Abstract
Mucins—large, highly glycosylated proteins—are important for the luminal protection of the gastrointestinal tract. Enterocytes have their apical surface covered by transmembrane mucins and goblet cells produce the secreted gel-forming mucins that form mucus. The small intestine has a single unattached mucus layer. In cystic fibrosis, this layer becomes attached, accounting for the intestinal manifestations of this disease. The stomach and colon have two layers of mucus; the inner layer is attached and the outer layer is less dense and unattached. In the colon, the outer mucus layer is the habitat for commensal bacteria. The inner mucus layer is impervious to bacteria and is renewed every hour by surface goblet cells. The crypt goblet cells have the ability to restitute the mucus layer by secretion, for example after an ischaemic challenge. Proteases of certain parasites and some bacteria can cleave mucins and dissolve the mucus as part of their pathogenicity. The inner mucus layer can, however, also become penetrable to bacteria by several other mechanisms, including aberrations in the immune system. When bacteria reach the epithelial surface, the immune system is activated and inflammation is triggered. This mechanism might occur in some types of ulcerative colitis.
Introduction
The gastrointestinal tract is an amazing organ: it can digest food but does not digest itself; it harbours more bacteria than there are cells in the human body, yet does not allow the bacteria to take over despite their rapid multiplication; and it can handle relatively strong hydrochloric acid without denaturing the stomach. The mechanisms behind these amazing skills vary, but a major reason is the uttermost defence line of the gastrointestinal tract—the mucus.1 The proximal part of the digestive tract, the mouth and oesophagus, is, like the skin, protected by multiple layers of tight and largely inert squamous epithelium, which is flushed by mucus from salivary and other glands. By contrast, the rest of the gastrointestinal tract has a single layer of very active cells. The major protection of this vulnerable cellular compartment is by mucus covering these cells and by the glycocalyx,2,3 which is both built by and around mucins.
The gastrointestinal tract mucus was studied relatively intensely during the 1960s–1980s,4,5 a period that is not covered here. However, more recently it has been less well appreciated or understood that the gut is covered with mucus. Here, we provide an overview of the mucus system along the gastrointestinal tract and discuss the role of mucus in health and disease.
Mucins
Mucin domain
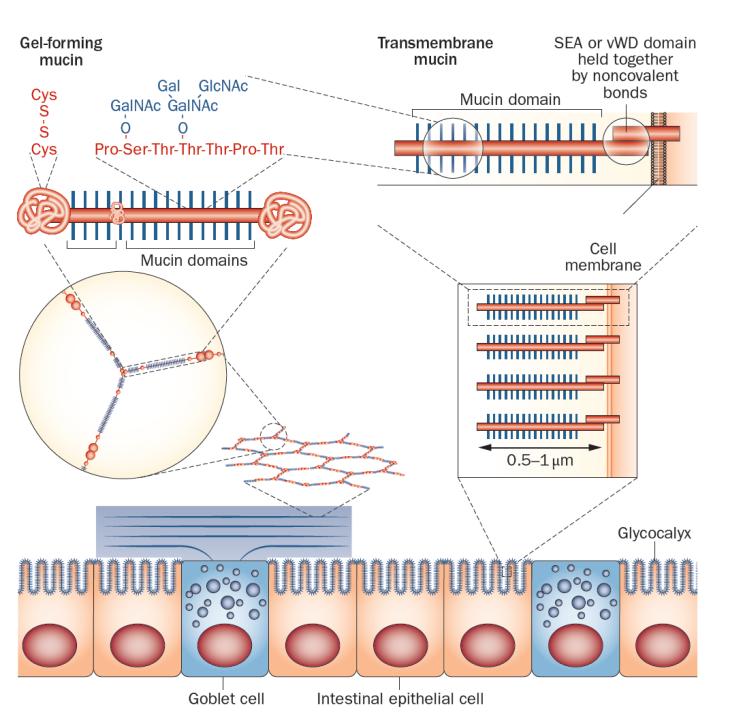
The major building blocks in mucus are mucins, which are large, highly glycosylated proteins (Figure 1).6–10 Typically, these mucins are >80% carbohydrate, and are concentrated into mucin domains.11 These domains are built on a protein core that is rich in the amino acids proline, serine and threonine (called PTS sequences). These sequences are often called VNTRs (variable number tandem repeats), as the amino acid sequences are often repeated in tandem, although this is not always the case. As the VNTR designation suggests, the length can vary, but as these are encoded within one exon the length is genetically determined.11,12 PTS sequences can be very long; for example the largest one in the MUC2 mucin is about 2,300 amino acids.13 The hydroxyl group of the amino acids threonine and serine become the attachment sites for GalNAc (N-acetylgalactosamine), which is added in the Golgi apparatus. The repertoire of peptidyl-GalNAc transferases is unique for each organ and cell type.14 These GalNAc residues are then extended and branched by a series of other glycosyltransferases, generating a very complex mixture of glycan epitopes.15,16 Once this glycosylation process is finished at the end of the Golgi apparatus, the mucin domains have extended into long rods. We have estimated that the 2,300 amino acids of the large MUC2 mucin domain make it about 0.45 μm long and the total length of the two mucin domains is 0.55 μm (Table 1).17 These long stiff rods are so densely covered with glycans that their protein core is totally protected from degradation by proteases. The mucin domain glycans bind a lot of water and thereby generate most of the typical gel-like properties of mucins.
Figure 1.
Simplified model of a gel-forming mucin produced by goblet cells and transmembrane mucins attached to the apical membrane of enterocytes. Left: the MUC2 mucin polymer is packed in the granulae (blue) of the goblet cells and during secretion expands to form flat ring-like structures that stack under each other. The enlarged ring shows the oligomeric nature of the MUC2 polymer, and above the MUC2 monomer is shown with its central mucin domains and exemplified O-glycans. The MUC2 N-termini and C-termini are held together with numerous disulphide bonds. Right: the apical side of the intestinal enterocytes are covered by a glycocalyx made up of mucins that are anchored in the cell membrane. The long and extended mucin domains make up the glycocalyx. The cleaved and associated SEA and vWD domains are indicated as well as the mucin domain. Abbreviations: SEA: sea urchin-enterokinase-agrin domain; vWD, von Willebrand domain.
Table 1.
Mucins in the gastrointestinal tract.
| Mucin* (and type) | Protein domains‡ |
Number of amino acids |
Number of mucin domains (and estimated length of mucin domains§) |
Cell type expression |
Function | Reference |
|---|---|---|---|---|---|---|
| MUC1 (transmembrane) | 1 SEA | ~1,250 | 1 (~200 nm) | Epithelial cells | Signalling, protection |
30, 118–124 |
| MUC2 (gel-forming) | 4 VWD, 2 CysD, 1 CK |
~5,200 | 2 (~550 nm) | Goblet cells Paneth cells |
Protection, lubrication, entrapment |
13, 50, 125, 126 |
| MUC3II (transmembrane) | 1 SEA | >2,550 | 1 (>350 nm) | Enterocytes | Apical surface protection |
127 – 129 |
| MUC4 (transmembrane) | 1 NIDO, 1 AMOP, 1 VWD |
~5,300 | 1 (~800 nm) | Epithelial cells Goblet cells |
Signalling, protection |
130-133 |
| MUC5AC (gel-forming) | 4 VWD, 11 CysD, 1 CK |
>5,050 | 11 (>350 nm) | Mucous cells | Protection, lubrication, entrapment |
57, 134–138 |
| MUC5B (gel-forming) | 4 VWD, 7 CysD, 1 CK |
~5,700 | 7 (~550 nm) | Mucous cells Goblet cells |
Protection, lubrication, entrapment |
139 – 141 |
| MUC6 (gel-forming) | 1 VWD, 1 CK |
~2,400 | 1 (~250 nm) | Mucous cells | Protection, lubrication, entrapment |
142 – 144 |
| MUC7 (gel-forming) | None | 377 | 1 (~230 nm) | Mucous cells | Protection | 41, 145 |
| MUC12 (transmembrane) | 1 SEA | ~5,500 | 1 (~1000 nm) | Enterocytes | Apical surface protection |
26 |
| MUC13 (transmembrane) | 1 SEA | 512 | 1 (~30 nm) | Enterocytes | Apical surface protection? |
27, 146 |
| MUC16 (transmembrane) | 33 SEA | ~22,000 | 1 (~2400 nm) | Epithelial cells | Apical surface protection |
147, 148 |
| MUC17 (transmembrane) | 1 SEA | ~4,500 | 1 (~800 nm) | Enterocytes | Apical surface protection |
149 |
Human mucin nomenclature is used. A curated database of mucin gene and protein sequences can be found at www.medkem.gu.se/mucinbiology/databases.
In addition to mucin domains (glycosylated PTS domains).
Total length of all mucin domains. Length = 0.2nm/amino acid.
