Abstract
Background and Objectives
Opioid titration is an effective strategy for treating pain. However, titration is generally impractical in the busy emergency department (ED) setting. Our objective was to test a rapid, 2-step, hydromorphone titration protocol against usual care in older patients presenting to the ED with acute severe pain.
Methods
This was a prospective, randomized clinical trial of patients 65 years of age and older presenting to an adult, urban, academic ED with acute severe pain. The study was registered at www.clinicaltrials.gov (NCT01429285). Patients randomized to the hydromorphone titration protocol initially received 0.5 mg intravenous (IV) hydromorphone. Patients randomized to usual care received any dose of any IV opioid. At 15 minutes, patients in both groups were asked, “Do you want more pain medication?” Patients in the hydromorphone titration group who answered “yes” received a second dose of 0.5 mg IV hydromorphone. Patients in the usual care group who answered “yes” had their treating attending notified, who then could administer any (or no) additional medication. The primary efficacy outcome was satisfactory analgesia defined a priori as the patient declining additional analgesia at least once when asked at 15 or 60 minutes after administration of initial opioid. Dose was calculated in morphine equivalent units (MEU: 1 mg hydromorphone = 7 mg morphine). Need for naloxone to reverse adverse opioid effects was the primary safety outcome.
Results
83.0% of 153 patients in hydromorphone titration group achieved satisfactory analgesia compared to 82.5% of 166 patients in the usual care group (p=0.91). Patients in the hydromorphone titration group received lower mean initial doses of opioids at baseline than patients in UC (3.5 MEU vs. 4.7 MEU respectively, p=<0.001) and lower total opioids through 60 minutes (5.3 MEU vs. 6.0 MEU, p=0.03). No patient needed naloxone.
Conclusions
Low-dose titration of IV hydromorphone in increments of 0.5 mg provides comparable analgesia to usual care with less opioid over 60 minutes.
1. BACKGROUND AND OBJECTIVE
Older adults are at high risk for untreated and undertreated pain, which is associated with depression, social isolation, immobility, and sleep disturbances.[1]
A pharmacologic regimen to treat acute pain in older adults must combine induction of clinically satisfactory analgesia with sufficient safety precautions, minimizing the incidence of potentially serious side effects such as respiratory depression and hypotension.[2] This is especially important for older adults because both pharmacokinetics and pharmacodynamics may be altered by age and comorbidity.[3] For example, drug distribution may be altered due to decreased lean body mass, decreased total body water, and increased proportion of body fat. These changes in body composition lead to a decrease in the volume of distribution and an increase in the serum concentrations of various drugs, potentially resulting in a higher incidence of side effects[3].
Titration to analgesic effect is a promising strategy because it optimizes the opportunity for the clinician to provide an adequate total amount of opioid to achieve induction of clinically satisfactory analgesia while minimizing the probability of an iatrogenic opioid overdose. Although frequent titration of IV opioids is routine in postoperative patients in recovery rooms, such a strategy is often impractical in a busy emergency department (ED) where both the physician and nurse may be caring for many acutely ill patients simultaneously.
Prior work in younger patients assessed the efficacy and safety of a rapid titration protocol in which patients 21 to 64 years of age received a bolus of 1 mg IV hydromorphone followed by a second 1 mg dose if patients wanted more analgesia 15 minutes after the first dose.[4] We found that 94% of the patients obtained adequate analgesia within 60 minutes using this strategy.
The objective of this study was to assess the efficacy and safety of a similar rapid IV titration protocol targeted at induction of clinically satisfactory analgesia in older adults presenting to the ED with acute severe pain. Our hypothesis was that this rapid, 2-step IV titration protocol modified for older patients would be more efficacious and as safe as usual care.
Analogous to the previous study undertaken in younger patients but following standard practice of administering smaller doses of opioids to older patients, we administered an initial dose of 0.5 mg IV hydromorphone followed by a second 0.5 mg dose 15 minutes later if the patient answered “yes” when asked, “Do you want more pain medication?” Thus, the maximum total dose given to older patients randomized to this rapid, 2-step hydromorphone titration protocol within the one hour study period was 1.0 mg. We believe that such a protocol will be more readily adaptable to the ED setting than typical postoperative protocols that require reassessment and titration of pain mediation as frequently as every 3 to 5 minutes.
