Introduction
Asthma is the most common chronic disorder in pediatrics affecting more than 1 out of 10 children in Maryland1 and as many as 1 out of 5 Baltimore City children.2 The current asthma treatment guidelines recommend that persistent asthma should be treated as a chronic illness with daily inhaled steroid therapy rather than emergently, when exacerbations occur.3 Improved asthma outcomes are clearly related to asthma controller medication use.4-6 Adherence to controller asthma medication regimens is less than ideal especially in underserved populations7-9. Theories as to the causes of secondary or tertiary non-adherence are well represented in the literature. Primary non-adherence, or not obtaining or filling the medication as prescribed, is not well understood. Adherence may be impacted by many variables including access to quality care10-11,12 noncompliance of prescribing physicians with asthma guidelines13-15 patient-physician relationships16, cost of asthma medications17-19, and patient/caregiver related medication preferences, beliefs or misconceptions.20,21-22 The purpose of this study is to evaluate primary non-adherence in underserved children with asthma cared for by an allergist, by determining if asthma medication prescriptions that are written are ever filled, if prescriptions for controllers are refilled and if families deploy a “selective” filling pattern.
Methods
Data for this study were collected from a convenience sample of subjects treated in an urban subspecialty allergy practice and enrolled in a nebulizer education (NEBS) intervention trial.23 Briefly, the NEBS study evaluated the impact of a nebulizer use focused asthma education intervention on asthma outcomes in inner city children age 2-8 years old with persistent asthma. Children were defined as persistent asthmatics based on national guideline3 criteria using caregiver reported daytime and nighttime symptom frequency. The study was approved by the Institutional Review Boards of The Johns Hopkins Medical Institution and the University of Maryland School of Medicine. Informed consent from all subjects was obtained in writing prior to enrollment in the study. As part of the intervention trial, caregivers completed administered questionnaires at baseline, 6, 12 and 18-months. They were also asked to provide the names of all the pharmacies used in the year prior to the start of the study and during the 18 month study period. Pharmacy records were collected from every pharmacy identified by the child's caregiver at each data collection point as described previously.24 Briefly, all pharmacies identified by the caregiver as used to fill their child's asthma medications were initially contacted via fax to request refill records. Those that did not respond to multiple fax requests were contacted by phone. Pharmacy records were considered complete for a child if responses were obtained from all pharmacies identified by the caregiver. Only subjects with complete pharmacy refill data were included in the analysis (n=53).
Children with persistent asthma, who had complete pharmacy records, and had at least one Allergy subspecialty visit in the year prior to the start of the study were included in the analysis. Subjects in the Allergy Practice were chosen since copies of all prescriptions written for the patients were kept in the medical record. Medical records were reviewed for the 53 subjects to compare asthma medication prescriptions written during the study period and compared with asthma prescription fill patterns from pharmacy records. Data presented were collected over an 18 month period (182 days prior to baseline and 366 days post baseline) for each subject over the 3 1/2 year study.
Chart Review
Medical records from subjects seen in the Pediatric Allergy practice were reviewed for evidence of an asthma medication prescription (including number of refills) written either during a clinic visit or in response to a phone call by the caregiver requesting asthma medication. Prescription fills from pharmacy records were categorized as “subspecialty” or “other prescriber”. Any prescription written by the Allergy subspecialty and filled during the study period was considered a “subspecialty” prescription. Prescriptions filled with no corresponding subspecialty prescription in the medical record were considered to be “other prescriber”. Prescriptions documented in the Allergy medical record with “no refills” that were subsequently refilled after the initial fill were considered to be in the “other prescriber” category.
Prescriptions that were documented in the Allergy medical record but had no corresponding pharmacy record were considered “never obtained or never filled”. Total number of potential medication fills was determined for each drug class, including inhaled corticosteroids (ICS), leukotriene modifiers (LTM), inhaled corticosteroids combined with long acting beta agonist (ICS/LABA), short-acting beta agonists (SABA) and oral corticosteroids (OCS). The total number of potential fills included the initial prescription with number of refills documented in the Allergy medical record. Thus, a medication written for an ICS medication with 5 refills would equal 6 potential medication fills. The time period difference between when the prescription was written and when the prescription was filled was calculated by drug class (ICS, LTM, ICS/LABA, SABA and OCS). All controller prescriptions written by the Allergy subspecialist and documented in the Allergy medical record were written in 30-day quantities with refills for all controller medications and short acting beta agonists were written as single inhalers with no refills. For medications with documented refills, the time period difference between the original prescription fill and subsequent refills was also calculated.
