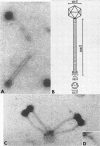
Abstract
Temperate Bacillus phage φ105 is serologically unrelated to previously described virulent Bacillus phages. Phage φ105 is incapable of generalized transduction. Prophage φ105 is inducible with mitomycin C. Phage φ105 contains double-stranded deoxyribonucleic acid (DNA) with a molecular weight of about 25 × 107 as determined by band sedimentation and electron microscopy. The per cent guanine plus cytosine of φ105 DNA is 43.5 as determined by buoyant density in CsCl and by thermal denaturation. Phage φ105 DNA may contain complementary single-stranded ends.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G., NICE S. J. Studies on lysogenesis. II. The effect of temperature on the lysogenization of Shigella dysenteriae with phage P1. J Bacteriol. 1954 Feb;67(2):202–209. doi: 10.1128/jb.67.2.202-209.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin R. L., Barrand P., Fritsch A., Goldthwait D. A., Jacob F. Cohesive sites on the deoxyribonucleic acids from several temperate coliphages. J Mol Biol. 1966 Jun;17(2):343–357. doi: 10.1016/s0022-2836(66)80146-8. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey A. D., Burgi E., Ingraham L. COHESION OF DNA MOLECULES ISOLATED FROM PHAGE LAMBDA. Proc Natl Acad Sci U S A. 1963 May;49(5):748–755. doi: 10.1073/pnas.49.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMERS K., PUTNEY F., STEINBERG I. Z., SCHACHMAN H. K. ULTRACENTRIFUGE STUDIES WITH ABSORPTION OPTICS. 3. A SPLIT-BEAM PHOTOELECTRIC, SCANNING ABSORPTION SYSTEM. Arch Biochem Biophys. 1963 Dec;103:379–400. doi: 10.1016/0003-9861(63)90428-4. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Meselson M., Stahl F. W., Vinograd J. EQUILIBRIUM SEDIMENTATION OF MACROMOLECULES IN DENSITY GRADIENTS. Proc Natl Acad Sci U S A. 1957 Jul 15;43(7):581–588. doi: 10.1073/pnas.43.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Mudd J. A., Mangan J., Huang W. M., Subbaiah T. V., Marmur J. Properties of the defective phage of Bacillus subtilis. J Mol Biol. 1968 Jun 28;34(3):413–428. doi: 10.1016/0022-2836(68)90169-1. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Mudd J. A., Mangan J., Huang W. M., Subbaiah T. V., Marmur J. Properties of the defective phage of Bacillus subtilis. J Mol Biol. 1968 Jun 28;34(3):413–428. doi: 10.1016/0022-2836(68)90169-1. [DOI] [PubMed] [Google Scholar]
- Peterson A. M., Rutberg L. Linked transformation of bacterial and prophage markers in Bacillus subtilis 168 lysogenic for bacteriophage phi 105. J Bacteriol. 1969 Jun;98(3):874–877. doi: 10.1128/jb.98.3.874-877.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades M., MacHattie L. A., Thomas C. A., Jr The P22 bacteriophage DNA molecule. I. The mature form. J Mol Biol. 1968 Oct 14;37(1):21–40. doi: 10.1016/0022-2836(68)90071-5. [DOI] [PubMed] [Google Scholar]
- Roth T. F., Helinski D. R. Evidence for circular DNA forms of a bacterial plasmid. Proc Natl Acad Sci U S A. 1967 Aug;58(2):650–657. doi: 10.1073/pnas.58.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg L., Hoch J. A., Spizizen J. Mechanism of transfection with deoxyribonucleic acid from the temperate Bacillus bacteriophage phi-105. J Virol. 1969 Jul;4(1):50–57. doi: 10.1128/jvi.4.1.50-57.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutberg L. Mapping of a temperate bacteriophage active on Bacillus subtilis. J Virol. 1969 Jan;3(1):38–44. doi: 10.1128/jvi.3.1.38-44.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Taylor M. J., Thorne C. B. Concurrent changes in transducing efficiency and content of transforming deoxyribonucleic acid in Bacillus subtilis bacteriophage SP-10. J Bacteriol. 1966 Jan;91(1):81–88. doi: 10.1128/jb.91.1.81-88.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINOGRAD J., BRUNER R., KENT R., WEIGLE J. Band-centrifugation of macromolecules and viruses in self-generating density gradients. Proc Natl Acad Sci U S A. 1963 Jun;49:902–910. doi: 10.1073/pnas.49.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]