2. METHODS
2a. Study design
This was a randomized clinical trial comparing the efficacy and safety of a rapid 2-step hydromorphone titration protocol in older adults to that of usual care for the ED treatment of acute severe pain. The study was approved by the Montefiore Medical Center institutional review board and is registered in www.clinicaltrials.gov (NCT01429285).
2b. Setting
The study took place in an academic inner city ED with an annual census of over 100,000 adults.
2c. Patients
Patients aged 65 years and older presenting to the ED with acute pain were enrolled if their pain was of sufficient severity to warrant use of IV opioids in the judgment of the ED attending physician.
Exclusion criteria were as follows: history of allergy to opioids; hypotension (systolic blood pressure < 90 mm Hg); room air oxygen saturation < 95%; heart rate < 50 beats per minute, alcohol or other drug intoxication as judged by the attending physician; use of other opioids within the past 7 days; use of a monoamine oxidase inhibitor; weight less than 100 pounds; history of a chronic pain syndrome (such as sickle cell disease or fibromyalgia); patients managed by an of the ED attending physicians who were investigators in this study, and cognitive impairment, as reflected by a score of less than 4 on the six-item screener[5]. In the six-item screener, patients repeat three words and then are asked for the year, month, and day of the week. They are then asked to recall the three words that they were told earlier. A point is awarded for each correct response with a maximum score of 6.
2d. Intervention
Research associates (RAs) consented and enrolled patients referred to them by ED physicians. The RAs were fluent in English and Spanish and enrolled patients 24 hours per day, 7 days per week, from July 2009 to June 2012.
Patients were randomized to the hydromorphone titration group or to usual care after signing a written informed consent form in either English or Spanish. Both versions were approved by our IRB. Random allocation was generated with www.randomization.com, using sealed opaque envelopes opened in sequential order by the RAs immediately following enrollment.
Patients who were randomly allocated to the hydromorphone titration group received an initial dose of 0.5 mg IV hydromorphone. Patients who were randomly allocated to usual care received an initial dose of IV opioid, the type and dose of which was determined by the treating ED attending physician. 15 minutes later patients in both groups were asked the question: “Do you want more pain medication?” If patients in the hydromorphone titration group answered “yes”, they received an additional 0.5 mg IV hydromorphone. If patients in the usual care group answered “yes”, the RA informed the attending physician of the patient’s request, and additional analgesia (if any) was administered at the attending physician’s discretion.
At 60 minutes after the initial administration of IV opioids, all patients were again asked the question, “Do you want more pain medication?”
Patients were also asked to rate their pain on a previously validated and reproducible[6] standard verbal numerical rating scale (NRS) ranging from 0 (“no pain”) through 10 (“worst pain possible”), immediately before the analgesic was administered (baseline), and repeatedly at 5, 15, 30, 45, and 60 minutes following administration of the initial bolus. Systolic blood pressure, oxygen saturation, nausea, vomiting, and pruritus were assessed at baseline and at 5, 15, 30, 45, and 60 minutes after initial administration of opioid.
Patients who experienced oxygen desaturation (defined as < 95%) were gently aroused if sleeping, asked to take several deep breaths, repositioned into a sitting position if they had been in a reclining position, and placed on 4 liters nasal cannula oxygen. The treating attending was notified and subsequent management, including the use of naloxone, was at the treating attending’s discretion.
Data were recorded on a standardized data collection instrument and entered into SPSS Data Entry (SPSS, Inc., Chicago, IL) by a trained data clerk. Double entry of key variables was performed by a second trained clerk and any discrepancies were reconciled by referral to the original data collection instrument.
2e. Main outcome measure
The primary efficacy outcome was the difference in the proportion of patients in each group that achieved satisfactory analgesia within 60 minutes of study enrollment, defined a priori as declining additional pain medication at least once when asked within 60 minutes of administration of initial opioid. Because we wished to compare the safety and efficacy of a rapid hydromorphone titration strategy to usual care in the induction of clinically satisfactory analgesia (considered separately from maintenance of analgesia after induction has been achieved), patients who did not want more medication on at least one occasion, when queried at 15 and 60 minutes, were considered to have achieved induction of clinically satisfactory analgesia within the one hour study period. Only those patients who responded affirmatively to the question posed to them at both 15 and 60 minutes were classified as not having achieved induction of clinically satisfactory analgesia within one hour. Further management after study termination at 60 minutes was based on attending discretion.