Two MUC3 genes A and B have been proposed to exist.150 Abbreviations: AMOP, adhesion associated domain in MUC4 and other proteins; CysD, Cystein D domain interrupting mucin domains; CK, cysteine knot; GF, Gelforming; SEA, sea urchin-enterokinase-agrin domain, and agrin domain; NIDO, nidogen-like domain; S, Secreted (not polymeric); VWD, von Willebrand.
Transmembrane mucins
In addition to one or several mucin domains, all mucins have domains that give them specific properties. Transmembrane mucins have a transmembrane domain that enables them to be anchored in the cell membrane (Figure 1, Table 1). These mucins have one large N-terminal mucin domain and a short cytoplasmic C-terminus.6,7 On the outer side of the membrane, these mucins either have a SEA-domain (sea urchin-enterokinase-agrin domain; the SEA-mucins are MUC1, MUC3, MUC12, MUC13 and MUC17) or NIDO-AMOP-vWD domains (only MUC4). Interestingly, both of these domains are cleaved during biosynthesis, but are held together by strong noncovalent bonds. The SEA domain is cleaved during folding by an autocatalytic mechanism that does not require additional enzymes.18–21 The two parts are held together by four β-pleated sheets—two from the outer part of the mucin domain and two from the membrane anchored part—these parts can be pulled apart by mechanical stress avoiding rupturing of the cell membrane as the force that can disrupt the SEA domain is smaller than the forces necessary for disrupting the membrane.21,22 The cleavage of MUC4 takes place in the vWD (von Willebrand D) domain. The mechanisms involved are less clear, but both enzymatic and autocatalytic processes have been suggested.23,24
The transmembrane mucins are found at the apical side of epithelial cells. MUC3, MUC12 and MUC17 probably build the glycocalyx, sometimes also called fuzz, on the microvilli of enterocytes.3,25,26 This hypothesis has not been shown formally, but the length of their extended mucin domains is in accordance with the thickness of the glycocalyx. MUC13 is also abundant in enterocytes, but has only a short mucin domain.27
The normal functions of the transmembrane mucins are only partly understood. The enterocyte glycocalyx probably forms a protective brush for the periciliary space on the ciliated cells of the lung,28 and probably acts as a diffusion barrier in the gastrointestinal tract, ensuring a stable apical cell membrane mileu and inhibiting the undesired passage of large molecules.3,7 MUC1, which is present in the stomach epithelium, has protective effects against Helicobacter pylori infection.29 MUC1 is also a well-known cancer cell antigen that can modulate growth and apoptosis; it relocalizes from the apical membrane and contributes to tumour cell behaviour with its large cytoplasmic tail interacting with β-catenin and other molecules involved in cancer development.6,30–33 MUC3, MUC12 and MUC17 all have cytoplasmic tails that interact with different PDZ-proteins, which are regulators of apical organization and inward and outward shuttling of proteins, especially ion channels.34–36 In addition to a role in protection, the transmembrane mucins are probably involved in apical cell surface sensing and signaling.7,37
Gel-forming mucins
The gel-forming mucins all have central mucin domains that are flanked by an N-terminal part (involved in oligomerization) and a C-terminal, forming dimeric structures (Figure 1, Table 1). This group of mucins uses their N-termini and C-termini to form large polymers that together with the mucin domains form the gels that are typical of mucus and are of paramount importance for protection of the gastrointestinal tract.1,8,13 In fact, the evolutionary appearance of this group of mucins probably coincides with the formation of a simple digestive system.11 This coincidental development could make sense considering that the intestine can digest all types of bonds between amino acids in food, whilst at the same time the intestine itself is left unaffected. The major intestinal mucin, MUC2, is resistant to endogenous proteases; the central mucin domains are protected by glycans, and the N-termini and C-termini are stabilized with numerous cross-links between cysteine amino acids. Notably, digestive enzymes are unable to digest glycans other than starch and some disaccharides, leaving the mucins intact. The main functions of the gel-forming mucins are to build the mucus that together with additional components protect and lubricate the gastrointestinal tract. Caldara et al.38 suggest that native fresh mucus is non-sticky and non-hydrophobic and that the sticky and hydrophobic properties of mucus appear after the mucus had been stored or purified.
An overview of the mucus systems
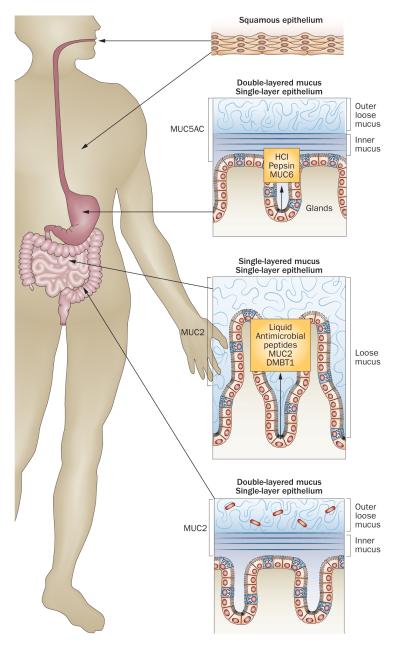
The organization of the protective mucus system varies markedly along the digestive tract (Figure 2, Table 2).39 In the mouth, the salivary glands produce MUC5B and MUC7, which lubricate ingested food for passage through the oesophagus.40–44 The stomach has a two-layered mucus system, composed of an inner, attached mucus and an outer, unattached, loose mucus layer, both built by the MUC5AC mucin produced by the superficial epithelium.39,45,46 Glands in the stomach and duodenum secrete the gel-forming mucin MUC6.47,48 The small intestine has, in contrast to the stomach and colon, only one type of surface mucus, again composed of MUC2. This mucus is unattached and easy to remove.13 Finally, the colon again has a two-layered mucus system, with an inner dense and attached mucus layer and an outer, loose and unattached mucus, both built by MUC2. The same mucin, MUC2, thus behaves differently in the small and large intestine.25,49,50
Figure 2.
The organization of epithelium and mucus in the gastrointestinal tract. The squamous epithelium of the mouth and oesophagus is washed by mucus from the salivary glands. The stomach and colon have a two-layered mucus system with an inner, attached mucus and an outer, unattached, loose mucus layer. The small intestine on the other hand has only one type of mucus that is unattached and loose.
Table 2.
Mucus along the gastrointestinal tract
| Mucus | Section of gastrointestinal tract | References | |||
|---|---|---|---|---|---|
| Mouth | Stomach | Small intestine | Large intestine | ||
| Mucus layers | None | Outer, unattached layer Inner, attached layer |
Single, unattached layer |
Outer, unattached layer Inner, attached layer |
39,50,57,151 |
Gel-forming mucins:
|
NF MUC5B NA |
MUC5AC MUC6 NA |
MUC2 MUC6 MUC2 |
MUC2 (MUC5B) NF NA |
25,42-50,152-154 |
| Transmembrane mucins:
|
MUC1, MUC16 |
MUC1 | MUC1, MUC3, MUC4, MUC12, MUC13, MUC17 |
MUC1, MUC3, MUC4, MUC12, MUC13, MUC17 |
25– 27,30,34,49,124,133,149,152,153,155–158 |
| Secreted mucins | MUC7 | NF | NF | NF | 40 – 42 |
Abbreviation: NA, not applicable. NF, not found.
Colon: mucus layer organization
Of the intestinal mucus layers, most is known about the colon and MUC2. This mucin is built by monomers that have a mass of about 2.5 MDa, of which 20% is the protein core and the rest is glycans. The central mucin domains make the monomers into ~0.6 μm long stiff rods.17 These monomers are covalently interlinked as dimers in their C-terminus and as trimers in their N-terminus, thus forming large net-like structures.51,52 Before release into the gut lumen, MUC2 is stored in the goblet cell granulae assembled on a ring-like platform formed by calcium dependent interactions between MUC2 N-termini.17 Upon release, the calcium is chelated, probably by bicarbonate, and the mucin unfolds like an umbrella and expands >1,000-fold in volume. The formed nets then spontaneously organize into flat sheets that have a tendency to stack on top of each other, like roof tiles, to form the lamellar inner mucus layer (Figure 1).53 This layer remains anchored to the epithelial cells: it cannot be aspirated off (thus originally called firmly attached); it is highly organized; and it does not allow bacteria to penetrate. The inner mucus layer is mainly formed and renewed by surface goblet cells in the colon.54 The thickness of this layer is several hundred micrometers in humans, but less in rodents. This inner layer is thus renewed from the epithelial side and has a turnover time of about an hour.54
At the luminal border of the inner mucus layer, it is converted into the outer mucus layer at a relatively sharp demarcation line (Figures 2 and 3). As the outer layer is formed from the inner layer, it contains the same components, but interestingly it has markedly different properties: it is loose, unattached, and allows bacteria to penetrate. The transition from firm to loose form is probably due to proteolytic cleavages within the MUC2 mucin as this transition can be partly inhibited by protease inhibitors.50 These cleavages allow the mucin to expand 3–4 times in volume without disrupting the polymeric network, which is still held together by cysteine disulfide bonds.50 The exact mechanisms of conversion from inner to outer mucus layer are still only poorly understood, but are known to be generated by the host itself and not by bacteria since the outer mucus layer also exists in germ-free mice.50
Figure 3.