Results
Overall, 53 child subjects out of 55 subjects receiving Allergy care in the NEBS study were enrolled. Two subjects were not included due to missing pharmacy data. There were no significant differences in baseline characteristics between the 2 subjects that were excluded and the overall study group. Subjects had a mean age of 4.6 years old (range 2-8), and were primarily male (53%), African American (81%) and Medicaid insured (72%). All subjects had persistent asthma, with a mean of 2.7 (range 1-7) subspecialty visits during the 18 month study period. As shown in Table 1, at baseline, this population did not have well controlled asthma (eligibility criteria for the study) with frequent daytime and nighttime symptoms, almost half with activity limitation and nearly 30% with an Emergency Department visit in the previous 6 months.
Table 1.
Study Population Baseline Characteristics (n=53)
| Asthma Morbidity | % |
|---|---|
| Daytime Symptoms > 2×/week | 36.7% |
| Nighttime Symptoms > 2×/month | 40.8% |
| Activity Limitation due to Asthma | 47% |
| Hospitalization in the prior 6 months | 4% |
| ED visit in the prior 6 months | 28.6% |
According to the Allergy medical records there were a total of 204 initial prescriptions for the 53 subjects generated by the Allergy subspecialty practice during the 18 month study period. Almost 30% and 7.3% of prescriptions written by the Allergy practice were for LTM (n=61) and ICS/LABA (n=15), respectively. The percentage of total prescriptions written by the Allergy practice for rescue medications was 22.5% (n=46) for SABA and 2.4% (n=15) for oral corticosteroids. Only 3 prescriptions were written for salmeterol and were not included in the analysis due to low prescribing in this young population. Of note, Maryland Medicaid only allows for dispensing of 30 day quantities of medication at a time.
Overall, 59/204 (28.9%) of the initial prescriptions documented in the Allergy medical records were never filled according to the pharmacy records. Unfilled prescriptions by type of medication are shown in Table 2 indicating that 31.3% (n=21/67) of ICS prescriptions, 21.3% (n=13/61) of LTM prescriptions, 40% (n=6/15) of ICS/LABA prescriptions, 28.3 % (n=13/46) of SABA prescriptions and 40% (n=6/15) of OCS prescriptions were never filled. For all filled prescriptions, the mean number of days between the date the prescription was written and filled by drug was: ICS 30 days (range 0-177), LTM 26.6 days (range 0-156), ICS/LABA 37.3 day (range 0-147), SABA 16.8 days (range 0-139) and OCS 37.5 days (range 0-157). For medications that were filled and refilled at least once, the average number of days between date of refill availability (day 1) and date of refill was: ICS 91.29 days (range=1-432), LTM 65.2 days (range=1-350), ICS/LABA 63.8 days (range 12-226), SABA 92.42 days (range 1-350) and OCS 104.96 days (range 2-400).
Table 2.
