Abstract
Cochlin, an extracellular matrix protein, shares homologies with the Factor C, a serine protease found in horseshoe crabs, which is critical for anti-bacterial responses. Mutations in the COCH gene are responsible for human DFNA9 syndrome, a disorder characterized by neurodegeneration of inner ear that leads to hearing loss and vestibular impairments. The physiological function of cochlin, however, is unknown. Here, we report that cochlin is specifically expressed by follicular dendritic cells, and selectively localized in the fine extracellular network of conduits in the spleen and lymph nodes. During inflammation, cochlin was cleaved by aggrecanases and secreted into blood circulation. In models of lung infection with Pseudomonas aeruginosa and Staphylococcus aureus, Coch−/− mice show reduced survival linked to defects in local cytokine production, recruitment of immune effector cells and bacterial clearance. By producing cochlin, FDCs thus contribute to the innate immune response in defense against bacteria.
Keywords: Cochlin, LCCL domain, conduit, follicular dendritic cells, aggrecanase, Pseudomonas aeruginosa, Staphylococcus aureus
Introduction
Cochlin, encoded by the gene Coch, is a highly abundant extracellular matrix protein in the cochlea and vestibule of the inner ear (Robertson et al., 1994; Robertson et al., 1997). Missense mutations and in-frame deletions in the COCH gene are etiologically linked to the autosomal dominant DFNA9 syndrome, a disorder characterized by late onset nonsyndromic hearing loss and vestibular dysfunctions with associated neurosensory degeneration in the inner ear (Manolis et al., 1996). Whereas the neurodegeneration associated with DFNA9 is believed to be caused by a gain-of-function by the mutated cochlin, the normal physiological function of cochlin remains unknown. The N-terminus of cochlin contains a LCCL domain also present in Factor C, a serine protease of the horseshoe crab Limulus involved in activating the coagulation cascade in response to LPS (Iwanaga et al., 1992). The expression of cochlin is highly selective; it is abundantly expressed in the inner ear and also found in the spleen (Robertson et al., 1997). To understand the physiological function of cochlin in the spleen, we investigated the splenic cell type that produces cochlin, the regulation of its production and its possible role in immunity.
Follicular dendritic cells, a type of stromal cells of the secondary lymphoid organs, are recognized as key organizers of B cell follicles and central to the development of germinal centers (GCs) where cooperation of multiple cell lineages leads to the formation of isotype-switched, high-affinity immunoglobulin and the establishment of humoral immune memory (Allen and Cyster, 2008). FDCs function by presenting native antigen in the form of immune complexes to B cells, supporting both T-dependent and - independent B cell activation, and suppressing apoptosis in B cells with high affinity antigen-binding receptors resulting from successful somatic hypermutation processes. In the secondary lymphoid organs, FDCs are tightly associated with a fine tubular extracellular matrix network – named conduits – used as an adhesion substrate for cell's crawling movement and involved in distributing small molecules through the splenic white pulp and lymph nodes, respectively (Bajenoff et al., 2006; Gretz et al., 2000; Nolte et al., 2003; Sixt et al., 2005). FDCs may secrete cytokines such as CXCL13 into the conduits, and capture small antigens from the circulation (Roozendaal et al., 2009). Although the function of FDCs in modulating the activation of B cells can be regulated by innate immunity, FDCs have no established role in modulating innate immunity (El Shikh et al., 2007; Garin et al., 2010; Suzuki et al., 2010).
The homology of cochlin to the Limulus Factor C protein led us to postulate that cochlin may participate in host resistance to infection. In the present study, we investigated the role for cochlin in immunity. We demonstrate that cochlin is produced specifically by the FDCs in the secondary lymphoid organs and exclusively present in the fine extracellular network of conduits. However, cochlin deficiency has no effect on acquired immunity. Instead, we show that cochlin LCCL domain is released into the blood after proteolytic cleavage in response to LPS and bacterial infection. Finally, we provide evidence that cochlin-deficiency leads to a defective innate responses and an increased sensitivity to P. aeruginosa infection. Our study demonstrates cochlin as the first immune modulator released from conduits to act at the systemic level, revealing a unique biological function of conduits and evidence for a role of FDCs in regulating innate immunity.
Results
Cochlin is expressed and secreted by follicular dendritic cells
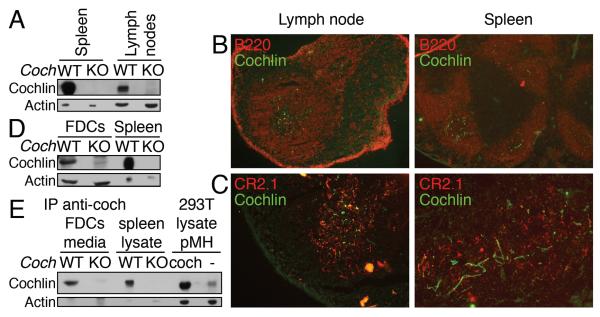
We investigated the expression of cochlin using a highly specific monoclonal antibody (Yao et al., 2010), and found that cochlin is expressed in spleens and lymph nodes (Figure 1A, S1A). However, because both protein and mRNA of cochlin are not detectable in isolated splenocytes, which contain mostly B and T cells (Figure S1B), we considered the possibility that cochlin is expressed by stromal cells in the spleens. Using in situ immunocytochemistry, we found that cochlin is present in the fine network-like structure located in the B cell zone in the white pulp of spleens and in the center of the B cell follicles in lymph nodes (Figure 1B, S1C). Because this localization corresponds to that of the follicular dendritic cells (FDCs), we examined the co-immunostaining of CR2.1, a marker for FDC, and cochlin. Indeed, cochlin is localized in the close proximity of the FDCs (Figure 1C). Cultured primary WT, but not Coch−/−, FDCs express and secrete cochlin in vitro (Figure 1D, E). We thus conclude that FDCs express and secrete cochlin in the spleen and the lymph nodes.
Figure 1.
Cochlin is produced and secreted by FDCs in the spleen and the lymph nodes.
A. Spleen and lymph node lysates from WT and Coch−/− mice were analyzed by WB.
B.C. Lymph node and spleen sections from WT mice were co-stained for cochlin and B cell (B220) (objective lens 10×) (B) or FDCs (CR2.1) (objective lens 20 ×) (C).
D.E. Primary FDCs were isolated from WT and Coch−/− mice. Lysates from in vitro cultured FDCs were analyzed by WB (D). Culture media were subjected to anti-cochlin IP and analyzed by WB (E). Spleen lysates from WT and Coch−/− mice, as well as lysates from 293T cells expressing cochlin were used as controls.
See also Figure S1.