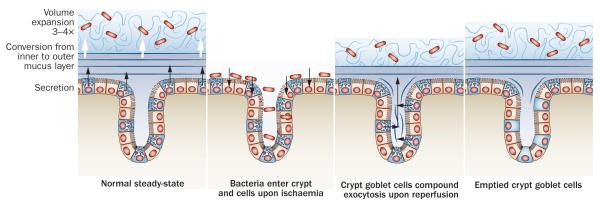
Mucus secretion from the colon surface epithelium and crypts during ischaemia and reperfusion. a ∣ Normal steady state renewal of the inner mucus layer by the surface goblet cells. This inner mucus layer is converted and expanded to the outer mucus layer. b ∣ Upon ischaemia the mucus layers are lost and bacteria can enter the crypt and epithelial cells. c ∣ Upon reperfusion, the crypt goblet cells release their stored mucins and a new inner and outer mucus layer are formed at the same time as the bacteria in the crypt are washed out. d ∣ After reperfusion, the crypt goblet cells remain emptied for several hours as repleneshing of the mucin granulae is slow. During this period, the tissue will be more vulnerable to another episode of ischemia.
Stomach: mucus and hydrochloric acid
Like the colon, the stomach has a two-layered mucus system.39 The inner mucus layer, built around MUC5AC, acts as a diffusion barrier for hydrochloric acid.55 The surface epithelial cells secrete bicarbonate, creating a pH gradient from the acidic lumen to neutral pH at the cell surface.56,57 Hydrochloric acid is produced in the glands and is secreted together with MUC6 and pepsin. Interestingly, these gland secretions are able to cross the surface inner mucus layer through what looks like temporary canals.58,59 These canals are subsequently immediately closed, but leave an imprint in the surface mucus. How the glands can withstand the very high proton concentration (pH 1–2) is not understood, but it is likely that MUC6 is important. Whether MUC6 also contributes to the formation of the inner gastric mucus layer is controversial.60,61
Small intestine: mucus and ion secretion
The small intestine has a loose, unattached mucus layer. Like the colon, this layer is formed by MUC2, but the small intestinal mucus layer has similar properties to the outer, not the inner colon mucus layer (Ermund et al. unpublished work . How the same mucin can behave so differently in these two intestinal segments is still not understood, although it is possible that MUC2 is processed differently.
To understand the ingenuity of the different organization of the mucus layers in the small intestine and colon, one has to remember their different functions and environment. The small intestine, except for the distal ileum, is kept sterile, whilst also enabling absorption of nutrients. By contrast, the colon is stably colonized with a complex flora that has an important role in metabolism. The small intestine normally limits bacterial exposure by flushing (that is, mucus and liquid secretions—mainly chloride through the CFTR channel) coordinated with intense motor activity. This motor activity is accomplished by the so-called migrating motor complex (MMC), generated by the enteric nervous system. The MMC generates long segments of maximal motor activity and secretion that move slowly down the small intestine.62,63 This activity probably physically moves the loose mucus and its bound bacteria distally into the colon.
To further minimize the risk of contact between virulent bacteria and the epithelium, the small intestinal mucus contains high concentrations of antibacterial peptides and proteins secreted by both Paneth cells and enterocytes.64 These antibacterial substances kill or entrap bacteria and thus keep the epithelial cells relatively free from bacterial contact.65,66 The mucus layer is an important component of this protection as it limits diffusion and generates a gradient of antibacterials from the epithelial side.67 The crypts of the small intestine, where stem cells are located, are especially vulnerable as there is no physical barrier present as in the colon. The Paneth cells at the bottom of these crypts not only secrete antibacterial peptides and lysozyme, but also scaffolding proteins such as MUC2 and DMBT1.68 Combined secretion of mucins and fluid from the crypts help to ensure that this compartment normally remains sterile.
Role of mucus in health and disease
Cystic fibrosis
The CFTR channel, which preferentially secretes chloride, has a key role in protecting the small intestine from bacterial invasion. The severe inherited disease cystic fibrosis is caused by a nonfunctional CFTR channel.69 This disease affects all organs that produce mucus. Problems with recurrent lung infections dominate the clinical course, although the gastrointestinal tract is also affected in many patients. Around 10% of newborns with cystic fibrosis are diagnosed with meconium ileus at birth and some adults with cystic fibrosis also have problems with distal intestinal obstruction syndrome (DIOS).70 These entities are characterized by an acute and complete or incomplete obstruction by inspissated mucus localized to the ileocaecum.71 Experimental animal models of cystic fibrosis (mice or pigs) tend to have a relatively severe intestinal phenotype.72–74 In mice with cystic fibrosis, the mucus was found to be attached to the epithelium.75 This mucus phenotype was normalized by exposing it to buffers containing about 100 mM bicarbonate. Conversely, in wild-type mice, the mucus could be turned into a cystic-fibrosis-like mucus phenotype by removing bicarbonate. Thus, the altered mucus in the cystic fibrosis mice was attributed to a lack of bicarbonate owing to the dysfunctional CFTR channel (in health, this channel also secretes bicarbonate). Bicarbonate probably acts by precipitating calcium ions, which seems to be necessary for the correct unfolding of stored mucins and formation of a proper gel.17
That the small intestinal mucus was found to remain attached to the epithelium in the absence of a functional CFTR channel could explain why patients with cystic fibrosis sometimes develop DIOS. If the mucus remains attached and cannot be moved forward by peristaltic movement, obstruction is easy to understand. Mouse models of cystic fibrosis can actually be kept alive with PEG (polyethylene glycol) in their drinking water, which is an osmotic laxative that is also used to treat DIOS.73,76 Given that the function of the small intestinal mucus is to trap and transport bacteria distally, one would predict an increased load of bacteria in the distal small intestine of patients with cystic fibrosis. In fact, an increased bacterial load has been observed both in mouse models of cystic fibrosis and in patients with cystic fibrosis (estimated from hydrogen breath tests).77 Furthermore, cystic fibrosis mice that become sick can be rescued with antibiotics,78,79 and anecdotal observations indicate that patients with cystic fibrosis often experience relief from intestinal pain during antibiotic treatment for lung disease. These observations from mice and humans elegantly illustrate the importance of the small intestinal mucus system for protecting the intestine from bacteria.
Commensal bacteria in the colon
If the small intestine is designed to limit the growth of bacteria and transport these organisms distally, the large intestine is made to harbour the 1013–1014 commensal bacteria. MMC movements do not occur in the colon; instead the enteric nervous system generates vigorous peristaltic waves (mass movements) that relentlessly move towards the rectum, eventually triggering the defecation reflex. These waves are physically powerful and carry mucus with them. Thus, the inner mucus layer that physically separates bacteria from the epithelial cells (by 50 μm in mouse and several hundred micrometers in humans) is of utmost importance for homeostasis in the colon.50
It should also be remembered that the outer mucus layer serves as the habitat and a partial food source for these bacteria. Complex polysaccharides in food, as well as MUC2, provide complex glycans that can be released by the numerous bacterial exoglycosidases that remove one monosaccharide at a time.80,81 Fortunately this is a slow process, but once the mucin protein core is reached the mucus gel is dissolved by bacterial proteases. This process is of mutual interest for bacteria and host because the bacteria make short-chain fatty acids from the degraded material and, depending on the bacterial species, deliver acetate, proprionate and butyrate as energy sources for the host epithelium.82
The commensal bacteria of humans is different from other animals and consists of about 50% Firmicutes and 30% Bacteroidetes.80 This composition is probably not random as each species seems to have mechanisms for selecting its microbiota, as illustrated by cross colonizing germfree zebra fish and mice.83 How such a selection can take place is not understood, but the observation that humans have a constant repertoire of MUC2 glycans in the colon suggests that glycan epitopes might be one such mechanism.16
Pathogenic organisms in the colon
An inner mucus layer in the colon that is impervious to bacteria is a major obstacle for pathogenic microorganisms. Infection requires highly specialized virulence systems, which explains the relatively limited number of such organisms. The rodent pathogen Citrobacter rodendium is an example of a bacteria that is able to pass and reside under the mucus layer,84 in a similar way as Helicobacter pylori has specialized mechanisms allowing it to reside under the inner gastric mucus layer.85
Helminth infections of the small intestine are a common problem worldwide, and at least one-quarter of the world’s population is infected with an intestinal worm of some sort. Clearance of the infection is associated with increased numbers of goblet cells and mucus secretion, as demonstrated in animal models infected with Trichinella spirlis, Nippostrongylus brasiliensis and Hymenolepis diminuta.86–90 The importance of mucus for clearance has also been shown in a mouse infected with Trichuris muris; this species is closely related to Trichuris trichuria, which infects the human colon.91,92 Protection is mediated by TH2 cytokines with microRNA as a regulator.93,94
The parasite Entamoeba histolytica is of particular as this large organism not only reaches but is also able to penetrate the colonic epithelium.95 Studies of its virulence factors have revealed that the parasite first uses a lectin-like adhesin to attach to the mucus, probably onto the outer side of the inner mucus layer. This adhesion triggers the secretion of a protease—specific to E. histolytica—that is capable of cleaving MUC2 at one of its most vulnerable positions (that lacks cysteine disulfide bond crosslinks).96,97 This mechanism means that this particular enzyme can dissolve the inner mucus layer, which is a necessary second step for invading the host. However, this vulnerable spot in MUC2 is not totally unprotected as there are glycosylation sites in the vicinity of the cleavage site. Glycosylation can block cleavage, but it seems that these sites are not always fully utilized (van der Post, personal communication). These glycosylation sites could be one explanation for the observation that only a minority of all patients infected with E. histolytica actually get invasive disease. It is possible that other microorganisms might also have developed clever methods to circumvent the protective function of the inner colon mucus layer formed by MUC2.