Medication Prescriptions Written by Allergist and Fill Patterns of Underserved Children with Asthma
| % of initial prescriptions written by allergist by drug class (N=204) | % of initial prescriptions written by allergist that were never filled by drug class | Mean # of days between prescription written and first fill (range) | Mean days between refills | |
|---|---|---|---|---|
| Inhaled Corticosteroids | 32.8% (n=67) | 31.3% (21/67) | 30 (0-177) | 91.3 (1-432) |
| Leukotriene Modifiers | 29.9% (n=61) | 21.3% (13/61) | 26.6 (0-156) | 65.2 (1-350) |
| Short Acting Beta Agonists | 22.5% (n=46) | 28.3% (13/46) | 16.8 (0-139) | 92.4 (1-350) |
| Oral Corticosteroids | 2.4% (n=15) | 40% (6/15) | 37.5 (0-157) | 105.0 (2-400) |
| Combination Inhaled Corticosteroid/Long Acting Beta Agonists | 7.3% (n=15) | 40% (6/15) | 37.3 (0-147) | 63.8 (12-226) |
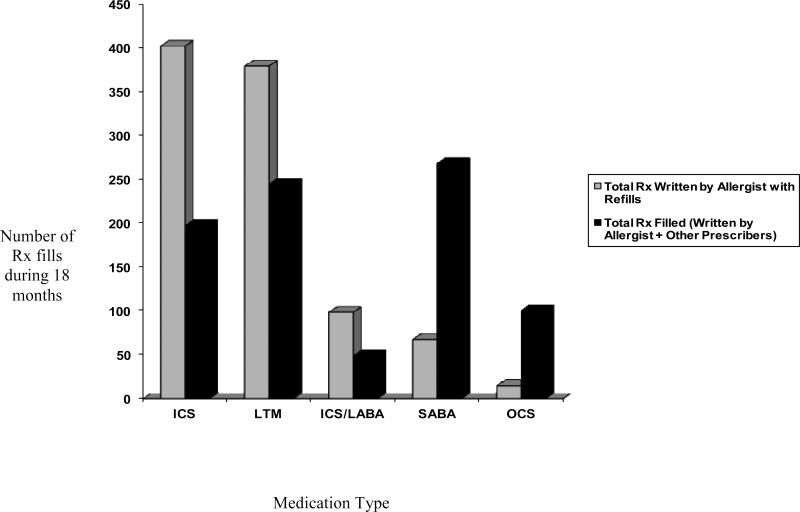
Of the prescriptions documented in the Allergy record, including all of the initial and refills for each of the medications, there were 965 potential medication fill opportunities for asthma medications. As shown in Figure 1, ICS and ICS/LABA fills were filled at rates less than 50% (ICS: 403 total potential medication fill opportunities, n=198 filled, 49.1% ; ICS/LABA: 99 potential fill opportunities, n=49 filled, 49.5%). For LTM, the percent filled was higher (380 potential fills, n=245 filled, 64.5%). For the SABA medications, there were 68 potential medication fills available through Allergy subspecialty and 269 were filled. For OCS, there were 15 potential medication fill opportunities and 101 were filled. Almost every subject (94.3%) had at least 1 SABA fill, yet only 52.8% (28/53) of subjects had prescriptions documented in the Allergy medical records for SABA. Subjects averaged 5.1 fills (range=0-14) of SABA during the study period even though only a mean of 1.28 (range 0-8) SABA prescriptions were generated by the Allergy subspecialty practice during the 18 month study period. For OCS, 81.5% of subjects filled at least one prescription for OCS during the 18 month study period, although only 28.3% were documented in the Allergy medical records. Subjects averaged 1.9 fills (range 0-8) of OCS although only 0.8 (range 0-2) were documented in the Allergy medical records.
Figure 1.
Number of prescriptions written by allergist (initial and refills) vs. prescriptions filled in underserved children with asthma
Discussion
Medicaid insured children with asthma have high asthma related morbidity and mortality and low adherence with controller medications significantly impacts asthma control.5-6, 9 Strikingly, in our sample of Medicaid insured children with poorly controlled asthma seen by an Allergy subspecialist over an 18 month study period, nearly 30% of all prescriptions written by an allergist were never filled. Higher rates of primary non-adherence (up to 40%) for controller medication prescriptions in this high risk study population were detected. Poor adherence has been reported in other Medicaid population studies of children with asthma; however, studies investigating primary non-adherence in this population are limited. .7, 25-27 Riekert, et al. studied primary non-adherence of controller medication in underserved children with asthma by comparing self-reported data from the caregivers of the children with the child's primary care provider self-report of prescribing. The study found that 38% of caregivers denied that their child had been prescribed a controller medication when the primary care provider claimed one had been prescribed.26 Bronstein, et al. reported that 30% of prescriptions noted in the medical record of asthma patients did not have a corresponding Medicaid claim, although the study did not specifically investigate specialty care provider claims.25 Watts reported only 30% of the asthma medication prescriptions written by a primary care provider were actually filled over a 6 month study period with higher rates of primary non-adherence in patients from lower socioeconomic background.28 Studies of non Medicaid populations have shown much higher rates of primary adherence.29-30 For example, Williams, et al. reported that 92% of patients with asthma from a large Michigan health maintenance organization database did fill their ICS prescription.29 However, details were not included in the paper regarding specific fill rates for children age 1-9 years and the population did not include underserved children. Studies specifically evaluating patients cared for by asthma subspecialists are limited. Sherman, et al. collected fill data from pharmacies for 116 Medicaid insured children followed in a Pulmonology practice and compared it to medical records. They reported a mean of 61.4% of patients prescribed ICS were ≥50% adherent with therapy.31 Details were not provided to determine how many subjects never filled their ICS prescription. Similar published studies in subjects receiving Allergy subspecialty care could not be found.
