and colleagues (Pickett et al., 2003) indicating that consistent pregnancy smokers were more likely to have less than high school education compared to intermittent smokers. Heavier persistent smokers were also more likely to be White, while moderate persistent smokers were more likely to have a paid job compared to the other groups.
Both nonpersistent moderate smokers and persistent heavy smokers had similar and higher depression symptoms during pregnancy compared to nonpersistent light smokers. A previous study examining changes in depression indicated that while prepregnancy depression scores were unrelated to changes in smoking during pregnancy, reduction in depression during pregnancy and postpartum was predictive of smoking cessation (Munafo et al., 2008). Perhaps persistent moderate smokers have higher levels of depression earlier in pregnancy or have higher overall symptoms throughout pregnancy, while non-persistent moderate smokers experience greater variability in symptoms of depression that could not be captured with one assessment. The result with regard to higher depression among persistent heavy smokers is supportive of a number of previous studies reporting significant associations between depression and smoking during pregnancy (Businelle et al., 2010; Ludman et al., 2000; Reitzel et al., 2010).
Moderate and heavy smokers reported higher stress and persistent smokers reported higher anger/hostility compared to nonpersistent light smokers. These results are supportive of several previous studies on the association between consistent smoking during pregnancy and higher stress (Businelle et al., 2010; Ludman et al., 2000; Reitzel et al., 2010). However, with one exception, (Schuetze et al., 2008), few studies examined issues of anger/hostility and smoking during pregnancy. The current results indicate the importance of examining anger as a significant correlate of continued smoking during pregnancy in addition to depression and stress.
Few studies examined smoking outcome expectancies during pregnancy, although both theory and empirical evidence highlight the importance of outcome expectancies as proximal predictors of continued smoking (Copeland et al., 1995; Wetter et al., 1994). Persistent heavy smokers were more likely to endorse smoking to reduce negative affect. This is supportive of the negative reinforcement model of drug addiction (Baker et al., 2004), emphasizing the role of negative affect as the single most important motivational factor driving continued substance use. Teaching heavy persistent pregnant smokers methods to reduce negative affect other than smoking may have promising effects. This may be of particular importance among pregnant low-income smokers with fewer resources for managing negative affect such as depression and anger.
In addition to negative affect reduction, heavy smokers were more likely to endorse stimulation/state enhancement motives for continued smoking and both groups of persistent smokers reported more craving compared to light smokers. The results with regard to stimulation/state enhancement is supportive of positive reinforcement models of substance use (Wills & Shiffman, 1985). This model suggests that smoking may serve as a source of positive reinforcement and perhaps as a way of coping with stress. This suggests that in addition to teaching pregnant smokers other methods of reducing negative affect, replacing smoking with other pleasurable activities may be important in interventions with low-income smokers.
The results with regard to craving are supportive of cognitive processing theory of substance use (Tiffany, 1990), suggesting that patterns of substance use are controlled by automatic processes among addicted users. Issues of craving may be particularly difficult to address in interventions with pregnant smokers. There is a great deal of controversy about nicotine replacement therapy during pregnancy, and there is no clear solution to address issues of craving and addiction among pregnant smokers. The current findings indicate that multifocused interventions that include a mental health component, suggest other sources of positive reinforcement, and incorporate behavioral therapies to address issues of craving may be more successful with chronic low-income pregnant smokers than any single intervention alone.
The study has several limitations. First, the sample size is limited to pregnant women who were current smokers at the first prenatal appointment. This sample excludes women who may have quit before conception. Second, it is possible that there may be more than the four trajectories identified in this study in a larger population of pregnant smokers. Future studies with larger samples of pregnant smokers may yield a larger number of smoking trajectories with different patterns of change. Third, the results are generalizable to primarily low-income smokers and higher income smokers may have a different trajectory of smoking during pregnancy. Fourth, it is possible that using a different unit other than number of cigarettes per month may have resulted in different smoking patterns. For instance, modeling cigarettes per day of pregnancy may have yielded different smoking trajectories. However, this would have resulted in an extremely large number of data points over the 9 months of pregnancy.
In spite of these limitations, it is clear that pregnant smokers are not a uniform group and static measures of smoking during pregnancy may be likely to lose valuable information with regard to points of intervention as well as child outcomes associated with different trajectories. The current results suggest that regardless of amount of use in the first trimester, a large number of women attempt to cut down on the number of cigarettes between the third and fourth month of pregnancy. This may be a potentially powerful moment for intervening with pregnant smokers. The results also indicate that heavier smokers may need additional support in their efforts to cut down on the number of cigarettes toward the end of the second trimester when they exhibited an increase in the number of cigarettes. Alternatively, smoking cessation interventions could ideally begin in the fourth or fifth month of pregnancy and continue through the end of pregnancy. Thus, the current results may speak to the ideal timing and length of interventions for low-income pregnant smokers.