Colonic ischaemia and compound exocytosis
Ischemia of the colon can be caused by disease or during surgery. Colonic ischemia has a more favourable outcome than ischemia of the small intestine.98,99 The reason for this difference has been addressed;100 1 h of colonic ischemia led to detachment of the inner mucus, enabling bacteria to reach the epithelium and penetrate into the normally sterile crypts. However, when the tissue was reperfused, the crypt goblet cells released their stored mucin granulae and the mucus cleaned the crypts of bacteria and restored a functional mucus layer (Figure 3). Goblet cells thus seem to tolerate ischemia relatively well and when circulation is restored, they release their mucins by so-called compound exocytosis.101 This mode of mass release can also be induced by other mechanisms, for example by acetylcholine.2,102 The mass release of mucins is in line with the fact that colon crypt goblet cells accumulate large amounts of MUC2. These mucin granulae have a slower turnover, about 6–8 h compared to about 3 h for surface goblet cells, and normally do not release their contents until the cells have reached the crypt opening.54 These observations suggest that crypt goblet cells are charged with large amounts of mucus available for release in the crypts when exposed to hazards. After ischaemia–reperfusion, the crypt goblet cells are totally emptied and restoration of the mucin granulae is slow.100 Repetitive challenges with short intervals will thus be difficult to handle for the colonic epithelium, with implications for colonic surgery.100,103
Ulcerative colitis
Ulcerative colitis, a form of IBD, is restricted to the colon and is associated with commensal microbiota. The current concept is that contact of bacteria and bacterial antigens with the lamina propria immune cells triggers and drives an inflammatory immune reaction.104 The vast majority of research into the pathophysiology of ulcerative colitis has concentrated on the immunological response and not many papers have addressed the alternative hypothesis of a defective inner mucus layer leading to increased bacterial contact with the epithelium.105 This idea is actually supported by the observation that mice lacking MUC2 develop severe colitis and eventually colon cancer.50,106,107 In mice without MUC2, there is no mucus layer at all and bacteria are in direct contact with epithelial cells. Bacteria are also found deep in the crypts and inside the epithelial cells of these animals, something that is never seen in healthy animals.50 Other mouse models with defective mucus all develop colitis.108–110 It is, therefore, conceivable that the main problem in at least some patients with ulcerative colitis is a defective mucus layer rather than a hyperactive immune system (Figure 4).
Figure 4.
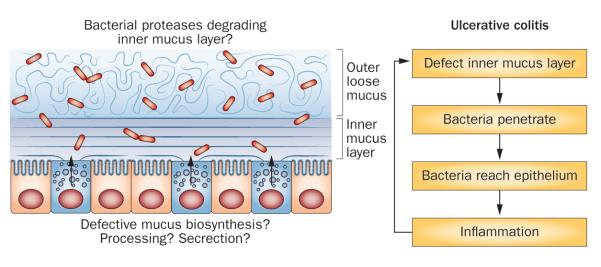
Model of mechanisms that could affect the inner mucus layer properties and potentially cause ulcerative colitis. The inner mucus layer normally separates the bacteria from the epithelial cells. If this inner mucus layer is absent or penetrable to bacteria, the load of bacteria on the epithelium increases, which will trigger an immune reaction by the subepithelial immune system.
The most common animal model for ulcerative colitis in mice or rats is to administer dextran sodium sulphate (DSS) in their drinking water. Normally, the animals develop inflammation after 3–5 days; it has been shown that the inner mucus layer, in both mouse and human colon, becomes penetrable to bacteria more or less directly after exposure to DSS.111 This enables bacteria to reach the epithelial cells. The observation that DSS increases the penetrability of the inner mucus layer to bacteria and beads the size of bacteria without changing its thickness shows that mucus properties can be altered. We have observed that several mouse models with defective ion transport (NHE3; sodium–hydrogen exchanger 3) or cytokine (for example, IL-10) production have seemingly intact mucus that turns out to be penetrable to bacteria.112 This phenomenon has also been observed in patients with active ulcerative colitis.112 Moreover, in the proximal colon at least, major changes in the epithelial reactivity to secretagogues are seen in patients with ulcerative colitis in stable remission.113 Together, these findings imply that the inner mucus layer is a dynamic system that can be affected by changes in local ion concentrations, thus participating in complex and poorly understood crosstalk with mucosal ion transport systems.
A number of other animal models that develop spontaneous colitis and ulcerative-colitis-like symptoms exist. A single unifying mechanism for these models does not exist, and it is likely that a number of defects lead to colonic inflammation. Of these models, several can be linked to dysfunction of the mucus system. The observation that mice that lack the master regulator XBP-1 of endoplasmic reticulum stress develop colitis has triggered a lot of interest.114 Given that the endoplasmic reticulum stress system is linked to inflammation, this connection is possible. Two mouse models that develop spontaneous colitis were shown to have mutations in MUC2 that caused misfolding and accumulation of this protein in the endoplasmic reticulum, something that triggers endoplasmic reticulum stress.108,115 AGR2 is assumed to be an endoplasmic reticulum resident disulfide isomerase that is essential for proper MUC2 production and its deletion also causes colitis.116,117 Together, the available information suggests that several components of the secretory pathway are important for colonic homeostasis, although the mechanisms are not clarified. One should remember that because of its high number of disulfide bonds and high degree of glycosylation, MUC2 is one of the most complex proteins synthesized in the body. Increased demand, as a result of aggressive bacteria for example, might ‘push’ this complex synthesis, leading to release of suboptimally assembled MUC2 and generation of a low-quality inner mucus layer (Figure 4). However, the colonic mucus system and layers also contain a number of other components that contribute to the mucus properties in as-yet undetermined ways.
Conclusions
The gastrointestinal mucus system is the first line of defence against bacteria, but its organization varies along the tract. The small intestinal mucus layer, with its antibacterial peptides and proteins, is freely transported distally together with trapped bacteria. These disarmed bacteria are then cleared by motor activity and fluid secretion. The colon handles its large bacterial load with a two-layered mucus system, in which the inner layer normally remains impenetrable to bacteria. Defective function of the inner colonic mucus layer might be a previously unexplored pathophysiological mechanism in ulcerative colitis and infectious diseases, knowledge that could lead to novel therapeutic principles.
Key points.
Gastrointestinal mucus is the first line of defence against bacteria
The mucus layer in the small intestine is freely movable and carries bacteria distally
In cystic fibrosis, the small intestinal mucus is not freely movable, which might explain the intestinal symptoms of this disease
The colon handles its large bacterial load with a two-layered mucus system, in which the inner layer normally remains impenetrable to bacteria.
Defective functioning of the inner mucus layer of the colon might be a pathophysiological mechanism for colitis and infectious diseases
Acknowledgments
This work was supported by the Swedish Research Council, The Swedish Cancer Foundation, The Knut and Alice Wallenberg Foundation, IngaBritt and Arne Lundberg Foundation, Sahlgren’s University Hospital (LUA-ALF), Wilhelm and Martina Lundgren’s Foundation, Torsten och Ragnar Söderbergs Stiftelser, The Sahlgrenska Academy, National Institute of Allergy and Infectious Diseases (U01AI095473, the content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH), and The Swedish Foundation for Strategic Research - The Mucus-Bacteria-Colitis Center (MBC) of the Innate Immunity Program.