Secondary efficacy outcomes included: 1) declining additional analgesics at 15 minutes and at 60 minutes; 2) between-group difference in mean change in NRS pain scores from 0 to 60 minutes; 3) difference in proportion of patients in each group achieving ≥ 50% decline in NRS from baseline to 60 minutes;[7, 8] 3) between-group difference in proportion of patients achieving an absolute NRS score ≤ 3 by 60 minutes;[9, 10] and 4) difference in median absolute pain score between groups at 60 minutes.
The primary safety outcome was use of naloxone at any point during the study, which could be given for any reason as determined by ED attending judgment. Secondary safety outcomes were oxygen saturation < 95%,[11] hypotension (systolic blood pressure <90 mm Hg), nausea, vomiting, or pruritus.
Information about additional administered medications, including name of medication, dose, route of delivery, and time of administration was abstracted from the medical record while the patient was still in the ED. In order to facilitate dose comparison across both arms of the trial, the amount of opioid received was converted into morphine equivalent units (MEU), in which 1 mg of hydromorphone was calculated as being equivalent to 7 mg of morphine.[12]
2f. Data analysis
All variables are shown as means with standard deviations (SD), medians with interquartile ranges (IQR), or proportions. Chi-square tests were used to compare proportions, Wilcoxon rank-sum tests to compare medians, and Student t-tests to compare means with adjustment for unequal variance when appropriate. We used Wilson’s method of calculating a 95% CI around differences between proportions.[13] Standard methods were used[14] to calculate confidence intervals around differences between means. SPSS version 17 (Chicago, IL.) was used to conduct all data analyses.
2g. Sample size calculation
We estimated that a sample size of 160 patients per group would be needed to detect at least a 10%[15, 16] absolute difference in proportion of patients declining additional pain medication when asked, using a 2-tailed alpha of 0.05 and power = 0.80. In order to ensure enrollment of a minimum of 320 patients for analysis, an additional 30 patients (approximately 10%) were enrolled to account for potential protocol violations and missing data. We used nQuery Advisor version 6.0 (Los Angeles, CA) to calculate the sample size.
3. RESULTS
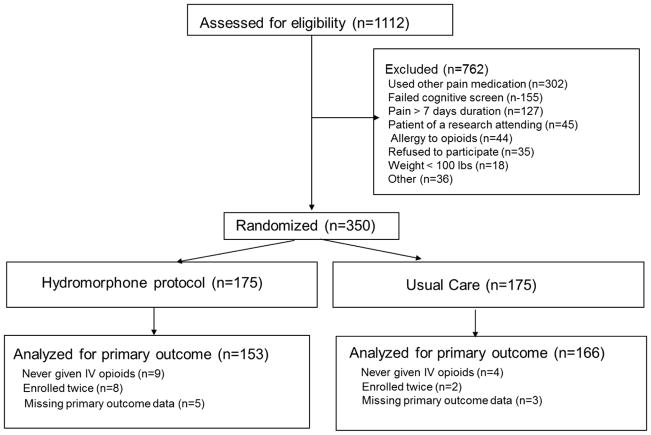
As shown in the CONSORT flow diagram (Figure 1), 1,112 patients were screened for eligibility. The most frequent reasons patients were excluded were use of opioids within the past 7 days, failing the cognitive screen, and pain present for more than a week. Since 13 patients (9 in the protocol group and 4 in the usual care group) never received intravenous opioids after being randomized, an intention-to-treat analysis could not be performed.
Figure 1.
CONSORT diagram
Table 1 displays baseline characteristics of the study cohort. The patients were predominantly female and Hispanic. Approximately two-thirds had abdominal pain and half reported their pain severity as 10 on the NRS pain scale.
Table 1.