Despite access to specialty asthma care that focused on prescribing and teaching appropriate medication usage, very few of the subjects had appropriate asthma medication fill rates over the period of the study. The high rate of non-adherence with controller medications in underserved populations is alarming and may be explained by multiple barriers that these families encounter such as Medicaid formulary limitations, access to pharmacies and other socioeconomic stressors competing with obtaining medications. Additional factors that may impact adherence in this population include misunderstanding or distrust of the medical providers regarding the asthma diagnosis and treatment and healthcare or cultural beliefs that question the need for preventive treatment. Parental concern regarding use of inhaled steroids was investigated in the NEBS study and was not found to impact prescription fill rates.23 Although our observational study was not designed to investigate the impact of interventions on primary adherence, development of more culturally sensitive educational materials that address some of these concerns may lead to improved adherence in this population.
Because all patients in our study were classified with persistent asthma, based on NAEPP guidelines3, their preventive care should include use of a daily controller medication. Although all subjects were prescribed controller medications by the Allergy subspecialist, fill rates for this high risk population were poor. On the other hand, in our study population only 28.3% of the OCS filled by the study participants were prescribed by the Allergy subspecialist. Thus, a majority of the participants received OCS from other health care providers, albeit not surprising since patients are more likely to see their primary care provider or urgent care site than their asthma specialist for acute exacerbations. More important is the high rate of SABA use noted in this population. Over the 18 month study period, participants received an average of 5.1 fills of SABA (510 doses for 547 days) or one dose every 1.07 days. This data indicates that most of the patients were not well controlled, which was in fact, required for study eligibility. As important, 80% of the rescue medication was provided by “other health care providers”. Other pharmacy refill studies of similar underserved populations have shown a high rate of rescue beta agonist fills and low rate of controller medication fills with a negative impact on asthma control.8, 18, 32 One hypothesized scenario based on a prior study is that patients may receive “long-term” beta agonist refills, or refills over the phone without the knowledge of the physician.33 Our study suggests that specialty and non-specialty providers should limit refills for rescue medications. If rescue inhalers are used at a rate of more than 1 inhaler equivalent every 6 months, controller therapy should be added or stepped up. Requiring patients to call for additional rescue medication refills also allows for an opportunity to reinforce the preventive management plan with the patient or advise the patient to attend clinic to reassess their asthma status. Not prescribing additional refills for rescue medication without evaluating the patient allows the provider to determine if the level of therapy is maintaining adequate control, with rescue beta agonist requirement as a key component of that assessment. Pharmacists may also play a role in tracking overuse of beta agonists by notifying providers if patients require >2 rescue inhalers per year.
Cost of medications is one of the many factors that may impact primary non-adherence18-19 However, at the time these data were collected, a co-payment fee was not required by the Medicaid system for any of the asthma medications. Furthermore, at that time most medications did not require prior authorization for access to ICS or LTM so neither cost, nor pre-authorization should have contributed to non-adherence for this subject population.
Jones, et al found a 34 found a difference in fill rates for types of controller medications in their study of a large population of children and adults with asthma with primary adherence for LTMs at 67.7% and for ICS, 33.8%. They suggested a potential “aversion” to inhaled steroids driving this type of filling pattern. Our study did not support a difference in ICS versus LTM fills for those filling their initial controller prescription, with 67.7% filling the first prescription for ICS and 77.7% filling for LTM.