Cochlin accumulates in the lumen of the conduits
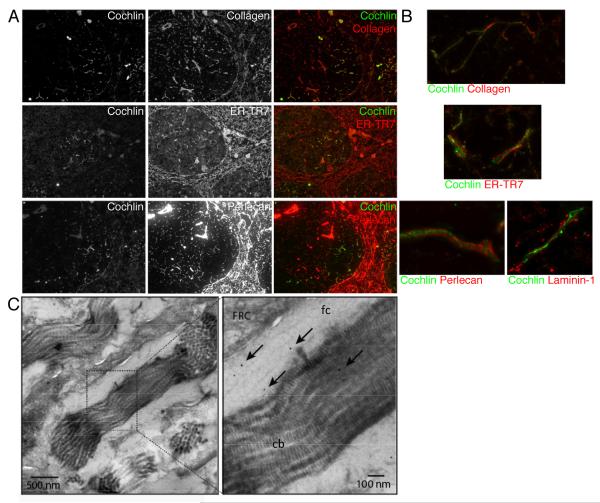
Because cochlin is an extracellular protein and distributed in a fine network-like structure in the spleen and lymph nodes (Figure 1), we considered the possibility that cochlin might be associated with the conduits, an extracellular matrix network involved in distributing chemokines and small blood and lymph-borne molecules through the splenic white pulp and lymph nodes respectively (Gretz et al., 2000; Nolte et al., 2003; Roozendaal et al., 2009; Sixt et al., 2005). We investigated whether cochlin co-localizes with known markers for conduits such as collagen, ER-TR7, and perlecan in the spleens. We found that cochlin colocalizes with the subset of conduits present in the B cell zone, but not in the periarteriolar lymphocyte sheath (PALS) (Figure 2A). While most of the conduit constituents are synthesized by enwrapping fibroblastic reticular cells (FRC), only conduits in the B cell zone are associated with FDCs in addition to FRCs (Gretz et al., 2000; Lokmic et al., 2008; Roozendaal et al., 2009; Sixt et al., 2005). The specific association of cochlin with the conduits in the B cell zone further supports that cochlin is produced by the FDCs.
Figure 2.
Cochlin concentrates in the lumen of conduits.
A.B. Spleen sections from WT mice were co-stained for cochlin and the conduits markers collagen, ER-TR7, perlecan and laminin-1. Objective lens 10× (A). Objective lens 100X, 3D reconstructions were performed using deconvolved Z-series (B).
C. Spleen fine sections were stained for cochlin (gold beads, black arrowheads; fc, fibrillar collagen; cb, collagen bundles).
See also Figure S2.
Conduits are formed by a central collagen bundle core surrounded by a microfibrillar layer which is enwrapped in a basement membrane. High resolution fluorescence microscopic analysis of splenic samples co-immunostained with anti-cochlin and antibodies for laminin-1 and perlecan, markers for the basement membrane, or with antibody for ER-TR7, a marker for the microfibrillar layer, demonstrate that cochlin is wrapped by the basement membrane and by the microfibrillar layer. Indeed, fluorescence microscopy analysis suggests that cochlin colocalizes with the central collagen core (Figure 2B). Using electron microscopic analysis, we found that cochlin colocalizes with the central collagen bundles and with fibrillar collagen in the conduits (Figure 2C). We thus concluded that cochlin is synthesized by the FDCs and secreted in the lumen of the FDCs-associated conduits.
We next assessed the function of cochlin by analyzing the FDC-associated conduits in the spleen and lymph nodes of Coch−/− mice. Cochlin deficiency did not modify the FDC-associated conduit's structure as seen in fluorescent and electronic microscopy (Figure S2A–C). In the spleen or the lymph nodes, immune cells crawl on the surface of the conduits to circulate (Bajenoff et al., 2006), and cochlin deficiency did not impair B cell, FDCs or T cell localization in the spleen or the lymph nodes of Coch−/− mice (Figure S2A–B, and data not shown). Conduits also allow for the flow of low molecular weight molecules (MW ≤ 70 kDa) such as cytokines and small antigens (Gretz et al., 2000; Nolte et al., 2003; Roozendaal et al., 2009) in their lumen. Cochlin deficiency did not modify either the flow of small molecules nor the molecular weight cut-off of the components circulating through the conduits, as observed by following the flow of 10, 70 and 500 kDa fluorescent dextran in Coch−/− spleens (Figure S2D–F). FDCs adhere to the surface of the conduits and capture circulating small antigens before transferring them to the B cells, or alternatively B cells directly sample small antigens circulating in the conduits (Roozendaal et al., 2009). However, cochlin deficiency in vivo did not impair the close association of FDCs with the conduits (Figure S3A). Thus, we conclude that cochlin deficiency has no effect on the adherence of FDCs to the conduits.
We next tested whether cochlin was involved in the transfer of small antigens from the conduits to the immune cells, but cochlin deficiency did not alter the ability of FDCs or B cells to capture small antigens at their surfaces in the lymph nodes (Figure S3B–C). In addition, Coch−/− mice did not show any defects in the immunoglobulin response to the small antigens TEL (turkey egg lysozyme, 12–14 kDa) trafficking through the conduits lumen, nor to the large antigen PE (phycoerytherin, 240 kDa) excluded from the conduits, as anticipated, and used here as a control (Figure S3D) (Roozendaal et al., 2009). Furthermore, Coch−/− mice showed no defect in germinal center formation (Figure S3E) or antibody affinity maturation during primary and secondary responses (Figure S3F–G). Taken together, we conclude that although cochlin is specifically associated with the conduits in the B cell zone, cochlin deficiency has no detectable effect on adaptive immune response in our experimental conditions.
Cochlin cleavage products are released in the blood during inflammation
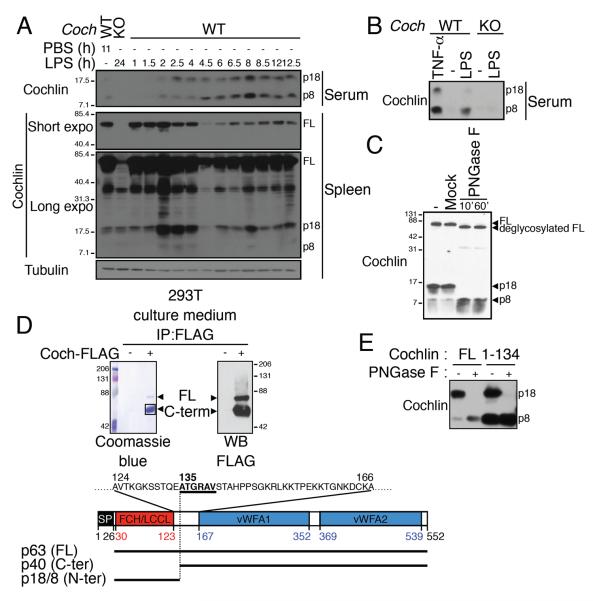
As the conduit lumens connect with the blood circulation (Gretz et al., 2000), we next tested whether cochlin is released into the blood stream using western-blot analysis of the serum for detection. In basal conditions, two weak immunoreactive bands reacting with the cochlin antibody were present in the blood, which were absent in the sera from Coch−/− mice (Figure 3A). We named them p8 and p18 according to their respective apparent molecular weights. Since the Limulus Factor C which contains a homologous LCCL domain is secreted into the hemolymph in response to LPS (Iwanaga et al., 1992), we next tested if cochlin p8 and p18 are released into the blood during LPS response. We found that the levels of cochlin p8 and p18 in sera greatly increased shortly after intraperitoneal (i.p.) injection of LPS in WT but not Coch−/− mice (Figure 3A). In accordance with cleavage followed by secretion, full-length cochlin is substantially reduced in the spleen starting 4 h after LPS injection, and p18 peaks transiently in the spleen from 2 h to 4 h following LPS injection before gradually decreasing but being maintained at a detectable level. Altogether these data suggest that full-length cochlin is processed into the p8 and p18 forms in the spleen prior to their release in the blood.
Figure 3.
Cochlin LCCL domain is released into the blood following proteolytic cleavage upon inflammation.