Footnotes
Author contributions All authors contributed to all aspects of the manuscript.
Competing interests The authors declare no competing interests
Review criteria We searched our in-house database of 9,600 references for suitable articles. In addition we searched PubMed using the following terms “mucin”, “mucus”, “gastrointestinal tract”, “intestine”, “colon” and “colitis”. We searched for articles dating back to the time of the cloning of the mucin genes in the 1990s.
References
- 1.Kim YS, HO SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr. Gastroenterol. Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neutra MR, O’Malley LJ, Specian RD. Regulation of intestinal goblet cell secretion. II. A survey of potentid secretugogues. Am. J. Physiol. Gastroint. Liver Physiol. 1982;242:G380–G387. doi: 10.1152/ajpgi.1982.242.4.G380. [DOI] [PubMed] [Google Scholar]
- 3.Ito S. Structure and function of the glycocalyx. Fed. Proc. 1969;28:12–25. [PubMed] [Google Scholar]
- 4.Soergel KH, Ingelfinger FJ. Composition of rectal mucus in normal subjects and patients with ulcerative colitis. Gastroenterology. 1964;47:610–616. [PubMed] [Google Scholar]
- 5.Rhodes JM. Colonic mucus and mucosal glycoproteins: the key to colitis and cancer? Gut. 1989;30:1660–1666. doi: 10.1136/gut.30.12.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollingsworth MA, Swanson BJ. Mucin in cancer: protection and control of the cell surface. Nat. Rev. Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 7.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Ann. Rev. Physiol. 2008;70:431–457. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 8.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Ann. Rev. Physiol. 2008;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 9.McGuckin MA, Linden SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011;9:265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 10.Johansson MEV, et al. Composition and functional role of the mucus layers in the intestine. Cell Mol. Life Sci. 2011;68:3535–3641. doi: 10.1007/s00018-011-0822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang T, Hansson GC, Samuelsson T. Gel-forming mucins appeared early in metazoan evolution. Proc. Natl Acad. Sci. USA. 2007;104:16209–16214. doi: 10.1073/pnas.0705984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowler J, Vinall L, Swallow D. Polymorphism of the human MUC genes. Front. Biosci. 2001;6:D1207–D1215. doi: 10.2741/A674. [DOI] [PubMed] [Google Scholar]
- 13.Gum JR, Hicks JW, Toribara NW, Siddiki B, Kim YS. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J. Biol. Chem. 1994;269:2440–2446. [PubMed] [Google Scholar]
- 14.Bennett EP, et al. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012;22:736–756. doi: 10.1093/glycob/cwr182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen PH, Kolarich D, Packer NH. Mucin-type O-glycosylation—putting the pieces together. FEBS J. 2010;277:81–94. doi: 10.1111/j.1742-4658.2009.07429.x. [DOI] [PubMed] [Google Scholar]
- 16.Larsson JM, Karlsson H, Sjovall H, Hansson GC. A complex, but uniform O-glycosylation of the human MUC2 mucin from colonic biopsies analyzed by nanoLC/MSn. Glycobiology. 2009;19:756–766. doi: 10.1093/glycob/cwp048. [DOI] [PubMed] [Google Scholar]
- 17.Ambort D, et al. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc. Natl Acad. Sci. USA. 2012;109:5645–5650. doi: 10.1073/pnas.1120269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ligtenberg MJL, et al. Cell-associated episialin is a complex containing two proteins derived from a common precursor. J. Biol. Chem. 1992;267:6171–6177. [PubMed] [Google Scholar]
- 19.Palmai-Pallag T, et al. The role of the SEA (sea urchin sperm protein, enterokinase and agrin) module in cleavage of membrane-tethered mucins. FEBS J. 2005;272:2901–2911. doi: 10.1111/j.1742-4658.2005.04711.x. [DOI] [PubMed] [Google Scholar]
- 20.Levitin F, et al. The MUC1 SEA module is a self-cleaving domain. J. Biol. Chem. 2005;280:33374–33386. doi: 10.1074/jbc.M506047200. [DOI] [PubMed] [Google Scholar]
- 21.Macao B, Johansson DGA, Hansson GC, Härd T. Auto-proteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin. Nat. Struct. Mol. Biol. 2006;13:71–76. doi: 10.1038/nsmb1035. [DOI] [PubMed] [Google Scholar]
- 22.Pelaseyed T, et al. Unfolding dynamics of the mucin SEA domain probed by force spectroscopy suggest that it acts as a cell protective device. FEBS J. doi: 10.1111/febs.12144. http://dx.doi.org/10.1111/febs.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soto P, Zhang J, Carraway KL. Enzymatic cleavage as a processing step in the maturation of Muc4/sialomucin complex. J. Cell. Biochem. 2006;97:1267–1274. doi: 10.1002/jcb.20718. [DOI] [PubMed] [Google Scholar]
- 24.Lidell ME, Hansson GC. Cleavage in the GDPH sequence of the C-terminal cysteine-rich part of the human MUC5AC mucin. Biochem. J. 2006;399:121–129. doi: 10.1042/BJ20060443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss AA, Babyatsky MW, Ogata S, Chen A, Itzkowitz SH. Expression of MUC2 and MUC3 mRNA in human normal, malignant, and inflammatory intestinal tissues. J. Histochem. Cytochem. 1996;44:1161–1166. doi: 10.1177/44.10.8813081. [DOI] [PubMed] [Google Scholar]
- 26.Williams SJ, et al. Two novel mucin genes down-regulated in colorectal cancer identified by differential display. Cancer Res. 1999;59:4083–4089. [PubMed] [Google Scholar]
- 27.Williams SJ, et al. MUC13, a novel human cell surface mucin expressed by epithelial and hemopoietic cells. J. Biol. Chem. 2001;276:18327–18336. doi: 10.1074/jbc.M008850200. [DOI] [PubMed] [Google Scholar]
- 28.Button B, et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337:937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linden SK, et al. MUC1 limits helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy. PLoS Pathog. 2009;5:e1000617. doi: 10.1371/journal.ppat.1000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gendler S. Muc1, The renaissance molecule. J. Mammary Gland Biol. Neoplasia. 2001;6:339–353. doi: 10.1023/a:1011379725811. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto M, Bharti A, Li Y, Kufe D. Interaction of the DF3/MUC1 breast carcinoma-associated antigen and beta-catenin in cell adhesion. J. Biol. Chem. 1997;272:12492–12494. doi: 10.1074/jbc.272.19.12492. [DOI] [PubMed] [Google Scholar]
- 32.Wen YF, Caffrey TC, Wheelock MJ, Johnson KR, Hollingsworth MA. Nuclear association of the cytoplasmic tail of MUC1 and beta-catenin. J. Biol. Chem. 2003;278:38029–38039. doi: 10.1074/jbc.M304333200. [DOI] [PubMed] [Google Scholar]
- 33.Schroeder JC, et al. MUC1 overexpression results in mammary gland tumorigenesis and prolonged alveolar differentiation. Oncogene. 2004;23:5739–5747. doi: 10.1038/sj.onc.1207713. [DOI] [PubMed] [Google Scholar]
- 34.Malmberg EK, et al. The transmembrane MUC17 mucin C-terminus binds to the scaffold protein PDZK1 that stably localizes it to the enterocyte apical membrane in the small intestine. Biochem. J. 2008;410:283–289. doi: 10.1042/BJ20071068. [DOI] [PubMed] [Google Scholar]
- 35.LaLonde DP, Garbett D, Bretscher AA. Regulated complex of the scaffolding proteins PDZK1 and EBP50 with ezrin contribute to microvillar organization. Mol. Biol. Cell. 2010;21:1519–1529. doi: 10.1091/mbc.E10-01-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donowitz M, Li X. Regulatory binding partners and complexes of NHE3. Physiol. Rev. 2007;87:825–872. doi: 10.1152/physrev.00030.2006. [DOI] [PubMed] [Google Scholar]
- 37.Singh PK, Hollingsworth MA. Cell surface-associated mucins in signal transduction. Trends Cell Biol. 2006;16:467–476. doi: 10.1016/j.tcb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Caldara M, et al. Mucin biopolymers prevent bacterial aggregation by retaining cells in the free-swimming state. Curr. Biol. 2012;22:2325–2330. doi: 10.1016/j.cub.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atuma C, Strugula V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol. 2001;280:G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 40.Khan SH, Aguirre A, Bobek LA. In-situ hybridization localized MUC7 mucin gene expression to the mucous acinar cells of human and MUC7-transgenic mouse salivary glands. Glycoconj. J. 1998;15:1125–1132. doi: 10.1023/a:1006955604501. [DOI] [PubMed] [Google Scholar]