Poor fill rates for controller medications and overfilling of beta agonist medications may explain the high morbidity detected in this underserved population. Without appropriate asthma medication use per national guidelines(NAEPP)3, these children will continue to have increased morbidity and ultimately, higher cost of care.35
Limitations
Our study used pharmacy fill data as a measure of adherence but cannot account for drug wasting by the patient, actual use of the medication, poor technique in administration of drug, drug sharing and drug lost. If a family did not identify a pharmacy, prescriptions that were actually filled would have been missed and be considered “written but not filled”. However, previous studies including our prior data have determined that pharmacy claims data correlate well with Medicaid claims and medical record data in this population.31,36
We could not directly correlate the prescriptions written by the Allergy subspecialist versus those written by non specialist providers since pharmacy records did not include information about the prescribing provider. Therefore, our controller medication fill rates for prescriptions written by the allergists may be overestimated. However, at best the rate remains low. Medical Assistance prescriptions are technically only valid for filling for 60 days after the date of the prescriptions.37 Since the ranges for days to fill a prescription varied from 0 to 177 days, it could be estimated that the percentage of Allergy subspecialist prescriptions never filled by class could be higher than what we report. Finally, this group of Medicaid insured patients receiving Allergy subspecialty services may not be representative of the population, since in order to be eligible for the study, the subjects had to have asthma that was not well-controlled. We did not have access to pharmacy data for ineligible (better controlled) asthmatics from the practice for comparison.
Conclusions
Our study demonstrates that poorly controlled underserved children with asthma receiving Allergy subspecialty care sub-optimally fill controller medications and overfill rescue albuterol medications written by other providers. Pharmacy records are a rich source of data to assess medication adherence and are a basis for practicing guideline based care. Our study suggests that keeping paper or electronic copies of prescriptions in the medical record or practice site is useful to track adherence. Limiting short-acting beta agonist refills and requiring parents to contact their child's health care provider for additional rescue medication refills, may allow an opportunity to address adherence with preventive asthma medications and improve asthma control. Incorporating tracking and notification procedures by pharmacies and/or insurance providers to make healthcare providers and caregivers of children with asthma aware of the inadequate use of controller medications and overuse of rescue medications may improve asthma control in these high risk children.
Acknowledgement
The authors would like to thank Amanda Manning for her assistance with data collection and entry for this study.
Funding Source: National Institute of Nursing Research NIH NR 05060
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asthma in Maryland. 2007 http://fha.maryland.gov/pdf/mch/asthmareport2007.pdf.
- 2.Amr S, Bollinger ME, Myers M, et al. Environmental allergens and asthma in urban elementary schools. Ann Allergy Asthma Immunol. 2003 Jan;90(1):34–40. doi: 10.1016/S1081-1206(10)63611-3. [DOI] [PubMed] [Google Scholar]
- 3.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007 Nov;120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 4.Castro-Rodriguez JA, Rodrigo GJ. The role of inhaled corticosteroids and montelukast in children with mild-moderate asthma: results of a systematic review with meta-analysis. Arch Dis Child. May. 2010;95(5):365–370. doi: 10.1136/adc.2009.169177. [DOI] [PubMed] [Google Scholar]
- 5.Lasmar L, Camargos P, Champs NS, et al. Adherence rate to inhaled corticosteroids and their impact on asthma control. Allergy. 2009 May;64(5):784–789. doi: 10.1111/j.1398-9995.2008.01877.x. [DOI] [PubMed] [Google Scholar]