A.B. WT and Coch−/− mice were injected i.p. with LPS (5 mg/kg) (A), TNF- α (15 μg, 8 h) (B) or PBS as control.
C. The p8 fragment is the deglycosylation product of p18. Serum free culture medium from 293T cells expressing cochlin was treated by PNGase F for 1 h (−, untreated; mock, 1 h w/o PNGase F).
D. The C-term fragment corresponds to a.a. 135–552. Anti-FLAG IPs from the culture medium of 293T cells expressing cochlin-FLAG.
E. The p8 and p18 fragments correspond to a.a. 26–134. Serum-free culture media of 293T cells expressing the full length or the N-terminal domain (a.a. 1–134) of cochlin treated with PNGase F as indicated.
See also Figure S3.
The levels of TNF-α, one of the first cytokines detectable in the blood, peaked at 1 h after LPS injection (Figure S4A). We observed that TNF-α injection is sufficient to induce the release of the cochlin p8 and p18 forms into the blood (Figure 3B). This suggests that cochlin p18/8 release in the blood is mediated downstream of TNF-α release following LPS injection. Thus, increased levels of cochlin p8 and p18 in the blood are not restricted to LPS stimulation per se but may be a common phenomenon under various inflammatory conditions involving TNF-α.
The cochlin p8 and p18 forms correspond to the LCCL domain
Because the cochlin antibody used for the WB analysis recognizes the N-terminal part of cochlin (Yao et al., 2010), which contains a LCCL domain, the p8 and p18 are most likely derived from the LCCL domain. Consistent with this possibility, we have previously shown that cochlin p8 and p18 forms are detectable in conditioned media of 293T cells transfected with a cochlin expression vector (Yao et al., 2010). To further characterize the identity of the cochlin p8 and p18, we treated conditioned media of 293T cells expressing cochlin with N-glycosidase F, since cochlin is known to be glycosylated (Kommareddi et al., 2007; Robertson et al., 2003). We observed that following N-glycosidase F treatment, the cochlin p18 disappeared while the p8 level correspondingly increased (Figure 3C), suggesting that cochlin p18 is the glycosylated form of p8.
We then determined the cleavage site of full-length cochlin by Edman degradation of the purified C-terminal FLAG-tagged cleavage product corresponding to that of p18 (Figure 3D); “ATGRAV” was found to be its N-terminal residues. As this sequence corresponds to a.a. 135–140 of full-length cochlin, we concluded that cochlin is cleaved between E134 and A135. Cochlin p8 and p18 fragments are recognized by the monoclonal antibody 9A10D2 raised against the N-terminal cochlin antigen corresponding to a.a. 7–227 (Yao et al., 2010). As a.a. 1–25 correspond to the signal peptide, we hypothesized that the p8 and p18 N-terminal fragment may correspond to the a.a. 26–134. We compared the migration profile in a SDS-PAGE gel of p8 and p18 in conditioned media of 293T cells expressing full-length cochlin, and in conditioned media of 293T cells expressing cochlin a.a. 1–134 following PNGase F treatment. The expression from the vector coding for cochlin a.a. 1–134 resulted in bands of the same apparent molecular weights as p8 and p18. Taken together, we concluded that p8 corresponds to cochlin N-terminal fragment a.a. 26–134, which is then glycosylated to form p18 (Figure 3E).
We next investigated the release of the C-terminal domain using the polyclonal antibody recognizing epitopes predominantly in this domain. The C-terminal domain remained undetectable in the serum even after LPS injection (Figure S4B), and IF staining using the same antibody showed no decrease in cochlin intensity in the spleen following LPS injection (Figure S4C–D). We concluded that, contrary to the LCCL domain, the C-terminal domain of cochlin remains in the spleen following cochlin cleavage.
To determine the expression of LCCL domains in sera under control or LPS stimulated conditions, we next used purified cochlin aa 26–134 fused with HA (hereafter named LCCL-HA) of known concentration as a standard. We found that the expression of p8 and p18 under basal conditions are too low to be precisely determined while 8 h after LPS injection the concentration of p8 and p18 reach ~0.4 μg/ml (Figure S4E–F).
Together these data demonstrate that cochlin LCCL domain is released from the spleen into the blood by cochlin proteolytic cleavage during inflammation.
Cochlin is processed by aggrecanase-1 and -2
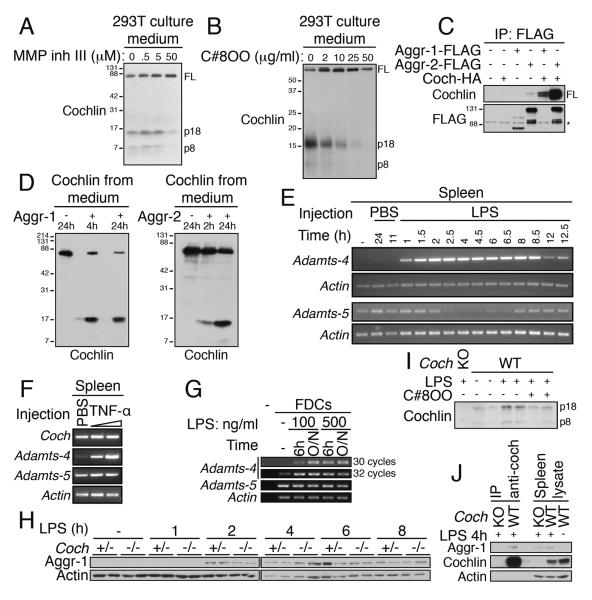
To explore the mechanism that mediates the cleavage of full-length cochlin, we incubated 293T cells expressing cochlin with chemical inhibitors of different proteases. We found that in the presence of MMP inhibitor III, a broad spectrum metalloprotease inhibitor, no p8 and p18 fragments were detected in the culture medium of 293T cells expressing cochlin (Figure 4A). Since cochlin is cleaved between E134–A135, we next investigated whether two glutamyl endopeptidases in the metalloprotease family known to cleave E-A bonds, namely aggrecanase-1 and -2, were involved in processing cochlin (Nagase and Kashiwagi, 2003). Compound 8OO, a specific inhibitor of aggrecanases (patent US2007/0043066) inhibits the release of p8 and p18 fragments in the culture medium of 293T cells expressing cochlin (Figure 4B). In addition, we observed that aggrecanase-1 and -2 physically interact with cochlin as shown by co-immunoprecipitation assay in 293T cells (Figure 4C) and that purified aggrecanase-1 or -2 can directly cleave full-length cochlin to the cochlin p18 fragment in an in vitro cleavage assay (Figure 4D).
Figure 4.
Cochlin is cleaved by the metalloproteases aggrecanase-1 and -2.
A–B. Aggrecanase inhibitors block cochlin cleavage. 293T cells expressing cochlin were cultured in serum free medium with the indicated concentrations of MMP inhibitor III or compound 8OO for 24 h.
C. Cochlin interacts with aggrecanase-1 and -2. Anti-FLAG IP from lysates of 293T cells expressing the indicated constructs (*, non specific band).
D. Aggrecanase-1 and -2 cleave secreted full length cochlin. Purified full length cochlin was treated with aggrecanase-1 (left panel) or aggrecanase-2 (right panel).
E–F. Adamts-4 gene expression is up-regulated in mice spleen upon LPS or TNF-α injection. Spleens from mice injected i.p. with LPS (5 mg/kg) (E), mTNF-α (5 μg or 15 μg, 8h) (F) or PBS (as control) were analyzed by RT-PCR for the expression of Adamts-4 and Adamts-5.