- 41.Bobek LA, Tsai H, Biesbrock AR, Levine MJ. Molecular cloning, sequence, and specificity of expression of the gene encoding the low molecular weight human salivary mucin (MUC7) J. Biol. Chem. 1993;268:20563–20569. [PubMed] [Google Scholar]
- 42.Nielsen PA, Mandel U, Therkildsen MH, Clausen H. Differential expression of human high-molecular-weight salivary mucin (MG1) and low-molecular-weight salivary mucin (MG2) J. Dental Res. 1996;75:1820–1826. doi: 10.1177/00220345960750110201. [DOI] [PubMed] [Google Scholar]
- 43.Thornton DJ, et al. Salivary mucin MG1 is comprised almost entirely of different glycosylated forms of the MUC5B gene product. Glycobiology. 1999;9:293–302. doi: 10.1093/glycob/9.3.293. [DOI] [PubMed] [Google Scholar]
- 44.Wickstrom C, Davies JR, Eriksen GV, Veerman ECI, Carlstedt I. MUC5B is a major gel-forming, oligomeric mucin from human salivary gland, respiratory tract and endocervix: identification of glycoforms and C-terminal cleavage. Biochem. J. 1998;334:685–693. doi: 10.1042/bj3340685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.HO SB, et al. Expression cloning of gastric mucin complementary DNA and localization of mucin gene expression. Gastroenterology. 1995;109:735–747. doi: 10.1016/0016-5085(95)90380-1. [DOI] [PubMed] [Google Scholar]
- 46.Nordman H, et al. Gastric MUC5AC and MUC6 are large oligomeric mucins that differ in size, glycosylation and tissue distribution. Biochem. J. 2002;364:191–200. doi: 10.1042/bj3640191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Debolos C, Garrido M, Real FX. MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology. 1995;109:723–734. doi: 10.1016/0016-5085(95)90379-8. [DOI] [PubMed] [Google Scholar]
- 48.Bartman AE, et al. The MUC6 secretory mucin genets expressed in a wide variety of epithelial tissues. J. Pathol. 1998;186:398–405. doi: 10.1002/(SICI)1096-9896(199812)186:4<398::AID-PATH192>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 49.Audie JP, et al. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J. Histochem. Cytochem. 1993;41:1479–1485. doi: 10.1177/41.10.8245407. [DOI] [PubMed] [Google Scholar]
- 50.Johansson MEV, et al. The inner of the two Muc2 mucin dependent mucus layers in colon is devoid of bacteria. Proc. Natl Acad. Sci. USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Godl K, et al. The N-termini of the MUC2 mucin form trimers that are held together within a trypsin-resistant core fragment. J. Biol. Chem. 2002;277:47248–47256. doi: 10.1074/jbc.M208483200. [DOI] [PubMed] [Google Scholar]
- 52.Lidell ME, et al. The recombinant C-terminus of the human MUC2 mucin forms dimers in CHO cells and heterodimers with full-length MUC2 in LS 174T cells. Biochem. J. 2003;372:335–345. doi: 10.1042/BJ20030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Round AN, et al. Lamellar structures of MUC2-rich mucin: a potential role in governing the barrier and lubricating functions of intestinal mucus. Biomacromolecules. 2012;13:3253–3261. doi: 10.1021/bm301024x. [DOI] [PubMed] [Google Scholar]
- 54.Johansson MEV. Fast renewal of the distal colonic mucus layers by the surface goblet cells as measured by in vivo labeling of mucin glycoproteins. PLoS ONE. 2012;7:e41009. doi: 10.1371/journal.pone.0041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schade C, Flemstrom G, Holm L. Hydrogen ion concentration in the mucus layer on top of acid-stimulated and -inhibited rat gastric mucosa. Gastroenterology. 1994;107:180–188. doi: 10.1016/0016-5085(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 56.Phillipson M, Atuma C, Henriksnas J, Holm L. The importance of mucus layers and bicarbonate transport in preservation of gastric juxtamucosal pH. Am. J. Physiol. 2002;282:G211–G219. doi: 10.1152/ajpgi.00223.2001. [DOI] [PubMed] [Google Scholar]
- 57.Allen A, Flemstrom G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am. J. Physiol. Cell Physiol. 2005;288:C1–C19. doi: 10.1152/ajpcell.00102.2004. [DOI] [PubMed] [Google Scholar]
- 58.Bhaskar KR, et al. Viscous fingering of HCl through gastric mucin. Nature. 1992;360:458–461. doi: 10.1038/360458a0. [DOI] [PubMed] [Google Scholar]
- 59.Johansson M, Synnerstad I, Holm L. Acid transport through channels in the mucous layer of rat stomach. Gastroenterology. 2000;119:1297–1304. doi: 10.1053/gast.2000.19455. [DOI] [PubMed] [Google Scholar]
- 60.HO SB, et al. The adherent gastric mucous layer is composed of alternating layers of MUC5AC and MUC6 mucin proteins. Dig. Dis. Sci. 2004;49:1598–1606. doi: 10.1023/b:ddas.0000043371.12671.98. [DOI] [PubMed] [Google Scholar]
- 61.Phillipson M, et al. The gastric mucus layers: constituents and regulation of accumulation. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G806–G812. doi: 10.1152/ajpgi.90252.2008. [DOI] [PubMed] [Google Scholar]
- 62.Baxter PS, et al. Abnormal jejunal potential difference in cystic fibrosis. Lancet. 1989;333:464–466. doi: 10.1016/s0140-6736(89)91366-4. [DOI] [PubMed] [Google Scholar]
- 63.Deloose E, Janssen P, Depoortere I, Tack J. The migrating motor complex: control mechanisms and its role in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2012;9:271–285. doi: 10.1038/nrgastro.2012.57. [DOI] [PubMed] [Google Scholar]
- 64.Ouellette AJ. Paneth cells and innate mucosal immunity. Curr. Opin. Gastroenterol. 2010;26:547–553. doi: 10.1097/MOG.0b013e32833dccde. [DOI] [PubMed] [Google Scholar]
- 65.Vaishnava S, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chu H, et al. Human defensin 6 promotes mucosal innate immunity through self-assembled peptide nanonets. Science. 2012;337:477–481. doi: 10.1126/science.1218831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johansson MEV, Hansson GC. Keeping bacteria at a distance. Science. 2011;334:182–183. doi: 10.1126/science.1213909. [DOI] [PubMed] [Google Scholar]
- 68.Rosenstiel P, et al. Regulation of DMBT1 via NOD2 and TLR4 in intestinal epithelial cells modulates bacterial recognition and invasion. J. Immunol. 2007;178:8203–8211. doi: 10.4049/jimmunol.178.12.8203. [DOI] [PubMed] [Google Scholar]
- 69.Riordan JR. CFTR function and prospects for therapy. Annu. Rev. Biochem. 2008;77:701–726. doi: 10.1146/annurev.biochem.75.103004.142532. [DOI] [PubMed] [Google Scholar]
- 70.O’Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009;373:1891–1904. doi: 10.1016/S0140-6736(09)60327-5. [DOI] [PubMed] [Google Scholar]
- 71.Houwen RH, et al. Defining DIOS and constipation in cystic fibrosis with a multicentre study on the incidence, characteristics, and treatment of DIOS. J. Pediatr. Gastroenterol. Nutr. 2010;50:38–42. doi: 10.1097/MPG.0b013e3181a6e01d. [DOI] [PubMed] [Google Scholar]
- 72.Grubb BR, Gabriel SE. Intestinal physiology and pathology in gene-targeted mouse models of cystic fibrosis. Am. J. Physiol. 1997;273:G258–G266. doi: 10.1152/ajpgi.1997.273.2.G258. [DOI] [PubMed] [Google Scholar]
- 73.French PJ, et al. A Delta F508 mutation in mouse cystic fibrosis transmembrane conductance regulator results in a temperature-sensitive processing defect in vivo. J. Clin. Invest. 1996;98:1304–1312. doi: 10.1172/JCI118917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rogers CS, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gustafsson JK, et al. Bicarbonate and functional CFTR channel is required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J. Exp. Med. 2012;209:1263–1272. doi: 10.1084/jem.20120562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Lisle RC, Roach E, Jansson K. Effects of laxative and N-acetylcysteine on mucus accumulation, bacterial load, transit, and inflammation in the cystic fibrosis mouse small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G577–G584. doi: 10.1152/ajpgi.00195.2007. [DOI] [PubMed] [Google Scholar]
- 77.Fridge JL, Conrad C, Gerson L, Castillo RO, Cox K. Risk factors for small bowel bacterial overgrowth in cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 2007;44:212–218. doi: 10.1097/MPG.0b013e31802c0ceb. [DOI] [PubMed] [Google Scholar]
- 78.Malmberg EK, et al. Increased levels of mucins in the cystic fibrosis mouse small intestine and modulator effects of the Muc1 mucin expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G203–G210. doi: 10.1152/ajpgi.00491.2005. [DOI] [PubMed] [Google Scholar]