- 6.Farber HJ, Chi FW, Capra A, et al. Use of asthma medication dispensing patterns to predict risk of adverse health outcomes: a study of Medicaid-insured children in managed care programs. Ann Allergy Asthma Immunol. 2004 Mar;92(3):319–328. doi: 10.1016/S1081-1206(10)61569-4. [DOI] [PubMed] [Google Scholar]
- 7.Rohan J, Drotar D, McNally K, et al. Adherence to pediatric asthma treatment in economically disadvantaged African-American children and adolescents: an application of growth curve analysis. J Pediatr Psychol. 2010 May;35(4):394–404. doi: 10.1093/jpepsy/jsp074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butz AM, Tsoukleris M, Donithan M, et al. Patterns of inhaled antiinflammatory medication use in young underserved children with asthma. Pediatrics. 2006 Dec;118(6):2504–2513. doi: 10.1542/peds.2006-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauman LJ, Wright E, Leickly FE, et al. Relationship of adherence to pediatric asthma morbidity among inner-city children. Pediatrics. 2002 Jul;110(1 Pt 1):e6. doi: 10.1542/peds.110.1.e6. [DOI] [PubMed] [Google Scholar]
- 10.Flores G, Snowden-Bridon C, Torres S, et al. Urban minority children with asthma: substantial morbidity, compromised quality and access to specialists, and the importance of poverty and specialty care. J Asthma. 2009 May;46(4):392–398. doi: 10.1080/02770900802712971. [DOI] [PubMed] [Google Scholar]
- 11.Lozano P, Grothaus LC, Finkelstein JA, Hecht J, Farber HJ, Lieu TA. Variability in asthma care and services for low-income populations among practice sites in managed Medicaid systems. Health Serv Res. 2003 Dec;38(6 Pt 1):1563–1578. doi: 10.1111/j.1475-6773.2003.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crain EF, Kercsmar C, Weiss KB, Mitchell H, Lynn H. Reported difficulties in access to quality care for children with asthma in the inner city. Arch Pediatr Adolesc Med. 1998 Apr;152(4):333–339. doi: 10.1001/archpedi.152.4.333. [DOI] [PubMed] [Google Scholar]
- 13.Cabana MD, Rand CS, Becher OJ, Rubin HR. Reasons for pediatrician nonadherence to asthma guidelines. Arch Pediatr Adolesc Med. 2001 Sep;155(9):1057–1062. doi: 10.1001/archpedi.155.9.1057. [DOI] [PubMed] [Google Scholar]
- 14.Navaratnam P, Jayawant SS, Pedersen CA, Balkrishnan R. Physician adherence to the national asthma prescribing guidelines: evidence from national outpatient survey data in the United States. Ann Allergy Asthma Immunol. 2008 Mar;100(3):216–221. doi: 10.1016/S1081-1206(10)60445-0. [DOI] [PubMed] [Google Scholar]
- 15.Jones EM, Portnoy JM. Modification of provider behavior to achieve improved asthma outcomes. Curr Allergy Asthma Rep. 2003 Nov;3(6):484–490. doi: 10.1007/s11882-003-0059-9. [DOI] [PubMed] [Google Scholar]
- 16.Greene J, Yedidia MJ. Provider behaviors contributing to patient self-management of chronic illness among underserved populations. J Health Care Poor Underserved. 2005 Nov;16(4):808–824. doi: 10.1353/hpu.2005.0097. [DOI] [PubMed] [Google Scholar]
- 17.Divertie V. Strategies to promote medication adherence in children with asthma. MCN Am J Matern Child Nurs. 2002 Jan-Feb;27(1):10–18. doi: 10.1097/00005721-200201000-00006. quiz 19. [DOI] [PubMed] [Google Scholar]
- 18.Mattke S, Martorell F, Hong SY, Sharma P, Cuellar A, Lurie N. Anti-inflammatory medication adherence and cost and utilization of asthma care in a commercially insured population. J Asthma. 2010 Apr;47(3):323–329. doi: 10.3109/02770900903497196. [DOI] [PubMed] [Google Scholar]
- 19.Blais L, Beauchesne MF, Levesque S. Socioeconomic status and medication prescription patterns in pediatric asthma in Canada. J Adolesc Health. 2006 May;38(5):607, e609–616. doi: 10.1016/j.jadohealth.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Yoos HL, Kitzman H, McMullen A. Barriers to anti-inflammatory medication use in childhood asthma. Ambul Pediatr. 2003 Jul-Aug;3(4):181–190. doi: 10.1367/1539-4409(2003)003<0181:btamui>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Conn KM, Halterman JS, Fisher SG, Yoos HL, Chin NP, Szilagyi PG. Parental beliefs about medications and medication adherence among urban children with asthma. Ambul Pediatr. 2005 Sep-Oct;5(5):306–310. doi: 10.1367/A05-004R1.1. [DOI] [PubMed] [Google Scholar]