G. Aggrecanase-1 and -2 are expressed by FDCs. In vitro FDC cultures were treated with LPS (100 ng/ml, 6 h or overnight (O/N)). Aggrecanase-1 and -2 expressions were analyzed by RT-PCR.
H. Aggrecanase-1 protein level is up-regulated in the spleen upon LPS injection with a kinetics similar to cochlin cleavage. Spleens from Coch+/− and Coch−/− mice injected i.p. with LPS (5 mg/kg) were analyzed by WB.
I. Aggrecanase-1 and -2 specific inhibitor C8OO inhibits LPS-induced p18 release into the blood. WT and Coch−/− mice were injected intraperitonealy with LPS (1 mg/kg) and C8OO (100 mg/kg) or vehicule only (1.3% DMSO+1.3% solutol) for 8 h. n=2 mice per group.
J. Aggrecanase-1 interacts with cochlin in vivo upon LPS injection. Spleen lysates from WT and Coch−/− mice injected i.p. with LPS (5 mg/kg, 4 h) were subjected to anti-cochlin IP.
See also Figure S4.
To investigate the mechanism that leads to the processing and secretion of cochlin following LPS or TNF-α stimulation, we assessed aggrecanase expression in the spleen following LPS or TNF-α injection. The Adamts-4 (A Desintegrin And Metalloproteinase with ThromboSpondin motifs) gene encoding aggrecanase-1 is not expressed in the spleen in basal conditions but its transcription is greatly activated as soon as 1 h after LPS injection (Figure 4E). Adamts-4 mRNA was also induced following TNF-α injection (Figure 4F). On the other hand, Adamts-5 gene encoding aggrecanase-2 is expressed in the spleen under basal conditions. However, the expression of Adamts-5 not only is not activated following LPS or TNF-α injection but actually decreases transiently following LPS injection (Figure 4E–F). Consistently, the expression of Adamts-4 mRNA in cultured FDCs was induced by LPS whereas that of Adamts-5 mRNA in FDCs was constitutive (Figure 4G).
At the protein level, splenic aggrecanase-1 was induced 2 h after LPS injection, corresponding exactly to the kinetics of the observed cochlin cleavage in the spleen and the secretion of cochlin p8 and p18 into the blood (Figure 4H, 3A). Consistent with the processing of cochlin by aggrecanases, the levels of p8 and p18 induced in the serum by LPS were reduced by the injection of compound 8OO (Figure 4I). We next tested the interaction of endogenous cochlin with aggrecanase-1 in vivo. Aggrecanase-1 co-immunoprecipitates with cochlin in spleen lysates from WT mice injected with LPS (Figure 4J). Neither aggrecanase-1 nor cochlin bands were detectable in the anti-cochlin immunoprecipitation from the splenic lysate of a Coch−/− mouse treated in the same conditions, demonstrating the specificity of the assay. Together with the in vitro data, our results strongly indicate that aggrecanase-1 cleaves cochlin in vivo during LPS and TNF-α responses.
Cochlin-deficiency leads to defects in anti-bacterial innate immunity
To characterize the role of cochlin in anti-bacterial innate immunity, we first examined the role of cochlin in LPS responses, based on the homology of its LCCL domain with the evolutionarily ancient related Factor C protein from the horseshoe crab Limulus. Factor C is critical in sensing LPS and in the consequent activation of the hemolymph coagulation cascade as well as in complement deposition onto the surface of bacteria (Ariki et al., 2008; Iwanaga et al., 1992; Koshiba et al., 2007). However, we did not detect any interaction between the cochlin LCCL domain and LPS (data not shown). Coch−/− macrophages showed no defect in the response to LPS in vitro, nor did the addition of recombinant cochlin LCCL domain have any impact on this response (Figure S5A–B). Moreover, Coch−/− mice showed normal coagulation and cytokine responses to LPS injection in vivo (Figure S5C–D), and their viability to LPS challenge was not modified as compared to that of WT mice (Figure S5E). We thus concluded that cochlin is not involved in the control of the LPS response.
We next tested whether cochlin took part in the detection of bacteria by pattern recognition receptors (PRRs) independently of LPS recognition by TLR4-MD2. In vitro, Coch−/− macrophages respond normally to peptidoglycans (PGN) which activate TLR2 and Nod-1 and -2 receptors, and the addition of recombinant cochlin LCCL domain did not impact this response (Figure S5A). We next investigated the role of cochlin in the detection of intact bacteria using P. aeruginosa (strain PA14) as a model. Since Coch−/− macrophages, in the presence or absence of recombinant cochlin LCCL domain, showed no difference in cytokine responses to the infection by PA14 in vitro (Figure S5F), we concluded that cochlin is not involved in the mechanism of bacterial detection per se.
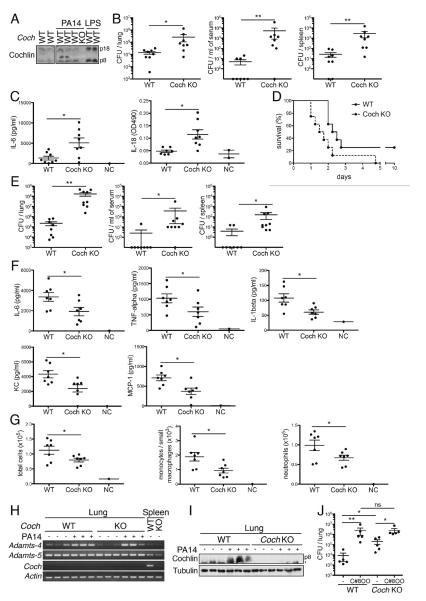
We next reasoned that while the molecular mechanisms used by cochlin and Limulus Factor C might have diverged, their function might nevertheless be conserved and we investigated the role of cochlin in response to bacteria in vivo. We examined how cochlin might impact infection caused by P. aeruginosa, a gram-negative bacteria usually not pathogenic in healthy individuals but which constitutes a major cause of morbidity for cystic fibrosis patients with chronic lower respiratory tract infection, as well as acute opportunistic pneumonias in immunocompromised individuals (Sadikot et al., 2005). Innate immune responses that recruit neutrophils to the lungs are the primary means by which mammals resist P. aeruginosa infections (Koh et al., 2009). Similar to that of LPS injection, intranasal (i.n.) infection with P. aeruginosa (strain PA14) triggered the release of cochlin p8 and p18 into mouse sera (Figure 5A). More importantly, 24 h after i.n. infection of PA14, Coch−/− mice showed higher bacterial loads in the lungs, blood and spleens compared to WT mice (Figure 5B). This defect in controlling infection with P. aeruginosa was associated with higher levels of cytokines such as IL-6 and IL-18 in the serum at 24 h (Figure 5C), and decreased survival of Coch−/− mice (Figure 5D). Similarly, Coch−/− mice showed higher bacterial loads in the lungs, blood and spleens as compared to WT mice 24 h following i.n. infection with the gram-positive bacteria Staphylococcus aureus (LAC strain) (Figure 5E), demonstrating that cochlin anti-bacterial function is not restricted to one specific bacteria strain, and definitively excluding a LPS-dependent mechanism. Since cochlin LCCL had direct effect neither on the in vitro viability and growth of PA14 and LAC nor on biofilm formation (Figure S5G–J), we concluded that cochlin is unlikely to be a bactericidal effector and hypothesized that it may regulate innate immune response. Indeed, analysis of the broncho-alveolar lavage of infected lungs showed that Coch−/− mice had a defect in the local secretion of IL-6, TNF-α, IL-1β and in the macrophage and neutrophil chemoattractants MCP-1 and KC at 8 h post-infection with PA14 (Figure 5F), followed by a defective recruitment of monocytes/small macrophages and neutrophils at 12 h post-infection (Figure 5G). Differentiated alveolar macrophage counts were similar in BAL of WT and Coch−/− mice at this time point (data not shown). Since cochlin was not expressed at the mRNA level in lung under basal condition nor following infection with PA14 (Figure 5H) but cochlin LCCL protein accumulated in the lung following infection with PA14 (Figure 5I), we hypothesized that cochlin cleavage by aggrecanases and the subsequent release of cochlin LCCL into the blood was necessary for cochlin to control bacteria loads in the lung. Indeed inhibition of aggrecanases by i.v. injection of compound 8OO greatly increased lung bacteria loads, demonstrating that aggrecanase activity participate in the control of bacteria growth (Figure 5J). More importantly, the effect of compound 8OO was significantly attenuated in Coch−/− mice compared to that of WT mice as shown by the statistical significance of the interaction in the two-way ANOVA. We thus conclude that cochlin cleavage accounts for a significant part of the anti-bacterial effect by aggrecanases, although aggrecanases also control bacteria growth through additional cochlin-independent mechanisms.