Baseline Characteristics
| Characteristic | Hydromorphone Rapid Titration Group (n=153) | Usual Care Group (n=166) | p-value |
|---|---|---|---|
| Sex n (%) | |||
| Female | 105 (68.6) | 108 (65.1) | 0.5 |
| Male | 48 (31.4) | 58 (34.9) | |
| Race/ethnicity n (%) | |||
| Hispanic | 97 (63.4) | 103 (62.0) | 0.6 |
| African-American | 35 (22.9) | 44 (26.5) | |
| White | 15 (9.8) | 16 (9.6) | |
| Other | 6 (3.9) | 3 (1.8) | |
| Age (years) Mean ± SD | 75 (7) | 74 (6) | 0.2 |
| Weight (lbs) Mean ± SD | 162 (33) | 165 (38) | 0.4 |
| Diagnosis n (%) | |||
| Nonspecific abdominal pain | 50 (33) | 57 (34) | 0.5 |
| Musculoskeletal pain | 24 (16) | 24 (14) | |
| Back pain | 14 (9) | 13 (8) | |
| Colitis/diverticulitis | 14 (9) | 10 (6) | |
| Small bowel obstruction | 12 (8) | 13 (8) | |
| Extremity fracture | 11 (7) | 7 (4) | |
| Biliary colic/cholecystitis | 6 (4) | 11 (7) | |
| Kidney stone/pyelonephritis | 7 (5) | 11 (7) | |
| Appendicitis | 0 (0) | 6 (4) | |
| Other (pancreatitis, perforated viscus, volvulus, pneumonia, rib fracture, gout, etc) | 15 (10) | 14 (8) | |
| Pain intensity n (%) | |||
| 3–6 | 12 (7.8) | 13 (7.8) | 0.9 |
| 7 | 14 (9.2) | 20 (12.0) | |
| 8 | 31 (20.3) | 28 (16.9) | |
| 9 | 20 (13.1) | 24 (14.5) | |
| 10 | 76 (49.7) | 81 (48.8) | |
| Nauseated or vomited before receiving opioids in ED n (%) | |||
| Yes | 65 (42.8) | 77 (46.7) | 0.5 |
| No | 87 (57.2) | 89 (53.6) | |
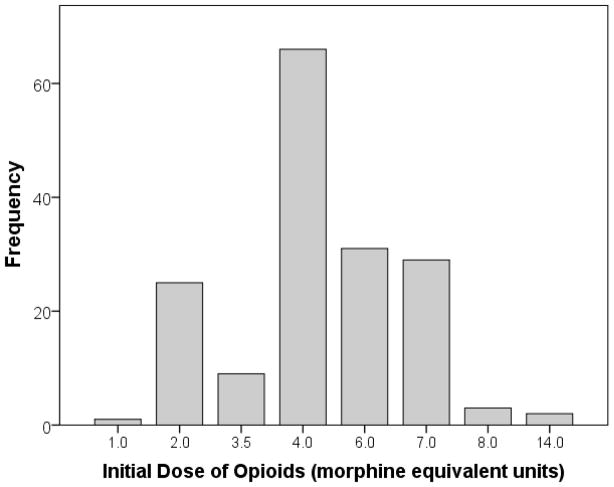
Figure 2 shows the distribution of initial IV opioids administered to the usual care group. The most frequent IV opioid and dose was 4 mg IV morphine. All usual care patients received either intravenous morphine or intravenous hydromorphone (although permitted, no alternative IV opioid, such as fentanyl or meperidine, was administered). The mean initial dose of IV opioid was 4.7 MEU in the usual care group, significantly higher than the initial dose of 3.5 MEU in the hydromorphone titration group [difference 1.2 MEU; 95% CI: 0.9 to 1.5 MEU; p<0.001)]. The total dose over the one hour period was also larger in the usual care group: 6.0 MEU v 5.3 MEU [difference: 0.7 MEU; 95% CI: 0.1 to 1.4 MEU; p=0.03]. 95.3% patients in the hydromorphone titration group who wanted more analgesics at 15 minutes received the second dose, while only 67.3% of usual care patients who wanted more analgesics at 15 minutes received additional pain medication (difference: 28.0%; 95% CI: 14.4% to 41.6%; p< 0.001).
Figure 2.
Frequency Distribution of Initial Dose of Opioids Given to Patients in the Usual Care Group. Amount of opioids shown in morphine equivalent units in which 1 mg hydromorphone = 7 mg morphine.
As seen in table 2, the two regimens had similar efficacy. Most patients in both groups (83.0% in the hydromorphone titration and 82.5% in the usual care) achieved the primary outcome of satisfactory induction of analgesia within 60 minutes. While the majority of patients received adequate self-defined analgesia when asked 15 minutes after their first dose, a substantial minority did not (41.8% of patients who received 0.5 mg hydromorphone, 33.2% of patients in the usual care group). At 60 minutes nearly three-quarters of the patients in both groups did not want additional medication (Table 2). Of the one-quarter that did want more pain medication at 60 minutes, 38.2% had achieved satisfactory analgesia at 15 minutes but failed to maintain it. This percentage was virtually identical in both groups: 38.1% in the hydromorphone titration group and 38.3% in the usual care group. The analyses of the efficacy outcomes based on more traditional NRS pain scores were consistent with the primary endpoint in that none favored one group over the other (see Table 2). Results did not change with regression analyses.