Acknowledgments
We thank the families who participated in the study and research staff who were responsible for data collection. Special thanks to Dr. Amol Lele for collaboration on data collection and Dr. Gerard Connors for collaboration on the larger study. The study was made possible by a grant from the National Institute on Drug Abuse, National Institutes on Health (R01DA019632).
Glossary
- Abruption
Separation of placenta from the uterine wall in pregnancy.
- Placenta previa
Abnormal placement of the placenta to partially or completely cover the cervix.
Biographies
Dr. Eiden received her doctoral degree in developmental psychology from the University of Maryland in 1992. She is currently a Senior Research Scientist at the Research Institute on Addictions, State University of New York at Buffalo. Her current research interest is in examining the impact of prenatal substance exposure and the postnatal environment on developmental outcomes and trajectories, specifically variations in parent-child interactions, attachment, and social-emotional development (self-regulation and peer interactions).

Dr. Homish is an Assistant Professor of Community Health and Health Behavior at the State University of New York at Buffalo. His research interests focus on social network influences on substance use and mental health. His work examines how adult intimate partner's substance use behaviors impact changes in each other's substance use over time. Additionally, he explores how larger social networks (e.g., families and friends) relate to changes in substance use and health.

Dr. Colder received his doctoral degree from Arizona State University in 1994 in clinical psychology, and completed postdoctoral training at Duke University Medical Center and The Health Policy and Researcher Centers at University of Illinois at Chicago. He is currently Professor of Psychology at the University at Buffalo. His area of interest is in childhood and adolescent behavior problems, and the development of substance abuse.

Dr. Gray received her doctoral degree in toxicology in 2010 from the University of Maryland-Baltimore. Her research focused on identifying prenatal drug exposure through analysis of meconium. She is currently a forensic toxicologist.

Dr. Huestis is Chief, Chemistry and Drug Metabolism at the IRP, National Institute on Drug Abuse. Her current research efforts are focused on mechanisms of action of cannabinoid agonists and antagonists, effects of in utero drug exposure, designer drugs, and the neurobiology and pharmacokinetics of stimulants. Her research group utilizes sensitive and specific liquid chromatography tandem mass spectrometry and gas chromatography mass spectrometry methods to address the problems of drug addiction and prevention and treatment of drug abuse.

Footnotes
Declaration of Interest: The authors report no conflicts of interest.
References
- Allen AM, Prince CB, Dietz PM. Postpartum depressive symptoms and smoking relapse. American Journal of Preventive Medicine. 2009;36:9–12. doi: 10.1016/j.amepre.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Baker TB, Morse E, Sherman JE. The motivation to use drugs: A psychobiological analysis of urges. Nebraska Symposium on Motivation. 1986;34:257–323. [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Internal consistencies of the original and revised Beck Depression Inventory. Journal of Clinical Psychology. 1984;40:1365–1367. doi: 10.1002/1097-4679(198411)40:6<1365::aid-jclp2270400615>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller I. Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors. 1998;12:101–112. [Google Scholar]
- Buschgens CJ, Swinkels SH, van Aken MA, Ormel J, Verhulst FC, Buitelaar JK. Externalizing behaviors in preadolescents: Familial risk to externalizing behaviors, prenatal and perinatal risks, and their interactions. European Journal of Child Adolescent Psychiatry. 2009;18:65–74. doi: 10.1007/s00787-008-0704-x. [DOI] [PubMed] [Google Scholar]
- Businelle MS, Kendzor DE, Reitzel LR, Costello TJ, Cofta-Woerpel L, Li Y, et al. Mechanisms linking socioeconomic status to smoking cessation: A structural equation modeling approach. Health Psychology. 2010;29:262–273. doi: 10.1037/a0019285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss AH, Perry M. The aggression questionnaire. Journal of Personality and Social Psychology. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Trends in smoking before, during, and after pregnancy -pregnancy risk assessment monitoring system (PRAMS),United States, 31 sites,2000–2005. Morbidity and Mortality Weekly Report. 2009;58(SS-4) [PubMed] [Google Scholar]