- 22.Laforest L, El Hasnaoui A, Pribil C, et al. Asthma patients’ self-reported behaviours toward inhaled corticosteroids. Respir Med. 2009 Sep;103(9):1366–1375. doi: 10.1016/j.rmed.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Butz AM, Eggleston P, Huss K, Kolodner K, Rand C. Nebulizer use in inner-city children with asthma: morbidity, medication use, and asthma management practices. Arch Pediatr Adolesc Med. 2000 Oct;154(10):984–990. doi: 10.1001/archpedi.154.10.984. [DOI] [PubMed] [Google Scholar]
- 24.Mudd K, Bollinger ME, Hsu VD, Donithan M, Butz A. Pharmacy fill patterns in young urban children with persistent asthma. J Asthma. 2006 Oct;43(8):597–600. doi: 10.1080/02770900600878537. [DOI] [PubMed] [Google Scholar]
- 25.Bronstein JM, Santer L, Johnson V. The use of Medicaid claims as a supplementary source of information on quality of asthma care. J Healthc Qual. 2000 Nov-Dec;22(6):13–18. doi: 10.1111/j.1945-1474.2000.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 26.Riekert KA, Butz AM, Eggleston PA, Huss K, Winkelstein M, Rand CS. Caregiver-physician medication concordance and undertreatment of asthma among inner-city children. Pediatrics. 2003 Mar;111(3):e214–220. doi: 10.1542/peds.111.3.e214. [DOI] [PubMed] [Google Scholar]
- 27.Smith MJ, Pawar V. Medical services and prescription use for asthma and factors that predict inhaled corticosteroid use among African-American children covered by Medicaid. J Asthma. 2007 Jun;44(5):357–363. doi: 10.1080/02770900701344355. [DOI] [PubMed] [Google Scholar]
- 28.Watts RW, McLennan G, Bassham I, el-Saadi O. Do patients with asthma fill their prescriptions? A primary compliance study. Aust Fam Physician. 1997 Jan;26(Suppl 1):S4–6. [PubMed] [Google Scholar]
- 29.Williams LK, Joseph CL, Peterson EL, et al. Patients with asthma who do not fill their inhaled corticosteroids: a study of primary nonadherence. J Allergy Clin Immunol. 2007 Nov;120(5):1153–1159. doi: 10.1016/j.jaci.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 30.Pando S, Lemiere C, Beauchesne MF, Perreault S, Forget A, Blais L. Suboptimal use of inhaled corticosteroids in children with persistent asthma: inadequate prescription, poor drug adherence, or both? Pharmacotherapy. 2010 Nov;30(11):1109–1116. doi: 10.1592/phco.30.11.1109. [DOI] [PubMed] [Google Scholar]
- 31.Sherman J, Hutson A, Baumstein S, Hendeles L. Telephoning the patient's pharmacy to assess adherence with asthma medications by measuring refill rate for prescriptions. J Pediatr. 2000 Apr;136(4):532–536. doi: 10.1016/s0022-3476(00)90019-2. [DOI] [PubMed] [Google Scholar]
- 32.Naureckas ET, Dukic V, Bao X, Rathouz P. Short-acting beta-agonist prescription fills as a marker for asthma morbidity. Chest. 2005 Aug;128(2):602–608. doi: 10.1378/chest.128.2.602. [DOI] [PubMed] [Google Scholar]
- 33.Goodman DC, Lozano P, Stukel TA, Chang C, Hecht J. Has asthma medication use in children become more frequent, more appropriate, or both? Pediatrics. 1999 Aug;104(2 Pt 1):187–194. doi: 10.1542/peds.104.2.187. [DOI] [PubMed] [Google Scholar]
- 34.Jones C, Santanello NC, Boccuzzi SJ, Wogen J, Strub P, Nelsen LM. Adherence to prescribed treatment for asthma: evidence from pharmacy benefits data. J Asthma. 2003 Feb;40(1):93–101. doi: 10.1081/jas-120017212. [DOI] [PubMed] [Google Scholar]
- 35.Bender BG, Rand C. Medication non-adherence and asthma treatment cost. Curr Opin Allergy Clin Immunol. 2004 Jun;4(3):191–195. doi: 10.1097/00130832-200406000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Mudd KE, Bollinger ME, Hsu VD, Manning A, Tsoukleris MG, Butz AM. Concordance of Medicaid and pharmacy record data in inner-city children with asthma. Contemp Clin Trials. 2008 Jan;29(1):13–20. doi: 10.1016/j.cct.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Hygeine MDoHaM Maryland Medicaid Pharmacy Fill Policy. 2009 http://www.dhmh.state.md.us/mma/mpap/pdf/2009/jul09/Policy_Refills_3_09.pdf.