Table 2.
Efficacy Measures by Group
| Outcomes | Hydromorphone Titration Group (n=153) | Usual care (n=166) | Differencea (95% CI) | p-value |
|---|---|---|---|---|
| Primary Outcome | ||||
| Achieved satisfactory analgesia within 60 minutes; n (%) | 127 (83.0) | 137 (82.5) | 0.5 (−7.9 to 8.8) | 0.91 |
| Secondary Outcomes | ||||
| Achieved satisfactory analgesia at 15 minutes post-baseline; n (%) | 89 (58.2) | 111 (66.8) | −8.7 (−19.1 to 1.9) | 0.11 |
| Achieved satisfactory analgesia at 60 minutes post-baseline; n (%) | 111 (72.5) | 119 (71.7) | 0.9 (−8.9 to 10.6) | 0.86 |
| Mean change in NRS pain score from baseline to 60 minutes; mean (SD) | 5.4 (2.9) | 5.2 (3.1) | 0.2 (−0.5 to 0.9) | 0.57 |
| Achieved 50% or greater decline in pain score baseline to 60 minutes; n (%) | 106 (69.3) | 107 (64.9) | 4.4 (−5.9 to 14.6) | 0.40 |
| Achieved pain score of 3 or less at 60 minutes; n (%) | 83 (54.3) | 87 (52.7) | 1.5 (−9.4 to 12.3) | 0.79 |
| Pain score at 60 minutes Median (IQR) | 3 (0 to 5) | 3 (0 to 6) | 0 | 0.79 |
The difference is the value of the outcome in the hydromorphone titration group minus the value in the usual care group. The 95% CI is the 95% confidence interval around this difference.
Table 3 summarizes the incidence of adverse events and side-effects by group. No patient required use of IV naloxone, which was our primary safety endpoint. A total of twenty patients experienced oxygen desaturation below 95%. All of these patients promptly returned above 95% after administration of nasal cannula oxygen as per our protocol.
Table 3.
Adverse events by group
| Adverse event | Hydromorphone Titration N=153 |
Usual Care N=166 |
Differencea (95% CI) | p-value |
|---|---|---|---|---|
| Administration of naloxone; n (%) | 0 (0.0) | 0 (0.0) | 0 (−2.3 to 2.5) | 1.0 |
| Oxygen saturation < 95%; n (%) | 7 (4.6%) | 13 (7.8%) | −3.2% (−8.9% to 2.3%) | 0.23 |
| Systolic blood pressure < 90 mm Hg; n (%) | 4 (2.6%) | 2 (1.2%) | 1.4% (−2.1% to 5.4%) | 0.43c |
| Pruritus; n (%) | 2 (1.3%) | 8 (4.8%) | −3.5% (−8.0% to 1.0%) | 0.11c |
| Nauseab; n (%) | 10/87 (11.5%) | 6/89 (6.7%) | 4.8% (−4.2% to 13.9%) | 0.27 |
| Vomitingb; n (%) | 4/87 (4.6%) | 3/89 (3.4%) | 1.2% (−5.5% to 8.2%) | 0.72c |
The difference is the value of the outcome in the hydromorphone titration group minus the value in the usual care group. The 95% CI is the 95% confidence interval around this difference.
n=87 for patients who were not nauseated or vomiting before receiving initial IV opioid
Fisher exact test
4. DISCUSSION
Older adults are at substantial risk for undertreatment of acute pain.[17–20] In one retrospective ED cohort study, older adults (age 70 and older) were less likely to receive pain medication (66% vs. 80%, p=0.02), received analgesics in a less timely fashion (74 vs. 52 minutes, p=0.02), and tended to receive smaller equivalent doses (44% vs. 19%, p=0.002) as compared to younger adults (age 20–50 years).[18] In another outpatient study, Federman[17] showed that older adults with pain and back or joint disorders used non-steroidal anti-inflammatory drugs (NSAIDs) more often and opioids less often as compared to younger patients, suggesting lower quality of pain management in older adults. This is particularly troubling because older adults experience disproportionately greater rates of NSAID-associated adverse effects such as reduction in renal function and gastrointestinal bleeding.[21]
In this randomized clinical trial we determined that a rapid, 2-step titration protocol that administers 0.5 mg IV hydromorphone followed by an optional 0.5 mg IV hydromorphone 15 minutes later, was neither clinically nor statistically more efficacious than usual care for the treatment of acute pain in older adults (≥ 65 years) presenting to the ED. Overall both groups received good initial pain management: 80% of patients in both groups achieved satisfactory induction of analgesia, approximately 66% had a 50% or greater reduction in the NRS pain score, and the median NRS pain score at 60 minutes was 3 in both groups (an NRS score of 3 is generally thought to represent “mild” pain[22] when categorizing pain scores into “mild”, “moderate”, and “severe” categories).