Figure 5.
Cochlin promotes anti-bacterial immunity against P. aeruginosa.
A. Release of cochlin p8 and p18 into the serum during infection with PA14. Sera from WT and Coch−/− mice i.n. infected with PA14 for 10 h were analyzed by WB. Sera from mice injected i.p. with LPS (5 mg/kg, 8 h) were used as positive controls.
B. Increased CFU number in lung and bacterial spread in Coch−/− mice. Coch−/− and WT mice were i.n. infected with PA14 for 24 h. n=8 per group; *, p<0.01; **, p<0.001); data are representative of 4 independent experiments. Error bars represent one SE from the mean.
C. Enhanced systemic cytokine burst in Coch−/− mice. Coch−/− and WT mice were i.n. infected with PA14 for 24 h. IL-6 and IL-18 concentrations in sera were measured by ELISA. Uninfected mice were used as negative controls (NC). n=8 per group; *, p<0.01. Error bars represent one SE from the mean.
D. Reduced survival of infected Coch−/− mice. Coch−/− and WT mice were i.n. infected with PA14. n=8 per group; Gehan-Breslow-Wilcoxon test p<0.05; data are representative of 2 independent experiments.
E. Increased CFU number in lung and bacterial spread in Coch−/− mice following S. aureus i.n. infection. Coch−/− and WT mice were i.n. infected with LAC for 24 h. n=8–9 per group; *, p<0.01; **p<0.001); data are representative of 2 independent experiments. Error bars represent one SE from the mean.
F. Decreased cytokine levels in the BAL fluid of Coch−/− mice. Coch−/− and WT mice were i.n. infected with PA14 for 8 h. Cytokines concentrations in the BAL fluid were determined by ELISA. n=7 per group; *, p<0.05. Error bars represent one SE from the mean.
G. Decreased lung recruitment of PMNs in Coch−/− mice. Coch−/− and WT mice were i.n. infected with PA14 for 12 h. Cell numbers and composition in the BAL was analyzed by flow cytometry. Neutrophils and monocyte/small macrophages are defined as CD11c−/CD11bhigh/GR-1+ and CD11c−/CD11bmild/GR-1− respectively. n=7 per group; *, p<0.05. Error bars represent one SE from the mean.
H.I. Cochlin is not produced but LCCL accumulates in the lung following P. aeruginosa i.n. infection. Coch−/− and WT mice were infected with PA14 for 8 h. Lung were analyzed for cochlin mRNA expression by RT-PCR (H) and cochlin LCCL protein accumulation by WB (I). *, unspecific band.
J. Reduced effect of aggrecanase inhibition on CFU number in the lung of Coch−/− mice. Coch−/− and WT mice were i.v. injected with C8OO (100 mg/kg) or vehicle only (−) and i.n. infected with PA14 for 24 h. n=5 per group; *, p<0.01; **, p<0.001; ns, non significant. The effect of cochlin deficiency on the increase in CFU following C8OO injection was assessed by a two-way ANOVA using “genotype”, “C8OO treatment” and the interaction between “genotype” and “C8OO treatment” as fixed factors. “genotype”: F1.18=11.68, p=0.0035; “C8OO”: F1.18=55.07, p<0.001; “genotype*C8OO”: F1.18=5.57, p=0.0313. Error bars represent one SE from the mean.
See also Figure S5.
Taken together, we conclude that cochlin, specifically expressed by the follicular dendritic cells and selectively localized in the fine extracellular network of conduits in the spleen and lymph nodes, is an important modulator of innate immune response. During infection and inflammation, cochlin is cleaved by aggrecanases and secreted into blood circulation and regulates local cytokine production, recruitment of immune effector cells and bacterial clearance (Figure S5K). We propose that by regulating the production of cochlin, the FDCs may have a previously unknown function in innate immune response in defense against bacteria.
Discussion
Our study describes cochlin as a modulator of innate immunity produced by the FDCs, the known critical modulators of humoral immunity promoting activation and maturation of B cells within follicles (Allen and Cyster, 2008). FDCs are tightly associated with conduits, a fine extracellular network in spleen and lymph nodes that is involved in small antigens and cytokines transport. While other conduit-associated cytokines have been shown to regulate local cell migration within the lymphoid organ, our study demonstrates cochlin as the first immune modulator locally deposited by the FDCs into the conduits and released upon inflammation into the systemic circulation. Our study supports the notion that the conduit is not only a channel mediating passive diffusion of small molecules, but also a reservoir for ready-to-be-released immune regulators to play active roles in modulating immunity. Moreover, our study suggests a unique role for the FDCs in modulating innate immunity.
We identified the FDCs as the producer of cochlin in the secondary lymphoid organs. FDCs play a critical role in germinal center formation and B cell activation, affinity maturation and differentiation (Allen and Cyster, 2008). Coch−/− mice showed no defects in germinal center formation, immunoglobulin responses or antibody affinity maturation, suggesting that cochlin in the FDCs may serve no function with regards to B cell activation. A previous study implicated cochlin in cell-adhesion, most likely through its two C-terminus von Willebrand factor A-like (vWFA) domains (Bhattacharya et al., 2005). However, cochlin deficiency does not modify FDC localization around the conduits or their ability to capture antigen flowing in the conduit's lumen, suggesting that cochlin does not play a critical role in mediating adhesion of FDCs. Although cochlin is associated with structures mediating fluid flow such as the trabecular meshwork in the eyes, the conduits in the secondary lymphoid organs, as well as in the inner ear (Bhattacharya et al., 2005; Nolte et al., 2003; Robertson et al., 2006; Roozendaal et al., 2009), cochlin-deficiency causes no impairment in the flow inside the conduit, suggesting that cochlin does not play a critical structural role in supporting the integrity of the fine tubular structure. As abnormal accumulation of cochlin in the inner ear and the eyes has been proposed to interfere with fluid flow and contribute to pathological conditions (Bhattacharya et al., 2005), a possible future study may be to identify any condition where abnormal accumulation of cochlin in the conduits may interfere with the flow which might provide further insights for the physiological function of the conduits in the secondary lymphoid organs.