- 79.Delisle RC, Roach EA, Norkina O. Eradicaion of small intestinal bacterial overgrowth in the cystic fibrosis mouse reduces mucus accumulation. J. Pediatr. Gastroenterol. Nutr. 2006;42:46–52. doi: 10.1097/01.mpg.0000189322.34582.3e. [DOI] [PubMed] [Google Scholar]
- 80.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host–bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 81.Sonnenburg JL, et al. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307:1955–1959. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 82.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 83.Rawls JF, Mahowald MA, Ley RE, Gordon JI. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell. 2006;127:423–433. doi: 10.1016/j.cell.2006.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bergstrom KSB, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6:e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moore ME, Boren T, Solnick JV. Life at the margins: modulation of attachment proteins in Helicobacter pylori. Gut Microbes. 2011;2:42–46. doi: 10.4161/gmic.2.1.14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Else KJ, Finkelman FD. Intestinal nematode parasites, cytokines and effector mechanisms. Int. J. Parasitol. 1998;28:1145–1158. doi: 10.1016/s0020-7519(98)00087-3. [DOI] [PubMed] [Google Scholar]
- 87.Ishikawa N, Horii Y, Nawa Y. Immune-mediated alteration of the terminal sugars of goblet cell mucins in the small intestine of Nippostrongylus brasiliensis-infected rats. Immunology. 1993;78:303–307. [PMC free article] [PubMed] [Google Scholar]
- 88.Ishikawa N, Wakelin D, Mahida YR. Role of T helper 2 cells in intestinal goblet cell hyperplasia in mice infected with Trichinella spiralis. Gastroenterology. 1997;113:542–549. doi: 10.1053/gast.1997.v113.pm9247474. [DOI] [PubMed] [Google Scholar]
- 89.Koninkx JF, Mirck MH, Hendriks HG, Mouwen JM, van Dijk JE. Nippostrongylus brasiliensis: histochemical changes in the composition of mucins in goblet cells during infection in rats. Exp. Parasitol. 1988;65:84–90. doi: 10.1016/0014-4894(88)90109-9. [DOI] [PubMed] [Google Scholar]
- 90.McKay DM, et al. Hymenolepis diminuta: intestinal goblet cell response to infection in male C57 mice. Exp. Parasitol. 1990;71:9–20. doi: 10.1016/0014-4894(90)90003-u. [DOI] [PubMed] [Google Scholar]
- 91.Hasnain SZ, et al. Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology. 2010;138:1763–1771. doi: 10.1053/j.gastro.2010.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hasnain SZ, et al. Muc5ac: a critical component mediating the rejection of enteric nematodes. J Exp. Med. 2011;208:893–900. doi: 10.1084/jem.20102057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Finkelman FD, et al. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol. Rev. 2004;201:139–155. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 94.Biton M, et al. Epithelial microRNAs regulate gut mucosal immunity via epithelium-T cell crosstalk. Nat. Immunol. 2011;12:239–246. doi: 10.1038/ni.1994. [DOI] [PubMed] [Google Scholar]
- 95.Petri WA, Haque R, Mann BJ. The bittersweet interface of parasite and host: lectin-carbohydrate interactions during human invasion by the parasite Entamoeba histolytica. Annu. Rev. Microbiol. 2002;56:39–64. doi: 10.1146/annurev.micro.56.012302.160959. [DOI] [PubMed] [Google Scholar]
- 96.Moncada D, Keller K, Chadee K. Entamoeba histolytica cysteine proteinases disrupt the polymeric structure of colonic mucin and alter its protective function. Infec. Immun. 2003;71:838–844. doi: 10.1128/IAI.71.2.838-844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lidell ME, Moncada DM, Chadee K, Hansson GC. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal part and dissolves the protective colonic mucus gel. Proc. Natl Acad. Sci. USA. 2006;103:9298–9393. doi: 10.1073/pnas.0600623103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brandt LJ, Feuerstadt P, Blaszka MC. Anatomic patterns, patient characteristics, and clinical outcomes in ischemic colitis: a study of 313 cases supported by histology. Am. J. Gastroenterol. 2010;105:2245–2252. doi: 10.1038/ajg.2010.217. [DOI] [PubMed] [Google Scholar]
- 99.[No authors listed] American Gastroenterological Association Medical Position Statement: Guidelines on intestinal ischemia. Gastroenterology. 2000;118:951–953. doi: 10.1016/s0016-5085(00)70182-x. [DOI] [PubMed] [Google Scholar]
- 100.Grootjans J, et al. Ischemia-induced mucus barrier loss and bacterial penetration are rapidly counteracted by increased goblet cell secretory activity in human and rat colon. Gut. 2013;62:259–258. doi: 10.1136/gutjnl-2011-301956. [DOI] [PubMed] [Google Scholar]
- 101.Specian D, Neutra MR. Mechanism of rapid mucus secretion in goblet cells stimulated by acetylcholine. J. Cell Biol. 1980;85:626–640. doi: 10.1083/jcb.85.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Plaisancie P, et al. Effects of neurotransmitters, gut hormones, and inflammatory mediators on mucus discharge in rat colon. Am. J. Physiol. 1998;275:G1073–G1084. doi: 10.1152/ajpgi.1998.275.5.G1073. [DOI] [PubMed] [Google Scholar]
- 103.Johansson MEV, Hansson GC. The goblet cell: a key player in ischemia–reperfusion injury. Gut. 2012;62:188–189. doi: 10.1136/gutjnl-2012-302582. [DOI] [PubMed] [Google Scholar]
- 104.Ordas I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. The Lancet. 2012;380:1609–1619. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 105.Swidsinski A, et al. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut. 2007;56:343–350. doi: 10.1136/gut.2006.098160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Velcich A, et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 107.Van der Sluis M, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 108.Heazlewood CK, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dawson PA, et al. Impaired intestinal function in the hyposulphataemic NaS1 null mouse. Gut. 2009;58:910–919. doi: 10.1136/gut.2007.147595. [DOI] [PubMed] [Google Scholar]
- 110.Fu J, et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis. J. Clin. Invest. 2011;121:1657–1666. doi: 10.1172/JCI45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Johansson MEV, et al. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS ONE. 2010;5:e12238. doi: 10.1371/journal.pone.0012238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Johansson MEV, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and in patients with ulcerative colitis. Gut. doi: 10.1136/gutjnl-2012-303207. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gustafsson JK, Hansson GC, Sjovall H. Ulcerative colitis patients in remission have an altered secretory capacity in the proximal colon despite macroscopically normal mucosa. Neurogastroenterol. Motil. 2012;24:e381–e391. doi: 10.1111/j.1365-2982.2012.01958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaser A, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eri RD, et al. An intestinal epithelial defect conferring ER stress results in inflammation involving both innate and adaptive immunity. Mucosal Immunol. 2011;4:354–364. doi: 10.1038/mi.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Park SW, et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc. Natl Acad. Sci. USA. 2009;106:6950–6955. doi: 10.1073/pnas.0808722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao F, et al. Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2−/− mice. Develop. Biol. 2010;338:270–279. doi: 10.1016/j.ydbio.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lan MS, Batra SK, Qi W, Metzgar RS, Hollingsworth MA. Cloning and sequencing of a human pancreatic tumor mucin cDNA. J. Biol. Chem. 1990;265:15294–15299. [PubMed] [Google Scholar]
- 119.Ligtenberg MJL, Vos HL, Gennissen AMC, Hilkens J. Episialin, a carcinoma-associated mucin, is generated by a polymorphic gene encoding splice variants with alternative amino termini. J. Biol. Chem. 1990;265:5573–5578. [PubMed] [Google Scholar]
- 120.Gendler SJ, et al. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J. Biol. Chem. 1990;265:15286–15293. [PubMed] [Google Scholar]
- 121.Lancaster CA, et al. Structure and expression of the human polymorphic epithelial mucin gene: an expressed VNTR unit. Biochem. Biophys. Res. Commun. 1990;173:1019–1029. doi: 10.1016/s0006-291x(05)80888-5. [DOI] [PubMed] [Google Scholar]