We chose to give a conservative dose of 0.5 mg IV hydromorphone followed by an additional 0.5 mg of hydromorphone primarily due to safety concerns and following standard practice of administering smaller doses of opioids to older patients. Contrary to our initial expectations, patients in the usual care group received more IV opioids (both initially and in total) in terms of morphine equivalent units (MEU) than patients randomly allocated to rapid hydromorphone titration.
Patients in both groups were asked whether they wanted more pain medication 15 minutes after their first dose. 41.8% in the hydromorphone titration group and 33.2% in the usual care group wanted more analgesia at 15 minutes. The large proportion of patients for whom the first dose was not sufficient highlights the variability of response to opioids. It suggests that patients should be asked after a first dose if they want more analgesia, whether or not a protocol is being followed. The findings also suggest that an initial dose of 0.5 mg of hydromorphone may be too low in older ED patients with acute severe pain. However, in light of the similar high degree of pain control in both groups within 60 minutes of the initial dose, 0.5 mg IV hydromorphone, coupled with offering additional dosing soon after the initial bolus, may adequately balance safety and analgesia. Approximately one-quarter of patients 60 minutes after baseline wanted more analgesics. Some of these patients had adequate analgesia induced by 15 minutes but failed to maintain it, while others did not achieve satisfactory pain control at any time in the 60 minute period. Again, this highlights the need to return to the patient at regular intervals rather than assuming that the initial bolus provided and maintained sufficient analgesia. Asking patients at 15 minutes if they need more pain medication is a modified titration strategy that is more feasible in the ED than initial titration to analgesic effect. However, in settings where adequate staffing exists, individual titration at the beginning of treatment may be optimal.
No patient needed administration of naloxone to reverse the opioids administered. There was a higher incidence of oxygen desaturation below 95% in the usual care group than in the hydromorphone titration group (7.8% vs. 4.6%), but this was not statistically significant.
The study has several limitations. Medical staff members were not masked to study group random allocation. Providers could not be blinded because they were responsible for choosing analgesic treatment for patients randomly allocated to usual care. Knowledge of the dose and potency of hydromorphone given to the hydromorphone titration group may have influenced ED physicians’ decisions to use higher doses of IV opioids in the usual care group than they may ordinarily use. In addition, we have conducted a series of opioid studies[4, 11, 15, 16, 23–25] in our ED such that our attendings are now more comfortable administering higher doses of opioids to patients with acute severe pain. The effect of this potential performance bias would be to underestimate the difference between the hydromorphone titration group and usual care. In other ED settings, where older patients may be treated more conservatively, the hydromorphone titration protocol might prove to be more efficacious than usual care.
Asking all patients if they wanted more analgesics 15 minutes after the initial dose is not usual care in most EDs, including our own, as studies have shown that ED pain assessment usually occurs only once (typically at triage).[26] Most studies that compared protocols for treating pain have used a visual analog scale (VAS) or numerical rating scale of pain. We used a non-traditional measure based on the patient’s desire for additional medication at 15 and 60 minutes after an initial dose of IV opioid to assess for adequacy of analgesic relief. Advantages of this simple yes/no approach (“Do you want more pain medication?”) is that it is patient centered, easily understood, easier to answer than a numerical rating scale, and may also transcend the mild confusional states that may accompany severe pain, especially in older patients. As it does not specifically ask for a rating of pain, it is an omnibus measure that reflects the individual’s assessment of the balance between pain relief and the undesirable effects of analgesics. However, the use of this non-traditional, categorical measure of efficacy did not affect the inference as the results of the analysis of pain scores were similar.