Our fluorescent and electron microscopy data consistently show that cochlin colocalizes with collagen-forming fibrillar and bundle structures in the conduits. A similar association with type II fibrillar collagen has previously been shown in the inner ear (Mizuta et al., 2008). Indeed, type I, II and IV collagens bind to the second vWFA domain but not the LCCL-domain of cochlin (Nagy et al., 2008). Our data reveal that the LCCL domain is released in the blood following cochlin cleavage between its LCCL domain and its C-terminal domain containing the two vWFA domains. While cochlin C-terminal domains (named p40 and p45) are found in the spleen (Rodriguez et al., 2004), they remained undetectable in the blood in both basal and inflammatory conditions. Thus, our working model is that cochlin binds to conduit collagen through its vWFA domains, and following cochlin cleavage by aggrecanases, the LCCL domain is released into the blood stream while the C-terminal domain remained bound to collagen in the conduits.
Multiple cochlin isoforms have been described that result from both alternative splicing and post-translational modifications, and their expression patterns show tissue specificity (Bhattacharya et al., 2005; Ikezono et al., 2001; Kommareddi et al., 2007; Mizuta et al., 2008; Robertson et al., 2001). Our study reports p8 and p18 as new circulating forms of cochlin, highly reminiscent of the N-terminal p16 fragment named cochlin tomoprotein (CTP) recently identified in the ear perilymph, but absent from inner ear tissue (Ikezono et al., 2004). Our data open perspectives in the identification of CTP, which may be identical to p8 and p18, and in its mechanism of secretion that may be dependent on aggrecanases. In addition, our finding that p18 is a glycosylated product of p8 is consistent with the residue N102 being a consensus site for N-linked glycosylation.
Our observation that the cochlin cleavage site [E-AtgRavsTA] was highly similar to the established aggrecanase-1 consensus cleavage site [E-(AFVLMY)-X(0,1)-(RK)-X(2,3)-(ST)-(VYIFWMLA)] lead us to identify cochlin as a new substrate for aggrecanase-1 and -2. These glutamyl-endopeptidases from the ADAMTS metalloprotease family play a key role in arthritis pathogenesis by cleaving the cartilage matrix proteoglycan aggrecan (Lin and Liu, 2010). The other substrates of the ADAMTS family include several components of the extracellular matrix such as COMP (cartilage oligomeris protein), biglycan, TIMP-4, matrillin-2 and -3 (Hills et al., 2007). We found that aggrecanase-2, but not aggrecanase-1, is constitutively expressed in the spleen suggesting that the low basal circulating level of cochlin p8 and p18 in the blood may result of aggrecanase-2 activity. Aggrecanase-1 expression is highly induced in the spleen following LPS or TNF-α injection with kinetics matching exactly these of cochlin cleavage. This observation is consistent with multiple reports claiming that aggrecanase-1 expression is up-regulated by inflammatory cytokines (Bondeson et al., 2006; Cross et al., 2006; Song et al., 2007; Tortorella et al., 2001; Yamanishi et al., 2002).
Our discovery that cochlin LCCL domain is a blood circulating protein with increased level during inflammation, led us to investigate a systemic role of the LCCL domain in the innate immune response. LCCL is an autonomous folding domain consisting of a central α helix wrapped by two β sheets named after the first three proteins identified to contain this domain (Limulus factor C, Cochlin and Lgl-1 (late gestation lung)) and later found in various secreted proteins with modular structures (Liepinsh et al., 2001). The horseshoe crab Limulus coagulation factor C is a serine protease secreted in the hemolymph, activated upon LPS binding and is critical in anti-bacterial immunity. In particular, factor C mediates the degranulation of the amoebocytes, the initiation of a protease cascade resulting in coagulation, and the activation of the complement system (Ariki et al., 2008; Iwanaga et al., 1992; Koshiba et al., 2007). The contribution of the LCCL domain in the function of factor C remains unknown, and in particular LCCL does not bind LPS (Koshiba et al., 2007; Tan et al., 2000). A role in anti-bacterial innate immunity has also been suggested for the mammalian protein lgl-1 (also named CRISPLD2 for cysteine-rich secretory protein LCCL domain containing 2) expressed in various organs and notably detected in the serum. Lgl-1 binds to LPS and sequesters it away from the PBMCs surface (Wang et al., 2009). Cochlin LCCL sequence and secretion pattern homologies with Limulus factor C and lgl-1 led us to hypothesize a conservation of their functions. Indeed, we discovered that cochlin is critical in innate immunity using models of P. aeruginosa and S. aureus i.n. infection. However, contrary to factor C or lgl-1, cochlin effector function is independent of LPS, as cochlin LCCL domain does not bind to LPS (data not shown and (Liepinsh et al., 2001)), and impacts LPS response neither in vitro nor in vivo. In addition, the low affinity of the P. aeruginosa LPS for TLR4 and the absence of phenotype of the Tlr4−/− mice in regards to P. aeruginosa infection predict that the TLR4-mediated LPS response is accessory in the immunity against this pathogen, and modulation of this pathway is not expected to impact the outcome of the infection (Feuillet et al., 2006; Ramphal et al., 2005). Finally cochlin role in controlling the gram-positive bacteria S. aureus definitely demonstrates a LPS-independent mechanism.
Our study shows a defect of Coch−/− mice in local cytokine and chemoattractant production in the lung and in the subsequent recruitment of immune cells leading to an impaired control of the bacteria and a consequent reduced viability following infection. These data confirm previous studies describing that the recruitment of neutrophils and macrophages to the lung is absolutely critical for the outcome of P. aeruginosa infection and that blunted early local inflammatory response in the lung leads to later higher bacteria load, uncontrolled systemic cytokine secretion and reduced survival (Horino et al., 2009; Koh et al., 2009). Taken together, our study demonstrates an unexpected function of FDC in modulating innate immunity and a role for secondary lymphoid organ and particularly local lymph nodes in the amplification of the innate immune response by the release of modulators such as cochlin.
Materials and Methods
Expression vectors
Cochlin expression vectors have already been described (Yao et al., 2010) Adamts-4 and Adamts-5 genes were cloned from total cDNA isolated from C57B6 mouse spleen using the primers adamts4-f 5′-CATTTTGGTGCCGCAGATG-3′, adamts4-r 5′-CGGGACAGTGAGGTTATTTCC-3′, adamts5-f 5′-CACTATGCGGCTCGAGTG-3′, adamts5-r 5′-CAGGCTAACATTTCTTCAGCAGAC-3′ in pcDNA3.
Reagents
Compound 8OO (patent US2007/0043066) was custom synthetized by Shanghai ChemPartner Co., Ltd.
Antibodies
The monoclonal anti-cochlin 9A10D2 raised against a.a. 7–227 of cochlin has been described (Yao et al., 2010) (WB analysis). Rabbit polyclonal antibody P13 was raised against cochlin a.a.7–227 (histology staining and immunoprecipitation). The rat monoclonal AD4/4D2E10 anti-aggrecanase-1 was raised against full-length murine aggrecanase-1.
FDC isolation
FDCs were isolated from LN as described (Sukumar et al., 2006) and cultured in FDCs media (DMEM, FCS 10%, Hepes 20 mM, glutamine 2 mM, gentamicin 50 μg/ml, non-essential amino acid 1×, β-mercaptoethanol 4×10−4 %).