- 122.Wreschner DH, et al. Human epithelial tumor antigen cDNA sequences. Differential splicing may generate multiple protein forms. Eur. J. Biochem. 1990;189:463–473. doi: 10.1111/j.1432-1033.1990.tb15511.x. [DOI] [PubMed] [Google Scholar]
- 123.McAuley JL, et al. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J. Clin. Invest. 2007;117:2313–2324. doi: 10.1172/JCI26705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Linden SK, Florin TH, McGuckin MA. Mucin dynamics in intestinal bacterial infection. PLoS ONE. 2008;3:e3952. doi: 10.1371/journal.pone.0003952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gum JR, et al. Molecular cloning of human intestinal mucin cDNAs sequence analysis and evidence for genetic polymorphism. J. Biol. Chem. 1989;264:6480–6487. [PubMed] [Google Scholar]
- 126.Gum JR, et al. The human MUC2 intestinal mucin has cysteine-rich subdomains located both upstream and downstream of its central repetitive region. J. Biol. Chem. 1992;267:21375–21383. [PubMed] [Google Scholar]
- 127.Gum JR, et al. Molecular cloning of cDNAs derived from a novel human intestinal mucin gene. Biochem. Biophys. Res. Commun. 1990;171:407–415. doi: 10.1016/0006-291x(90)91408-k. [DOI] [PubMed] [Google Scholar]
- 128.Gum JR, et al. MUC3 human intestinal mucin. J. Biol. Chem. 1997;272:26678–26686. doi: 10.1074/jbc.272.42.26678. [DOI] [PubMed] [Google Scholar]
- 129.Crawley SC, et al. Genomic organization and structure of the 3′region of human MUC3: alternative splicing predicts membrane-bound and soluble forms of the mucin. Biochem. Biophys. Res. Commun. 1999;263:728–736. doi: 10.1006/bbrc.1999.1466. [DOI] [PubMed] [Google Scholar]
- 130.Moniaux N, Nollet S, Degand P, Laine A, Aubert JP. Complete sequence of the human mucin MUC4: a putative cell membrane-associated mucin. Biochem. J. 1999;338:325–333. [PMC free article] [PubMed] [Google Scholar]
- 131.Escande F, et al. Genomic organization of MUC4 mucin gene. Towards the characterization of splice variants. Eur. J. Biochem. 2002;269:3637–3644. doi: 10.1046/j.1432-1033.2002.03032.x. [DOI] [PubMed] [Google Scholar]
- 132.Price-Schiavi SA, Perez A, Barco R, Carraway KL. Cloning and characterization of the 5′ flanking region of the sialomucin complex/rat Muc4 gene: promoter activity in cultured cells. Biochem. J. 2000;349:641–649. doi: 10.1042/0264-6021:3490641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rong M, et al. Expression and localization of Muc4/sialomucin complex (SMC) in the adult and developing rat intestine: implications for Muc4/SMC function. J. Cell. Physiol. 2005;202:275–284. doi: 10.1002/jcp.20121. [DOI] [PubMed] [Google Scholar]
- 134.Buisine MP, et al. Genomic organization of the 3′-region of the human MUC5AC mucin gene: additional evidence for a common ancestral gene for the 11p15.5 mucin gene family. Biochem. J. 1998;332:729–738. doi: 10.1042/bj3320729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Escande F, Aubert JP, Porchet N, Buisine MP. Human mucin gene MUC5AC: organization of its 5′-region and central repetitive region. Biochem. J. 2001;358:763–772. doi: 10.1042/0264-6021:3580763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li DZ, Gallup M, Fan N, Szymkowski DE, Basbaum CB. Cloning of the amino-terminal and 5′-flanking region of the human MUC5AC mucin gene and transcriptional up-regulation by bacterial exoproducts. J. Biol. Chem. 1998;273:6812–6820. doi: 10.1074/jbc.273.12.6812. [DOI] [PubMed] [Google Scholar]
- 137.Klomp LWJ, Vanrens L, Strous GJ. Cloning and analysis of human gastric mucin cDNA reveals two types of conserved cysteine-rich domains. Biochem. J. 1995;308:831–838. doi: 10.1042/bj3080831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Meerzaman D, et al. Cloning and analysis of cDNA encoding a major airway glycoprotein, human tracheobronchial mucin (Muc5) J. Biol. Chem. 1994;269:12932–12939. [PubMed] [Google Scholar]
- 139.Keates AC, Nunes DP, Afdhal NH, Troxler RF, Offner GD. Molecular cloning of a major human gall bladder mucin: complete C-terminal sequence and genomic organization of MUC5B. Biochem. J. 1997;324:295–303. doi: 10.1042/bj3240295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Offner GD, Nunes DP, Keates AC, Afdhal NH, Troxler RF. The amino-terminal sequence of MUC5B contains conserved multifunctional D domains: implications for tissue-specific mucin functions. Biochem. Biophys. Res. Commun. 1998;251:350–355. doi: 10.1006/bbrc.1998.9469. [DOI] [PubMed] [Google Scholar]
- 141.Troxler RF, Offner GD, Zhang F, Iontcheva I, Oppenheim FG. Molecular cloning of a novel high molecular weight mucin (MG1) from human sublingual gland. Biochem. Biophys. Res. Commun. 1995;217:1112–1119. doi: 10.1006/bbrc.1995.2884. [DOI] [PubMed] [Google Scholar]
- 142.Toribara NW, et al. Human gastric mucin. J. Biol. Chem. 1993;268:5879–5885. [PubMed] [Google Scholar]
- 143.Toribara NW, et al. The carboxyl-terminal sequence of the human secretory mucin, MUC6. J. Biol. Chem. 1997;272:16398–16403. doi: 10.1074/jbc.272.26.16398. [DOI] [PubMed] [Google Scholar]
- 144.Rousseau K, et al. The complete genomic organization of the human MUC6 and MUC2 mucin genes. Genomics. 2004;83:936–939. doi: 10.1016/j.ygeno.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 145.Situ H, Wei G, Smith CJ, Mashhoon S, Bobek LA. Human salivary MUC7 mucin peptides: effect of size, charge and cysteine residues on antifungal activity. Biochem. J. 2003;375:175–182. doi: 10.1042/BJ20030779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sheng YH, et al. The MUC13 cell-surface mucin protects against intestinal inflammation by inhibiting epithelial cell apoptosis. Gut. 2011;60:1661–1670. doi: 10.1136/gut.2011.239194. [DOI] [PubMed] [Google Scholar]
- 147.Yin BWT, Lloyd K. Molecular cloning of the CA125 ovarian cancer antigen. J. Biol. Chem. 2001;276:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 148.O’Brien TJ, Beard JB, Underwood LJ, Shigemasa K. The CA125 gene: a newly discovered extesion of the glycosylated N-terminal domain doubles the size of this extracellular superstructure. Tumor Biol. 2004;23:154–169. doi: 10.1159/000064032. [DOI] [PubMed] [Google Scholar]
- 149.Gum JR, Crawley SC, Hicks JW, Szymkowski DE, Kim YS. MUC17, a novel membrane-tethered mucin. Biochem. Biophys. Res. Commun. 2002;291:466–475. doi: 10.1006/bbrc.2002.6475. [DOI] [PubMed] [Google Scholar]
- 150.Pratt WS, et al. Multiple transcripts of MUC3: evidence for two genes MUC3A and MUC3B. Biochem. Biophys. Res. Commun. 2000;275:916–923. doi: 10.1006/bbrc.2000.3406. [DOI] [PubMed] [Google Scholar]
- 151.Pullan RD, et al. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut. 1994;35:353–359. doi: 10.1136/gut.35.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Debailleul V, et al. Human mucin genes MUC2, MUC3, MUC4, MUC5AC, MUC5B, and MUC6 express stable and extremely large mRNAs and exhibit a variable length polymorphism. J. Biol. Chem. 1998;273:881–890. doi: 10.1074/jbc.273.2.881. [DOI] [PubMed] [Google Scholar]
- 153.Ogata S, Uehara H, Chen A, Itzkowitz SH. Mucin gene expression in colonic tissues and cell lines. Cancer Res. 1992;52:5971–5978. [PubMed] [Google Scholar]
- 154.Vanklinken BJW, et al. MUC5B is the prominent mucin in human gallbladder and is also expressed in a subset of colonic goblet cells. Am. J. Physiol. 1998;37:G871–G878. doi: 10.1152/ajpgi.1998.274.5.G871. [DOI] [PubMed] [Google Scholar]
- 155.Peat N, Gendler SJ, Lalani N, Duhig T, Taylor-Papadimitriou J. Tissue-specific expression of a human polymorphic epithelial mucin (MUC1) in transgenic mice. Cancer Res. 1992;52:1954–1960. [PubMed] [Google Scholar]
- 156.Zhang J, Yasin M, Carraway CA, Carraway KL. MUC4 expression and localization in gastrointestinal tract and skin of human embryos. Tissue Cell. 2006;38:271–275. doi: 10.1016/j.tice.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 157.Gonzalez-Begne M, et al. Proteomic analysis of human parotid gland exosomes by multidimensional protein identification technology (MudPIT) J. Proteome Res. 2009;8:1304–1314. doi: 10.1021/pr800658c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Senapati S, et al. Expression of intestinal MUC17 membrane-bound mucin in inflammatory and neoplastic diseases of the colon. J. Clin. Pathology. 2010;63:702–707. doi: 10.1136/jcp.2010.078717. [DOI] [PMC free article] [PubMed] [Google Scholar]