- Chung T, Maisto SA, Cornelius JR, Martin CS, Jackson KM. Joint trajectory analysis of treated adolescents' alcohol use and symptoms over 1 year. Addictive Behaviors. 2005;30:1690–1701. doi: 10.1016/j.addbeh.2005.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Jones BL, Wood DS, Cornelius JR. Substance use disorder trajectory classes: Diachronic integration of onset age, severity, and course. Addictive Behaviors. 2006;31:995–1009. doi: 10.1016/j.addbeh.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Copeland AL, Brandon TH, Quinn EP. The smoking consequences questionnaire–adult: Measurement of smoking outcome expectancies of experienced smokers. Psychological Assessment. 1995;7:484–494. [Google Scholar]
- Cornelius MD, Day NL. The effects of tobacco use during and after pregnancy on exposed children. Alcohol Research and Health. 2000;24:242–249. [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Leech SL, Goldschmidt L. Characteristics of persistent smoking among pregnant teenagers followed to young adulthood. Nicotine and Tobacco Research. 2004;6:159–169. doi: 10.1080/14622200310001656975. [DOI] [PubMed] [Google Scholar]
- Cornelius MD, Leech SL, Goldschmidt L, Day NL. Prenatal tobacco exposure: Is it a risk factor for early tobacco experimentation? Nicotine and Tobacco Research. 2000;2:45–52. doi: 10.1080/14622200050011295. [DOI] [PubMed] [Google Scholar]
- Edwards EP, Homish GG, Eiden RD, Grohman KK, Leonard KE. Longitudinal prediction of early childhood discipline styles among heavy drinking parents. Addictive Behaviors. 2008;34:100–106. doi: 10.1016/j.addbeh.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen C, Baker T. Treating tobacco use and dependence: 2008 update Clinical practice guidelines. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. [Google Scholar]
- Fried P. Cigarette smoke exposure and hearing loss. Journal of American Medical Association. 1998;280(11):963. [PubMed] [Google Scholar]
- Goldman MS. Expectancy operation Cognitive-neural models and architecture. Washington, D.C: American Psychological Association; 1999. [Google Scholar]
- Gray TR, Eiden RD, Leonard KE, Connors GJ, Shisler S, Huestis MA. Identifying prenatal cannabis exposure and effects of concurrent tobacco exposure on neonatal growth. Clinical Chemistry. 2010a;56:1442–1450. doi: 10.1373/clinchem.2010.147876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TR, Eiden RD, Leonard KE, Connors G, Shisler S, Huestis MA. Nicotine and metabolites in meconium as evidence of maternal cigarette smoking during pregnancy and predictors of neonatal growth deficits. Nicotine and Tobacco Research. 2010b;12:658–664. doi: 10.1093/ntr/ntq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston BK, Vavak CR. Developmental factors, psychosocial correlates, and health behaviors. Health Psychology. 1991;10:9–17. doi: 10.1037//0278-6133.10.1.9. [DOI] [PubMed] [Google Scholar]
- Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods and Research. 2001;29:374–393. [Google Scholar]
- Kendzor DE, Businelle MS, Costello TJ, Castro Y, Reitzel LR, Cofta-Woerpel LM. Financial strain and smoking cessation among racially/ethnically diverse smokers. American Journal of Public Health. 2010;100:702–706. doi: 10.2105/AJPH.2009.172676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkus IM, Barefoot JC, Williams RB, Siegler IC. Personality measures as predictors of smoking initiation and cessation in the UNC Alumni Heart Study. Health Psychology. 1994;13:149–155. doi: 10.1037//0278-6133.13.2.149. [DOI] [PubMed] [Google Scholar]
- Ludman EJ, McBride CM, Nelson JC, Curry SJ, Grothaus LC, Lando HA, et al. Stress, depressive symptoms, and smoking cessation among pregnant women. Health Psychology. 2000;19:21–27. doi: 10.1037//0278-6133.19.1.21. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S. Births: Final data for 2004. National Vital Statistics Reports. 2006;55:1–88. [PubMed] [Google Scholar]
- McCartney JS, Fried PA, Watkinson B. Central auditory processing in school-age children prenatally exposed to cigarette smoke. Neurotoxicology and Teratology. 1994;16:269–276. doi: 10.1016/0892-0362(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Miller TQ, Smith T, Turner C, Guijarro M, Hallet A. A meta-analytic review of research on hostility and physical health. Psychological Bulletin. 1996;119:322–348. doi: 10.1037/0033-2909.119.2.322. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Heron J, Araya R. Smoking patterns during pregnancy and postnatal period and depressive symptoms. Nicotine and Tobacco Research. 2008;10:1609–1620. doi: 10.1080/14622200802412895. [DOI] [PubMed] [Google Scholar]