In vitro cleavage assay
The C-term FLAG-tagged aggrecanase-1 and -2 were purified by IP from culture media of 293T cells transfected with the murine Adamts-4 (aggr-1) or Adamts-5 (aggr-2) constructs. The full length C-term FLAG-tagged cochlin was similarly purified by IP from culture media of transfected 293T cells in the presence of MMP inhibitor III (50 μM). Proteins were eluted by FLAG peptide, and dialyzed in cleavage buffer (Tris HCl pH 7.5 50 mM, NaCl 100 mM, CaCl2 10 mM, NaN3 0.02%, NP-40 0.02%). Cochlin and aggrecanase-1 or -2 were incubated in cleavage buffer at 37 °C, and reactions were stopped by Laemmli buffer.
Bacteria infection
Gender-matched 6- to 9-week old Coch−/− and C57B6 control mice were sedated with ketamine hydrochloride (65 mg/kg) and xylazine (13 mg/kg), and infected i.n. with PA14 (1–3×107 CFU) or LAC (1–3×108 CFU). For CFU enumeration, lungs, blood, and spleens were harvested and homogenized in LB, and dilutions platted on LB-agar plates. Broncho-alveolar lavages (BAL) were collected in 2 ml of PBS and spun at 1500 rpm for 5 min.
Supplementary Material
Acknowledgment
We thank Colin L. Stewart (National Cancer Institute, Frederick, Maryland) for providing Coch−/− mice; Bjorn R. Olsen and Rodrick Bronson for scientific advices and discussions; Adeline Bernier and Melissa Marinelli for technical assistance; James Kenny, Lisa A. Pitcher, Caroline N. Herndon, Sloan Siegrist, Ying Li and Michael Boyce for technical advices; Stéphanie Bedhomme for statistical analysis; Jennifer Walters and the staff at the Nikon imaging center, Maria Ericsson and the staff at the electronic microscopy facility and the rodent histology facility (Harvard Medical School) for help with image analysis. This work was supported in part by the National Institutes of Health Director's Pioneer Award (to J. Y.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin Immunol. 2008;20:14–25. doi: 10.1016/j.smim.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariki S, Takahara S, Shibata T, Fukuoka T, Ozaki A, Endo Y, Fujita T, Koshiba T, Kawabata S. Factor C acts as a lipopolysaccharide-responsive C3 convertase in horseshoe crab complement activation. J Immunol. 2008;181:7994–8001. doi: 10.4049/jimmunol.181.11.7994. [DOI] [PubMed] [Google Scholar]
- Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya SK, Rockwood EJ, Smith SD, Bonilha VL, Crabb JS, Kuchtey RW, Robertson NG, Peachey NS, Morton CC, Crabb JW. Proteomics reveal Cochlin deposits associated with glaucomatous trabecular meshwork. J Biol Chem. 2005;280:6080–6084. doi: 10.1074/jbc.M411233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther. 2006;8:R187. doi: 10.1186/ar2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AK, Haddock G, Stock CJ, Allan S, Surr J, Bunning RA, Buttle DJ, Woodroofe MN. ADAMTS-1 and -4 are up-regulated following transient middle cerebral artery occlusion in the rat and their expression is modulated by TNF in cultured astrocytes. Brain Res. 2006;1088:19–30. doi: 10.1016/j.brainres.2006.02.136. [DOI] [PubMed] [Google Scholar]
- El Shikh ME, El Sayed RM, Wu Y, Szakal AK, Tew JG. TLR4 on follicular dendritic cells: an activation pathway that promotes accessory activity. J Immunol. 2007;179:4444–4450. doi: 10.4049/jimmunol.179.7.4444. [DOI] [PubMed] [Google Scholar]
- Feuillet V, Medjane S, Mondor I, Demaria O, Pagni PP, Galan JE, Flavell RA, Alexopoulou L. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci U S A. 2006;103:12487–12492. doi: 10.1073/pnas.0605200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garin A, Meyer-Hermann M, Contie M, Figge MT, Buatois V, Gunzer M, Toellner KM, Elson G, Kosco-Vilbois MH. Toll-like receptor 4 signaling by follicular dendritic cells is pivotal for germinal center onset and affinity maturation. Immunity. 2010;33:84–95. doi: 10.1016/j.immuni.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med. 2000;192:1425–1440. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills R, Mazzarella R, Fok K, Liu M, Nemirovskiy O, Leone J, Zack MD, Arner EC, Viswanathan M, Abujoub A, et al. Identification of an ADAMTS-4 cleavage motif using phage display leads to the development of fluorogenic peptide substrates and reveals matrilin-3 as a novel substrate. J Biol Chem. 2007;282:11101–11109. doi: 10.1074/jbc.M611588200. [DOI] [PubMed] [Google Scholar]
- Horino T, Matsumoto T, Ishikawa H, Kimura S, Uramatsu M, Tanabe M, Tateda K, Miyazaki S, Aramaki Y, Iwakura Y, et al. Interleukin-1 deficiency in combination with macrophage depletion increases susceptibility to Pseudomonas aeruginosa bacteremia. Microbiol Immunol. 2009;53:502–511. doi: 10.1111/j.1348-0421.2009.00143.x. [DOI] [PubMed] [Google Scholar]
- Ikezono T, Omori A, Ichinose S, Pawankar R, Watanabe A, Yagi T. Identification of the protein product of the Coch gene (hereditary deafness gene) as the major component of bovine inner ear protein. Biochim Biophys Acta. 2001;1535:258–265. doi: 10.1016/s0925-4439(00)00101-0. [DOI] [PubMed] [Google Scholar]
- Ikezono T, Shindo S, Li L, Omori A, Ichinose S, Watanabe A, Kobayashi T, Pawankar R, Yagi T. Identification of a novel Cochlin isoform in the perilymph: insights to Cochlin function and the pathogenesis of DFNA9. Biochem Biophys Res Commun. 2004;314:440–446. doi: 10.1016/j.bbrc.2003.12.106. [DOI] [PubMed] [Google Scholar]
- Iwanaga S, Miyata T, Tokunaga F, Muta T. Molecular mechanism of hemolymph clotting system in Limulus. Thromb Res. 1992;68:1–32. doi: 10.1016/0049-3848(92)90124-s. [DOI] [PubMed] [Google Scholar]
- Koh AY, Priebe GP, Ray C, Van Rooijen N, Pier GB. Inescapable need for neutrophils as mediators of cellular innate immunity to acute Pseudomonas aeruginosa pneumonia. Infect Immun. 2009;77:5300–5310. doi: 10.1128/IAI.00501-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommareddi PK, Nair TS, Raphael Y, Telian SA, Kim AH, Arts HA, El-Kashlan HK, Carey TE. Cochlin isoforms and their interaction with CTL2 (SLC44A2) in the inner ear. J Assoc Res Otolaryngol. 2007;8:435–446. doi: 10.1007/s10162-007-0099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T, Hashii T, Kawabata S. A structural perspective on the interaction between lipopolysaccharide and factor C, a receptor involved in recognition of Gram-negative bacteria. J Biol Chem. 2007;282:3962–3967. doi: 10.1074/jbc.M609198200. [DOI] [PubMed] [Google Scholar]