Our inclusion criteria included patients with pain of sufficient severity to warrant use of IV opioids in the judgment of the ED attending physician. The factors that influence the decision to use IV opioids are complex and extensive. An approach that is commonly taken to address the issue of patient selection in drug trials is to use a specific condition (e.g. renal colic) or treatment (e.g. post-hysterectomy) that would generally be thought to be appropriately treated with an IV opioid analgesic, thereby eliminating individual judgment about eligibility for the study. However in order to assess the role of IV opioids with the widest generalizability in the ED setting, we decided to enroll patients with a variety of diagnoses, all with a complaint of acute pain. Opioids are not an appropriate treatment for all patients who present with a complaint of pain (e.g., gastroenteritis, migraine). Therefore, unless there is a restriction to patients with a specific diagnosis, either a comprehensive list of diagnoses and situations in which opioids are indicated must be specified, or clinical judgment needs to be used. We have opted for the latter alternative.
Since the patients in the study were largely Hispanic (approximately 60%), African-American (approximately 25%), urban, and poor, it is possible that the effect of the hydromorphone titration regimen might be different than in other patient populations. Requesting additional pain medication may be influenced by many factors, including culture and expectation of pain relief.[27, 28] If other patient groups are more likely to request additional analgesics, the rapid hydromorphone titration regimen might be more effective than it was in our patient population. Thus our results should not be generalized to other patient populations with different sociodemographic characteristics without independent validation. In addition, our results cannot be generalized to the patients who met our exclusion criteria, 75% of whom fell into one of three categories: pain greater than 1 week in duration, used other pain medication, or failed the cognitive screen.
5. CONCLUSIONS
In summary, a low-dose, 2-step, hydromorphone titration protocol was very similar to usual care with respect to both efficacy and safety for treatment of acute pain in older adults presenting to the ED. The study highlights the variability in response to opioids and the necessity of returning to the patient to assess adequacy of pain control.
Footnotes
Author contributions:
AKC: Created study concept and design, managed acquisition of subjects, prepared manuscript.
PEB: Provided all statistical analyses, edited manuscript
MD: Assisted in data collection, auditing, and edited manuscript
EJG: Involved in design of study, edited manuscript.
Conflict of interest: Dr. Chang is supported by a grant from the NIA (K23 AG033100-01A2). None of the other authors have any financial or personal conflicts of interest.
References
- 1.Cavalieri TA. Pain management in the elderly. J Am Osteopath Assoc. 2002 Sep;102(9):481–5. [PubMed] [Google Scholar]
- 2.McCarberg BH. Introduction. Pain and the elderly. Clin J Pain. 2004 Jul-Aug;20(4):205–6. doi: 10.1097/00002508-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Blanda MP. Pharmacologic issues in geriatric emergency medicine. Emergency medicine clinics of North America. 2006 May;24(2):449–65. viii. doi: 10.1016/j.emc.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Chang AK, Bijur PE, Davitt M, et al. Randomized clinical trial comparing a patient-driven titration protocol of intravenous hydromorphone with traditional physician-driven management of emergency department patients with acute severe pain. Ann Emerg Med. 2009 Oct;54(4):561–7. e2. doi: 10.1016/j.annemergmed.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Callahan CM, Unverzagt FW, Hui SL, et al. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002 Sep;40(9):771–81. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2003 Apr;10(4):390–2. doi: 10.1111/j.1553-2712.2003.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 7.Bijur PE, Kenny MK, Gallagher EJ. Intravenous morphine at 0.1 mg/kg is not effective for controlling severe acute pain in the majority of patients. Ann Emerg Med. 2005 Oct;46(4):362–7. doi: 10.1016/j.annemergmed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Birnbaum A, Esses D, Bijur PE, et al. Randomized double-blind placebo-controlled trial of two intravenous morphine dosages (0.10 mg/kg and 0.15 mg/kg) in emergency department patients with moderate to severe acute pain. Ann Emerg Med. 2007 Apr;49(4):445–53. 53e1–2. doi: 10.1016/j.annemergmed.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 9.Aubrun F, Langeron O, Quesnel C, et al. Relationships between measurement of pain using visual analog score and morphine requirements during postoperative intravenous morphine titration. Anesthesiology. 2003 Jun;98(6):1415–21. doi: 10.1097/00000542-200306000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Galinski M, Dolveck F, Borron SW, et al. A randomized, double-blind study comparing morphine with fentanyl in prehospital analgesia. Am J Emerg Med. 2005 Mar;23(2):114–9. doi: 10.1016/j.ajem.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Chang AK, Bijur PE, Napolitano A, et al. Two milligrams i.v. hydromorphone is efficacious for treating pain but is associated with oxygen desaturation. J Opioid Manag. 2009 Mar-Apr;5(2):75–80. doi: 10.5055/jom.2009.0008. [DOI] [PubMed] [Google Scholar]
- 12.Hardman JG, Limbird LE, Gilman AG. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 10. New York: McGraw-Hill Professional; 2001. [Google Scholar]
- 13.Wilson E. Probable Inference, the Law of Succession, and Statistical Inference. Journal of the American Statistical Association. 1927;22:209–12. [Google Scholar]
- 14.Leon RV. The concept of the confidence interval for the mean. [cited 2012 April 19]; Available from: http://web.utk.edu/~leon/stat201/Confidence%20Interval%20Concept.html.