- Nagin DS. Analyzing developmental trajectories: A semi-parametric, group-based approach. Psychological Methods. 1999;4:139–157. doi: 10.1037/1082-989x.6.1.18. [DOI] [PubMed] [Google Scholar]
- O'Callaghan FV, Al Mamun A, O'Callaghan M, Alati R, Najman JM, Williams GM, Bor W. Maternal smoking during pregnancy predicts nicotine disorder (dependence or withdrawal) in young adults – a birth cohort study. Australian and New Zealand Journal of Public Health. 2009;33:371–377. doi: 10.1111/j.1753-6405.2009.00410.x. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Rathouz PJ, Kasza K, Wakschlag LS, Wright R. Self-reported smoking, cotinine levels, and patterns of smoking in pregnancy. Pediatric and Perinatal Epidemiology. 2005;19:368–376. doi: 10.1111/j.1365-3016.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Wakschlag LS, Dai L, Leventhal BL. Fluctuations of maternal smoking during pregnancy. Obstetrics and Gynecology. 2003;101:140–147. doi: 10.1016/s0029-7844(02)02370-0. [DOI] [PubMed] [Google Scholar]
- Porath AJ, Fried PA. Effects of prenatal cigarette and marijuana exposure on drug use among offspring. Neurotoxicology and Teratology. 2005;27(2):267–277. doi: 10.1016/j.ntt.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Reitzel LR, Vidrine JI, Businelle MS, Kendzor DE, Costello TJ, Li Y, et al. Preventing postpartum smoking relapse among diverse low-income women: A randomized clinical trial. Nicotine and Tobacco Research. 2010;12:326–335. doi: 10.1093/ntr/ntq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze P, Lopez FA, Granger DA, Eiden RD. The association between prenatal exposure to cigarettes and cortisol reactivity and regulation in 7-month-old infants. Developmental Psychobiology. 2008;50:819–834. doi: 10.1002/dev.20334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp LK, Lipsky MS. Screening for depression across the lifespan: A review of measures for use in primary care settings. American Family Physician. 2002;66:1001–1008. [PubMed] [Google Scholar]
- Siegler IC, Peterson BL, Barefoot JC, Williams RB. Hostility during late adolescence predicts coronary risk factors at mid-life. Americal Journal of Epidemiology. 1992;136:146–154. doi: 10.1093/oxfordjournals.aje.a116481. [DOI] [PubMed] [Google Scholar]
- Silberg JL, Parr T, Neale MC, Rutter M, Angold A, Eaves LJ. Maternal smoking during pregnancy and risk to boys' conduct disturbance: An examination of the causal hypothesis. Biological Psychiatry. 2003;53(2):130–135. doi: 10.1016/s0006-3223(02)01477-4. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students' recent drinking history: Utility for alcohol research. Addictive Behaviors. 1986;11:149–161. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Paster RL, Papandonatos GD, Niaura R, Salisbury AL, Battle C, et al. Maternal smoking during pregnancy and newborn neurobehavior: Effects at 10 to 27 days. Journal of Pediatrics. 2009;154:10–16. doi: 10.1016/j.jpeds.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Henry DB, Blair RJ, Dukic V, Burns J, Pickett KE. Unpacking the association: Individual differences in the relation of prenatal exposure to cigarettes and disruptive behavior phenotypes. Neurotoxicology and Teratology. 2011;33:145–154. doi: 10.1016/j.ntt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter DW, Smith SS, Kenford SL, Jorenby DE, Fiore MC, Hurt RD. Smoking outcome expectancies: Factor structure, predictive validity, and discriminant validity. Journal of Abnormal Psychology. 1994;103:801–811. doi: 10.1037//0021-843x.103.4.801. [DOI] [PubMed] [Google Scholar]
- Whiteman MC, Fowkes FG, Deary IJ, Lee AJ. Hostility, cigarette smoking and alcohol consumption in the general population. Social Science Medicine. 1997;44:1089–1096. doi: 10.1016/s0277-9536(96)00236-5. [DOI] [PubMed] [Google Scholar]
- Wills TA, Shiffman S, editors. Coping and substance use: A conceptual framework. Orlando, FL: Academic Press; 1985. [Google Scholar]
- Witkiewitz K, Marlatt GA. Relapse prevention for alcohol and drug problems: That was Zen, this is Tao. American Psychologist. 2004;59:224–235. doi: 10.1037/0003-066X.59.4.224. [DOI] [PubMed] [Google Scholar]
- Zhu SH, Sun J, Hawkins S, Pierce J, Cummins S. A population study of low-rate smokers: Quitting history and instability over time. Health Psychology. 2003;22:245–252. doi: 10.1037/0278-6133.22.3.245. [DOI] [PubMed] [Google Scholar]