- Liepinsh E, Trexler M, Kaikkonen A, Weigelt J, Banyai L, Patthy L, Otting G. NMR structure of the LCCL domain and implications for DFNA9 deafness disorder. EMBO J. 2001;20:5347–5353. doi: 10.1093/emboj/20.19.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EA, Liu CJ. The role of ADAMTSs in arthritis. Protein Cell. 2010;1:33–47. doi: 10.1007/s13238-010-0002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokmic Z, Lammermann T, Sixt M, Cardell S, Hallmann R, Sorokin L. The extracellular matrix of the spleen as a potential organizer of immune cell compartments. Semin Immunol. 2008;20:4–13. doi: 10.1016/j.smim.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Manolis EN, Yandavi N, Nadol JB, Jr., Eavey RD, McKenna M, Rosenbaum S, Khetarpal U, Halpin C, Merchant SN, Duyk GM, et al. A gene for non-syndromic autosomal dominant progressive postlingual sensorineural hearing loss maps to chromosome 14q12-13. Hum Mol Genet. 1996;5:1047–1050. doi: 10.1093/hmg/5.7.1047. [DOI] [PubMed] [Google Scholar]
- Mizuta K, Ikezono T, Iwasaki S, Arai M, Hashimoto Y, Pawankar R, Watanabe T, Shindo S, Mineta H. Ultrastructural co-localization of cochlin and type II collagen in the rat semicircular canal. Neurosci Lett. 2008;434:104–107. doi: 10.1016/j.neulet.2008.01.036. [DOI] [PubMed] [Google Scholar]
- Nagase H, Kashiwagi M. Aggrecanases and cartilage matrix degradation. Arthritis Res Ther. 2003;5:94–103. doi: 10.1186/ar630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy I, Trexler M, Patthy L. The second von Willebrand type A domain of cochlin has high affinity for type I, type II and type IV collagens. FEBS Lett. 2008;582:4003–4007. doi: 10.1016/j.febslet.2008.10.050. [DOI] [PubMed] [Google Scholar]
- Nolte MA, Belien JA, Schadee-Eestermans I, Jansen W, Unger WW, van Rooijen N, Kraal G, Mebius RE. A conduit system distributes chemokines and small blood-borne molecules through the splenic white pulp. J Exp Med. 2003;198:505–512. doi: 10.1084/jem.20021801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R, Balloy V, Huerre M, Si-Tahar M, Chignard M. TLRs 2 and 4 are not involved in hypersusceptibility to acute Pseudomonas aeruginosa lung infections. J Immunol. 2005;175:3927–3934. doi: 10.4049/jimmunol.175.6.3927. [DOI] [PubMed] [Google Scholar]
- Robertson NG, Cremers CW, Huygen PL, Ikezono T, Krastins B, Kremer H, Kuo SF, Liberman MC, Merchant SN, Miller CE, et al. Cochlin immunostaining of inner ear pathologic deposits and proteomic analysis in DFNA9 deafness and vestibular dysfunction. Hum Mol Genet. 2006;15:1071–1085. doi: 10.1093/hmg/ddl022. [DOI] [PubMed] [Google Scholar]
- Robertson NG, Hamaker SA, Patriub V, Aster JC, Morton CC. Subcellular localisation, secretion, and post-translational processing of normal cochlin, and of mutants causing the sensorineural deafness and vestibular disorder, DFNA9. J Med Genet. 2003;40:479–486. doi: 10.1136/jmg.40.7.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson NG, Khetarpal U, Gutierrez-Espeleta GA, Bieber FR, Morton CC. Isolation of novel and known genes from a human fetal cochlear cDNA library using subtractive hybridization and differential screening. Genomics. 1994;23:42–50. doi: 10.1006/geno.1994.1457. [DOI] [PubMed] [Google Scholar]
- Robertson NG, Resendes BL, Lin JS, Lee C, Aster JC, Adams JC, Morton CC. Inner ear localization of mRNA and protein products of COCH, mutated in the sensorineural deafness and vestibular disorder, DFNA9. Hum Mol Genet. 2001;10:2493–2500. doi: 10.1093/hmg/10.22.2493. [DOI] [PubMed] [Google Scholar]
- Robertson NG, Skvorak AB, Yin Y, Weremowicz S, Johnson KR, Kovatch KA, Battey JF, Bieber FR, Morton CC. Mapping and characterization of a novel cochlear gene in human and in mouse: a positional candidate gene for a deafness disorder, DFNA9. Genomics. 1997;46:345–354. doi: 10.1006/geno.1997.5067. [DOI] [PubMed] [Google Scholar]
- Rodriguez CI, Cheng JG, Liu L, Stewart CL. Cochlin, a secreted von Willebrand factor type a domain-containing factor, is regulated by leukemia inhibitory factor in the uterus at the time of embryo implantation. Endocrinology. 2004;145:1410–1418. doi: 10.1210/en.2003-1361. [DOI] [PubMed] [Google Scholar]
- Roozendaal R, Mempel TR, Pitcher LA, Gonzalez SF, Verschoor A, Mebius RE, von Andrian UH, Carroll MC. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity. 2009;30:264–276. doi: 10.1016/j.immuni.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2005;171:1209–1223. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, Pabst R, Lutz MB, Sorokin L. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Song RH, Tortorella MD, Malfait AM, Alston JT, Yang Z, Arner EC, Griggs DW. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56:575–585. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- Sukumar S, Szakal AK, Tew JG. Isolation of functionally active murine follicular dendritic cells. J Immunol Methods. 2006;313:81–95. doi: 10.1016/j.jim.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Maruya M, Kawamoto S, Sitnik K, Kitamura H, Agace WW, Fagarasan S. The sensing of environmental stimuli by follicular dendritic cells promotes immunoglobulin A generation in the gut. Immunity. 2010;33:71–83. doi: 10.1016/j.immuni.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Tan NS, Ng ML, Yau YH, Chong PK, Ho B, Ding JL. Definition of endotoxin binding sites in horseshoe crab factor C recombinant sushi proteins and neutralization of endotoxin by sushi peptides. FASEB J. 2000;14:1801–1813. doi: 10.1096/fj.99-0866com. [DOI] [PubMed] [Google Scholar]
- Tortorella MD, Malfait AM, Deccico C, Arner E. The role of ADAM-TS4 (aggrecanase-1) and ADAM-TS5 (aggrecanase-2) in a model of cartilage degradation. Osteoarthritis Cartilage. 2001;9:539–552. doi: 10.1053/joca.2001.0427. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Xing WM, Fan HH, Wang KS, Zhang HK, Wang QW, Qi J, Yang HM, Yang J, Ren YN, et al. The novel lipopolysaccharide-binding protein CRISPLD2 is a critical serum protein to regulate endotoxin function. J Immunol. 2009;183:6646–6656. doi: 10.4049/jimmunol.0802348. [DOI] [PubMed] [Google Scholar]
- Yamanishi Y, Boyle DL, Clark M, Maki RA, Tortorella MD, Arner EC, Firestein GS. Expression and regulation of aggrecanase in arthritis: the role of TGF-beta. J Immunol. 2002;168:1405–1412. doi: 10.4049/jimmunol.168.3.1405. [DOI] [PubMed] [Google Scholar]
- Yao J, Py BF, Zhu H, Bao J, Yuan J. Role of protein misfolding in DFNA9 hearing loss. J Biol Chem. 2010;285:14909–14919. doi: 10.1074/jbc.M110.106724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.