- 15.Chang AK, Bijur PE, Gallagher EJ. Randomized clinical trial comparing the safety and efficacy of a hydromorphone titration protocol to usual care in the management of adult emergency department patients with acute severe pain. Ann Emerg Med. 2011 Oct;58(4):352–9. doi: 10.1016/j.annemergmed.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Chang AK, Bijur PE, Lupow J, et al. Randomized Clinical Trial of Efficacy and Safety of a Single 2 mg IV Dose of Hydromorphone vs. Usual Care in the Management of Acute Pain. Acad Emerg Med. 2012 doi: 10.1111/acem.12071. in press. [DOI] [PubMed] [Google Scholar]
- 17.Federman AD, Litke A, Morrison RS. Association of age with analgesic use for back and joint disorders in outpatient settings. Am J Geriatr Pharmacother. 2006 Dec;4(4):306–15. doi: 10.1016/j.amjopharm.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Jones JS, Johnson K, McNinch M. Age as a risk factor for inadequate emergency department analgesia. Am J Emerg Med. 1996 Mar;14(2):157–60. doi: 10.1016/S0735-6757(96)90123-0. [DOI] [PubMed] [Google Scholar]
- 19.Hwang U, Richardson LD, Harris B, et al. The quality of emergency department pain care for older adult patients. J Am Geriatr Soc. 2010 Nov;58(11):2122–8. doi: 10.1111/j.1532-5415.2010.03152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platts-Mills TF, Esserman DA, Brown DL, et al. Older US emergency department patients are less likely to receive pain medication than younger patients: results from a national survey. Ann Emerg Med. 2012 Aug;60(2):199–206. doi: 10.1016/j.annemergmed.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenwald DA. Aging, the gastrointestinal tract, and risk of acid-related disease. Am J Med. 2004 Sep 6;117(Suppl 5A):8S–13S. doi: 10.1016/j.amjmed.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Forchheimer MB, Richards JS, Chiodo AE, et al. Cut point determination in the measurement of pain and its relationship to psychosocial and functional measures after traumatic spinal cord injury: a retrospective model spinal cord injury system analysis. Archives of physical medicine and rehabilitation. 2011 Mar;92(3):419–24. doi: 10.1016/j.apmr.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 23.Chang AK, Bijur PE, Baccelieri A, et al. Efficacy and safety profile of a single dose of hydromorphone compared with morphine in older adults with acute, severe pain: a prospective, randomized, double-blind clinical trial. Am J Geriatr Pharmacother. 2009 Feb;7(1):1–10. doi: 10.1016/j.amjopharm.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Chang AK, Bijur PE, Campbell CM, et al. Safety and efficacy of rapid titration using 1mg doses of intravenous hydromorphone in emergency department patients with acute severe pain: the “1+1” protocol. Ann Emerg Med. 2009 Aug;54(2):221–5. doi: 10.1016/j.annemergmed.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Chang AK, Bijur PE, Meyer RH, et al. Safety and efficacy of hydromorphone as an analgesic alternative to morphine in acute pain: a randomized clinical trial. Ann Emerg Med. 2006 Aug;48(2):164–72. doi: 10.1016/j.annemergmed.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Todd KH, Ducharme J, Choiniere M, et al. Pain in the emergency department: results of the pain and emergency medicine initiative (PEMI) multicenter study. J Pain. 2007 Jun;8(6):460–6. doi: 10.1016/j.jpain.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Todd KH, Lee T, Hoffman JR. The effect of ethnicity on physician estimates of pain severity in patients with isolated extremity trauma. JAMA. 1994 Mar 23–30;271(12):925–8. [PubMed] [Google Scholar]
- 28.Todd KH, Samaroo N, Hoffman JR. Ethnicity as a risk factor for inadequate emergency department analgesia. JAMA. 1993 Mar 24–31;269(12):1537–9. [PubMed] [Google Scholar]